Abstract
Objective:
The goal of the performed study was to evaluate the possibility of a three-dimensional endoscope to become a combined microscope-endoscope device in one. We analyzed the ergonomy of the device, the implementation into the surgical workflow, the image quality, and the future perspectives such devices could have for the next generation of neurosurgeons.
Materials and Methods:
Within 6 months, 22 patients (10 male, 12 female, 20-65 age) underwent surgery in neuroaxis using the new 3D-microendoscope (ME). The new 3D-ME has (a) the ability to visualize the surgical field from out- to inside with all advantages offered by a microscope, and in the same moment, (b) its design is like a small diameter endoscope that allows stereoscopic views extracorporal, intracorporal, and panoramic “para-side” of the lesion.
Results:
In general, transcranial 3D-“microendoscopy” was performed in all patients with high-resolution 3D quality. No severe complications were observed intra- or postoperatively. With the addition of depth perception, the anatomic structures were well seen and observed.
Conclusion:
The 3D-microendoscopy is a very promising surgical concept associated with new technological developments. The surgeon is able to switch to a modern visualization instrument reaching the most optimal surgical approach without compromising safety, effectiveness, and visual information.
Keywords: Endoscopy, microendoscopy, skull base surgery, three dimensionality
Introduction
The primary focus in key-hole neurosurgery is never the size of a craniotomy per se, but to evaluate individual lesions with utmost care and determine the exact position of the craniotomy so as to approach the lesion with a minimum of damage to surrounding tissue and reaching the maximum effective treatment. To reach this goal, “light and sight” is of essential importance. Over a period of 4 decades, neurosurgical treatment modalities involving the surgical microscope as the most important “light and sight” spending device in neurosurgery. As we know, operative techniques need refinement and continuous improvement. Therefore, another tool smaller, accurate spending light intracranial in front of the lesion, the endoscope, came soon to evolve the most important adjunctive visualization instrument next to the microscope in the operation theater.[1] The so-called “endoscope-assisted neurosurgical technique” was a combination of both visualization tools during a surgical procedure, respectively.[2,3,4,5,6] The perspective of different anatomical structures under those both visualization tools may appear very different. Thus, intraoperative light and optimum sight to achieve visual control are still fundamental conditions for the performance of minimally invasive, maximally effective operative procedures. This so-called “optimally invasive surgical strategy” could be reached with development and application of new miniaturized combined visualization tools with microscopic and endoscopic favorable optical characteristics in one device. We called this new instrument “micro-endoscope” and the surgical technique “microendoscopy,” because of the ability of the tool to be microscope and endoscope in the same time, in the same tool. We describe the surgical performance and our experience with such a three-dimensional miniaturized micro-endoscope (3D-ME), which could be used as a microscope,[7,8] endoscope[9,10,11,12,13] or both in one tool during a neurosurgical microendoscopic procedure, like shown in this study. Furthermore, we describe our experience with the usability, ergonomy, and advantages of this new 3D-ME used for performing optimal invasive transcranial procedures in different brain areas, like the skull base and convexity. We performed a series of endoscope-assisted intracranial neurosurgical procedures where we used the microscope or the 2D endoscope in the past as a visualization tool alone or in combination. In this study, we used the 3D-ME as the only one visualization tool.
In our knowledge, this study is the first one described a series of 3D-micro-endoscopic-assisted procedures in different areas of the brain supra-, and infratentorial using the 3D-ME as the only one visualization tool.
Materials and Methods
Patient population
Within 6 months, 22 patients (10 male, 12 female, 20-65a) underwent surgery in neuroaxis using the 3D-ME. Pathologies included were located in neurocranium (n = 20) and spine (n = 2), and were detailed analyzed in Table 1. We will here analyze and focus on our experience with the intracranial surgical performance. In detail, we performed surgery on patients having olfactory groove meningiomas (n = 4), sphenoid wing meningiomas (n = 4), tuberculum sellae meningiomas (n = 2), posterior fossa meningiomas located in tentorial edge, petroclival, and foramen magnum (n = 3), cavernomas (n = 3), epidermoid tumor (n = 1), craniopharyngioma (n = 1), and pinealis tumors (n = 2). All patients underwent within 3, 6, and 12 months their postoperative follow-up via clinical examination and MRI scan.
Table 1.
Case analysis operated by the 3D-ME device
Ethical approval for performing the study was not needed, because the instrument described here is a CE-approved instrument for neurosurgical procedures used as an endoscope during brain tumor surgeries.
“3D Micro-endoscope”- definition and technical description
As a micro-endoscope, we defined a visualization tool what offers the advantages of a microscope and an endoscope in one tool. We called this 3D tool, described here, a 3D-ME, because it has (a) the ability to visualize the surgical field from out- to inside with all advantages offered by a microscope e.g. focus, zoom, 3D view bringing the light and sight from an “outside to inside” direction above and into the surgical field, and in the same moment, (b) its shape is like an endoscope, small enough to bring light and sight from an inside, outside, and “para-side” around the lesion, direction, if the surgeon placed the tool into the surgical corridor in front of the point of interest, like an endoscope.
The new 3D-ME described here, VSII (Visionsense, USA), with its single 3-D sensor merely 3.3 mm in diameter, imitates an insect's compound eye using advanced image-processing algorithms. The resulting natural stereoscopic vision enhances neurosurgical accuracy, and the depth perception with no trade-off in magnification and focus offers distinct advantages. We used two various configurations of cameras with 3.3 mm CCD sensor located within the scope housing. The telescope length used was 200 mm, and the degree of viewing 0° and 30°. With an, into the telescope, integrated button was possible to switch (if necessary) from 3D to 2D view during the procedure.
The VSII uses its own handle and an integrated, miniature LED light source with a built-in, automatic gain control, which illuminates the surgical field with just a few watts of power. The camera handle is lightweight and has an ergonomic design to facilitate tool maneuvering and extended periods of handling. It delivers a single video connection to the console and has programmable buttons for easy endoscope control and menu activation.
Surgical performance
All skull base lesions located in the frontal skull base (meningioma, craniopharyngioma) were operated via a supraorbital craniotomy. During the whole surgical performances, we used almost only one visualization tool, namely the 3D-ME, compared it with the conventional 2D endoscope (Storz, Tuttlingen, GE), which we placed next to the operation field for fast switch between both if necessary. The 3D-ME was placed on a holding arm for fixation above the surgical field [Figure 1a]. At the end of the surgeries, we controlled our surgical field with the conventional microscope only to be sure that we performed the surgery in a correct way without leaving tumor behind.
Figure 1.
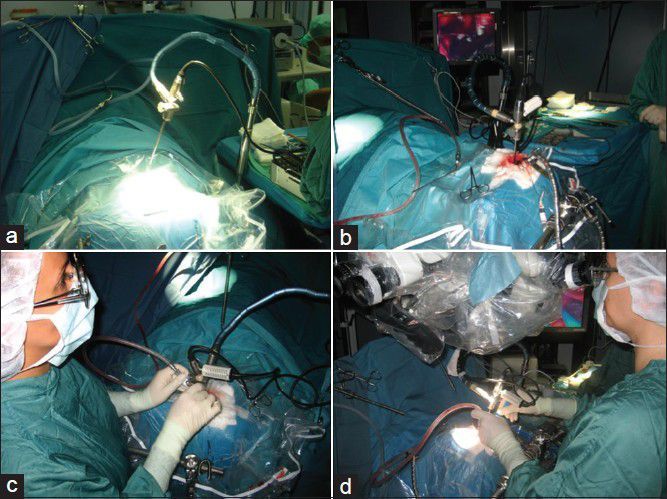
(a) Set-up of the 3D microendoscope in front of the surgical field using it as a microscope, and (b) in the depth of the surgical corridor as an endoscope in the same time, and (c) as a miniaturized combined micro-, and endoscope tool in one at the same procedure. (d) At the end of the surgery controlling the field with the microscope
All skull base lesions located in the posterior fossa (tentorial edge, petroclival, and foramen magnum meningiomas) were operated via a retrosigmoid, respectively, a paramedian far lateral approach. Here, we also performed the surgeries exact in the same conditions described above used as visualization tools the 3D-ME, as a combination tool of micro- and endoscope. The 3D-ME was safely fixed to the Mayfield headholder, thus allowing the neurosurgeon to perform the procedures with both hands. After the craniotomy was done, the 3D-ME was placed above the surgical field like a microscope, and the optical information was given to the surgeon via the integrated monitor placed in front of the surgeon's eyes. Next step was to creating the surgical corridor, placed the 3D-ME intracranial in front of the lesion and getting the function of an endoscope helping the surgeon to remove the lesion with spending light and sight in front of the target point [Figure 1b].
Both lesions in the pineal region were reached via a midline, infratentorial, supracerebellar approach.
The convexity lesions, like the epidermoid tumor and the cavernomas, were reached via a navigated, exact above the lesion placed, craniotomy. All those surgeries were performed using only the 3D-ME as a miniaturized combined micro-, and endoscope tool [Figure 1c]. Here, we transformed the monitor of the 3D-ME unit to a kind “occular” of the surgical microscope. The light and sight in the surgical region of interest came from the positioning of the telescope above the surgical field. The operating microscope, respectively the 2D endoscope were placed next to the 3D-ME only for controlling the operative field at the end of the surgery [Figure 1d].
Results
In general, transcranial “microendoscopy” was performed in all patients with high image quality using the 3D-ME. The technical system tower did not require special preparation. The tool was ready to use after connecting the camera to the display tower without considerable delay in pre-operative preparation. No complications were observed intra- or postoperatively, except one post-operative wound infection [Table 1]. Operative handling regarding weight and ergonomic features of the tool was considered comfortable. By addition of depth perception (3rd dimension like a microscope), the anatomic structures were displayed comparable to operation microscope views. Blurred vision by fogging of the endoscope tip like the 2D endoscopes was negligible with the 3D-ME. Blood drops on the camera tip occasionally led to a loss of three-dimensional perception, but they were very fast eliminated with a few drops of saline solution. This has to be considered by the surgeon as a possible reason for sudden loss of three-dimensional view, easy to overcome by simply cleaning the tool tip. A significant decrease in picture quality due to loss of focus level was observed in case of object-to-endoscope distances below 5 mm. To overcome this problem, a constant individual changing of the endoscope tip in relation to lesion distance was necessary, via manipulating the holding device during the progress of the surgery.
To demonstrate the particular benefit of 3D-ME instrumentation during open microsurgery, we present three patient examples.
Patient I: Supraorbital removal of olfactory groove meningioma
A 44-year-old woman suffered 2 months from bitemporal hemianopsia and headaches. The MRI scan showed a big olfactory groove meningioma laying bifrontal as shown in Figure 2a and b. She had a right supraorbital craniotomy with 3D-ME visualization of the tumor [Figure 3a], and gross total removal [Figure 3b] was performed. Three months after surgery, her vision improves near to normal. Follow-up MRI scans showed no residual tumor as shown in Figure 2c and d.
Figure 2.
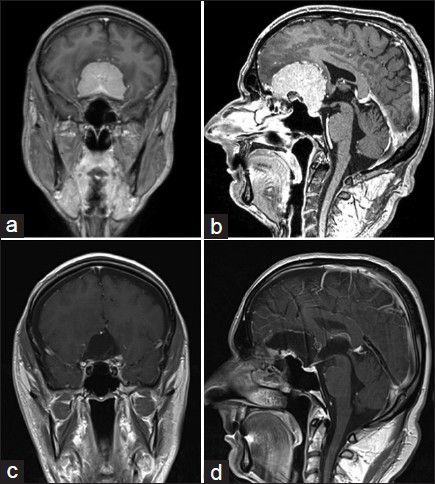
Olfactory groove meningioma: Preoperative MRI scans (a) coronar, and (b) sagittal slices. Postoperative MRI scans showed no tumor residual, (c) coronar, and (d) sagittal slices
Figure 3.
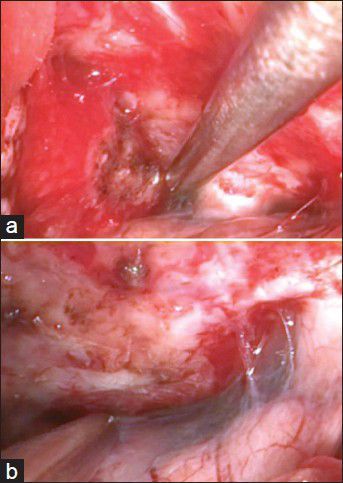
(a) Intraoperative view of the suprasellar cistern with tumor, and (b) without tumor
Patient II: Pterional approach and pure 3D endoscopic removal of the temporal epidermoid mass
A 55-year-old woman had headache and speech impairment. The MRI scans showed left temporal epidermoid mass associated with a cystic lesion temporodorsal as shown in Figure 4a and b. She underwent a left pterional craniotomy and tumor visualization under 3D-ME-assisted technique [Figure 5a]. Total removal under pure 3D-ME technique [Figure 5b] was performed. Intraoperative normal microscope was used only for control the tumor resection. The speech impairment improved immediately postoperative, and the follow-up MRI scans showed no residual mass [Figure 4c and d].
Figure 4.
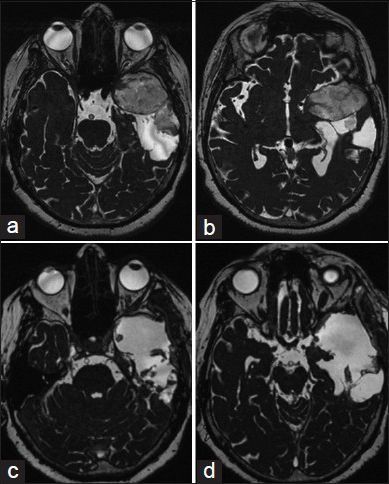
Left-sided epidermoid: Preoperative MRI scans, (a, b) axial slices. (c, d) Postoperative MRI scans showed no tumor residual
Figure 5.
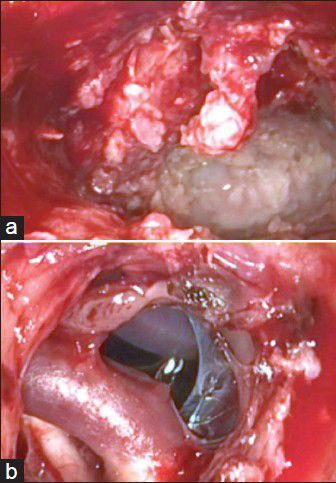
(a) Intraoperative microendoscopic view with epidermoid mass. (b) After opening the suprasellar cistern, carotic - basilar artery complex and optic nerve is visible
Patient III: Far lateral approach on the left craniocervical junction and removal of foramen magnum meningioma
A 65-year-old man with a 6-month history of left-sided weakness underwent an MRI scan. This showed left foramen magnum meningioma as shown in Figure 6a-c. He underwent a left far lateral craniotomy on the craniocervical junction with tumor 3D-ME visualization [Figure 7a], and gross total removal under pure 3D-ME-controlled technique [Figure 7b] was performed. Neither, microscope or normal 2D endoscope was used. Three months postoperative, the left-sided weakness improved and 6 months later, patient neurological deficit totally disappears. The follow-up MRI scans showed no residual or recurrent tumor as shown in Figure 6d and f.
Figure 6.
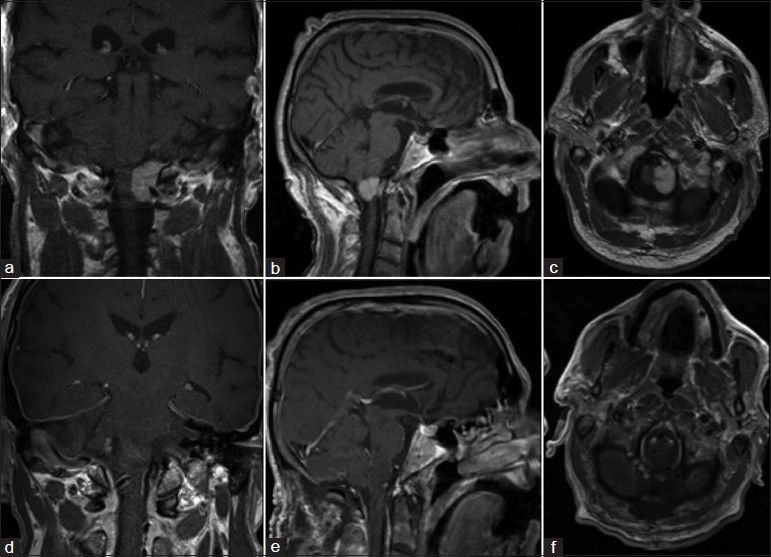
Left-sided foramen magnum meningioma: (a-c) Preoperative MRI scans showed the tumor left sided on the craniocervical junction. (d-f) Postoperative MRI scans showed no tumor residual
Figure 7.
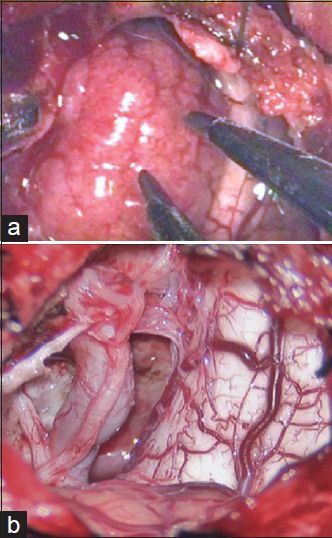
(a) Intraoperative view of the tumor on cerebellomedular angle. (b) After tumor removal, vertebral artery, XI, XII, and C1 nerve roots were visible
Discussion
Keyhole surgery does not imply that the size of the craniotomy is a keyhole, but that the choice of the correct individual craniotomy has a “key” function to enter a particular intracranial room and to work there with minimum trauma and maximum effectiveness, namely optimal invasive to deal only with the lesion and not with the surrounding normal tissue.[3,14,15] To reach this goal presumed that enough (optimal) light and sight is bringing in front of the lesion, to make it clear visible. This was achieved with developing and applying the endoscope-assisted micro-neurosurgical techniques, used today in a row of daily routine surgical procedures.[2,3] However, endoscope-assisted technique comprises the use of two visualization tools in interchange.[2] First is used the big, very costly, ergonomically difficult to manipulate and handle microscope for getting 3-D deep perception during the procedure. In addition, comes the small also very costly, ergonomic also difficult to use endoscope, to explore hidden corners within the surgical field. The endoscope unit needs extra light source, camera and monitor equipment, independent of the microscope, which takes time to set-up and consumes additional place in a moderate big operation room. With introduction of those surgical techniques and development new additional medical equipment from the one hand, the procedures became more safe and effective with lower complication rates, but from the other hand, the surgeons have to deal simultaneously with a lot of different equipment during a surgery like microscope, endoscope, often navigation, neuromonitoring (e.g. skull base surgery), etc., which makes either surgery more uncomfortable and complicate, and hindered the work flow in every modern operation room due to a lot of simultaneously used equipment.
However, having in mind all this daily routine aspects, the question is how we could keep alive and improve one side the safety of the surgical procedures and on the other side optimize the ergonomy and work flow in the surgical theaters with the target to improve: (a) comfort for the surgeon, which is needed during long-lasting procedures for holding constant the ability of the surgeon to act and reflect appropriately in every minute of the surgery, (b) safety for the patients minimizing furthermore the trauma and complications during surgery, and (c) the work flow in the operation room reducing the account of the used equipment without affecting the quality and result of the surgical procedure. Furthermore, all this would help in addition saving time with improving the work flow and money due to reduced financial expenses for different kind of equipment.
But, how we could change the way of thinking and acting during surgical procedures? Which instruments were really needed during a neurosurgical procedure? Which equipment could help improve and solve a lot of the problems mentioned before?
The biggest argument ever against a pure endoscopic technique during a surgery was that an endoscope offers only a fish eye view with lack of the third dimension, and the biggest advantage was that endoscopes could be introduced into the surgical fields to show hidden corners, which is not possible to achieve looking through a microscope. From the other side, the biggest argument to operate with a microscope was its offer of depth perception, but it was never possible to insert this huge tool intracranial in front or beside to the pathology, like an endoscope.
In respect of all this arguments, questions, and needs for improvement described above, why not try to combine those both tools in one? Not only in a well described concept and in intraoperative surgical interchange, described by Axel Perneczky's endoscope-assisted microsurgical technique, but going one step beyond and create in addition a technological very well sophisticate and sufficient platform as a series of new surgical tools combined different modalities in one.
To reach this goal, surgeons need sophisticate designed, smart, and easy-to-use equipment, ergonomically and maximal effective working instruments for visualization of the intraoperative target point, as more and clear as possible, in a desirable three-dimensional 360° panoramic view, getting all the important information needed to achieve the best surgical results.[7,8,16] To improve the ergonomic aspects in the operation theater, the desire for smaller, easier and uncomplicated usable, financially acceptable instruments increase in different surgical disciplines.[17]
The demographic and political socio-economic global changes make these developments more actually and needed as never before. As an evolution process of the endoscope-assisted microsurgical procedures for brain surgery performed with 2 different visualization tools, and having in mind all the other aspects described before for perfect optimizing the surgical performance, we start to think about fusion of both visualization abilities in one small in size, ergonomically worked, smart applicable instrument, which could be able to bring light and sight effectively and without losing important information like depth perception (microscope), ability to look around the corner of the targeted lesion (endoscope).
At this stage, the idea of the 3D micro-endoscope and microendoscopy-assisted head and neck surgery was born. Furthermore, we defined the principle of microendoscopy and the microendoscopic-performed neurosurgery, which means that you could approach from the beginning till the end of a procedure the targeted lesion only with the use of one, small smart, ergonomic, and sophisticate designed visualization tool which involve and acts with the ability of both micro-, and endoscope.
We started to use the VSII 3D-ME a few years ago performed initially simple, short-lived procedures, like small convexity brain tumors, superficially located cavernomas, etc., At the beginning, we used the new tool in addition to the micro-, or/and endoscope to became more familiar with the technology, 3D effect, ergonomic usability, and application of the tool. In the course of time, we refined our technique became more familiar with it and start to use 3D-ME in more complicated lesions located deeper in the skull base even approaching those endonasal in cooperation with ENT[18] or transcranial. As described before, we used the ability of the tool in different regions of the head, in skull base (via an endonasal or transcranial route) and in convexity, to insert it in the same manner, like a microscope with the disposition to transform it to an endoscope every time we introduced it intracranial into the surgical field. We realized that surgeons could have both visualization possibilities, micro-, and endoscopic in one small ergonomic and cost-effective device, without changing between visualization tools during the surgical performance. The application of such combined tools, especially in developed countries where the financial potential to have a lot of very expensive instruments in the operation theater, is very limited; solutions like 3D-ME, representing and acting as a fusion technology of micro-, and endoscope simultaneously, were very attractive. In general, surgeries with such a tool as the only one visualization source could be done with lesions lying even on the surface or deeper in the skull base, because of the ability of such systems to combine both, micro-, and/or endoscope in one tool.
With increasing experience, refinement through intensive training in the combined microendoscopic operative technique, familiarity with the technology and application options, microendoscopic-assisted neurosurgery is a very promise full surgical technique with the long-term potential to replace big, and in use complicate, expensive apparatus and strongly improve ergonomic conditions for surgeons and personal in the operation theatres without losing the safety, effectiveness, and information ability needed to get as surgeon from a modern visualization tool during a surgical performance for reaching the individual most optimal surgical result for the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fries G, Perneczky A. Intracranial endoscopy. Adv Tech Stand Neurosurg. 1999;25:21–60. doi: 10.1007/978-3-7091-6412-9_2. [DOI] [PubMed] [Google Scholar]
- 2.Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery. 1998;43:1330–6. doi: 10.1097/00006123-199812000-00037. [DOI] [PubMed] [Google Scholar]
- 3.Perneczky A, Fries G. Endoscope-assisted brain surgery: Part 1 – evolution, basic concept, and current technique. Neurosurgery. 1998;42:219–24. doi: 10.1097/00006123-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Perneczky A, Boecher-Schwarz HG. Endoscope-assisted microsurgery for cerebral aneurysms. Neurol Med Chir (Tokyo) 1998;38:33–4. doi: 10.2176/nmc.38.suppl_33. [DOI] [PubMed] [Google Scholar]
- 5.van Lindert E, Hopf N, Perneczky A. Endoscopic treatment of mesencephalic ependymal cysts: Technical case report. Neurosurgery. 1998;43:1234–41. doi: 10.1097/00006123-199811000-00135. [DOI] [PubMed] [Google Scholar]
- 6.Fratzoglou M, Leite dos Santos AR, Gawish I, Perneczky A. Endoscope-assisted microsurgery for tumors of the septum pellucidum: Surgical considerations and benefits of the method in the treatment of four serial cases. Neurosurg Rev. 2005;28:39–43. doi: 10.1007/s10143-004-0332-y. [DOI] [PubMed] [Google Scholar]
- 7.Mamelak AN, Nobuto T, Berci G. Initial clinical experience with a high-definition exoscope system for microneurosurgery. Neurosurgery. 2010;67:476–83. doi: 10.1227/01.NEU.0000372204.85227.BF. [DOI] [PubMed] [Google Scholar]
- 8.Mamelak AN, Drazin D, Black KL, Berci G. Infratentorial supracerebellar resection of a pineal tumor using a high definition video exoscope (VITOM®) J Clin Neurosci. 2012;19:306–9. doi: 10.1016/j.jocn.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Roth J, Fraser JF, Singh A, Bernardo A, Anand VK, Schwartz TH. Surgical approaches to the orbital apex: Comparison of endoscopic endonasal and transcranial approaches using a novel 3D endoscope. Orbit. 2011;30:43–8. doi: 10.3109/01676830.2010.543004. [DOI] [PubMed] [Google Scholar]
- 10.Roth J, Singh A, Nyquist G, Fraser JF, Bernardo A, Anand VK, et al. Three-dimensional and 2-dimensional endoscopic exposure of midline cranial base targets using expanded endonasal and transcranial approaches. Neurosurgery. 2009;65:1116–28. doi: 10.1227/01.NEU.0000360340.85186.7A. [DOI] [PubMed] [Google Scholar]
- 11.Tabaee A, Anand VK, Fraser JF, Brown SM, Singh A, Schwartz TH. Three-dimensional endoscopic pituitary surgery. Neurosurgery. 2009;64:288–93. doi: 10.1227/01.NEU.0000338069.51023.3C. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JF, Allen B, Anand VK, Schwartz TH. Three-dimensional neurostereoendoscopy: Subjective and objective comparison to 2D. Minim Invasive Neurosurg. 2009;52:25–31. doi: 10.1055/s-0028-1104567. [DOI] [PubMed] [Google Scholar]
- 13.Brown SM, Tabaee A, Singh A, Schwartz TH, Anand VK. Three-dimensional endoscopic sinus surgery: Feasibility and technical aspects. Otolaryngol Head Neck Surg. 2008;138:400–2. doi: 10.1016/j.otohns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M, Perneczky A. Subtemporal keyhole approach to the suprasellar and petroclival region: Microanatomic considerations and clinical application. Neurosurgery. 1997;41:592–601. doi: 10.1097/00006123-199709000-00017. [DOI] [PubMed] [Google Scholar]
- 15.van Lindert E, Perneczky A, Fries G, Pierangeli E. The supraorbital keyhole approach to supratentorial aneurysms: Concept and technique. Surg Neurol. 1998;49:481–9. doi: 10.1016/s0090-3019(96)00539-3. [DOI] [PubMed] [Google Scholar]
- 16.Shirzadi A, Mukherjee D, Drazin DG, Paff M, Perri B, Mamelak AN, et al. Use of the video telescope operating monitor (VITOM) as an alternative to the operating microscope in spine surgery. Spine (Phila Pa 1976) 2012;37:E1517–23. doi: 10.1097/BRS.0b013e3182709cef. [DOI] [PubMed] [Google Scholar]
- 17.Carlucci C, Fasanella L, Ricci Maccarini A. Exolaryngoscopy: A new technique for laryngeal surgery. Acta Otorhinolaryngol Ital. 2012;32:326–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Al Kadah B, Bumm K, Charalampaki P, Schick B. First experience in endonasal surgery using a new 3D-Chipendoscope. Laryngorhinootologie. 2012;91:428–33. doi: 10.1055/s-0032-1309051. [DOI] [PubMed] [Google Scholar]


