Abstract
Background:
Reports from middle- and high-income countries suggest that the improved health outcome from highly active antiretroviral therapy (HAART) in people living with human immunodeficiency virus (PLWHIV) is being mitigated by increase in deaths from cardiovascular disease (CVD).
Aims:
This study was to determine the prevalence of traditional cardiovascular risk factors (CVRFs) and the 10-year cardiovascular risk using three risk equations in PLWHIV with no overt vascular disease.
Materials and Methods:
This cross-sectional study involved 265 PLWHIV. We classified the subjects as having low, moderate or high cardiovascular risk using the Framingham, World Health Organization/International Society of Hypertension (WHO/ISH) and Systematic Coronary Risk Evaluation (SCORE) equations.
Results:
The mean age of the cohort was 38.7 ± 8.7 years; 179 (67.5%) were females and 214 (80.8%) were on HAART. The prevalent traditional CVRFs in our cohort were low physical activity (66%), low HDL-C (49.1%), hypercholesterolaemia (33.6%), BMI ≥ 25 kg/m2 (32.8%) and elevated LDL-C (28.3%). The prevalence of smoking was very low (1.9%). The prevalence of moderate to high 10-year coronary risk was 11.7, 12.8, and 12.8% according to the Framingham, WHO/ISH and SCORE risk equations, respectively.
Conclusion:
Most of our patients had low overall cardiovascular risk according to the three risk equations.
Keywords: Cardiovascular risk factors, Risk scores, Human immunodeficiency virus
Introduction
The introduction of highly active antiretroviral therapy (HAART) has led to significant improvement in the morbidity, mortality, and quality of life of people living with human immunodeficiency virus (HIV).[1,2] However, this improved outcome is being mitigated by increase in deaths from liver disease, cancer and cardiovascular disease (CVD).[3,4] The increase in CVD in people living with HIV (PLWHIV) has been attributed to increased prevalence of traditional cardiovascular risk factors (CVRFs), induction of potentially atherogenic dyslipidemia and vascular and immune changes by the virus and some anti-retroviral agents.[5] Reports suggest that there is excess risk of cardiovascular diseases (CVDs) among PLWHIV.[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] These reports have come mainly from high- and middle-income countries[6,7,8,9,10,11,12,13,14,15,16,17,18] with very few reports from low-income countries, particularly sub-Saharan Africa (SSA).[19,20,21] Although reports from SSA indicated that tuberculosis, sepsis, advanced HIV disease and pulmonary infections are the leading causes of death,[22,23,24,25] the opportunity to nip in the bud the emergence of CVD as an important cause of death in PLWHIV in SSA will be missed if attention is not given to the surveillance and control of CVRFs in this population.
Nigeria, with an estimated population of PLWHIV of approximately 3.5 million, has the second largest population of PLWHIV in the world, second only to South Africa, and is responsible for 10% of the global burden of HIV.[26,27] Due to the differences in the prevalence of CVD and the socio-demographic characteristics of PLWHIV (which are known to influence to CVD) in different economic regions of the world, reports from high- and middle-income countries cannot be extrapolated to SSA. Also, failure to appreciate possible emerging problems of CVD in PLWHIV in SSA may mitigate the gains from HAART in this region. We, therefore, embarked on this study to determine the prevalence of traditional CVRFs in our patients with no previously known CVRFs or CVD. Also, we determined the 10-year cardiovascular risk in our cohort using the Framingham,[28] the World Health Organization/International Society of Hypertension (WHO/ISH)[29,30] and the Systematic Coronary Risk Evaluation (SCORE)[31] equations.
Materials and Methods
Ethical approval for the study was obtained from the Institutional Research Ethics Committee. The patients involved gave both verbal and written consent after the study was duly explained to them.
This cross-sectional study involved 265 consecutive patients with documented HIV infection aged ≥ 18 years seen at the outpatient clinic dedicated to PLWHIV in our facility over a 4 month period. We excluded participants with the following features: Acute illness necessitating medical or surgical intervention; clinical and biochemical profile indicative of liver disease; clinical thyroid disease; pregnancy; known personal history of hypertension, diabetes mellitus (DM), ischemic heart disease (IHD), stroke; patients on lipid-lowering medication; and those that declined to be part of the study.
We used the STEP-wise approach to Surveillance (STEPS) questionnaire developed by the WHO for non-communicable disease.[32] The questionnaire was used to obtain information such as age; gender; educational status; use of cigarette; alcohol intake; physical activity; dietary habit; and family history of hypertension, DM, IHD, and stroke. Intake of HAART was noted and duration of HAART was calculated from the time of commencement of HAART to the time of inclusion of the patient in the study. HAART was defined as the use of ≥2 nucleoside reverse transcriptase inhibitors (NRTIs) and at least one non-nucleoside reverse transcriptase inhibitor (NNRTI); or ≥2 NRTIs and at least one protease inhibitor (PI) or one NRTI in combination with one PI and at least one NNRTI.[33]
The weight (kilogram) and height (metres) of the participants were measured in light clothing and with the shoes off. The body mass index (BMI) of the participants was calculated from weight (kg)/height2 (m2).[34] The waist circumference (WC) in (cm) and the hip circumference (HC) in (cm) were measured without clothing with a tape measure in light contact with but not compressing the skin midpoint between the lowest rib and the iliac crest and at the levels of the greater trochanters, respectively. The waist: Hip ratio (WHR) was calculated from WC/HC. Blood pressure (BP) was taken in a sitting position using the A and D UA 767 automated digital manometer which had been validated by the British Hypertension Society.[35] Three BP and pulse rate (PR) readings were obtained for each participants and the average of the last two readings were used for current BP and PR readings for analysis. The participants’ latest CD4 count was obtained from the folder.
Blood samples were drawn after an 8- to 12-h fast to determine the serum total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG). The TC and the different cholesterol fractions were determined using commercially available reagents (RandoxR laboratories Ltd, UK).[36] The concentration of the low-density lipoprotein cholesterol (LDL-C) was determined using the Friedwald equation for participants with a TG < 4.5 mmol/L.[37] The fasting plasma glucose (FPG) was determined using the glucose oxidase method.
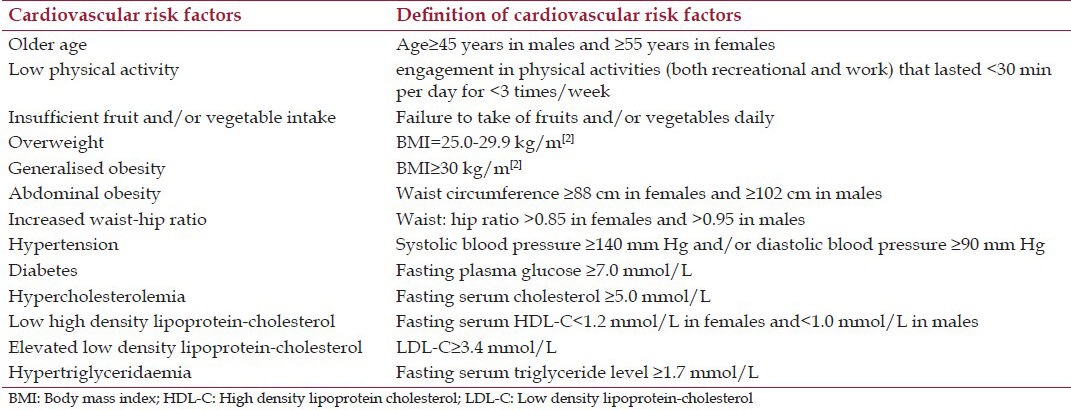
The presence of various traditional CVRFs was determined using cut-off values based on guidelines of the National Cholesterol Education Program (NCEP ATP III), the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC7) and the American Diabetes Association[28,38,39] and are as shown in Table 1. Cardiovascular risk was estimated for each subject using the ATP III – Framingham,[28] the WHO/ISH Risk Prediction Chart for Africa, sub-region D (which included Nigeria)[29,30] and the SCORE equations.[31] In view of the relatively low incidence of CHD in Nigeria,[40] we adopted the SCORE chart designed for low-risk regions of Europe.[31] We classified the subjects as having low, moderate or high cardiovascular risk using Framingham and WHO/ISH (<10%, 10-20%, and >20%, respectively) and SCORE (<3%, 3-4% and ≥5%, respectively) equations.[28,29,30,31] Patients with markedly raised levels of single risk factors i.e. TC ≥ 8 mmol/L (SCORE and WHO/ISH equations), LDL-C ≥ 6 mmol/L (SCORE), BP ≥ 180/110 mmHg (SCORE), BP ≥ 160/100 mmHg (WHO/ISH), and FPG ≥ 7 mmol/L [all risk equations] were defined as having coronary risk equivalents and classified as having high risk.[28,29,30,31]
Table 1.
Definition of cardiovascular risk factors
Statistical analysis
Quantitative variables are presented as means ± standard deviation. Categorical variables are presented as percentages. Differences between two means were assessed using the student's t-test while the Chi-square was used to assess the degree of association of categorical variables with Fishers test applied as appropriately. Cohen's kappa (κ) coefficient was used to assess agreements between two risk scores. The level of agreement was considered poor if κ = 0.20, fair if κ = 0.21-0.40, moderate if κ = 0.41-0.60, substantial if κ = 0.61-0.80, and very good if κ > 0.80.[41] All P values were two-tailed and P < 0.05 was considered statistically significant. All statistical analyses were done using the Statistical Package for Social Sciences software, version 15 (SPSS, Chicago, IL).
Results
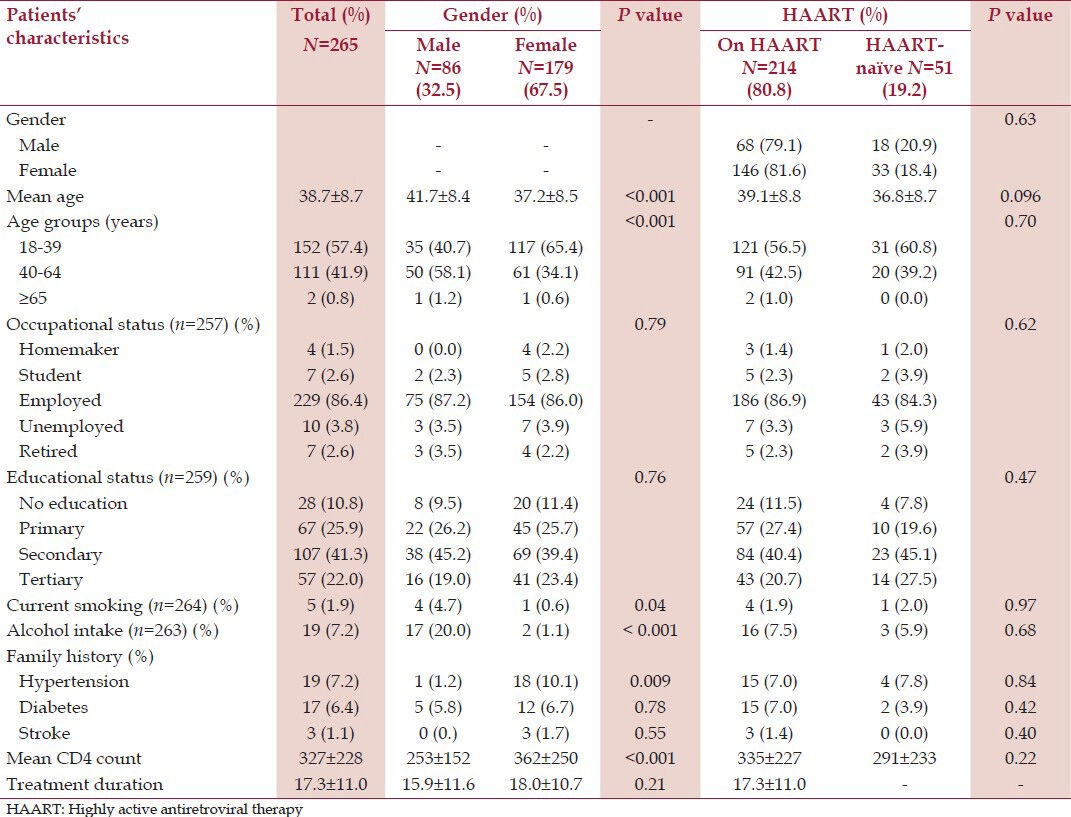
The study population consisted of 179 (67.5%) females and 86 (32.5%) males. Table 2 shows the demographic and social characteristics of the study population according to gender and HAART use. The mean age of the study population was 38.7 ± 8.7 years and the males were significantly older than the females. Current cigarette smoking and alcohol intake were found in 5 (1.9%) and 19 (7.2%) participants, respectively, and were commoner in the males. The males were significantly taller and heavier than the females. Two hundred and fourteen (80.8%) participants were on HAART with 194 (90.7%) on zidovudine/lamivudine/nevirapine), 7 (3.3%) on stavudine/lamivudine/nevirapine, 6 (2.8%) on tenofovir/lamivudine/efavirenz, 6 (2.8%) on tenofovir/emtricitabine/efavirenz, and 1 (0.4%) on zidovudine/lamivudine/efavirenz combinations. Participants on HAART were older than HAART-naïve patients and females had significantly higher CD4 count when compared to males [Table 2].
Table 2.
Demographic and social characteristics of the study population according to gender and HAART use
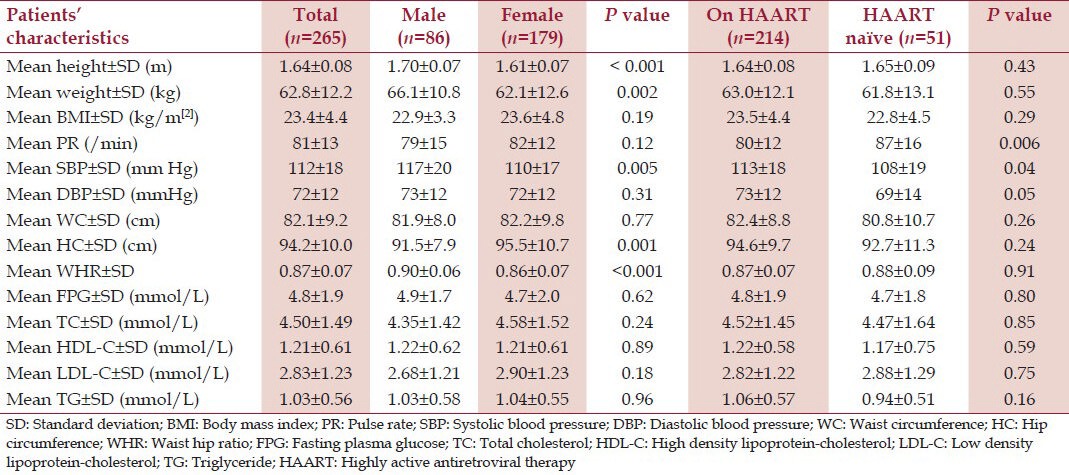
Males had significantly higher SBP and WHR than the females while the females had significantly wider HC [Table 3]. There was no statistically significant gender difference in the mean values of the WC, FPG, TC, HDL-C, LDL-C, and TG [Table 3]. Participants on HAART had higher SBP and DBP when compared to those not on HAART [Table 3]. Although those on HAART had higher mean values of TC, HDL-C, and TG when compared to HAART-naïve participants, the differences were not statistically significant [Table 3].
Table 3.
Anthropometric, clinical and laboratory characteristics of the study population according to gender and HAART use
Tables 4 and 5 show respectively the prevalence of the traditional CVRFs and cardiovascular risk scores in the study population and their comparison by gender and HAART intake. Older age and current smoking were significantly present in males compared to females. Low physical activity was present in 175 (66%) of the study participants with no significant gender difference. Overweight, generalized obesity, abdominal obesity, and increased WHR were present in 69 (26%), 18 (6.8%), 48 (13.1%), and 108 (40.8%) participants, respectively, and these variables were significantly higher in females compared to males. Hypertension and diabetes were found in 30 (11.3%) and 28 (10.6%) participants respectively with no significant gender difference. Hypercholesterolemia (TC ≥ 5 mmol/L), hypertriglyceridemia, and elevated LDL-C were found in 89 (33.6%), 34 (12.8%) and 75 (28.3%) participants respectively with no significant gender difference. Low HDL-C was present in 130 (49.1%) participants and was significantly higher in females compared to males (55.3% vs. 36.0%, P = 0.003). There was no statistically significant difference in the prevalence of hypertension, diabetes, hypercholesterolemia and cholesterol fractions in participants on HAART when compared with HAART-naïve participants [Table 4].
Table 4.
Traditional cardiovascular risk factors in the study population according to gender and HAART use
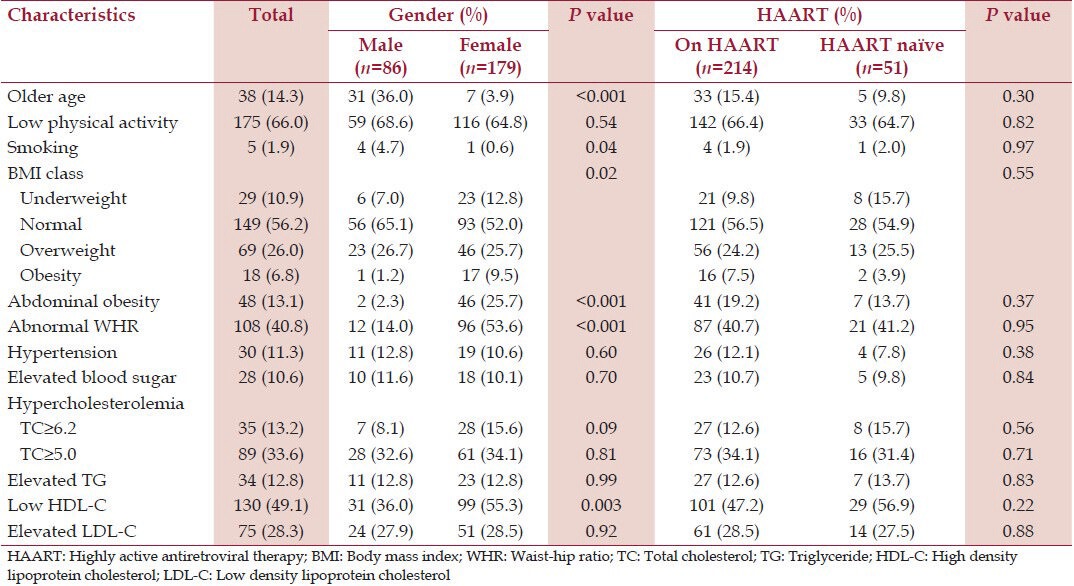
Table 5.
Cardiovascular risk assessment using Framingham, WHO/ISH and score risk equations according to gender and HAART use
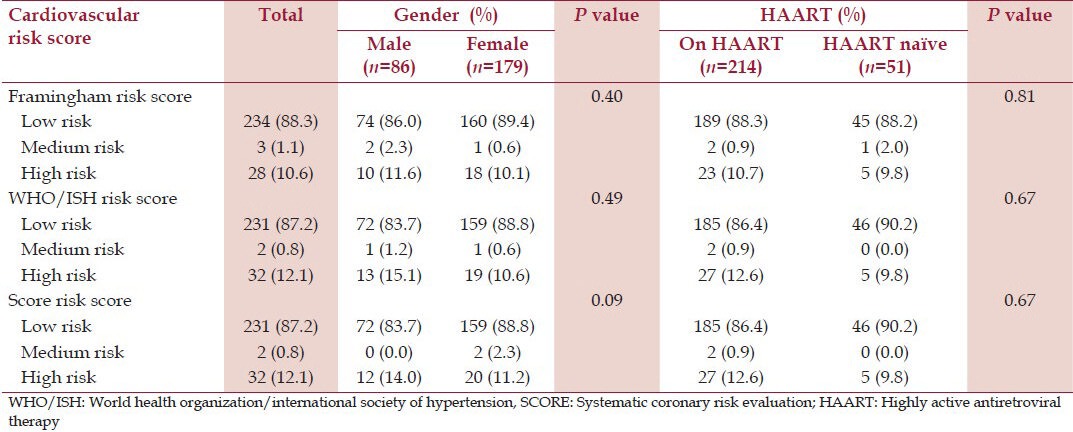
The 10 year-cardiovascular risk was low in 234 (88.3%), 231 (87.2%), and 231 (87.2%) participants according to the Framingham, WHO and SCORE risk equations, respectively [Table 5]. The prevalence of moderate to high 10-year coronary risk in the present study was 11.7%, 12.8% and 12.8% using the Framingham, WHO and SCORE risk equations, respectively. The prevalence of high cardiovascular risk according to the three risk scores was insignificantly higher in males when compared with females and in those on HAART when compared to HAART-naïve participants. The observed agreement between Framingham and WHO risk equations was substantial (κ =0.670); very good between Framingham and SCORE (κ = 0.809) and substantial between WHO and SCORE risk equations (κ = 0.732).
Discussion
The traditional CVRFs observed in our study population in decreasing order of frequency were low physical activity (66.0%), low HDL-C (49.1%), increased WHR (40.8%), overweight and obesity (32.8%), hypercholesterolemia (TC ≥ 5.0 mmol/L) (33.6%), elevated LDL-C (28.3%), older age (14.3%), abdominal obesity (13.1%), elevated TG (12.8%), hypertension (11.3%), diabetes (10.6%), alcohol consumption (7.2%), and smoking (1.9%). The prevalence of low HDL-C in our cohort of 49.1% is comparable to 49.5% by Cahn et al.,[16] in Latin-American cohort receiving HAART but higher than 44.9% and 36.3% reported by Saves et al.,[7] and Edwards-Jackson et al.,[18] respectively. The prevalence of BMI ≥ 25kg/m2 in our cohort of 32.8% is higher than 30.2% and 19.0% reported in SIMONE study[9] and Malawians,[19] respectively. Many published reports have drawn attention to the increasing prevalence of overweight and obesity in PLWHIV.[9,17,19,42] This is due to the fact that being overweight removes/reduces the suspicion of HIV positivity and stigma, and allows acceptability and integration into the society.
Hypertriglyceridaemia was present in 12.8% of our cohort and this is much less than 30-60% reported in non-African cohorts.[7,12,16] Blacks and people of African descent tend to have low prevalence of elevated TG, even in the presence of low HDL-C, the so-called “triglyceride paradox.”[43] The explanations adduced to this low prevalence of elevated TG in people of African descent include higher activity of lipoprotein lipase (LPL), LPL activity uninhibited by insulin resistance and lower levels of apolipoprotein C III activity which inhibits LPL activity in blacks compared to Caucasians.[43,44]
The prevalence of hypertension in our cohort of 11.3% falls within the reported prevalence of 5.2-31.5% from published studies.[7,9,10,14,15,16,17] The higher SBP and DBP seen in patients receiving HAART when compared with HAART-naïve patients may be explained by the older age and slightly higher prevalence of overweight/obesity of patients on HAART. The prevalence of elevated blood sugar in our cohort of 10.1% is higher than 2.0-7.3% reported by various workers in PLWHIV.[7,9,10,14,15,16,17] The higher prevalence reported in our cohort may not be unconnected with the fact FPG was done once in our cohort.
Smoking is estimated to cause 10% of CVD and its prevalence is estimated to be lowest in the WHO African Region.[45] Unlike reports from middle- and high-income countries and certain parts of West Africa (Mali, Cote d’ Ivoire) where the prevalence of smoking is reported to be between 13.2-66.8%,[46] the prevalence of smoking in our cohort is remarkably low at 1.9%. Reports from high-income countries have shown that tobacco smoking is higher in PLWHIV than in the general population and this is particularly related to the presence of a specific subpopulation of multidrug users.[47] We found a higher prevalence of smoking in males consistent with other published reports.[7,9,10,12,13,14,15,16,17,18] The low prevalence of smoking in our cohort can be explained partly by the low prevalence of smoking in our population, the higher prevalence of females (which reflects of our patient population) in our study population unlike reports from middle- and high-income countries with higher male population,[7,9,10,12,13,14,15,16,17,18] the continuous counselling of PLWHIV by health care workers of the danger of smoking and the fact that our patients do not share the same addiction characteristics common in PLWHIV in certain high-income countries.[47]
Patients on HAART, particularly those on protease inhibitors (PIs) present more metabolic alterations such as dyslipidaemia and metabolic syndrome than do HAART-naïve patients.[1,5,8] Also, these alterations tend to be prevalent with increased duration of treatment.[1,5,17,18] The absolute values of TC, HDL-C, TG and FPG were higher in people on HAART compared to HAART-naïve patients similar to other published studies.[12,14,17,21] However, unlike these studies, these differences were not statistically significant. Our findings can partly be explained by the fact that the HAART regimens in our cohort were different from that in high-income countries in that none of our patients was on PIs and the duration of treatment in our cohort was relatively shorter when compared with other studies,[16,17,18] with the mean duration of treatment of 17.3 months.
In this cohort of Nigerians living with HIV/AIDS, we found low overall cardiovascular risk as predicted by the Framingham, WHO and SCORE risk equations. This study showed a high prevalence of Nigerians living with HIV/AIDS at low cardiovascular risk regardless of the coronary risk equation used. Previous reports from Nigeria have documented a low prevalence of coronary heart disease[40] though there are suggestions that the incidence of CHD will increase as Nigerians continue to adapt western lifestyle. The low prevalence of CVD risk factors in our population can be explained by the relatively young age of the study cohort and the low incidence of CHD in our population.
The 10-year coronary risk greater than 10% according to Framingham score of 11.7% obtained in this study is slightly higher than 9.0% and 9.9% reported in the Data Collection on Adverse Events of Anti-HIV Drugs (D: A: D)[10] and by Edwards-Jackson et al.,[18] in Thais patients, respectively. However, our value is much lower than 23.4% reported by Knobel et al.,[13] in Spain, 24.1% by Moreira Guimaraes et al.,[14] in Brazil, 29.1% by Hadigan et al.,[48] and 16.5% by Glass et al.,[12] in the Swiss HIV cohort.
We found the levels of agreement between various risk scores to be quite good. However, which of the three risk equations will best predict cardiovascular risk in HIV-infected Nigerians can only be determined by a longitudinal study. Studies have shown that how well the predicted outcomes agree with the actual outcome (i.e. the calibration) using the various risk systems may vary widely.[49] Reasons adduced to this poor calibration include differences in the baseline rates of CVD in the different geographic regions which in turn may lead to overestimation or underestimation of risk; change in the secular trend of the baseline prevalence of CVD; assumption of constant effects of the risk factors at different ages and levels of the other risk factors by risk estimation systems; and variation in the different end-point definition of various risk systems.[49] Despite the shortcomings in the risk equation systems, clinicians must be encouraged to regularly evaluate and manage patients for CVD risk in order to improve CVD outcomes.
Our study has some limitations which should be taken into consideration when interpreting our results. First, our cohort consisted of consecutive patients with no overt cardiovascular disease attending a sub-urban HIV care center. Thus, our sample may not be representative of the whole population of Nigerians living with HIV/AIDS. However, we are unaware of any other study in our population that has attempted to assess cardiovascular risk using three CVD risk scores as done in this study. Our study therefore provides a useful comparison with other published studies from middle- and high-income countries. Second, the exclusion of patients with known personal history of hypertension, diabetes and use of lipid-lowering medications which are important CVRFs could explain the low prevalence of medium cardiovascular risk in this study cohort. Third, we did not include controls of HIV-negative people from the population. This is because our goal was to show the current status of CVD risk in Nigerians living with HIV/AIDS. Fourth, we did not assess for altered body compositions such as lipodystrophy and lipoatrophy which had been shown to be associated with increased CVD risk in some published studies.[9] Fifth, the risk equations were not developed in the Nigerian population and an overestimation of CVD risk in our population with lower incidence of CHD is possible. Lastly, we cannot make causal relationships between the CVRFs, HIV infection, and treatment with HAART since our study is cross-sectional.
Conclusion
Most of the study participants had low overall cardiovascular risk according to the three risk equations. Whilst the frequencies of certain CVRFs such as low physical activity, low HDL-C, overweight and hypercholesterolemia were high, others such as smoking was remarkably low in our cohort. As more Nigerians have access to HAART with consequent improvement in longevity, the emergence of CVDs as an important health challenge cannot be ruled out. Thus, clinicians involved in taking care of PLWHIV must not only pre-occupy themselves with achieving and maintaining virological control, but also assess and control for CVRFs.
Acknowledgement
Members of staff of dedicated clinic to people living with HIV/AIDS, Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Osun State, Nigeria.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Blanco F, Román JS, Vispo E, López M, Salto A, Abad V, et al. Management of metabolic complications and cardiovascular risk in HIV-infected patients. AIDS Rev. 2010;12:231–41. [PubMed] [Google Scholar]
- 2.Beard J, Feeley F, Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: A systematic literature review. AIDS Care. 2009;21:1343–56. doi: 10.1080/09540120902889926. [DOI] [PubMed] [Google Scholar]
- 3.Sackoff J, Hanna D, Pfeiffer M, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lewden C, Salmon D, Morlat P, Bevilacques S, Jougla E, Bonnet F, et al. Mortality 2000 study group. Causes of death among human immunodeficiency virus (HIV) - infected adults in the era of potent antiretroviral therapy: Emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–30. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 5.Grinspoon SK, Grunfeld C, Kotler DP, Currier JS, Lundgreen JD, Dubé MP, et al. State of the Science Conference: Initiative to decrease cardiovascular risk and increase quality of care of patients living with HIV/AIDS: Executive summary. Circulation. 2008;118:198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with HIV disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savès M, Chêne G, Ducmetrière P, Leport C, Le Moal G, Amouyel P, et al. French WHO MONICA Project and the APROCO (ANRS EP 11) Study Group. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–8. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 8.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Québec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 9.De Socio GV, Parruti G, Quirino T, Ricci E, Schillaci G, Adriani B, et al. CISAI study group. Identifying HIV patients with an unfavorable cardiovascular risk profile in the clinical practice: Results from the SIMONE study. J Infect. 2008;57:33–40. doi: 10.1016/j.jinf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Law MG, Friis-Møller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A, et al. for D: A: D Study Group. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: Comparison with observed events in the D: A: D study. HIV Med. 2006;7:218–30. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Møller N, Sabin CA, Weber R, D’Arminio Monforte A, El-Sadr WM, Reiss P, et al. For Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [Google Scholar]
- 12.Glass TR, Ungsedhapand C, Wolbers M, Weber R, Vernazza PL, Rickenbach M, et al. Swiss HIV Cohort Study. Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: The Swiss HIV Cohort Study. HIV Med. 2006;7:404–10. doi: 10.1111/j.1468-1293.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 13.Knobel H, Jericó C, Montero M, Sorli ML, Velat M, Guelar A, et al. Global cardiovascular risk in patients with HIV infection: Concordance and differences in estimates according to three risk equations (Framingham, SCORE and PROCAM) AIDS Patient Care STDS. 2007;21:452–7. doi: 10.1089/apc.2006.0165. [DOI] [PubMed] [Google Scholar]
- 14.Moreira Guimarães MM, Barolomeu Greco D, Ingles Garces AH, de Oliveira AR, Jr, Bastos Fóscol RB, de Campos Machado LJ. Coronary heart disease risk assessment in HIV-infected patients: A comparison of Framingham, PROCAM and SCORE risk assessment functions. Int J Clin Pract. 2010;64:739–45. doi: 10.1111/j.1742-1241.2009.02248.x. [DOI] [PubMed] [Google Scholar]
- 15.da Silva EF, Bassichetto KC, Lewi DS. Lipid profile, cardiovascular risk factors and metabolic syndrome in a group of AIDS patients. Arq Bras Cardiol. 2009;93:107–11. doi: 10.1590/s0066-782x2009000800008. [DOI] [PubMed] [Google Scholar]
- 16.Cahn P, Leite O, Rosales A, Cabello R, Alvarez CA, Seas C, et al. Metabolic profile and cardiovascular risk factors among Latin-American HIV-infected patients receiving HAART. Braz J Infect Dis. 2010;14:158–66. doi: 10.1590/s1413-86702010000200008. [DOI] [PubMed] [Google Scholar]
- 17.Aboud M, Elgalib A, Pomeroy L, Panayiotakopoulos G, Skopelitis E, Kulasegaram R, et al. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: Implications for clinical management: The CREATE 1 Study. Int J Clin Pract. 2010;64:1252–9. doi: 10.1111/j.1742-1241.2010.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards-Jackson N, Kerr SJ, Tieu HV, Ananworanich J, Hammer SM, Ruxrungtham K, et al. for HIV-NAT 006 Study Team. Cardiovascular risk assessment in persons with HIV infection in the developing world: Comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011;12:510–5. doi: 10.1111/j.1468-1293.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 19.Muronya W, Sanga E, Talama G, Kumwenda JJ, van Oosterhout JJ. Cardiovascular risk factors in adult Malawians on long-term antiretroviral therapy. Trans R Soc Trop Med Hyg. 2011;105:644–9. doi: 10.1016/j.trstmh.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6:222288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pefura Yone EW, Betyoumin AF, Kengne AP, Kaze Folefack FJ, Ngogang J. First line antiretroviral therapy and dyslipidaemia in people living with HIV-1 in Cameroon: A cross-sectional study. AIDS Res Ther. 2011;8:33. doi: 10.1186/1742-6405-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sani MU, Mohammed AZ, Adamu B, Yusuf SM, Samaila AA, Borodo MM. AIDS mortality in a tertiary health institution: A four year review. J Natl Med Assoc. 2006;98:862–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas S. Causes of death in the HAART era. Curr Opin Infect Dis. 2012;25:36–41. doi: 10.1097/QCO.0b013e32834ef5c4. [DOI] [PubMed] [Google Scholar]
- 24.Groenewald P, Bradshaw D, Dorrington R, Bourne D, Laubscher R, Nannan N. Identifying deaths from AIDS in South Africa: An update. AIDS. 2005;19:744–5. doi: 10.1097/01.aids.0000166105.74756.62. [DOI] [PubMed] [Google Scholar]
- 25.Bane A, Yohannes AG, Fekade D. Morbidity and mortality of adult patients with HIV/AIDS at Tikur Anbessa Teaching Hospital, Addis Abbaba, Ethiopia. Ethiop Med J. 2003;41:131–40. [PubMed] [Google Scholar]
- 26.UNAIDS World AIDS Day Report. 2012. [Accessed April 30, 2013]. at http://www.unaids.org/en/media/unaids/contenetassets/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf .
- 27.National Agency for the Control of AIDS (NACA), Federal Republic of Nigeria. Global AIDS Response Country Progress Report (GARPR) 2012. [Accessed April 30, 2012]. at http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012/country/Nigeria.pdf .
- 28.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 29.Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, Lim S, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: Assessment of cardiovascular risk for prevention and control of cardiovascular disease in low- and middle-income countries. J Hypertens. 2007;25:1578–82. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 30.Geneva: World Health Organization; 2007. [Accessed January 31, 2012]. Prevention of cardiovascular disease. at http://www.who.int/cardiovascular_diseases/guidelines/POCKETGL.ENGLISH.AFR-D-E.rev1.pdf . [Google Scholar]
- 31.de Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Daltongeville J, et al. European Society of Cardiology, American Heart Association, American College of Cardiology. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts) Atherosclerosis. 2004;173:381–91. [PubMed] [Google Scholar]
- 32.WHO Steps Instrument. [Accessed January 2, 2010]. at http://www.who.int/entity/chp/steps/STEPS_Instrument_v2.1.pdf .
- 33.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society – USA Panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 34.Obesity and overweight. [Accessed January 10, 2010]. at http://www.who.int.mediacentre/factsheets/fs311/en/index.html .
- 35.British Hypertension Society. Validated monitors. [Accessed January 10, 2012]. at http://www.bhsoc.org/blood_pressure_list.stm/bp_monitors/automatic/stm .
- 36.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 37.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 38.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falase AO, Oladapo OO, Kanu EO. Relatively low incidence of myocardial infarction in Nigerians. Cardiol Trop. 2001;27:45–7. [Google Scholar]
- 41.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 42.Boodram B, Plankey MW, Cox C, Tien PC, Cohen MH, Anastos K, et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women's Interagency HIV Study. AIDS Patient Care STDS. 2009;23:1009–16. doi: 10.1089/apc.2009.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu SS, Castilo DC, Courville AB, Sumner AE. The triglyceride paradox in people of African descent. Metab Syndr Relat Disord. 2012;10:77–82. doi: 10.1089/met.2011.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Summer AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, et al. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans with metabolic syndrome: Implications for cardiovascular prevention. CVD Prev Control. 2010;5:75–80. doi: 10.1016/j.cvdpc.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Global Atlas on Cardiovascular Diseases Prevention and Control. [Accessed January 2, 2012]. at http://www.whqlibdoc.who.int/publications/2011/9789241564373_eng.pdf .
- 46.Jaquet A, Ekouevi DK, Aboubakrine M, Bashi J, Messou E, Maiga M, et al. Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in West African countries. Int J Tuberc Lung Dis. 2009;13:1433–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Bénard A, Tessier JF, Ambeloarisoa J, Bonnet F, Fossoux H, Neau D, et al. HIV infection and tobacco smoking behavior: Prospects for prevention? ANRS CO3 Aquitaine Cohort 2002. Int J Tuberc Lung Dis. 2006;10:378–83. [PubMed] [Google Scholar]
- 48.Hadigan C, Meigs JB, Wilson PW, D’Agostino RB, Davis B, Basgoz N, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–16. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 49.Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk. J Am Coll Cardiol. 2009;54:1209–27. doi: 10.1016/j.jacc.2009.07.020. [DOI] [PubMed] [Google Scholar]


