Abstract
Azadirachta indica leaves indicated the presence of active principles with proven antioxidants, antiinflammatory, immunomodulatory, free radical scavenging and healing properties. In the present study we evaluated the healing effects of 50% ethanol extract of dried leaves of Azadirachta indica on trinitrobenzene sulfonic acid-induced colitis in rats. Azadirachta indica extract (500 mg/kg) was administered orally, once daily for 14 days and studied for its effects on diarrhoea, food and water intake, body weight changes, colonic damage and inflammation, histology, antibacterial activity and free radicals (nitric oxide and lipid peroxidation), antioxidants (superoxide dismutase, catalase and reduced glutathione) and myeloperoxidase activities in colonic tissue. Intracolonic trinitrobenzene sulfonic acid increased colonic mucosal damage and inflammation, diarrhea, but decreased body weight which were reversed by Azadirachta indica extract and sulfasalazine (positive control) treatments. Azadirachta indica extract showed antibacterial activity. Azadirachta indica extract and sulfasalazine enhanced the antioxidants but decreased free radicals and myeloperoxidase activities affected in trinitrobenzene sulfonic acid-induced colitis. Azadirachta indica extract, thus seemed to be effective in healing trinitrobenzene sulfonic acid-induced colitis in rats.
Keywords: Azadirachta indica, TNBS-colitis, myeloperoxidase, antioxidants, free radicals
Irritable bowel diseases (IBD) including ulcerative colitis (UC) is disorder with recurrent inflammatory involvement of large intestine. In UC, the colon becomes inflamed, often causing recurring abdominal pain including diarrhoea, blood in the stool and weight loss. Though exact etiology and pathophysiology is not known with certainty but genetic, immunological, reactive oxygen species (ROS) and environmental factors play a crucial role in the development of UC[1].
Azadirachta indica (Malvacea, AI) is popularly known as neem in the Indian subcontinent. Various parts of the neem tree like leaves, flowers, seeds, fruits, roots and bark have been used traditionally for the treatment of inflammation, microbial infections, antiseptic and skin diseases. Antiinflammatory, antiarthritic, hypoglycemic, antiulcer, antifungal, spermicidal, antibacterial, diuretics, antioxidant and immunomodulator actions have been reported in the extract of AI[2]. Considering the traditional uses of AI, the role of oxidative stress in the pathogenesis of UC and the presence of a number of compounds with antioxidant and anti-inflammatory properties prompted us to investigate the healing effect of 50% ethanol extract of dried leaves of AI (AIE) on trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
CF strain albino rats (180-220 g) of either sex were obtained and kept in the departmental animal house following the principles of laboratory animal care (NIH publication no. 82-23, revised 1985) guidelines. Approval from the Institutional Animal Ethical Committee was taken prior to the experimental work (Dean/2009-10/741 dated 11.12.2009). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma-Aldrich, St. Louis, MO; USA; sulfasalazine (SS) was procured from Wallace, Mumbai, India.
AI leaves were collected in the months of March to May from Ayurvedic Gardens, Banaras Hindu University. A total of 50% ethanol extract, of shade dried and powdered AI leaves (AIE), was prepared by cold decoction and the % yield was 10.2 (w/w).
Rats were given intracolonic normal saline (NS, 0.4 ml/rat, negative control) or TNBS alone (40 mg/0.4 ml of 40% ethanol/rat)[3] or intracolonic TNBS plus oral AIE (test extract) or standard colitis protective drug, SS (positive control). AIE (500 mg/kg)[4] and SS (100 mg/kg)[5,6] were suspended in 0.5% carboxymethyl cellulose (CMC) in distilled water and given orally, once daily for 14 days after induction of colitis with TNBS. Diarrhoea, food and water intake and body weight changes were calculated from their respective day 0 value and compared at day 7 and 14 of the experiment between TNBS and TNBS+AIE/SS groups.
The animals were killed on 15th day of experiment. Pathological changes (macroscopic) were seen by examination of 8 cm distal part of rat colon for severity and number of ulcers in terms of tissue damage score, thickening and adhesions[3]. A piece of colon was fixed in 10% buffered formalin and paraffin embedded. 4-6 μm thick sections were stained with haemotoxylin and eosin stain for histological evaluation.
Antioxidant properties using superoxide dismutase (SOD)[7], catalase (CAT)[8], and reduced glutathione (GSH)[9], free radicals scavenging by lipid peroxidation (LPO)[10] and nitric oxide (NO)[11] and acute inflammatory marker, myeloperoxidase (MPO)[12] and protein[13] was estimated in colonic mucosal homogenates. Antibacterial susceptibility was done against Escherichia coli ATCC 25922, Shigella boydii, Shigella sonnei and Shigella flexneri following the disk diffusion method while minimum inhibitory concentration (MIC) was performed by micro dilution method[4].
Adult Swiss albino mice of either sex, weighing between 25 to 30 g fasted overnight, were used for acute toxicity study. Suspension of AIE was orally administered at 2 g/kg start dose (4 times of the optimal effective dose of 500 mg/kg) to mice. Subsequent to AIE administration, animals were observed closely for first 4 h, for any toxicity manifestation, like increased motor activity, salivation, convulsion, coma and death. The animals were under further investigation up to a period of 14 days[14].
Statistical comparison was performed using either unpaired t-test or Chi-square test (for adhesion parameter only) or one way analysis of variance (ANOVA) and for multiple comparisons versus control group followed by Dunnett's test. All statistical analysis was performed using SPSS statistical version 16.0 software package (SPSS® Inc., USA). P<0.05 was considered statistically significant.
TNBS rats showed increase in faecal output by 43.1 and 52.7% (P<0.05) from day 0 (0 day faecal output of 2.33±0.13 g/100 g body weight), while TNBS+AIE/SS-treated rats showed decrease by 18.7/14.9% and 34.1/37.2% compared with TNBS group at day 7 and 14, respectively. TNBS-treated rats showed decrease in body weight from 199.4±2.19 g at day 0 to 182.8±3.77 (8.3% decrease, P<0.05) and 171.1±2.71 (14.2% decrease, P<0.05) at day 7 and 14, respectively, while AIE/SS-treated colitis rats showed reversal of their body weight by 7.1/8.5% and 10.9/12.7% at day 7 and 14 (P<0.05), respectively. However, little or no change was observed in the food and water intake amongst the groups at day 7 and 14, respectively. Normal saline (NS) did not show any colonic mucosal damage, inflammation or adhesion (0/6), while the colonic weight was 158.3±6.4 mg/cm. TNBS increased colonic mucosal damage score (5.17±0.31, P<0.05), adhesions (5/6 rats, 83.3%) and weight to 248.8±6.7 mg/cm (57.2% increase, P<0.05) compared with NS group while, TNBS+AIE/SS-treated rats showed decrease in colonic damage score, colonic weight and adhesions by 64.1/77.4%, 26.7/33.5% and 60/80.0%, respectively, (P<0.05), when compared with TNBS (fig. 1). Microscopic study of colon normal rats showed normal structure with intact epithelia, while TNBS rat showed severe mucosal erosion and transmural inflammation. AIE/SS treatment in TNBS-colitis rat showed the regenerative mucosa with mild lympho-plasmacytic infiltrate in the lamina propria (fig. 2).
Fig. 1.
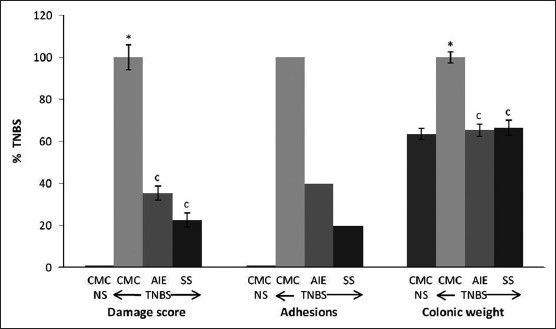
Effect of AIE and SS on rat colonic damage score, adhesions and colonic weight.
Results are percent mean ±SEM of TNBS (n=6). *P<0.05 compared to normal saline (NS) group (unpaired t-test), and cP<0.05 compared to respective TNBS group (one way ANOVA followed by Dunnett's test).
Fig. 2.
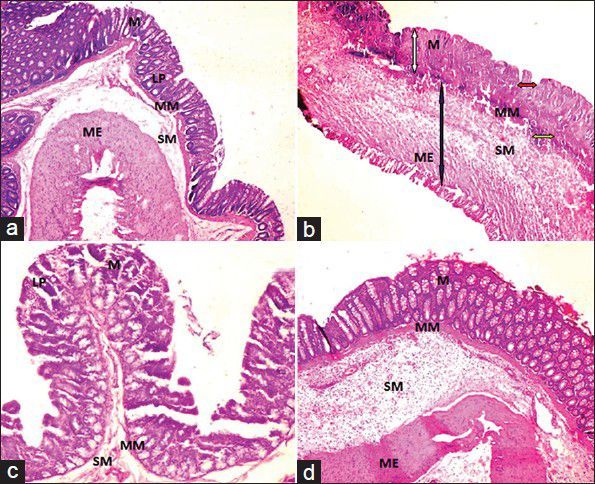
Photomicrographs of the rat colon (H and E, ×100).
(a) NS+CMC showing normal structure and clear with intact mucosa and sub mucosa. (b) TNBS+CMC showing ulcerated and eroded mucosa shown by white arrow, crypt destruction with severe cryptitis shown by red arrow, lymphoplasmacytic infiltrate shown by yellow arrow and transmural inflammation (predominantly-lymphocytes and plasma cells) shown by blue arrow. (c) TNBS+AIE showing regenerative mucosa with mild crypt distortion and mild lympho-plasmacytic infiltrate in the lamina propria with oedematous submucosa and (d) TNBS+SS showing intact mucosa with minimal lymphoplasmacytic infiltrate in the lamina propria. M: Mucosa; SM: Submucosa; LP: Lamina propria; MM: Muscularis mucosa; ME: Muscularis externa.
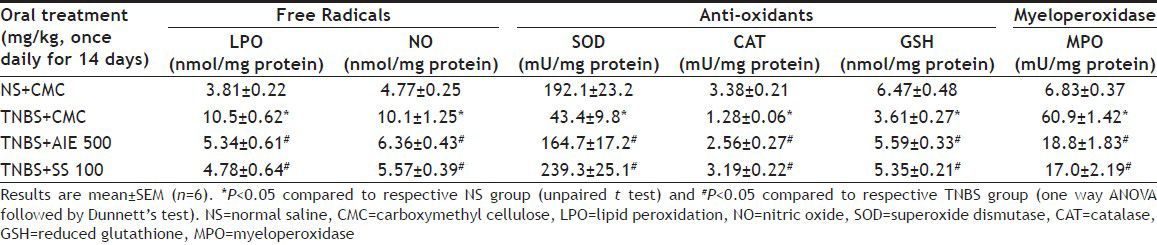
TNBS-treated animals showed a significant decrease in SOD, CAT and GSH enzymes activities in the colonic mucosal incubates when expressed either as mU (SOD and CAT) or nmol (GSH) per mg protein compared to NS-treated rats. Both AIE and SS when given for 14 days after TNBS induction of colitis reversed the above changes in SOD, CAT and GSH enzymes activities near to NS group. TNBS enhanced both LPO (LPO level was estimated in terms of malondialdehyde released during lipid peroxidation) and NO (nitrites and nitrates are formed as end products of reactive nitrogen products during NO formation which were measured by using Griess reagent) when expressed as nmol/mg protein compared to NS group. AIE and SS showed reversal of levels of both LPO and NO near to NS group. The effect on free radicals by AIE was comparable with SS. TNBS-treated animals showed significant increase in MPO enzyme activity in the colonic mucosal incubates when expressed as mU/mg protein compared to NS group. AIE and SS when given for 14 days after TNBS-induction of colitis reversed the above changes in MPO enzyme activity near to NS group (Table 1). AI extract showed positive susceptibility test against E. coli ATCC 25922, Shigella boydii, Shigella sonnei and Shigella flexneri at 200 mg/ml against all the above gram negative intestinal bacteria showing zone of inhibition ≥10 mm. MIC value against the above intestinal microorganism ranged from 12.5-25.0 mg/ml.
TABLE 1.
EFFECTS OF AIE AND SS ON TNBS-INDUCED COLONIC FREE RADICALS, ANTIOXIDANTS AND MYELOPEROXIDASE
Azadirachta indica extract even at 2 g/kg oral single dose did not show any acute toxicity manifestation like increased motor activity, salivation, colonic convulsion, coma and death, observed up to a period of 2 week.
The diarrhea and weight loss with TNBS could be due to alterations in the GIT absorptive functions produced, either directly or indirectly by products released from activated mast cells[15]. Treatment of colitis rats with AIE therefore reduced the colonic damage with concomitant increase in body weight, decrease in diarrhoea, fewer incidences of adhesions and decreased lympho-plasmacytic infiltration indicating healing effects in TNBS-induced colitis. TNBS has been reported to cause chronic inflammation of colon with changes in inflammatory mediators like prostaglandins, leukotrienes, platelet activating factor, MPO and interleukins[16]. Experimental and clinical evidences suggest that the inflamed colon undergoes substantial oxidative stress by neutrophils derived oxidants and MPO activity, both of which contribute markedly to tissue damage during chronic intestinal inflammation[17]. Increase in ROS and NO production and impaired antioxidant defense mechanisms are postulated to be causative factors in inflammatory diseases. Therefore, elimination of ROS could be an important strategy in treatment of colitis and antioxidants have been postulated to hasten the process of healing by destroying the free radicals[18]. TNBS-treated animals showed decrease in antioxidants and increase in free radicals levels and myeloperoxidase activity compared to NS-treated rats, while AIE possessed significant antioxidant activity reducing free radicals stress and decrease in colonic MPO activity which helped to prevent oxidative damage and promote the healing process.
AI contains important bioactive compounds such as phytosterols (sitosterols, sigmasterol, and campasterol) and flavonoids (rutin and quercetin), commonly known for their antioxidant activity, antiinflammatory and antimicrobial activities[4]. Role of microbial content of the gastrointestinal tract in the pathogenesis of colitis has been well established[19]. The activity exhibited by AIE at 200 mg/ml concentration was ≥10 mm, which have been considered as active dose[20] and this could be due to the presence of tannins and phenolic compounds in the extract which have been reported to have antimicrobial effect. The results of the present study with extract of leaves of AI do indicate promising healing effects in colitis.
ACKNOWLEDGEMENTS
This work was supported by Central Council for research in Ayurveda and Siddha (CCRAS), Department of AYUSH, Ministry of Health and Family Welfare, Govt. of India, New Delhi (BHU Research project no.: P-15-30).
Footnotes
Gautam, et al.; A. indica Attenuates Experimental Colitis
REFERENCES
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Maithani A, Parcha V, Pant G, Dhulia I, Kumar D. Azadirachta indica (neem) leaf: A review. J Pharm Res. 2011;4:1824–7. [Google Scholar]
- 3.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 4.Ghatule RR, Goel S, Gautam MK, Singh A, Joshi VK, Goel RK. Effect of Azadirachta indica leaves extract on AA-Induced colitis in rats: Role of antioxidants, free radicals and myeloperoxidase. Asian Pac J Trop Dis. 2012:S651–7. [Google Scholar]
- 5.Gautam MK, Goel S, Ghatule RR, Singh A, Nath G, Goel RK. Curative effect of Terminalia chebula extract on acetic acid-induced experimental colitis: Role of antioxidants, free radicals and acute inflammatory marker. Inflammopharmacology. 2013;21:377–83. doi: 10.1007/s10787-012-0147-3. [DOI] [PubMed] [Google Scholar]
- 6.Gautam MK, Ghatule RR, Singh A, Purohit V, Gangwar M, Kumar M, et al. Healing effects of Aegle marmelos (L.) Correa fruit extract on experimental colitis. Indian J Exp Biol. 2013;51:157–64. [PubMed] [Google Scholar]
- 7.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 8.Aebi HU. New York, USA: Academic Press; 1983. Catalase; Methods in enzymatic analysis; pp. 276–86. [Google Scholar]
- 9.Sedlak J, Lindsay RH. Estimation of total protein bound and nonprotien sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 11.Miranda KM, Epsey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Biol Chem. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 12.Bradley PD, Friebal DA, Christensen RD. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosenborough NJ, Farr AL, Randal RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Gautam MK, Singh A, Rao CV, Goel RK. Toxicological evaluation of Murraya paniculata (L.) leaves extract on rodents. Am J Pharm Toxicol. 2012;7:62–7. [Google Scholar]
- 15.Stein J, Ries J, Barret K. Disruption of intestinal barrier function associated with experimental colitis: Possible role of mast cells. Am J Physiol. 1998;274:G203–9. doi: 10.1152/ajpgi.1998.274.1.G203. [DOI] [PubMed] [Google Scholar]
- 16.Zea-Iriarte WL, Makiyama K, Goto S, Murase K, Urata Y, Sekine I, et al. Impairment of antioxidants in colonic epithelial cells isolated from trinitrobenzene sulphonic acid-induced colitis rats. Protective effect of rebamipide. Scand J Gastroenterol. 1996;31:985–92. doi: 10.3109/00365529609003118. [DOI] [PubMed] [Google Scholar]
- 17.Harris ML, Schiller HJ, Reilly PM, Donowitz M, Grisham MB, Bulkley GB. Free radicals and other reactive oxygen metabolites in inflammatory bowel disease: Cause, consequence or epiphenomenon. Pharmacol Ther. 1992;53:375–408. doi: 10.1016/0163-7258(92)90057-7. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Albumin: An important extracellular antioxidant. Biochem Pharmacol. 1988;37:569–71. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 19.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 20.Gautam MK, Gangwar M, Nath G, Rao CV, Goel RK. In vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata (L.) leaves. Asian Pac J Trop Biomed. 2012;2(suppl 3):S1660–3. [Google Scholar]


