Abstract
Backround:
Practical scenario in trauma neurosurgery comes with multiple challenges and limitations. It accounts for the maximum mortality in neurosurgery and yet the developing countries are still ill-equipped even for an emergency set-up for primary management of traumatic brain injuries. The evolution of modern neurosurgical techniques in traumatic brain injury has been ongoing for the last two centuries. However, it has always been a challenge to obtain a satisfactory clinical outcome, especially those following severe traumatic brain injuries. Other than the well-established procedures such as decompressive hemicraniectomy and those for acute and or chronic subdural hematomas and depressed skull fractures, contusions etcetera newer avenues for development of surgical techniques where indicated have been minimal. We are advocating a replacement for decompressive hemicranictomy, which would have the same indications as decompressive hemicraniectomy. The results of this procedure has been compared with the results of decompressive hemicraniectomy done in our institution and elsewhere and has been proven beyond doubts to be superior to decompressive hemicraniectomy. This procedure is elegant and can replace decompressive hemicraniectomy because of low morbidity and mortality. However, there is a steep learning curve and the microscope has to be used.
Materials and Methods:
Based on the clinical experience and observation of acute neurosurgical service in tertiary medical centers in a developing country, the procedure of cisternostomy in the management of trauma neurosurgery have been elucidated in the current study. The study proposes to apply the principles of microvascular surgery and skull base surgery in selected cases of severe traumatic brain injuries, thus replacing decompressive hemicraniectomy as the primary modality of treatment for indicated cases.
Conclusion:
Extensive opening of cisterns making use of skull base techniques to approach them in a swollen brain is a better option to decompressive hemicraniectomy for the same indications.
Keywords: Brain swelling, cisterns, decompressive hemicraniectomy, intra brain pressure, intra cisternal pressure
Introduction
Traumatic brain injury (TBI) is a major public health concern world-wide with no significant change in its epidemiology over the last 30 years.[1] The burden of death or disability from TBI has been increasing world-wide. According to the World Health Organization report, TBI is expected to surpass many other diseases, such as ischemic heart disease and cerebrovascular disease as a major cause of death and disability by the year 2020 AD.[2] The outlook in trauma neurosurgery in most centers world-wide has not made significant progress in the past several decades. The most significant step in this direction has been the introduction of decompressive hemicraniectomy and supportive management in an intensive care unit with intracranial pressure monitoring.
The poor results in trauma may be attributed partly to poor prognosis of the disease related to its severity as well as practical circumstances surrounding the scenario such as inexperienced surgeons or peri and intraoperative care facilities in different centers. In addition, trauma neurosurgery has been mostly looked upon as an extension of the general trauma team and therefore less appealing to aspiring neurosurgeons who aim to be trained in the skull base, vascular or spinal surgical specialties. Furthermore, interventions for traumatic intracranial lesions and or raised intracranial pressure have been fairly standard, e.g., medical management of raised intracranial pressure or ventricular catheterization, decompressive craniectomy (DHC), surgical evacuation of hematoma etc.[3]
Depending on surgical indication in a given case scenario, it is imperative that patients be managed with optimal care. However, the research and improvement of surgical technique in trauma has remained to be almost non-existent while non-surgical ways of treating TBI is fast gaining popularity. Research in conservative management of TBI with therapeutic options for neuroprotection has been rigorously pursued over the last 40 years.[4]
It is important that an appropriate surgical algorithm and technique be applied to the management of TBI, where indicated, for better patient outcome. Therefore in the current study, a novel technique, which incorporates the discipline of skull base and microvascular approach of opening the sub-arachnoid cisterns, has been described. Furthermore, it has been recommended that the technique can be reproduced in any well-equipped tertiary care center where the on-duty neurosurgery consultant is adequately trained and is cognizant of the pathophysiology of trauma neurosurgery and its approach to management. It is note-worthy that the microvascular approach described in this study has been similar to previously well-described cases of aneurysmal sub-arachnoid hemorrhage and related surgical procedures.
Materials and Methods
The study was started in 2007. This was conducted in a stepwise manner since the surgeon modified the existing treatment for severe head injury with the addition of cisternostomy first and after analyzing the results, cisternostomy was used as a substitute for decompressive hemicraniectomy.
Indications for cisternostomy are the same as that for decompressive hemicraniectomy. However, in the authors’ experience of more than 1000 cases, in the following instances, adding cisternostomy to the definitive procedure has been found to decrease the morbidity and mortality.
Cute subdural hematomas (SDH) with mass effect and midline shift more than 1 cm, even if they present as mild or moderate head injuries
Multiple contusions in combination with SDH and mass effect
Pediatric brain injuries with severe brain swelling.
This study was conducted by a single surgeon in three centers. The study was done in a stepwise manner, wherein DHC was done initially, then cisternostomy was added to DHC and then DHC was replaced by cisternostomy after seeing that the brain was lax and it remained so after cisternostomy.
Results [Graphs 1–5]
Graph 1.
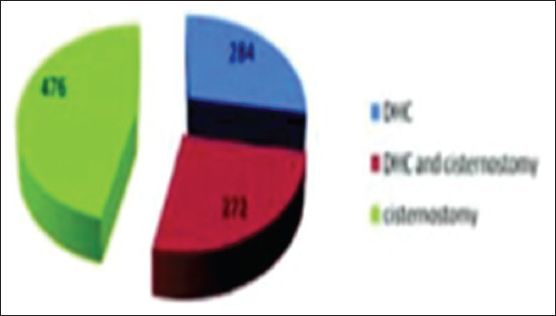
A total of 284 patients underwent decompressive hemicraniectomy, 272 of them underwent decompressive hemicraniectomy with cisternostomy and 476 of them underwent cisternostomy alone
Graph 5.
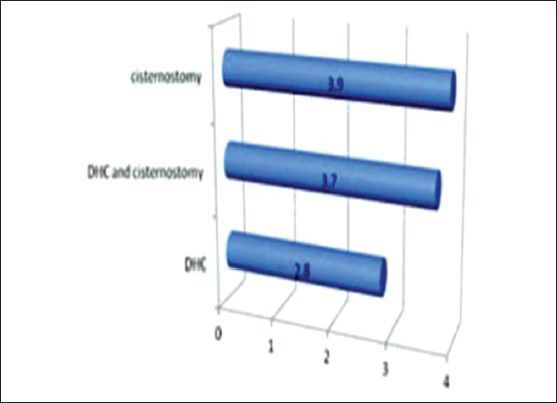
Mean GOS at 6 weeks for DHC arm was 2.8, for DHC and cisternostomy arm it was 3.7 and for cisternostomy alone arm it was 3.9
Graph 2.
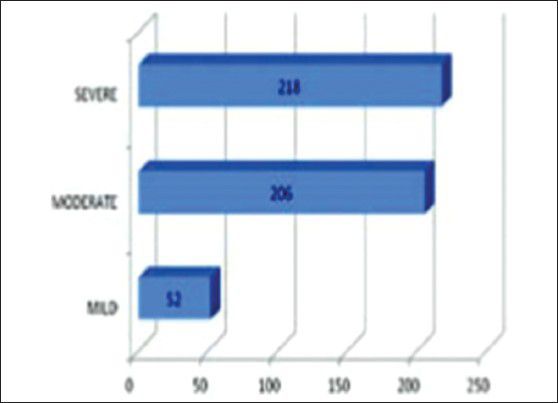
Among the cisternostomy group, 52 patients had mild head injury, 206 patients had moderate head injuries and 218 patients had severe head injury
Graph 3.
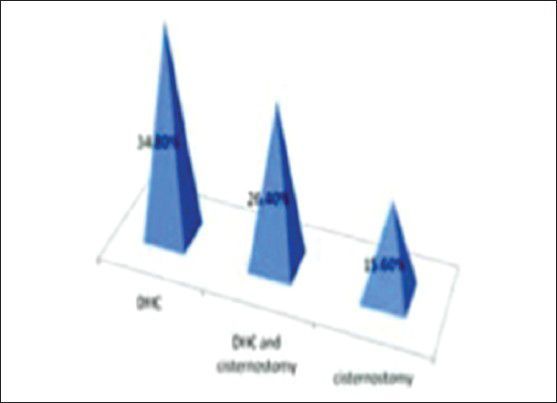
For severe head injuries, the mortality for cisternostomy was 15.6%, for DHC was 34.8% and for DHC with cisternostomy it was 26.4%
Graph 4.
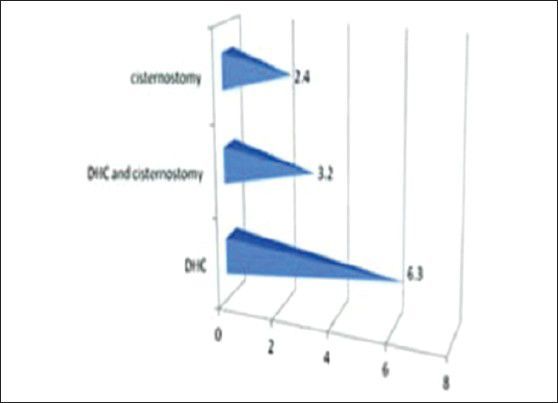
The mean days on the ventilator for the three groups were 2.4, 3.2 and 6.3 respectively for cisternostomy, decompressive hemicraniectomy with cisternostomy and decompressive hemicraniectomy
Number of patients in each subgroup
A total of 284 patients underwent decompressive hemicraniectomy, 272 of them underwent decompressive hemicraniectomy with cisternostomy and 476 of them underwent cisternostomy alone.
Presenting Glasgow Coma Scale of the patients in cisternostomy group
Among the cisternostomy group, 52 patients had mild head injury, 206 patients had moderate head injuries and 218 patients had severe head injury.
Mortality
For severe head injuries, the mortality for cisternostomy was 15.6%, for DHC was 34.8% and for DHC with cisternostomy it was 26.4%.
Days on ventilator
The mean days on the ventilator for the three groups were 2.4, 3.2 and 6.3 respectively for cisternostomy, decompressive hemicraniectomy with cisternostomy and decompressive hemicraniectomy.
Mean GOS at 6 weeks
Mean GOS at 6 weeks for DHC arm was 2.8, for DHC and cisternostomy arm it was 3.7 and for cisternostomy alone arm it was 3.9.
Discussion
Trauma and acute subarachnoid hemorrhage looks similar in more than one way is an observation, which most of the neurosurgeons will agree to. This led us to think that opening cisterns as in aneurysm surgery gets the brain to be lax in trauma as well. Cutting the tent to reach cisterns in trauma was practiced and still in practice.[5,6,7]
In severe traumatic head injuries, many modalities have been tried out; surgical steps such as tentoriotomy, decompressive hemicraniectomy along with uncusectomy and intracranial pressure (ICP) targeted therapy, cerebrospinal fluid (CSF) drainage procedures and even ongoing researches on medical management of severe head injuries.[8,9,10,11,12,13,14]
Cisternostomy was also carried out in the mild head injury as a prophylactic measure in patients with acute SDH associated with mass effect and midline shift. Studies have shown that prophylactic craniectomy for TBI favors lower therapeutic intensity scores and shorter intensive care unit days. Early craniectomy may provide a survival benefit and does not increase surgical complications. Prophylactic DHC may be considered as up-front treatment for TBI in very few selected cases.
The protocol was started off with a standard modality of treatment, which was decompressive hemicraniectomy and then the cisternostomy was added to it, after which only cisternostomy was practiced. This was in agreement with the fact that patients who underwent decompressive hemicraniectomy for TBI achieved good long-term outcome although they experienced significant difficulties in health status.[15]
We wanted to avoid the morbidities associated with removal of the bone flap in decompressive hemicraniectomy and also the need of doing cranioplasty later on. After cisternostomy, brain was quite lax and we were confident of putting the bone flap back without further complications. The argument for later brain swelling prompted us to keep a careful watch for any swelling, but this did not happen. First the temporal part of the bone flap was totally removed and the bone flap, was kept floating and then over a period of time, we started fixing the bone flap.
Mechanism
Our hypothesis works probably on the role of Virchow Robin (VR) spaces [Figures 1 and 2] whose presence has already been highlighted.[16,17] Iliff et al. have documented in the experimental study a paravascular pathway that facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, which they named glymphatic pathway.[18] In trauma, we believe that the cerebral edema may be due to egress of CSF from the cisternal space into the brain parenchyma via the VR spaces [Figure 3]. In decompressive hemicraniectomy removal of bone helps to reduce intracerebral pressure but at the expense of external cerebral herniation and its effect on CSF and on hemodynamic flow alteration [Figure 4]. Decompressive hemicraniectomy only brings the intracranial pressure to atmospheric pressure and it fails to deal with the intra-brain pressure, which causes severe brain swelling [Figure 4] and herniation although the intracranial pressure is reduced. Cisternostomy opens the basal cisterns to atmospheric pressure and causes a “back-shift” of CSF thru the VR spaces, thereby reducing the intra-brain pressure [Figure 5].
Figure 1.
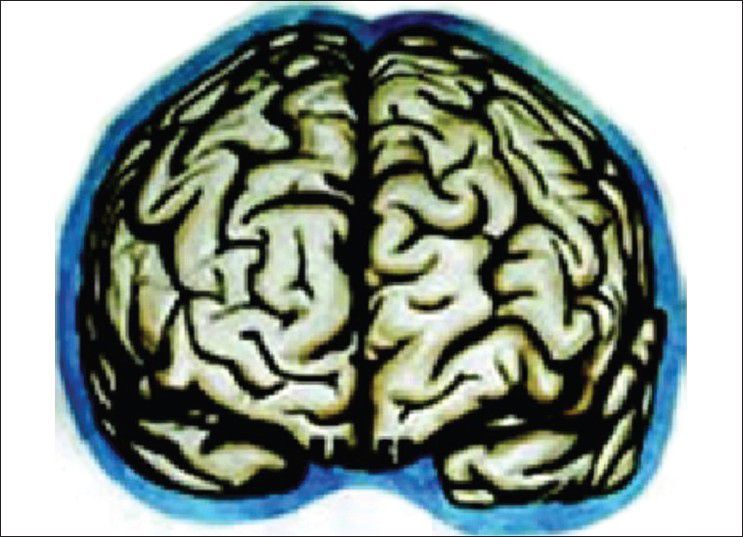
Virchow Robin spaces type 2 and 3
Figure 2.
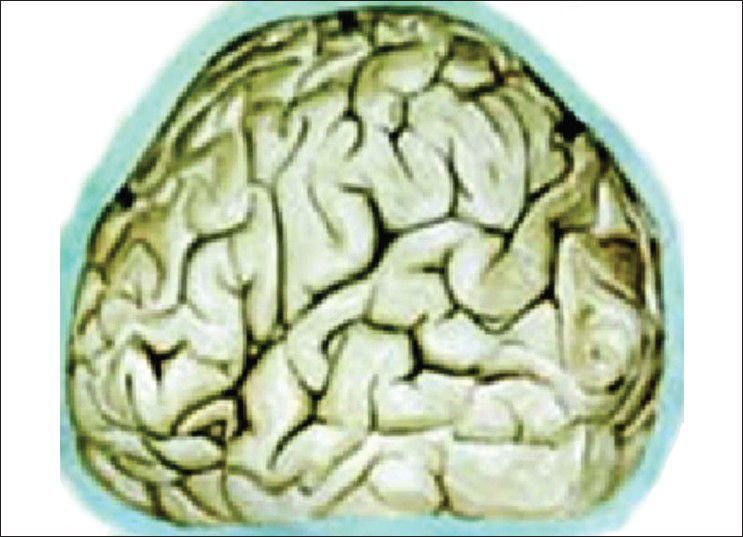
Virchow Robin spaces type 1
Figure 3.
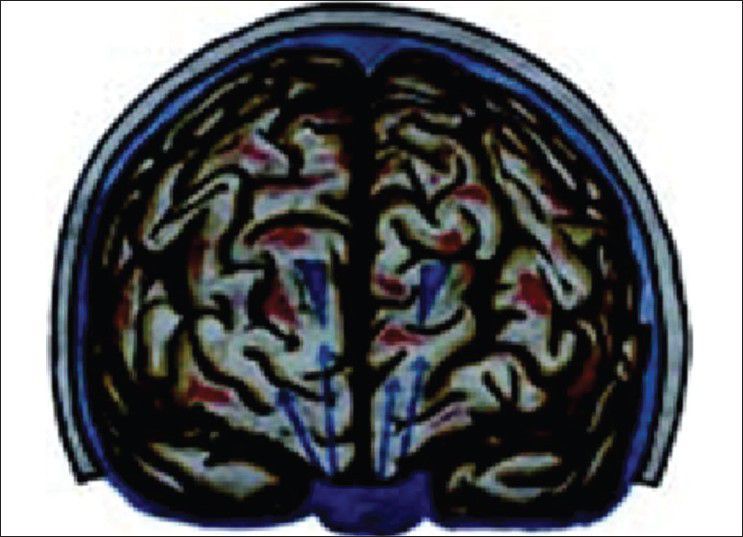
Cerebrospinal fluid migration in trauma
Figure 4.

The role of decompressive craniectomy
Figure 5.
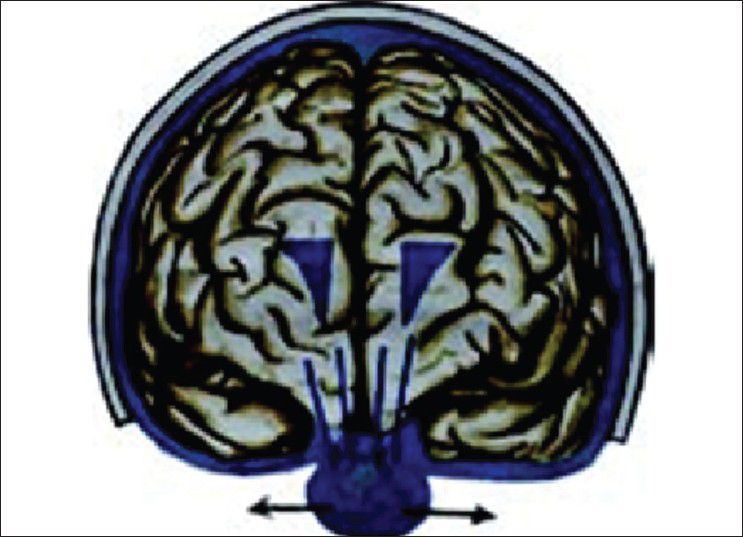
Cisternostomy causing the reverse shift of cerebrospinal fluid
The time taken for the entire cisternostomy from dural opening after the learning curve is about 10-20 min depending on the case, the surgeon and the facilities. We do not do a lobectomy and if there is a surface hematoma with significant mass effect, we make a minimal cortical incision and ask the Anesthesiologist to assist in evacuation of the clot by a Valsalva maneuver. Bipolar coagulation is almost never used and hemostasis is done with the help of oxidized cellulose and continuous irrigation.
There have been questions regarding the feasibility of subfrontal retraction in the setting of severe trauma. This is a highly controversial topic and we do agree that once ischemic changes have set in, it is difficult to contain the brain swelling even with cisternostomy. However, a basal approach and using the “2 min window” one gets after removing the subdural hematoma from the base (which is present in most of the cases) does help to get into the interoptic cisterns. Once that cistern is opened, the brain swelling comes down in dramatic fashion [Figures 6-8]. Furthermore, it is to be noted that the pediatric brain may not be compliant and a sliver of basifrontal lobe may need to be removed in a subpial fashion, at times to reach the cisterns. However, after cisternostomy the brain become lax in most instances. Mortality, morbidity, ventilator stay was significantly reduced. Its major disadvantages are a steep learning curve and need for operating microscope (especially for opening the membrane of Lilliquist). There is also predisposition for the formation of pseudomeningocele as we leave the dura open so as to facilitate the drainage of CSF from the cisterns to the subgaleal space where it could be absorbed and there is no need for a cisternal drain.
Figure 6.
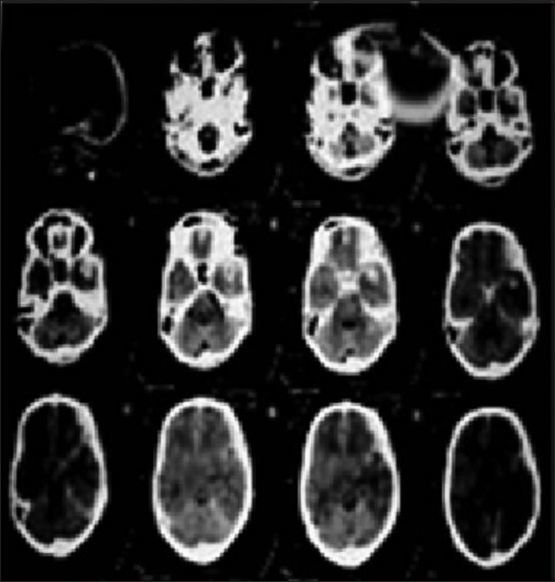
The pre-operative computed tomography scan of a male patient brought to the emergency with a Glasgow Coma Scale of 8
Figure 8.
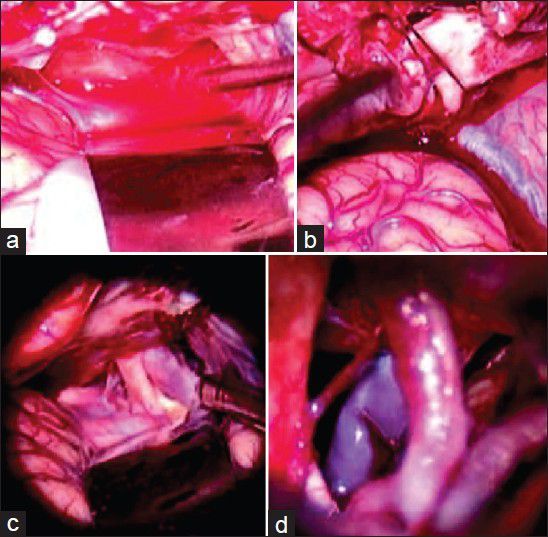
(a) Immediately after the dural opening and evacuation of the acute subdural hematoma. The surgeon gets a window period of 2-3 min of slightly lax brain before it starts swelling again. (b) In this “window” the surgeon should open the interoptic cistern. (c) The brain becomes lax sufficiently to expose and dissect the opticocarotid space and the lateral carotid space. (d) And through either of these spaces or both, the membrane of Lilliquist is sharply dissected to reach the interpeduncular and the prepontine cisterns
Figure 7.
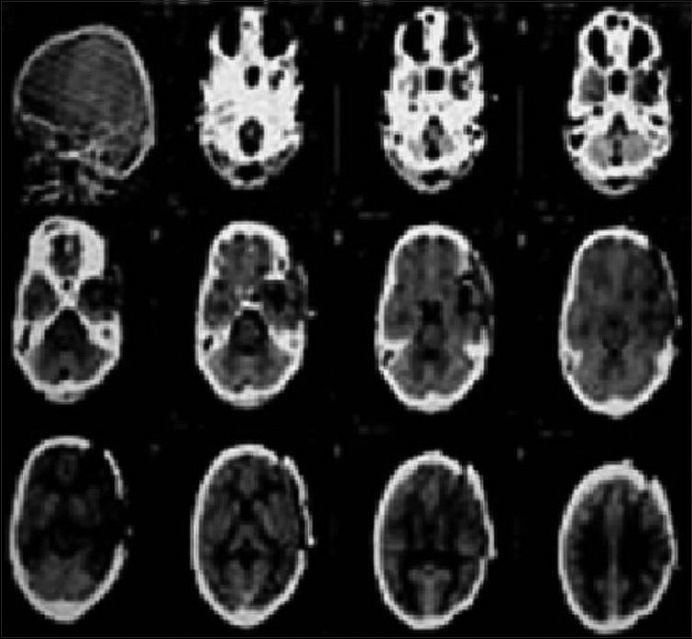
The post-operative scan after 2 weeks patient had a Glasgow Coma Scale of 15 with moderate cognitive deficit
Further confirmations and the usage of other modalities like MR elastography[19] may be helpful proving the increased compliance of the brain after cisternostomy may be helpful in corroborating this study. We are also working on International Consulting Group,[20] laser speckled contrast imaging[21] to confirm improved blood flow after the cisternostomy. Putting in an external ventricular drain to measure post-operative ICP to confirm the role of cisternostomy may be another step forward, albeit this being an invasive procedure. A radionucleotide scan to show the CSF transfer through VR Spaces would also be helpful in corroboration of the theory.
Decompressive hemicraniectomy was described by Kocher in 1901… It is over 110 years. It was the workhorse of Trauma Neurosurgery until so far… it's time to hand over the baton. However, we do agree that a lot work needs to be done before cisternostomy is accepted.
Conclusion
The best surgical treatment for severe TBI still so far has been decompressive hemicraniectomy. However, cisternostomy could replace decompressive for the same indications.
Footnotes
This work is dedicated to the memory of Miss. Mingwei Tan, who was an exchange student posted with us from Cambridge, UK she wanted to become a Neurosurgeon. Unfortunately, she passed away in a tragic accident and the whole team remembers her for her undying spirit.
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Evidence-based health policy – Lessons from the Global Burden of Disease Study. Science. 1996;274:740–3. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 3.Li LM, Timofeev I, Czosnyka M, Hutchinson PJ. Review article: The surgical approach to the management of increased intracranial pressure after traumatic brain injury. Anesth Analg. 2010;111:736–48. doi: 10.1213/ANE.0b013e3181e75cd1. [DOI] [PubMed] [Google Scholar]
- 4.Vink R, Bullock MR. Traumatic brain injury: Therapeutic challenges and new directions. Neurotherapeutics. 2010;7:1–2. doi: 10.1016/j.nurt.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sindou M. Favourable influence of opening the lamina terminalis and Lilliequist's membrane on the outcome of ruptured intracranial aneurysms. A study of 197 consecutive cases. Acta Neurochir (Wien) 1994;127:15–6. doi: 10.1007/BF01808539. [DOI] [PubMed] [Google Scholar]
- 6.John JC, Matt P, Michelle M, Holland MC, Manley GT. Prophylactic craniectomy for traumatic brain injury: Clinical results and complications: 901. Neurosurgery. 2005;57:430–1. [Google Scholar]
- 7.Saribekian AS, Lebedev VV, Sumskiĭ LI, Klimov AG. Tentoriotomy in severe craniocerebral injuries. Zh Vopr Neirokhir Im N N Burdenko. 1985;4:30–6. [PubMed] [Google Scholar]
- 8.Morrell RM. Effect of cutting the tentorium cerebelli on the response to craniocerebral trauma. J Neurosurg. 1960;17:374–84. doi: 10.3171/jns.1960.17.3.0374. [DOI] [PubMed] [Google Scholar]
- 9.Chibbaro S, Marsella M, Romano A, Ippolito S, Benericetti E. Combined internal uncusectomy and decompressive craniectomy for the treatment of severe closed head injury: Experience with 80 cases. J Neurosurg. 2008;108:74–9. doi: 10.3171/JNS/2008/108/01/0074. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Ishimaru S, Maeda M. Unco-parahippocampectomy for direct surgical treatment of downward transtentorial herniation. Acta Neurochir (Wien) 1998;140:1239–44. doi: 10.1007/s007010050244. [DOI] [PubMed] [Google Scholar]
- 11.Bao YH, Liang YM, Gao GY, Pan YH, Luo QZ, Jiang JY. Bilateral decompressive craniectomy for patients with malignant diffuse brain swelling after severe traumatic brain injury: A 37-case study. J Neurotrauma. 2010;27:341–7. doi: 10.1089/neu.2009.1040. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita K, Sakurai A, Utagawa A, Ebihara T, Furukawa M, Moriya T, et al. Importance of cerebral perfusion pressure management using cerebrospinal drainage in severe traumatic brain injury. Acta Neurochir Suppl. 2006;96:37–9. doi: 10.1007/3-211-30714-1_9. [DOI] [PubMed] [Google Scholar]
- 13.Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. 2007;24:927–35. doi: 10.1089/neu.2005.356E. [DOI] [PubMed] [Google Scholar]
- 14.Tran HT, Sanchez L, Brody DL. Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice. J Neuropathol Exp Neurol. 2012;71:116–29. doi: 10.1097/NEN.0b013e3182456aed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983. doi: 10.1002/14651858.CD003983.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Inglese M, Bomsztyk E, Gonen O, Mannon LJ, Grossman RI, Rusinek H. Dilated perivascular spaces: Hallmarks of mild traumatic brain injury. AJNR Am J Neuroradiol. 2005;26:719–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Inglese M, Grossman RI, Diller L, Babb JS, Gonen O, Silver JM, et al. Clinical significance of dilated Virchow-Robin spaces in mild traumatic brain injury. Brain Inj. 2006;20:15–21. doi: 10.1080/02699050500309593. [DOI] [PubMed] [Google Scholar]
- 18.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse SA, Dresner MA, Rossman P, Felmlee JP, Jack CR, Ehman RL. “Palpation of the brain” using magnetic resonance elastography. Neuroimage. 2008;1:231–7. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller E, Nadler A, Alkadhi H, Kollias SS, Yonekawa Y, Niederer P. Noninvasive measurement of regional cerebral blood flow and regional cerebral blood volume by near-infrared spectroscopy and indocyanine green dye dilution. Neuroimage. 2003;20:828–39. doi: 10.1016/S1053-8119(03)00315-X. [DOI] [PubMed] [Google Scholar]
- 21.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]


