Abstract
Melothria heterophylla (Lour.) Cogn., (family-Cucurbitaceae) popularly known as kundari, has been shown to exhibit antioxidant effects. The main objective was to isolate active constituents of the plant extract. In this study, the ability of M. heterophylla to induce apoptosis was studied in Ehrlich ascites carcinoma cells. Treatment of the Ehrlich ascites carcinoma cells with a variety of concentrations of the ethanol extracts of M. heterophylla and gallic acid (100-1000 μM), to determine the sequences of events marked by apoptosis, assayed by the spectrofluorometric method. Gallic acid and rutin were isolated from plant extract which were quantified by high-performance liquid chromatography. Our results indicate that ethanol extracts of M. heterophylla and gallic acid-induced apoptotic cell death in a dose dependent manner could be due to the generation of reactive oxygen species, especially H2O2, which is confirmed by caspase 3 activation. Treatment of Ehrlich ascites carcinoma bearing Swiss albino mice with varied doses (200 and 400 mg/kg, b.w.) of plant extract significantly reduced tumor volume and viable tumor cell count and improved hemoglobin content, RBC count, mean survival time, tumor inhibition, and percentage life span. The enhanced antioxidant status in extract-treated animals were evident from the decline in the levels of lipid peroxidation and increased levels of glutathione, catalase, and superoxide dismutase. The data suggest that M. heterophylla exerts anticancer activity, valuable for application in food and drug products.
Keywords: Melothria heterophylla; 2’,7’-dichlorofluorescein diacetate assay; EAC cells; gallic acid; rutin
Accumulation of reactive oxygen species (ROS) coupled with an increase in oxidative stress has been implicated in the pathogenesis of several disease states such as aging, inflammation, and neurodegenerative disorders. The role of oxidative stress in vascular diseases, diabetes, renal ischemia, atherosclerosis, pulmonary pathological states, inflammatory diseases, and cancer have been well established. Free radicals and other reactive species are constantly generated in vivo after exposure to drugs, xenobiotics, or ionizing radiation[1] and causes oxidative damage to biomolecules, a process held in check by the existence of multiple antioxidant and repair systems as well as the replacement of damaged nucleic acids, proteins, and lipids. Measuring the effect of antioxidant therapies and ROS activity intracellularly is crucial for suppressing or treating oxidative stress inducers.
The major biochemical change associated with cancer cells after treatment with anticancer drugs is the increase in ROS generation which is often considered as a cancer-promoting factor[2]. Studies have demonstrated that high levels of ROS can cause cellular damage and play an important role in mediating apoptosis. Interestingly, ROS has been demonstrated to selectively kill cancer cells[3].
Melothria heterophylla Lour. Cogn., Family- Cucurbitaceae, popularly known as kundari, a scandent herb with tuberous roots found throughout India ascending up to 2100 m in the hills. They are used by the tribals of Orissa for their stimulant, invigorating, antidiabetic, purgative property[4,5,6], and antioxidant activity[7]. The juice of the leaves is applied to the parts inflamed by the application of the marking nut juice (from Semecarpus anacardium Linn). Anticancer effects of polyphenolic compounds in plants are well established[8]. Therefore, based on the main components of M. heterophylla extract and its antioxidant properties, we hypothesized that ethanol extract might have anticancer activities against Ehrlich ascites carcinoma cells in vivo. However, that there is no scientific evidence to support the anticancer effect of M. heterophylla (Lour.) Cogn. Hence, the objective of this study was to evaluate the anticancer activity of M. heterophylla extract, and its active constituents.
Among all of the chromatographic methods, HPLC is applied widely to determine the chemical constituent present in the plant extract. In the study, HPLC with DAD were used for the determination of gallic acid (GA) and rutin (RU) present in the ethanol extract of aerial parts of M. heterophylla. The results indicate that this method is suitable for quantitative assessment of the ethanol extract of M. heterophylla.
MATERIALS AND METHODS
Male Swiss albino mice weighing 20±2 g were used in the present investigation. They were housed in clean polypropylene cages and were fed with a standard pellet diet (Hindustan Lever, Kolkata, India) and water ad libitum. The animals were acclimatized to laboratory condition for 1 week before the start of the experiment.
The aerial parts of Melothria heterophylla were collected from young matured plants during the month of August-September, from the rural belt of Mayurbhanj district, Odisha, India and identified by taxonomists at Botanical Survey of India, Howrah, India. A voucher specimen (CNH/I-I (65) 2006/Tech.II/1661) was deposited in the Department of Pharmaceutical Technology, Jadavpur University. The collected plant material was washed, shade-dried, and then milled to a coarse powder by a mechanical grinder for further studies.
HPLC grade methanol and water used in HPLC analysis were procured from Merck (Darmstadt, Germany). All other chemicals were of analytical grade purchased locally. Intracellular ROS Assay Kit was purchased from Cell Biolabs Inc., (San Diego, USA). The IR spectra were measured on a FT-IR Shimadzu spectrophotometer. 1H nuclear magnetic resonance spectra were recorded in CDCl3 using FT-NMR Bruker Avance 300 MHz spectrometers. The EI-MS mass spectra were obtained using a MAT 95×L mass spectrometer (Thermo Finnigan, San Jose, CA, USA).
Extraction and isolation of active compounds:
The powdered plant material (40 mesh size) was extracted with petroleum ether (40-60°), ethanol using soxhlet apparatus. The solvent was then removed under reduced pressure, to obtain petroleum ether (PEMH, yield 4.38%), ethanol (EEMH, yield 5.30%), respectively. The ethanol extract (3 g) was fractioned over silica gel column eluted with dichloromethan:methonol with gradual increase in the methanal content and forty fractions were collected. Fraction 19-21 are mixed together which shows a single spot having similar Rf and was rechromatographed over silica gel column eluted with 100% methanol to afford 40 mg of compound 1.
The ethanol fraction (2 g) was fractionated by column chromatography over silica gel with dicholormethane:methanol:water in ratio from 7:1:0.5 to 12.5:6:2 to yield subfractions (Fr. 1–Fr. 30). Subfraction Fr. 22 (372 mg) was further purified on a silica gel column with ethyl acetate:methanol:water (93:4:3) to give compound 2 which was recrystallized from MeOH to yield pure compound 2 (100 mg) as yellowish amorphous powder.
Liquid chromatography and identification of known active compounds:
The HPLC system consisted of Millennium32 system software (Waters company, USA), Waters 717 plus Autosampler (Waters company, USA), Model Waters Delta 600 pump (Waters company, USA), and Model Waters 2996 Diode Array Detector (Waters company, USA), HiQ Sil C18 V column (250×4.6 mm, KYA Technologies Corporation, Japan).
The dried ethanol extract was dissolved in the mobile phase (10 mg/ml). After filtering through a filter paper and a 0.45 μm membrane filter (Millipore), the extract was injected directly. The chromatographic analysis of the samples was carried out by HiQ Sil C18V reversed-phase column (250×4.6 mm) packed with 5 μm diameter particles. The conditions for HPLC: The mobile phase was methanol:water (25:75 v/v) containing 1.0% acetic acid. This mobile phase was filtered through a 0.45 μm membrane filter (Millipore), then deaerated ultrasonically prior to use. Compounds 1 and 2 were quantified by DAD following RP-HPLC separation. Flow rate and injection volume were 1.0 ml/min and 5 μl, respectively. The elution was monitored by the UV detector and the detection wavelength was set at 254 nm and the temperature was 20° during the HPLC run. Each sample was in triplicates. Compounds 1 and 2 were identified by HPLC with a comparison of retention times with reference compounds.
Acute toxicity study:
This was performed for the extract according to the acute toxic classic method as per the method of Litchfield and Wilcoxon[9]. The Institutional Animal Ethical Committee (CPCSEA/ORG/CH/2006/Reg.No. 95) permitted the use of the animals for this purpose. Healthy male Swiss albino mice were fasted overnight, but with free access to water. Animals were randomly divided into five groups (n=10/group). The first group serves as the control group which received normal saline orally. Groups 2, 3, 4, and 5 were treated with suspension of ethanol extract of M. heterophylla at doses of 0.250, 0.500, 1.0, and 2.0 g/kg, p.o., respectively. Animals were observed for mortality, general behavioral, neurological, autonomic profiles, and hazardous symptoms for a period of 72 h after treatment.
In vivo antitumor activity:
The antitumor activity of the EEMH was evaluated using ascites tumor models. Ehrlich ascites carcinoma (EAC) cells were obtained from Chittaranjan National Cancer Institute, Kolkata, India. The EAC cells were maintained in vivo in Swiss albino mice, by intraperitoneal (ip) transplantation of 2×106 cells/mouse after every 10 days interval. EAC cells of 9 days old were used for the screening of ethanol extract of M. heterophylla Lour. Cogn (EEMH).
Animals were divided into five groups of ten animals each. All the groups were injected intraperitonially (ip) with 2×106 viable EAC cells with ice-cold normal saline (0.9% w/v) (aspirated from EAC bearing mice at the log phase 7-8th day) except Group I (served as normal control). Group I served as saline control (5 ml/kg 0.9% NaCl w/v, po). Group II served as EAC control. Twenty-four hours after EAC transplantation, Groups III and IV received EEMH (200 and 400 mg/kg, ip), and Group V received reference drug 5-fluorouracil (5-FU, 20 mg/kg, ip) daily for 14 consecutive days[10]. Food and water were withheld 18 h before killing the animals. On the 15th day, six mice from each group were killed 24 h after the last dose and the rest were kept with food and water ad libitum to check the increase in the lifespan of the tumor hosts. After killing the animals, blood was collected to evaluate the hematological and biochemical parameters. The antitumor activity of the EEMH was measured in EAC animals with respect to the following parameters.
Tumor volume and percentage increase in life span:
The ascitic fluid was collected from the peritoneal cavity, and volume was measured by taking it in a graduated centrifuge tube. The effect of MECM on percentage increases in life span was calculated on the basis of mortality of the experimental mice. ILS% = [(Mean survival time of treated group/Mean survival time of untreated group)−1]×100 and Mean survival time=(First death+last death)/2.
Tumor cell count:
The ascitic fluid was taken in an RBC pipette and diluted until the cells become freely separated in the slide and countable easily. The dilution factor was mentioned and a drop of the diluted cell suspension was placed in the Neubauer counting chamber and the number of cells in the 64 small squares was counted in a compound microscope under 10×magnification.
Viable/nonviable tumor cell count:
The cells were taken from ascitic fluid and stained with trypan blue (0.4% in normal saline) dye. The cells that did not take up the dye were viable and those, which took the stain, were nonviable. These viable and nonviable cells were counted. The cell count was calculated by using the formula, Cell count=[(No. of cells×Dilution)/(Area×Thickness of liquid film)].
Hematological studies:
The effect of EEMH on peripheral blood was investigated. RBC, WBC counts, and estimation of hemoglobin was done with standard procedures. Red blood cell count (RBC), hemoglobin content[11], and white blood cell count (WBC)[12], were measured from the blood obtained intracardially.
In vivo antioxidant study:
The antioxidant study was performed on liver and evaluated by measuring the level of lipid peroxidation and the amount of enzymatic and nonenzymatic antioxidant. Microsomal lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Okhawa et al.[13]. The nonenzymatic antioxidant such as reduced glutathione (GSH) level was measured by Mulder et al.[14] method. The nonenzymatic antioxidant such as catalase (CAT) activity and superoxide dismutase (SOD) were assayed by the method of Luck[15] and Kakkar et al.[16] respectively.
Processing of tumor cells collected from tumor-transplanted mice:
EAC cells were collected from the animal's body after giving the last dose and kept at 37° for 1 h for macrophage adherence. Tumor cells isolated were treated with 0.83% ammonium chloride to remove blood cell contamination. Cells were used for assays after four washes with PBS.
Measurement of reactive oxygen species generation:
Intracellular ROS production was detected by flow cytometry using 2’,7’-dichlorofluorescein-diacetate (DCFH-DA) which is a specific probe for the presence of hydrogen peroxide[17]. EAC cells collected 12 h after treatment with the last dose of EEMH and gallic acid were used to measure ROS (long lived secondary radicals). Cells (1×106) harvested from all groups of mice were counted and labeled with 1 μl of H2 DCFDA (13 mM) fluorescence probe for measuring ROS and incubated at 37° for 30 min in the dark. Cells were then collected, washed, and resuspended in PBS and analyzed immediately using flow cytometry Becton–Dickinson FACS-Calibur flow cytometer (Becton Dickinson, NJ) with the excitation and emission wavelengths of 480 and 530 nm, respectively.
Caspase 3 activity assay:
Effect of treatment on caspase 3 activation was determined by using ApoAlert caspase colorimetric assay kit from Clontech following the manufacturer's protocol, using 100 μg of whole-cell lysate. Absorbance was read at 405 nm in a microtiter plate reader.
Statistical analysis:
Data were statistically evaluated using one-way analysis of variance (ANOVA) followed by Tuckey's test using Graphpad Prism computer software, version 5. The data expressed as the mean±SD and P<0.05 were considered to be significant.
RESULTS
After separation, the purity of the two compounds was confirmed by analytical HPLC. Compounds 1 and 2 were eluted with retention time of 12.14 and 14.34 min, respectively (figs. 1 and 2). Their relative purity was 88.78 and 72.33% whereas their concentration is 0.17% and 0.12%, respectively. Final structure was confirmed by analyzing the spectra of IR, MS, 1H, and 13C NMR.
Fig. 1.
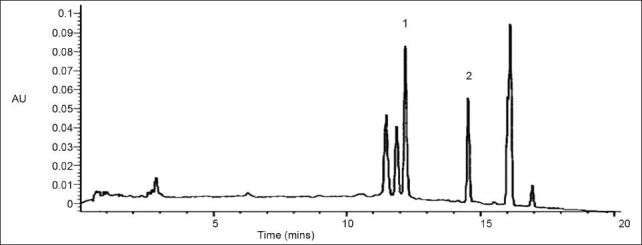
HPLC chromatogram of the ethanol extract of Melothria heterophylla.
(1) gallic acid (2) rutin
Fig. 2.
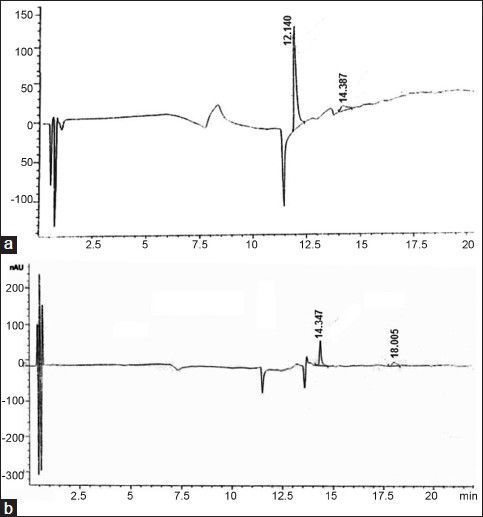
HPLC chromatogram of isolated gallic acid and rutin.
High-performance liquid chromatography profile og isolated gallic acid (a, Rt=12.14) and rutin (b, Rt=14.34).
Compound 1: White colored; TLC (hexane:ethyl acetate, 2:1 v/v) Rf, 0.46; UV λmax (ethanol) nm: 220, 271; IR (KBr) v cm-1: 3368, 3287, 3065, 1704, 1618, 1542, 1447, 1247 cm-1; MSES+ (m/z) 171.05 [M-H]- (calcd. for C7H6O5, 170.00). 1H NMR (CD3OD): δ H 7.097 (2H, s, H-3 and H-7); 13C NMR (CD3OD): δ C 170.44 (C-1), 145.83 (C-4 and C-6), 139.31 (C-5), 121.79 (C-2), 110.65 (C-3 and C-7), which confirms it to be gallic acid.
Compound 2: yellowish amorphous powder. ESI-MS: m/z 633.1 [M+Na]+ in positive providing the molecular weight of 610, which is in agreement with the molecular formula of rutin (C27H30O16). IR Vmax=3421 (OH), 1601.59, 1504.2, 1361.5 (arom. C=C), 1294, 1063.55 (glycosidic C-O) cm-1. 1H NMR (500 MHz; MeOH-d3):δ 1.15 (3H, d, J=9 Hz, H-18), 3.34 (2H, dd, J=6 Hz, H-8), 3.43 (1H, d, H-31), 3.46 (1H, s, H-32), 3.51 (1H, d, H-30), 3.55 (1H, d, 9 Hz, H-17), 3.57- 3.65 (1H, m, H-5, 9, 14), 3.65 (1H, d, H-15), 4.63 (1H, s, H-14), 5.13 (1H, d, J=6 Hz, H-11), 6.21 (1H, d, H-11), 6.40 (1H, d, H-27), 6.90 (1H, d, J=9Hz, H-29), 7.63 (1H, dd, J=3Hz, H=39), 7.66 (1H, dd, J=3Hz, H-38), 7.68 (1H, d, H-35). 13C NMR: 179.23 (C-21), C-28 (165.85), C-23 (159.22), C-25 (158.32), C-36 (145.68), C-39 (123.56), C-38 (117.69), C-2 (104.76), C-9 (99.89), C-5 (71.28), C-4 (72.16), C-13 (73.87), C-9 (75.66), C-18 (17.86).
Acute toxicity study:
In the acute toxicity assay it was found that no mortality was observed up to doses of 2000 mg/kg, orally and hence it was considered as safe. Also there were no signs of any toxic reaction found till the end of the study period.
Antitumor activity:
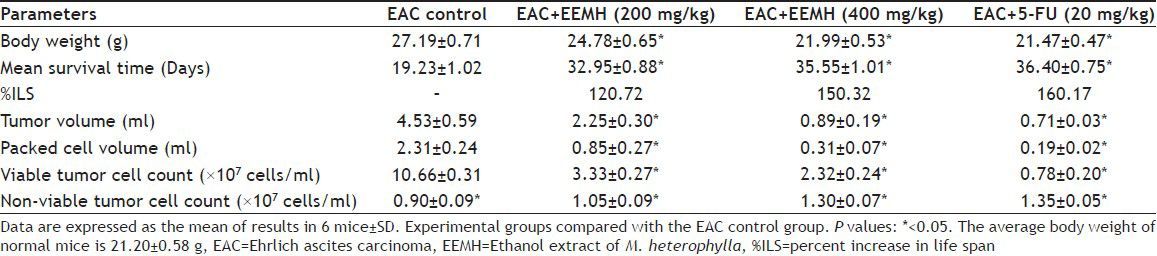
The antitumor activity of EEMH against EAC tumor bearing mice was assessed by the parameters such as tumor volume, packed cell volume, cell count (viable and non viable), mean survival time, and a percentage increase of life span. The antitumor activity of EEMH on EAC bearing mice is shown in Table 1. There is a significant increase in body weights in EAC bearing mice (27.19±0.71 g) as compared with normal group (21.20±0.58 g). Treatment with EEMH (200 and 400 mg/kg) significantly (P<0.05) reduced the increase in the body weight of EAC bearing mice. There was a significant increase in survival time in treating with EEMH at 200 and 400 mg/kg. A significant (P<0.05) decrease of tumor volume and packed cell volume was observed in EEMH treated mice compared to the EAC control group. The number of viable cell count was decreased whereas the number of nonviable cell count was increased on treatment with EEMH.
TABLE 1.
EFFECT OF EEMH ON BODY WEIGHT, SURVIVAL TIME, TUMOR GROWTH OF EAC BEARING MICE
Hematological parameters:
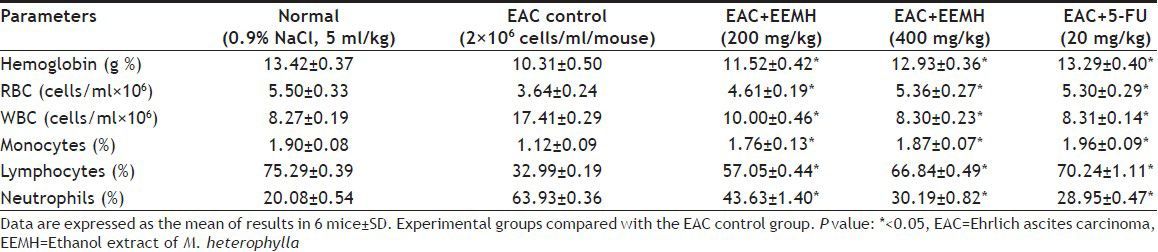
Hematological parameters of EEMH treated EAC bearing mice are presented in Table 2. The total WBC count was found to increase along with a reduction in the RBC count and hemoglobin content in tumor-bearing mice. A reduction in hemoglobin has been observed in an EAC control group with respect to normal mice, but in all the treated groups the hematological parameters were significantly (P<0.05) restored toward the normal.
TABLE 2.
EFFECT OF EEMH ON HEMATOLOGICAL PROFILES OF EAC BEARING MICE
Effect of EEMH on lipid peroxidation and different antioxidant enzyme activity:
Malondialdehyde (MDA) is the common marker of lipid peroxidation. MDA, the end product of lipid peroxidation, was reported to be higher in the cancer cell. The MDA content was significantly increased in EAC treated mice (Table 3). On treatment with EEMH significantly decreased the MDA content. The glutathione, SOD and catalase activity was decreased in EAC treated mice (Table 3). All the parameters were restored significantly (P<0.05) toward the normal on treatment with EEMH.
TABLE 3.
EFFECT OF EEMH ON LIPID PEROXIDATION AND DIFFERENT ANTIOXIDANT ENZYMES ACTIVITIES IN EAC-TREATED MICE
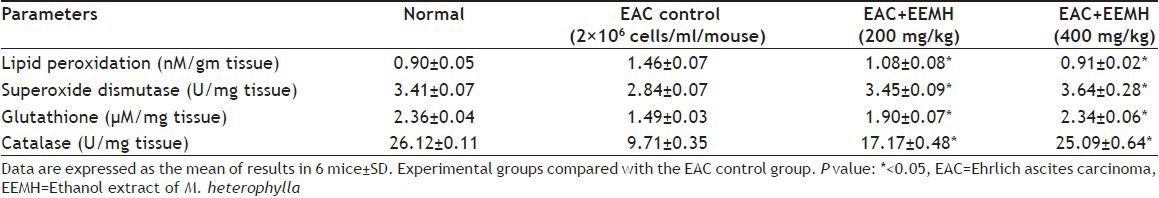
Effect of EEMH and Gallic acid on intracellular ROS generation:
2’,7’-dichlorofluorescein-diacetate (DCF-DA) is a specific superoxide tracing dye, which is frequently used to monitor H2O2 and hydroxyl radical levels in cells. Fluorescent intensity units directly represent the amount of ROS production. GA (100-1000 μM) concentration dependent changes in DCF fluorescence were observed within 12 h after GA treatment (fig. 3). As shown in figs. 3 and 4, treatment with EEMH and gallic acid markedly increased the DCF-DA-derived fluorescence (530 nm). Fig. 5 shows that DCF-fluorescence intensity after EEMH and gallic acid exposure at 12 h. Treatment of EAC cells with PEG-catalase to degrade intracellular H2O2 suppresses the increase in H2O2 generation.
Fig. 3.
Effects of GA on ROS generation in EAC cells.
GA (100-1000 μM) concentration dependent changes in DCF fluorescence. Results are mean values±SD (n=3).
Fig. 4.
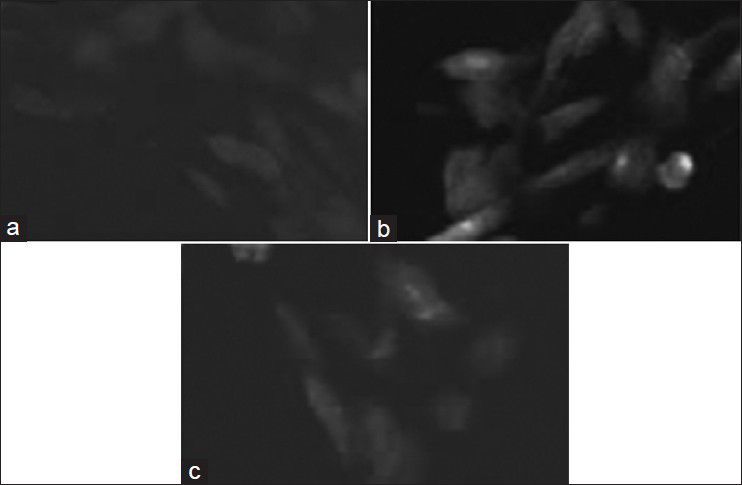
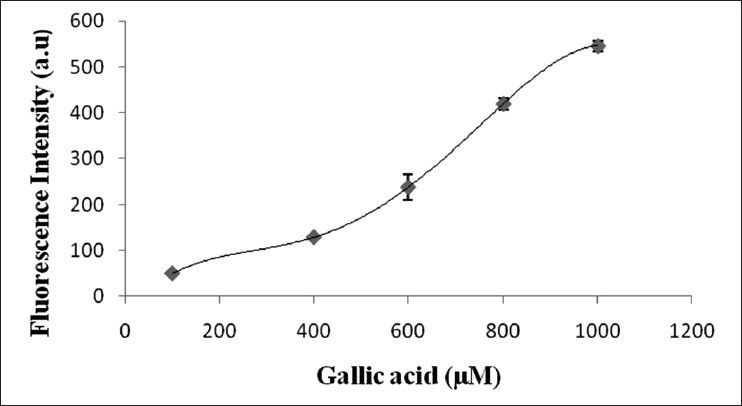
DCF fluorescence for ROS estimation.
Dichlorofluorescein fluorescence in untreated (a) EEMH (b) and GA (c) treated EAC cells after 12 h.
Fig. 5.
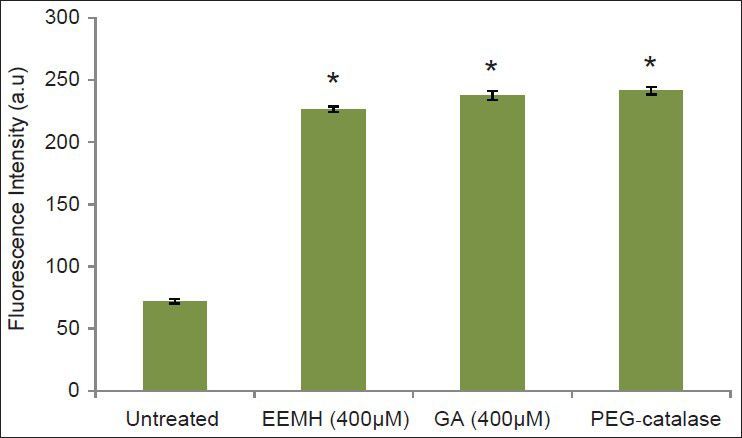
The effects of EEMH and GA on formation of DCF fluorescence in EAC cells preloaded with DCF.
Results are mean values±SD from three separate experiments. Statistical significance is indicated as (*) for P<0.05.
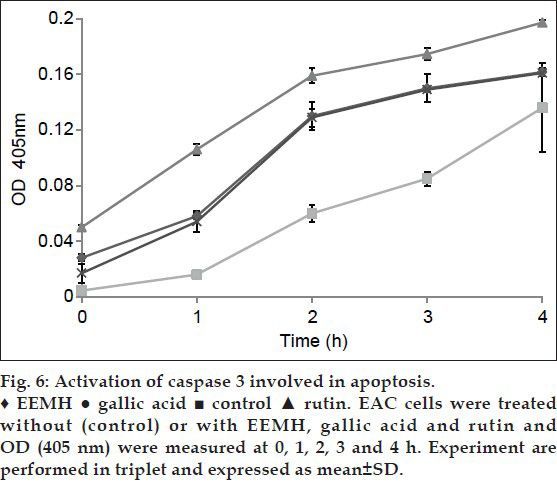
Caspase 3 activity assay:
Adding EEMH, gallic acid, and rutin that possess an inhibitory effect on caspase 3-like proteases abolished the increase in caspase 3 activity in the EAC cell lysates (fig. 6). These data suggest that activation of caspase 3-like proteases was involved in apoptosis.
Fig. 6.
DISCUSSION
The preliminary phytochemical screening of the extract showed the presence of steroids, glycosides, saponin, flavonoids and phenolic components. Gallic acid and rutin, the major components of ethanol extract of M. heterophylla (EEMH) were isolated and characterized in comparison with a standard one. The reliable criteria for judging the value of anticancer drugs are prolongation of life span, decrease of the WBC from the blood, and the decrease of tumor volume. The reduction in solid tumor volume indicated that EEMH plays a direct role in killing tumor cells and enhance the curative effect tumor chemotherapy. The level of lipid peroxidation, catalase, and protein contents were summarized in Table 3. Lipid peroxidation mediated by free radical considered being a primary mechanism of cell membrane destruction and cell damage. The present study indicated that EEMH significantly reduced the elevated levels of lipid peroxidation, and thereby it may act as an antitumor agent. A decrease in tumor volume and viable tumor cell count as mentioned above reduced the tumor burden and might have enhanced the life span of EAC bearing mice.
In cancer chemotherapy the major problem is of myelosuppression and anemia. The anemia encountered in tumor bearing mice is mainly due to reduction in RBC or hemoglobin percentage and this may occur either due to iron deficiency or due to hemolytic or myelopathic conditions. Treatment with EEMH brought back the hemoglobin content, RBC and WBC cell count near to normal values. This indicates that EEMH possess protective action on the heamatopoietic system. Excessive production of free radicals resulted in oxidative stress, which leads to the damage of macromolecules such as lipids that can induce lipid peroxidation in vivo. Increased lipid peroxidation would cause degeneration of tissues. Lipid peroxide formed in the primary site would be transferred through the circulation and provoke damage by propagating the process of lipid peroxidation. MDA, the end product of lipid peroxidation was reported to be higher in carcinomatous tissues than in nondiseased organs.
Glutathione, a potent inhibitor of a neoplastic process plays an important role of endogenous antioxidant system that is found particularly in high concentration in the liver and is known to have a key function in the protection process. EEMH reduced the elevated levels of lipid peroxidation and increased glutathione content in EAC bearing mice.
The DCHF-DA assay employs the cell-permeable fluorogenic probe whose fluorescence intensity is proportional to the ROS levels within the cell cytosol. The effect of antioxidant or free radical compounds on DCF-DA can be measured against the fluorescence of the DCF standard. The fluorescent intensity was measured spectrofluorimetrically with a fluorescence plate reader at 480 nm excitation/530 nm emission. These above results revealed that GA and EEMH induced ROS generation, and resulted in apoptosis. We propose that the gallic acid and rutin present in EEMH is responsible for its potent antitumor activity which can be inferred from the increased life span of EAC tumor bearing mice.
In conclusion, gallic acid and rutin were isolated from M. heterophylla and gallic acid worked as an antiproliferative agent in cancer cells, through the generation of ROS. The present study demonstrates that the ethanol extract of M. heterophylla Lour Cogn. increased the life span of EAC tumor bearing mice and decreased lipid peroxidation and thereby augmented the endogenous antioxidant enzymes in the liver. Overall, our results demonstrate that the fluorescence technique used in combination with the DCHF-DA assay is a suitable method for the direct quantitation of intracellular ROS levels in EAC cancer cells. It is very easy to use, extremely sensitive and inexpensive. The data suggest that M. heterophylla exerts antiproliferative action and growth inhibition of EAC cells through apoptosis induction confirming that it possesses anticancer properties valuable for application in food and drug products.
Footnotes
Mondal, et al.: Antitumor Potential of Melothria heterophylla
REFERENCES
- 1.Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin JP, Mankhetkorn S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2’,7’- dichlorofluorescein diacetate assay. Radiat Phys Chem. 2005;72:323–31. [Google Scholar]
- 2.Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, et al. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–71. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Tobar N, Cáceres M, Santibáñez JF, Smith PC, Martínez J. RAC1 activity and intracellular ROS modulate the migratory potential of MCF-7 cells through a NADPH oxidase and NFκB-dependent mechanism. Cancer Lett. 2008;267:125–32. doi: 10.1016/j.canlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kirtikar KR, Basu BD. 3rd ed. New Delhi: Sri Satguru Publication; 2000. Indian Medicinal Plants; p. 1618. [Google Scholar]
- 5.Anonymous. Vol. 6. New Delhi: Publications and Information Directorate, CSIR; 1962. The Wealth of India; p. 335. [Google Scholar]
- 6.Pant S, Samant SS. Ethanobotanical observations in the mornaula reserve forest of Kumoun, West Himalaya India. Ethnobot Leafl. 2010;14:193–217. [Google Scholar]
- 7.Mondal A, Maity TK, Pal DK, Sannigrahi S. In vitro antioxidant activity of the roots of Melothria heterophylla Lour Cogn. Pharmacologyonline. 2009;2:499–509. [Google Scholar]
- 8.Wang X, Wei Y, Yuan S, Liu G, Zhang YL, Wang W. Potential anticancer activity of litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2006;239:144–50. doi: 10.1016/j.canlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Litchfield JT, Wilcoxon FA. Simplified method of evaluating dose effect experiments. J Pharmacol Exp Ther. 1949;96:99–133. [PubMed] [Google Scholar]
- 10.Gupta M, Mazumder UK, Haldar PK, Kandar CC, Manikandan L, Senthil GP. Anticancer activity of Indigofera aspalathoides and Wedelia calendulaceae in Swiss albino mice. Iran J Pharm Res. 2007;6:141–5. [Google Scholar]
- 11.D’Amour FE, Blood FR, Belden DA. 3rd ed. Chicago: The University of Chicago Press; 1965. Manual for laboratory Work in Mammalian Physiology; pp. 4–6. [Google Scholar]
- 12.Wintrobe MM, Lee GR, Boggs DR. 5th ed. Philadelphia: Lea and Febiger; 1961. Clinical Hematology; p. 326. [Google Scholar]
- 13.Okhawa H, Oishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Mulder TP, Manni JJ, Roelofs HM, Peters WH, Wiersma A. Glutathione-S-transferases and glutathione in human head and neck cancer. Carcinogenesis. 1995;16:619–24. doi: 10.1093/carcin/16.3.619. [DOI] [PubMed] [Google Scholar]
- 15.Luck H. Methods of Enzymatic Analysis. In: Bergmeyer HV, editor. Vol. 3. New York: Academic Press; 1963. pp. 886–8. [Google Scholar]
- 16.Kakkar P, Das B, Vishwanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 17.Bhosle SM, Huilgol GN, Mishra KP. Enhancement of radiation-induced oxidative stress and cytotoxicity in tumor cells by ellagic acid. Clin Chim Acta. 2005;359:89–100. doi: 10.1016/j.cccn.2005.03.037. [DOI] [PubMed] [Google Scholar]


