Abstract
Context:
Brain metastases are the most common type of intracranial neoplasm, with the total number outnumbering primary brain tumors by a ratio of 10:1 and occur in about 25% of cancer patients. However, controversies exist regarding demographic and clinical profile of brain metastases.
Aims:
The purpose of this study was to analyze retrospectively the demographic and clinical profile of patients with brain metastases.
Settings and Design:
Retrospective, single institutional study.
Materials and Methods:
A retrospective study of 72 patients with brain metastasis was carried out from November 2010 to October 2012. The data pertaining to these patients was entered in a standardized case record form. These include History; clinical examination and other investigations including computed tomography/magnetic resonance imaging scan of the brain.
Statistical Analysis:
A statistical analysis was performed on the data collected using the MedCalc version 11.
Results:
Brain metastases were more common in male and occur in 6th decade of life mostly. There was no relationship of occupation or socio-economic status with the incidence of brain metastases. Carcinoma lung was the most common primary giving rise to brain metastases followed by breast. Adenocarcinoma accounts for most common histology of the primary that give rise to metastases. Multiple metastases were more common than the single group. Supratentorial lesions were more common than infratentorial lesions. Among them, parietal lobe was the most common site of involvement.
Conclusions:
The present study highlights that the incidence of brain metastasis is common in elderly population and mostly due to primary lung. Adenocarcinoma was the most common histology of primary. Majority of lesions has been observed at parietal lobe.
Keywords: Brain metastases, carcinoma lung, demographic profile
Introduction
Brain metastases are the most common type of intracranial neoplasm, with the total number diagnosed annually outnumbering all other intracranial tumors combined.[1] Brain metastases increases morbidity and mortality in cancer patients. They outnumber primary brain tumors by a ratio of 10:1 and occur in about 25% of all patients with cancer.[2] Conservative estimates suggest that 100,000-170,000 new cases of brain metastases are diagnosed every year in the united states. Between 20% and 40% of all patients with metastatic cancer will have brain metastases at autopsy.[3]
The majority of patients who develop brain metastases have a known primary cancer (metachronous presentation). Most brain metastases originate from lung (40-50%), breast (15-25%), melanoma (5-20%) and kidney (5-10%).[2] No primary site of cancer is detected in 5-10% of patients with brain metastases.[4] Brain metastases are located in the cerebral hemispheres in about 80%, in the cerebellum in 15% or in the brainstem in 5% of patients.[5]
In recent years, there is an apparent increase in cases of brain secondaries due to increasing incidence of lung cancer, improved detection by more sensitive imaging techniques, development in anticancer treatment resulting in prolonged survival.[6,7,8]
The clinical presentation of brain metastases is similar to any intracranial mass lesion and include headache (70%), seizures (30-60%), cognitive impairment (30%), papilledema (8%) and miscellaneous focal neurological deficits.[3,9]
Advances in neuroradiology have contributed greatly to the diagnosis and management of patients with suspected neoplastic diseases of central nervous system (CNS). Contrast-enhanced computed tomography (CECT) is used widely because of its easy accessibility and low cost. Contrast enhanced magnetic resonance imaging (MRI) is more sensitive than enhanced computed tomography (CT) scanning in detecting brain metastases, particularly small lesions or metastases situated in the posterior fossa.[10,11] MRI is particularly recommended for patients with an apparently single metastasis on a CT or for patients with limited disease (i.e., lung tumors) in whom the detection of asymptomatic brain metastases would alter the therapeutic management.[12] Radiographically, metastases are ring-enhancing lesions, most often located at the grey-white matter junction surrounded usually by significant edema. Unlike primary brain tumors, metastatic lesions rarely involve the corpus callosum or cross the midline. The radiographic appearance of brain metastases is non-specific and may mimic other processes, such as infection. Tissue confirmation is necessary in patients even with a history of prior cancer, in those whose history of cancer is remote and in those for whom clinical or neuroimaging features may suggest an alternative diagnosis, such as a primary brain tumor.
The purpose of this study was to analyze retrospectively the demographic and clinical profile of patients with brain metastases.
Materials and Methods
A retrospective study of 72 patients with brain metastasis was carried out in the Department of Radiotherapy, Institute of Postgraduate Medical Education and Research and SSKM Hospital, Kolkata from November 2010 to October 2012. The Institutional Ethical Committee cleared the protocol and the data pertaining to these patients was entered in a standardized case record form. These includes history; Clinical examination including neurological evaluation; fundoscopy and perimetry (in selected cases); complete blood count, liver and kidney function test; imaging - CT/MRI scan of the brain and other different imaging studies (chest X-ray posterior- anterior view, ultrasonography whole abdomen, CECT scan of chest, CECT scan of the abdomen and pelvis, CECT of the face and neck), which were done to detect the primary; histopathological biopsy of the primary site as well as intracranial SOL. A statistical analysis was performed on the data collected using the MedCalc version 11 software and the result were formulated.
Results
There were 72 patients with brain metastases between November 2010 and October 2012. Out of the total 72 patients with brain metastases majority were in 6th decade constituting 44.44% as evidenced in Figure 1. In this study, there was no significant difference in distribution of sex although Males were slightly more in number constituting 52.78% as evidenced in Figure 2. There was no significant difference in distribution of patients according to their occupation. Majority of patients were day laborer constituting 29.2% followed by service holder and farmer each constituting 20.8% [Figure 3]. There was no significant difference in distribution of patients according to socio-economic status by modified Kuppuswamy scale. Nearly 27.8% were in upper socio-economic status and 23.6% were in the lower socio-economic status [Figure 4]. Out of total 72 patients 40.3% had bilateral involvement in brain. In 34.7% of patients only left side of the brain was involved compared with 25% of patients where only right side of the brain was involved [Figure 5]. In 72 patients, total 150 metastatic lesions were found in the brain on an imaging study. Most of the tumor were supratentorial (97.2%) in location out of which parietal lobe was the most common site of involvement constituting 48% [Figure 6]. Only two patients had infratentorial brain metastasis as evidenced in Figure 7. Out of 72 patients 77.8% had multiple lesions and 22.2% had single lesion as shown in Figure 8. As observed carcinoma of the lung was the commonest primary that metastatizes to brain i.e. 51.4%, followed by breast. In 4.2% of patients, the primary was unknown, but they had evidence of metastases in lymph node/lung, which were proved on cytology/biopsy shown in Figure 9. In the majority of patients, histology of primary malignancy was adenocarcinoma constituting 38.9%. Squamous cell carcinoma accounts for 27.8%. In 4.2% of cases histology of the primary site was unknown [Figure 10]. Headache was the most common symptom followed by vomiting and neurological deficit, which was observed in this study [Figure 11]. At the time of detection of metastases, 29.2% of patients had controlled primary whereas in 70.8% of patients, the primary disease was not controlled shown in Figure 12.
Figure 1.
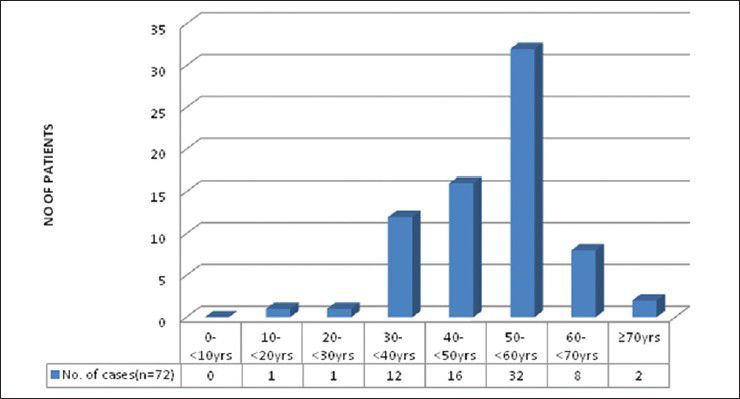
Bar diagram showing age distribution of patients (P < 0.0001)
Figure 2.
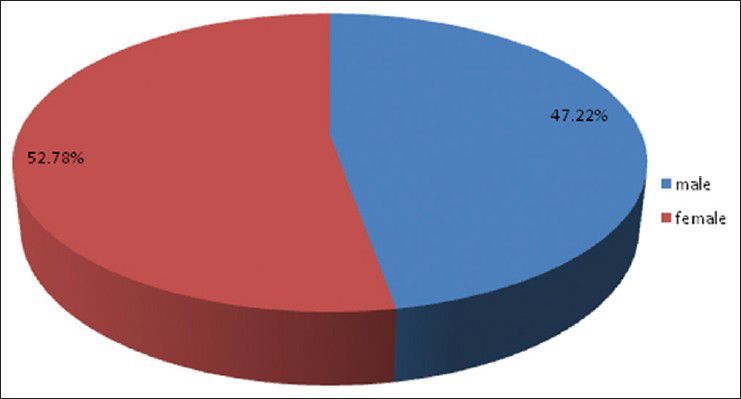
Pie diagram showing sex distribution of patients (P= 0.7237)
Figure 3.
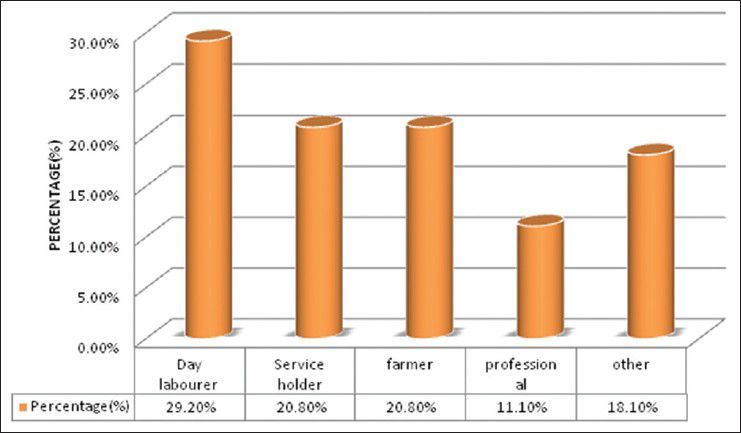
Bar diagram showing distribution of patients according to their occupation (P= 0.1950)
Figure 4.
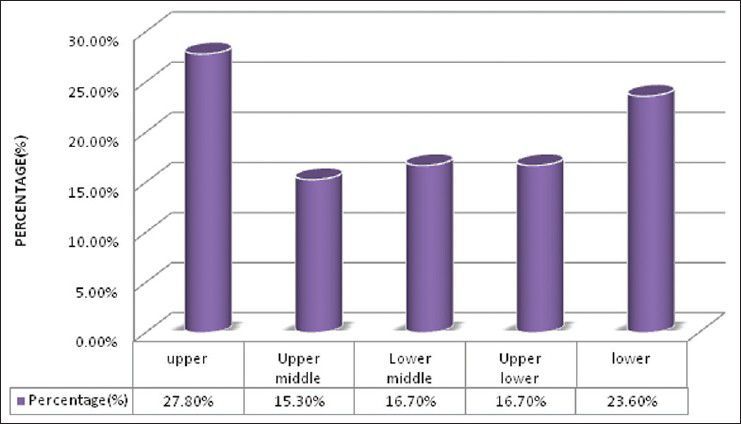
Bar diagram showing distribution of patients according to socioeconomic status by modified Kuppuswamy scale (P= 0.3732)
Figure 5.
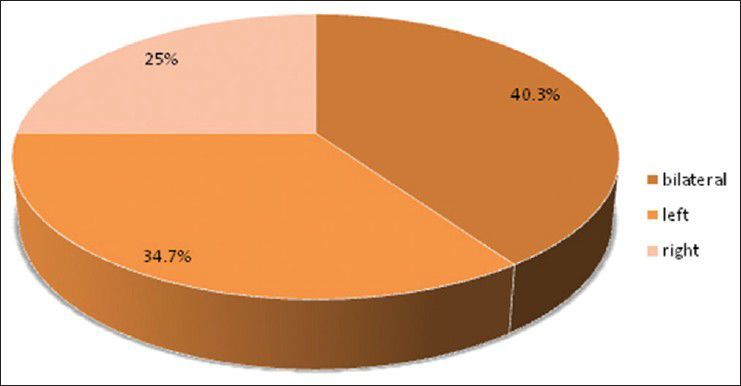
Pie diagram showing distribution of patients according to brain side involved (P= 0.2748)
Figure 6.
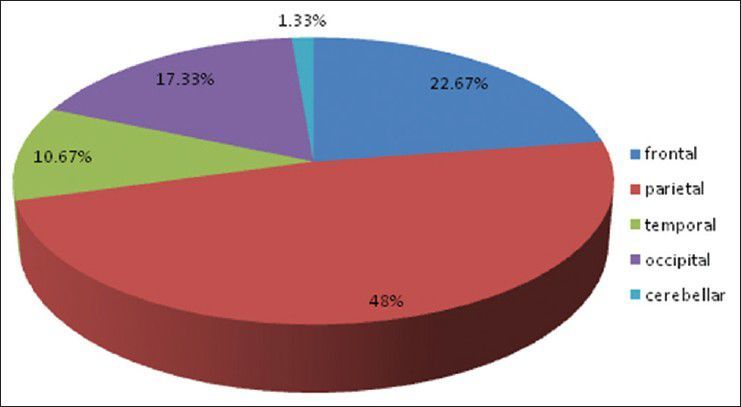
Pie diagram showing distribution of site of lesion in brain
Figure 7.
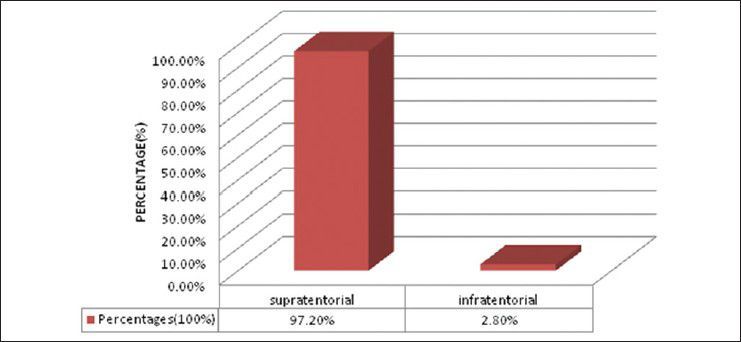
Bar diagram showing distribution of patients according to location of tumour (supratentorial vs. infratentorial) (P < 0.0001)
Figure 8.
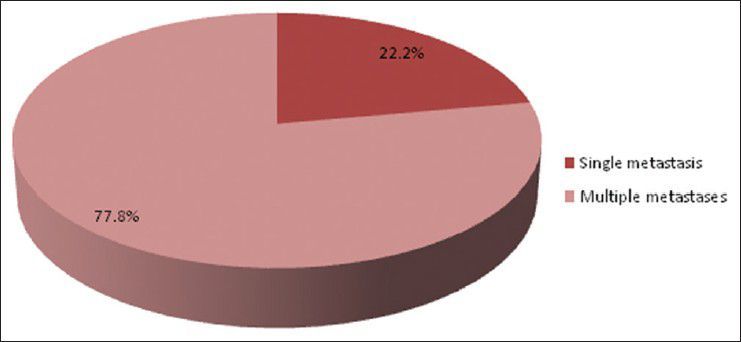
Bar diagram showing type of lesion (single vs. multiple). (P < 0.0001)
Figure 9.
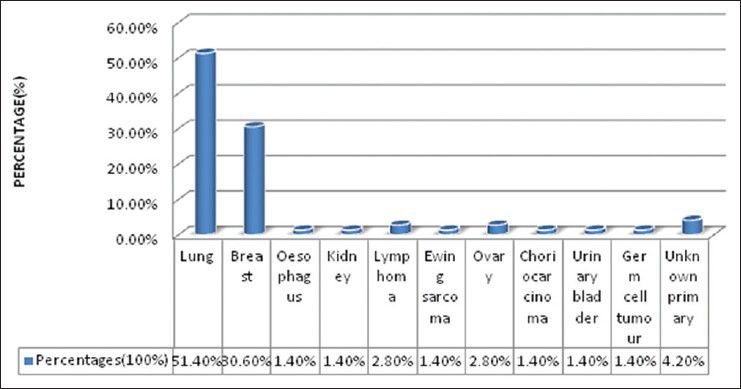
Bar diagram showing site of primary giving rise to metastasis (P < 0.0001)
Figure 10.
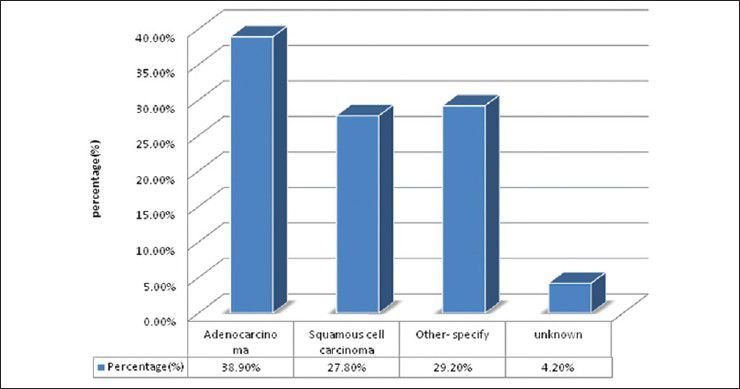
Bar diagram showing distribution of patients according to histology of primary (P= 0.0003)
Figure 11.
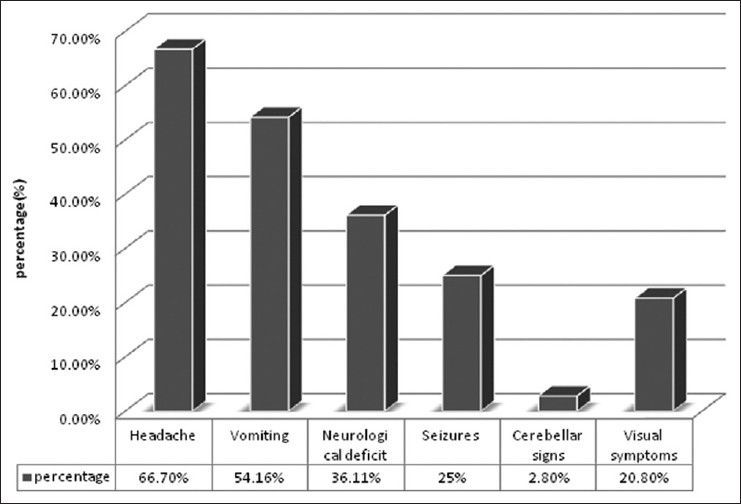
Bar diagram showing patients with different clinical symptoms
Figure 12.
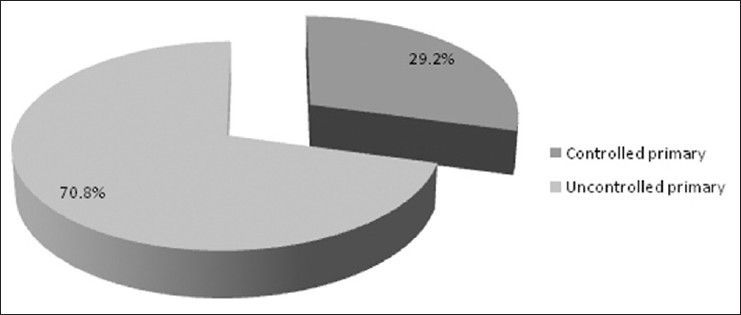
Pie diagram showing controlled primary versus uncontrolled primary (P= 0.0006)
Discussion
Brain metastases are the most common type of intracranial neoplasm, with the total number outnumbering primary brain tumors by a ratio of 10:1 and occur in about 25% of all patients with cancer. Brain metastases significantly increases morbidity and mortality in cancer patients.[1,2] Conservative estimates suggest that 100,000-170,000 new cases of brain metastases are diagnosed every year in the united states.[3] Treatment of brain metastases is multidisciplinary with radiation forming the cornerstone of treatment.[13,14]
Study by Victor[15] showed that about 60% of patients of brain metastasis are aged between 50 and 70 years. Metastasis is not common in children; accounts for 6% of all CNS tumor in children. Leukemia accounts for most metastatic CNS lesions in young patients – followed by lymphoma, osteogenic sarcoma and rhabdomyosarcoma. Germ cell tumors are common in adolescents and young adults between 15 and 21 years. Takokura et al. viewed that the age of onset of brain metastases in male is 56 years and in females 40 years.[16] In the present study, 44.44% of patients were in the 6th decade and 22.22% of patients were in 5th decade of life; which correlates with above study. The incidence of brain metastasis was also uncommon up to 3rd decade, in the present study. Victor[15] showed that Although melanoma spreads to the brain more commonly in males than in females, gender does not affect the overall incidence of brain metastases. Present study showed that there is almost equal distribution of sex male versus female (52.78% vs. 47.22%).
Debnath et al.[17] showed that the highest occupational group were day laborers (31.43%) followed by service-holders (22.85%) and farmers (20%). In our study, 29.2% of patients were day laborer followed by service holder and farmer both constituting 20.8%, which correlates with above study. There were no significant difference in distribution of patients according to socio-economic status by modified Kuppuswamy scale.[18] Nearly 27.8% were in upper socioeconomic status and 23.6% were in the lower socio-economic status. Debnath et al.[17] in their study showed that in the majority of patients with brain metastases histology of the primary was adenocarcinoma (40.00%) followed by small cell carcinoma of lungs (28.57%) and squamous cell carcinoma (22.86%). In the present study in the majority of patients histology of primary malignancy was adenocarcinoma constituting 38.9%. Squamous cell carcinoma accounts for 27.8%. In 4.2% of cases histology of the primary site was unknown, which correlates with above study.
Approximately 80% of lesions found in the cerebrum, 15% in the cerebellum and 5% in the brainstem as opined by Nussbaum et al.[19] and Delattre et al.[2] In the present study supratentorial involvement (97.2%) was much more commoner than infratentorial involvement (2.8%) and of which parietal lobe was the commonest site of involvement constituting 48% of all lesions. In the present study, out of total 72 patients 40.3% had bilateral involvement in brain. In 34.7% of patients only left side of the brain was involved compared with 25% of patients where only right side of the brain was involved.
Multiple secondaries predominant over solitary metastasis, which was opined by Posner et al.[9] Studies using CT scan data indicated that metastases to the brain are multiple in more than 50% of cases as shown by Delattre et al.[2] Recent experience with MRI indicates that proportion of multiple metastasis is higher and in the range of two-third to three-fourth of patients with brain metastases.[20] In our study, 77.8% of patients had multiple lesion and 22.2% had single lesion, which strongly correlates with above studies.
Lassman and De Angelis[21] reviewed nine studies and found the following variation in reported percentages of patients developing brain metastases for specific primary histologies: 18-64% (lung cancer), 2-21% (breast cancer), 2-12% (colorectal cancer), 4-16% (melanoma), 1-8% (kidney), 1-10% (thyroid) and 1-18% (unknown primary). In 2700 cases from the Memorial Sloan-Kettering Cancer Center in New York, Victor. showed the distribution of primary cancers as follows: 48% lung, 15% breast, 9% melanoma, 1% lymphoma (mainly non-Hodgkin), 3% gastrointestinal (GI) (3% colon and 2% pancreatic), 11% genitourinary (21% kidney, 46% testes, 5% cervix, 5% ovary), 10% osteosarcoma, 5% neuroblastoma and 6% head and neck tumor.[15] According to Takokura et al.[16] most common primary producing brain metastases are ca lung (48%), carcinoma breast (25%), GI tract (8%), genitourinary tract (6%), melanoma (6%) and others (13%). In the present study, carcinoma of the lung was the most common primary that metastatizes to brain (51.4%), followed by carcinoma breast (30.6%). In 4.2% of patients, the primary was unknown, but they had evidence of metastases in lymph node/lung, which were proved on cytology/biopsy. Our observation also correlates with the above study.
Approximately 60% of patients with brain metastases have subacute symptoms. Symptoms are usually related to the location of the tumor. Clinical symptoms or presentation of a patient with brain metastases have been described by Posner et al.[18] In his series, headache was the commonest clinical presentation observed in 49% of patients followed by mental changes in 32%, focal weakness in 30% and seizures in 18% of patients. Victor[15] found that headache (42%) and seizure (21%) are the 2 most common presenting symptoms. In addition, 35% of patients have cognitive dysfunction and 30% have motor dysfunction. In present study Headache (66.7%) was the most common symptom followed by vomiting (54.16%), neurological deficit (36.11%) and seizure (25%).
Victor[15] showed that maximum number of brain secondaries found in patients whose primary disease was not controlled at the time of presentation. In our study, at the time of detection of metastases, 29.2% of patients had controlled primary whereas in 70.8% of patients, the primary disease was not controlled, which also correlates with above study.
Acknowledgments
I would like to thank my patients who braved their disease and suffering during the course of my work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sawaya R, Ligon BL, Bindal RK. Management of metastatic brain tumors. Ann Surg Oncol. 1994;1:169–78. doi: 10.1007/BF02303562. [DOI] [PubMed] [Google Scholar]
- 2.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–4. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya R, Bindal RK, Lang FF, Abi-Said D. 2nd ed. New York: Churchill Livingstone; 2001. Metastatic brain tumors. [Google Scholar]
- 4.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 5.Patchell RA. Metastatic brain tumors. Neurol Clin. 1995;13:915–25. [PubMed] [Google Scholar]
- 6.Auchter RM, Lamond JP, Alexander E, Buatti JM, Chappell R, Friedman WA, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27–35. doi: 10.1016/s0360-3016(96)85008-5. [DOI] [PubMed] [Google Scholar]
- 7.Cherryman G, Golfieri R. Comparison of spin echo T1-weighted and FLASH 90 degrees gadolinium-enhanced magnetic resonance imaging in the detection of cerebral metastases. Br J Radiol. 1990;63:712–5. doi: 10.1259/0007-1285-63-753-712. [DOI] [PubMed] [Google Scholar]
- 8.Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470–6. doi: 10.1002/(sici)1097-0142(19961001)78:7<1470::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Posner JB. Brain metastases: 1995. A brief review. J Neurooncol. 1996;27:287–93. doi: 10.1007/BF00165486. [DOI] [PubMed] [Google Scholar]
- 10.Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: Double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:293–300. [PMC free article] [PubMed] [Google Scholar]
- 11.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44:275–81. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Yamamoto M, Hasegawa Y, Ando M, Shima K, Sako C, et al. Magnetic resonance imaging and computed tomography in the diagnoses of brain metastases of lung cancer. Lung Cancer. 2004;46:357–60. doi: 10.1016/j.lungcan.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Mintz A, Perry J, Spithoff K, Chambers A, Laperriere N. Management of single brain metastasis: A practice guideline. Curr Oncol. 2007;14:131–43. doi: 10.3747/co.2007.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JE, Robins HI, Mehta MP. Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol. 2007;5:54–64. [PubMed] [Google Scholar]
- 15.Victor TS. Brain metastasis. Medscape reference. [Last updated on 2011 Oct 7]. Available from: http://www.emedicine.medscape.com/article .
- 16.Takokura K, Ho SH, et al. Metastatic tumour of CNS Tokyo egarku, shoal. 1982 [Google Scholar]
- 17.Debnath H, Barua KK, Hossain MA, Khair MA, Islam MA. Outcome and prognosis of metastatic brain tumour: A study of 35 cases. Bangladesh J Neurosci. 2008;24:17–23. [Google Scholar]
- 18.Kumar N, Gupta N, Kishore J. Kuppuswamy's socioeconomic scale: Updating income ranges for the year 2012. Indian J Public Health. 2012;56:103–4. doi: 10.4103/0019-557X.96988. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–8. [PubMed] [Google Scholar]
- 20.Sze G, Milano E, Johnson C, Heier L. Detection of brain metastases: Comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990;11:785–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21:1–23. doi: 10.1016/s0733-8619(02)00035-x. vii. [DOI] [PubMed] [Google Scholar]


