Abstract
Seaweed extracts of Sargassum cinereum was used as a reducing agent in the eco-friendly extracellular synthesis of silver nanoparticles from an aqueous solution of silver nitrate (AgNO3). High conversion of silver ions to silver nanoparticles was achieved with a reaction temperature of 100° and a seaweed extract concentration of 10% with a residential time of 3 h. Formation of silver nanoparticles was characterised by spectrophotometry and the scanning electron microscope. The average particles size was ranging from 45 to 76 nm. Antimicrobial activities indicate the minimum inhibitory concentration of biologically synthesised nanoparticles tested against the pathogen Staphylococcus aureus with 2.5 μl (25 μg/disc). High inhibitions over the growth of Enterobacter aerogenes, Salmonella typhi and Proteus vulgaris were witnessed against the concentrations of 100 μg/disc. Promising potential and the future prospects of S. cinereum nanoparticles in pharmaceutical research are the highlights in this paper.
Keywords: Silver nanoparticles, Sargassum cinereum, AgNO3, SEM, antimicrobial activity
Seaweeds or marine algae constitute one of the commercially important renewable marine living resources. Work done for the last three decades were mainly on screening biologically active compounds in different seaweeds against various human pathogenic viruses, bacteria and fungi[1,2,3,4,5,6,7,8,9]. Many scientists have been attracted recently towards the biosynthesis of gold and silver nanoparticles using various plant sources and obtained a good nanoparticles with an average size of 20-30 nm[10,11,12,13]. Green synthesis of nanoparticles provides advancement over chemical and physical methods as it is cost effective, environment friendly, easily scaled up for large scale synthesis and further there is no need to use high pressure, energy, temperature and toxic chemicals. Biosynthetic methods employing either biological microorganism or plant extracts have emerged as simple and viable alternative to chemical methods but most of the methods are still in developmental stages[14].
Antimicrobial activity of silver has been recognised by clinicians for over 100 years[15]. In addition, reports suggest that hygienic benefits have been associated with the use of silver for considerably longer. Records show that Hippocrates recognised the role of silver in the prevention of disease and accounts exist that, the Romans stored wine in silver vessels to prevent spoilage[16]. Silver nanoparticles have also been demonstrated to exhibit antimicrobial properties both against bacteria[17] and viruses[18] with close attachment of the nanoparticles themselves with the microbial cells. Recent studies[19] showed the antimicrobial activity of Ag nanoparticles against yeast, Escherichia coli and Staphylococcus aureus. The importance of bactericidal nanomaterials study is increasing due to the increase of new resistant strains of bacteria against most potent antibiotics. This has promoted research in the well known activity of silver ions and silver-based compounds, including silver nanoparticles[20]. Current study discusses the silver nanoparticles synthesised by the aqueous extract of sea weed S. cinereum and its merits towards bactericidal activity.
Seaweed samples were freshly collected from the coastal areas of Goa, mainly Anjuna, Vagator and Dona Paula during May-June 2011 washed thoroughly with distilled water and identified as S. cinereum using the methods suggested by Jha et al[21]. The leaves were dried in an incubator for 2 days at 37°. Dried seaweed (25 g) was taken in a 500 ml conical flask added with 200 ml distilled water, boiled for 30 min and then filtered through Whatman no. 1 filter paper. This extract was dried with a rotary evaporator; 10 mg of the above was dissolved in 1 ml of sterile distilled water and stored in a refrigerator. This extract was later used to test antibacterial activities.
Aqueous solution (1 mM) of silver nitrate (Sigma, MO, USA, 58157) was prepared in milli-Q water and used for synthesising silver nanoparticles. Seaweed extract (5 ml, 10%) was added to 45 ml of 1 mM silver nitrate for reduction in to Ag+ ions. The mixed solution was subjected to vigorous stirring for 3 h using a magnetic stirrer. The reaction mixtures were continuously monitored for its colour change from yellowish green to orange. This indicates the formation of silver particles and the products were tested.
UV/Vis spectral analysis was done using Schimadzu UV-2450 spectrophotometer with a resolution of 1 nm between 200 and 600 nm possessing the scanning speed of 500 nm/min. The reduction of Ag+ ion was monitored by measuring the UV/Vis spectrum of the reaction medium. Nanoparticle solution showed the peak areas between 380 and 450 nm indicated the presence of silver nanoparticles.
To optimise the time required for the completion of nanoparticle formation, the reactions were monitored from 0 to 150 min. At every 30 min interval the solutions were removed from the reaction flasks and scanned between 200 and 600 nm with a spectrophotometer.
Solutions containing the silver nanoparticles were centrifuged and filtered using a 0.2 μm Whatman filter paper. The supernatant was taken on a glass plate and heat fixed using an incubator. The dried powder fixed to the glass plate was then mounted on the specimen holder and used for characterisation studies. The sample was gold coated to make the sample conducting, using a sputtered and analysed by a scanning electron microscope (SEM, Joel JSM-6360A, Japan). SEM data were worked out with ImageJ software available in the internet to analyse the sizes of the nanoparticles.
Clinical isolates of both Gram-positive (Staphylococcus aureus) and Gram-negative (Enterobacter aerogenes, Salmonella typhi and Proteus vulgaris) bacterial strains were obtained from the Department of Microbiology, Goa Medical College and Hospital, Goa. The pathogens were maintained on nutrient agar (NA) (Hi-Media Laboratories Pvt. Ltd., Mumbai) and used for the antibacterial assay by standard disc diffusion method. Care was taken while handling these microbes in connection to biosafety and our institute instructions were followed strictly. Overnight grown inoculum 100 μl were spread plated on NA. Sterile paper discs of 5 mm diameter containing the silver nanoparticles (i.e., 2.5, 5, 7.5 and 10 μl) equivalent to the concentrations of 25, 50, 75 and 100 μg/disc were placed in each plate. The plates were incubated at room temperature for 24 h and the zone of clearance around the disc was measured. Standard antibiotics like ampicillin (25 μg/disc); streptomycin (300 μg/disc) and tetracycline (10 μg/disc) (Hi-Media, India) were also tested against the silver nanoparticles to compare with.
Reduction of silver ions to silver nanoparticles by exposing the silver nitrate to seaweed extract was tracked by monitoring the changes in the colour with UV/Vis spectroscopy. Silver nanoparticles are known to exhibit a UV/Vis absorption maximum in the range of 380-450 nm due to their size-dependent optical properties[22]. S. cinereum extract showed colour change from brown to reddish-yellow and had a peak at 342-408 nm, this may be due to the excitation of surface plasmon vibrations and it provides a convenient spectroscopic signature to indicate the formation of silver nanoparticles. Metals like silver, gold, palladium exhibit unique optical properties due to the property of surface plasmon resonance (SPR) which is the collective oscillation of the conduction electrons in resonance with the wavelength of irradiated light. The size and shape of metal nanoparticles determine the spectral position of plasmon band absorption as well as its width. Gold and silver nanoparticles also exhibit size-dependent optical properties[23]. The nanoparticles synthesised using seaweed S. cinereum gave a particle size varying from 46 to 76 nm.
UV/Vis spectrum of silver nanoparticles formed with S. cinereum extract is given in fig. 1. The UV spectrum showed characteristic absorption at 408 nm for silver nanoparticles, 220 nm for silver nitrate and 301 nm for sea weed extracts. The series of peaks show the formation of silver nanoparticles of different sizes and shapes. The time for completion of the reaction was 3 h. As the duration of reaction increases, more silver nanoparticles are formed. Due to the instability of the silver nanoparticles formed an agglomeration may happened that leads to larger particle sizes. So the maximum time required for the completion of this process was stopped with 3 h. The formation of silver nanoparticles has been observed only after 60 min (fig. 2). Before that there is no indication of nanoparticle formation. When the time increased from 60 to 180 min with an interval of 30 min the peak area was increasing this indicate high amount of nanoparticle formations.
Fig. 1.
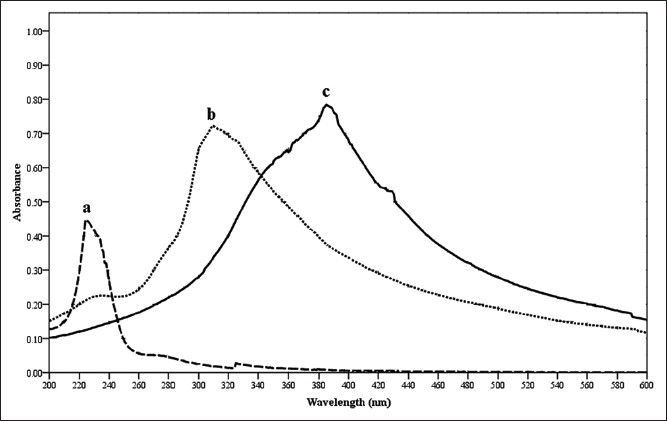
UV spectra.
Overlaid UV spectrum of silver nitrate (a), seaweed extract (b) and silver nonoparticle solution (c) during preliminary screening of Silver nanoparticles.
Fig. 2.
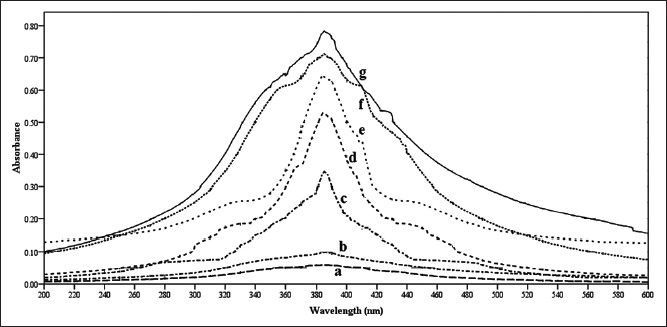
Various reaction times tested for silver nanoparticle formation.
Different reaction times were evaluated for optimization of silver nanoparticles synthesis like, a:0; b:30; c:60; d:90; e:120; f:150; g:180 min.
Antimicrobial activity tested with silver nanoparticles synthesised from S. cinereum showed good activity against the pathogenic bacteria Enterobactor aerogens, Proteus vulgaris and Salmonella typhi. Staphylococcus aureus was shown slightly lower resistance while comparing with other pathogens. In general S. cinereum extract exhibited the zone of inhibitions from 10 to 29 mm. Even the minimum concentration of 2.5 μl sample tested could give the best activity against these pathogens (Table 1). The antibacterial effect of nanoparticles can be attributed to the stability in the medium as colloid, which modulates the phosphotyrosine profile of the bacterial proteins and arrest bacterial growth. Silver nanoparticles synthesised by us were showing much better antibacterial activity against the multidrug-resistant organism Enterobactor aerogens. Equivalent resistance with standard ampicillin (25 μg/disc) were shown by nanoparticles; the strain Proteus vulgaris observed to have more resistance to our nanoparticles than the ampicillin and slightly better than streptomycin (300 μg/disc) and tetracycline (10 μg/disc) Staphylococcus aureus indicated low resistance while comparing with the standard antibiotics tested. Salmonella typhi showed its resistance with our silver nanoparticles in equivalent to standard antibiotics, in some cases it behaved much better resistance than the standards (Table 2).
TABLE 1.
ANTIBIOTIC SENSITIVITY TEST FOR THE SILVER NANOPARTICLES
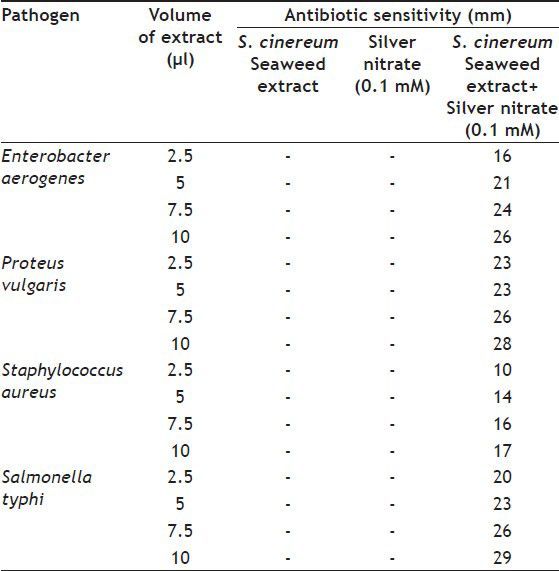
TABLE 2.
ANTIBIOTIC SENSITIVITY TEST OF THE STANDARDS
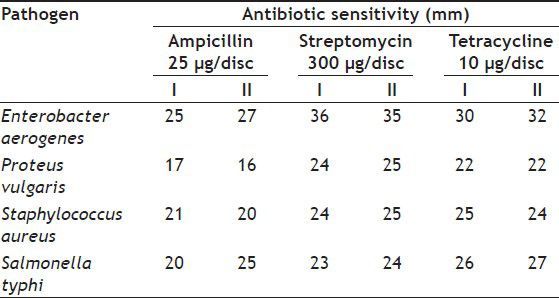
Fig. 3a and 3b shows the results of the silver nanoparticles formed from the seaweed extract captured by means of SEM. Fig. 3a represents the view of the sample with the magnification of 20,000× could notice from the particles size of 45-300 nm; however, we could not manage to examine the structure of the observed nanoparticles less than 45 nm because of difficulties connected with getting higher magnification. But huge amount of nanoparticles ranging the sizes between 45 and 75 nm can be visible in fig. 3a. The factor responsible for difficulties connected with getting higher magnification was high susceptibility of nanoparticles to aggregate into larger conglomerates. Fig. 3b shows the formation of triangular silver nanoparticles. The size of these triangular nanoparticles ranged from 200 nm and we could be able to see a large number of particles belonging to the sizes ranging from 45 to 60 nm.
Fig. 3.

SEM of silver nanoparticles. Scanning electron micrographs of silver nanoparticles synthesized with 5 ml of S. cinereum seaweed extract, 1.0 mM silver nitrate: (a) magnified ×20,000 inset bar represents 10 μm (b) magnified ×10,000 insert bar represents 6 μm.
Developing a reliable eco-friendly process for synthesising silver nanoparticles for pharmaceutical applications are off critical need in the field of nanotechnology in recent times. The marine seaweed S. cinereum can produce silver nanostructures through efficient green nanochemistry avoiding the presence of hazardous and toxic solvents and waste. The biosynthesised silver nanoparticles using S. cinereum extract proved excellent antimicrobial activity and the activity is well demonstrated by considerable zone of inhibition against the multidrug resistant organisms Enterobactor aerogens, Staphylococcus aureus, Salmonella typhi and Proteus vulgaris. Applications of these eco-friendly nanoparticles with bactericidal and other medical applications will have high potentiality for large scale synthesis in future.
ACKNOWLEDGEMENTS
The authors would like to thank the Director, NIO for the facilities provided and Mr. V. Khedekar for providing SEM facility. We wish to acknowledge Dr. Jose and Dr. Ragya, Microbiology Department, Goa Medical College and Hospital for providing pathogenic bacterial strains. We also wish to acknowledge Dr. N. Ramaiah, Project Leader Marine Biotechnology for his constant support. RR and SS acknowledge CSIR and UGC, respectively for the fellowship. This is CSIR-NIO contribution number 5476
Footnotes
Mohandass, et al.; Silver Nanoparticles and Antibacterial Activity
REFERENCES
- 1.Rao P, Parekh KS. Antibacterial activity of Indian seaweed extracts. Bot Mar. 1981;23:216–21. [Google Scholar]
- 2.Glombitza KW, Klapperich K. Antibiotics from Algae. Cleavage of the high-molecular-weight methylated phlorotannin fraction from the brown alga Pelvetia canaliculata. Bot Mar. 1985;28:139–44. [Google Scholar]
- 3.De Campos-Takaki GM, Du MB, Koening MI, Pereira EC. Screening of marine algae from Brazilian north east coast for antimiorobial activity. Bot Mar. 1988;31:375–7. [Google Scholar]
- 4.Rao PS. Biological investigation of Indian Phaeophyceae XI. Screening of different parts of Sargassum wighti Greville for this antibacterial activity. Indian Bot Rep. 1990;9:52–5. [Google Scholar]
- 5.Vidyavathi N, Sridhar KR. Seasonal and Geographical variation in the antimicrobial activity of seaweeds from the Mangalore coast of India. Bot Mar. 1991;34:279–84. [Google Scholar]
- 6.Kamat SY, Wahidulla S, D’Souza L, Naik CG, Ambiye V, Bhakuni DS, et al. Bioactivity of marine Organisms VI. Antiviral evaluation of marine algal extracts from the Indian coast. Bot Mar. 1992;35:161–4. [Google Scholar]
- 7.Premnathan M, Chandra K, Bajpai SK, Kathiresan K. A survey of some Indian Marine plants for antiviral activity. Bot Mar. 1992;35:321–4. [Google Scholar]
- 8.Sastry VM, Rao GR. Antibacterial substances from marine algae: successive extraction using benzene, chloroform and methanol. Bot Mar. 1994;37:357–60. [Google Scholar]
- 9.Robles-Centeno PO, Ballantine DL, Gerwick WH. Dynamics of antibacterial activity in three species of Caribean marine algae as a function of habitat and life history. Hydrobiologia. 1996;326:457–62. [Google Scholar]
- 10.Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langnmuir. 2003;19:1357–61. [Google Scholar]
- 11.Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19:1627–31. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- 12.Rai A, Chaudhary M, Ahmed A, Bhargava S, Sastry M. Synthesis of triangular Au core-Ag shell nanoparticles. Mater Res Bull. 2007;42:1212–20. [Google Scholar]
- 13.Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B Biointerfaces. 2007;57:97–101. doi: 10.1016/j.colsurfb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Veerasamy R, Xin TZ, Gunasagaran S, Xiang TF, Yang EF, Jayakumar N, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15:113–20. [Google Scholar]
- 15.Lansdown AB. Silver I: Its antibacterial properties and mechanism of action. J Wound Care. 2002;11:125–30. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 16.Sturgis S. Biomechanics. San Francisco: CMP Healthcare Media Publication; 2005. Precious metal - foot care takes a shine to silver; pp. 1–6. [Google Scholar]
- 17.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:1–10. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez Padilla C. PVP coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnol. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha B, Reddy CR, Thakur MC, Rao MU. Dordrecht, Netherlands: Springer; 2009. Seaweeds of India: The diversity and distribution of seaweeds of gujarat coast; p. 89. [Google Scholar]
- 22.Duran N, Marcato PD, Alves OL, De Souza GI, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol. 2005;3:1–7. doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcoxon JP, Martin JE, Parsapour F, Wiedenman B, Kelley DF. Photoluminescence from nano sized gold clusters. J. Chem Phys. 1998;108:9137–43. [Google Scholar]


