Abstract
We have discovered a microbial interaction between yeast, bacteria, and nematodes. Upon coculturing, Saccharomyces cerevisiae stimulated the growth of several species of Acinetobacter, including, A. baumannii, A. haemolyticus, A. johnsonii, and A. radioresistens, as well as several natural isolates of Acinetobacter. This enhanced growth was due to a diffusible factor that was shown to be ethanol by chemical assays and evaluation of strains lacking ADH1, ADH3, and ADH5, as all three genes are involved in ethanol production by yeast. This effect is specific to ethanol: methanol, butanol, and dimethyl sulfoxide were unable to stimulate growth to any appreciable level. Low doses of ethanol not only stimulated growth to a higher cell density but also served as a signaling molecule: in the presence of ethanol, Acinetobacter species were able to withstand the toxic effects of salt, indicating that ethanol alters cell physiology. Furthermore, ethanol-fed A. baumannii displayed increased pathogenicity when confronted with a predator, Caenorhabditis elegans. Our results are consistent with the concept that ethanol can serve as a signaling molecule which can affect bacterial physiology and survival.
In the natural environment, microbes exist in a state of constant interaction with other microbes. Most microbial interactions are antagonistic. Microbes compete for nutrients and inhibit the growth of their competitors by producing antimicrobial compounds. The most notable example of antibiosis was the discovery of penicillin by Fleming, in which Penicillium notatumin was found to inhibit the growth of Staphylococcus aureus (9). Positive interactions also occur: a reciprocal transfer of required nutrients has been demonstrated using Lactobacillus arabinosus and Streptococcus faecalis in minimal medium which enabled both to grow (29). While much effort has been devoted to describing bacterial cell-cell interactions, particularly antagonistic ones, surprisingly little attention has been devoted to yeast-microbe interactions.
Saccharomyces cerevisiae is an ideal organism in which to study how eukaryotic cells interact with other microorganisms. Yeasts are ubiquitous in nature, soil dwelling, and are also opportunistic pathogens (13, 26, 35, 37). The wealth of information about this fungus lends itself to the genetic, molecular biological, and microbiological techniques required for dissecting this eukaryote-microbe interaction.
We examined the interactions between naturally occurring strains of the budding yeast, S. cerevisiae, and a wide variety of bacteria. We found that the pathogenic isolates of yeast had the ability to affect the growth of the human pathogens Acinetobacter baumannii and Acinetobacter haemolyticus as well as several natural isolates of Acinetobacter spp. Acinetobacters are commonly found in soil, water, and sewage (15). It has been estimated that acinetobacters comprise as much as 0.001% of the population of heterotrophic aerobic bacteria in soil and water (1), illustrating their prevalence and versatility. Acinetobacters are best known for their ability to transform with DNA readily (18), their ability to utilize and degrade a wide range of carbon sources including petroleum (1, 3, 28), and for their rising incidence of multidrug-resistant, nosocomial-derived strains infecting immunocompromised individuals in hospitals worldwide (5, 10, 41).
Here we describe antagonistic interactions and an unexpected synergistic relationship between S. cerevisiae and Acinetobacter. This synergistic relationship involves the production of a diffusible factor by yeast that allows the bacteria to grow to higher cell density. The diffusible factor responsible for this synergistic relationship was explored further and found to be ethanol. Ethanol-fed acinetobacters can withstand salt stress and are more virulent to the bacterial predator Caenorhabditis elegans. These results suggest that ethanol can act not only as a carbon source but also as a signal which triggers one or more pathways that result in an alteration of a bacterium's physiology and survival.
MATERIALS AND METHODS
Strains and media.
Yeast and bacterial strains used in this study are listed in Table 1. Yeast growth conditions were as described in Guthrie and Fink (13). YPAD contains 1% yeast extract, 2% Bacto-Peptone, 2% glucose, and 300 μM adenine. LB contains 1% Bacto-Tryptone, 0.5% yeast extract, and 1% NaCl. Lactic acid mineral medium (LAMM) contains 0.5% lactic acid, 10 mM KH2PO4, 100 mM Na2HPO4, 0.2% NH4Cl, 1 mM MgSO4, 0.001% CaCl2, 0.0005% FeSO4 (pH 6.65) (17).
TABLE 1.
Strains used in this study
| Strain | Sourcea |
|---|---|
| Yeast | |
| S288C | Snyder lab collection |
| W303 | Snyder lab collection |
| Σ1278b | Snyder lab collection |
| Y800 | Snyder lab collection |
| Y2423 | Snyder lab collection |
| Y2424 | Snyder lab collection |
| YJM145 | John McCusker |
| YJM189 | John McCusker |
| YJM195 | John McCusker |
| YJM244 | John McCusker |
| YJM280 | John McCusker |
| YJM326 | John McCusker |
| YJM339 | John McCusker |
| YJM441 | John McCusker |
| YJM450 | John McCusker |
| YJM453 | John McCusker |
| YJM456 | John McCusker |
| YJM470 | John McCusker |
| YJM553 | John McCusker |
| YJM556 | John McCusker |
| YJM627 | John McCusker |
| YJM681 | John McCusker |
| YJM682 | John McCusker |
| YJM835 | John McCusker |
| YJM939 | John McCusker |
| YJM940 | John McCusker |
| 91-917.1 | Andre Lachance |
| 87-2421.1 | Andre Lachance |
| 83-883.3 | Andre Lachance |
| 79-65 | Andre Lachance |
| Bacteria | |
| Acinetobacter spp. | D. Young, N. Ornston |
| (A, B, C, D, 01B0, 19B2, 48A1, 59A1, 62A1, 63A1, 66A1, 71A1, 85A1, 89A1, 93A2, A3-1, AA1-1, AC423D, AC511B, AD321, ADP1, ADP230, ADP7594, AZR2865, AZR3517, AZR583, BWB1, ISA25, LUH540, P1-3, P1-6) | |
| Acinetobacter baumannii | ATCC 17928 |
| Acinetobacter haemolyticus | ATCC 17906 |
| Agrobacterium (merlot) | Thomas Burr |
| Agrobacterium (reisling) | Thomas Burr |
| Agrobacterium vitis | Thomas Burr |
| Citrobacter | Arnold Barton |
| Escherichia coli (A) | Arnold Barton |
| Escherichia coli (B) | Arnold Barton |
| Enterobacter aerogenes | Arnold Barton |
| Enterobacter spp. | Arnold Barton |
| Enterococcus spp. | Arnold Barton |
| Klebsiella spp. | Arnold Barton |
| Listeria monocytogenes | Arnold Barton |
| Morganella spp. | Arnold Barton |
| Proteus spp. | Arnold Barton |
| Providencia spp. | Arnold Barton |
| Pseudomonas putida (A) | Arnold Barton |
| Pseudomonas putida (B) | Arnold Barton |
| S. enterica serovar cholerasuis | Arnold Barton |
| S. enterica serovar enteritidis | Arnold Barton |
| Shigella flexneri | Arnold Barton |
| Shigella sonnei | Arnold Barton |
| Staphylococcus aureus (A) | Arnold Barton |
| Staphylococcus aureus (B) | Arnold Barton |
| Staphylococcus epidermidis | Arnold Barton |
| Staphylococcus sapprophyticus | Arnold Barton |
| Pseudomonas aeruginosa PA14 | Stefan Pukatzki |
| Pseudomonas aeruginosa 12A1 | Stefan Pukatzki |
| Pseudomonas aeruginosa PA103 | Stefan Pukatzki |
| Pseudomonas aeruginosa PA103::exoU | Stefan Pukatzki |
Affiliations: J. McCusker, Duke University; A. Lachance, University of Western Ontario; D. Young and N. Ornston, Yale University; T. Burr, Cornell University; A. Barton, The University of Medicine and Dentistry of New Jersey; S. Pukatzki, Harvard University.
Gene deletions.
Deletions of the genes encoding alcohol dehydrogenases were performed using a PCR-based method described by Goldstein and McCusker (12). Primers for ADH1 were 5′-CACAA TATTT CAAGC TATAC CAAGC ATACA ATCAA CTATC TCATA TACAA CGTAC GCTGC AGGTC GAC-3′ and 5′-GAAAG AGTTA CTCAA GAATA AGAAT TTTCG TTTTA AAACC TAAGA GTCAC ATCGA TGAAT TCGAG CTCG-3′. Primers for ADH3 were 5′-TCTGT TCACA GTTAA AACTA GGAAT AGTAT ATCAT AAGTC GTACG CTGCA GGTCG AC-3′ and 5′-CTGGT ACTGC TTCTT GATTT AGTGA TTAAT CTTTG CTCCA ATCGA TGAAT TCGAG CTCG-3′. Primers for ADH5 were 5′-GCAAA CTACT GCTTT ACTGT CTCAC AATGT CTATG ATTGG CGTAC GCTGC AGGTC GAC-3′ and 5′-CACTC GCTAT TTACT GAAGT TCAGA AATGG AGTAA TTCTC ATCGA TGAAT TCGAG CTCG-3′. Gene deletions were selected using 200 μg of G418 (Sigma Chemical Co., St. Louis, Mo.)/ml, 300 μg of hygromicin B (Sigma Chemical Co.)/ml, and 100 μg of nourseothricin (Werner Bioagents, Jena-Cospeda, Germany)/ml as appropriate. All antibiotics were filter sterilized and added to autoclaved medium.
Microbial interaction assay.
Bacteria and yeast were grown separately overnight in YPAD at room temperature with shaking. The bacteria were then diluted and added to molten YPAD with agar (60°C) and overlaid onto cooled YPAD plates. Yeast were diluted and spotted onto the lawns of bacteria, and the plates were incubated overnight at room temperature. For liquid-based assays, yeast were grown to an optical density at 600 nm (OD600) of 5 to 7 in YPAD, the cells were pelleted by centrifugation, and the conditioned medium (CY) was filter sterilized using a 0.22-μm-pore-size filter. Conditioned media were added to fresh media to a final volume of 10%. Bacterial precultures were grown overnight at room temperature in YPAD and diluted to an OD600 of 0.01 in fresh YPAD, YPAD plus 10% conditioned medium, or YPAD plus 0.1% ethanol. Bacteria were incubated overnight at room temperature with shaking, and the OD600 was measured to determine cell density. Aliquots were also removed, serially diluted, and plated onto YPAD plates to determine the number of CFU present in the samples. Pronase E (Sigma Chemical Co.) was added directly into molten agar (or to CY) to a final concentration of 50 μg/ml.
Ethanol determination.
Ethanol concentrations were determined using a kit developed by Boehringer Mannheim (Darmstadt, Germany). Briefly, samples were diluted 1:1,000 in water and 100 μl was added to 3 ml of potassium diphosphate buffer. An absorbance reading was taken at 340 nm. An enzyme suspension containing alcohol dehydrogenase and aldehyde dehydrogenase was added, and a second OD340 measurement was taken. The difference in the absorbances (ΔA) was then factored into the following equation: concentration (in grams per liter) of ethanol = V × MW(g)/ɛ × d × v × 2,000 × ΔA, where V is the final volume (in milliliters), v is the sample volume (in milliliters), MW is the molecular mass of the substance to be assayed (in grams), d is the light path (in centimeters), and ɛ is the extinction coefficient.
C. elegans killing assay.
Escherichia coli (strain OP50) cells grown on NGM medium (38) were fed to C. elegans (strain N2) worms. L3/L4-stage worms were placed onto lawns of Acinetobacter spp. grown on NGM, NGM plus 1% ethanol, PGS (1% Bacto-Peptone-1% NaCl-1% glucose-0.15 M sorbitol-1.7% Bacto-Agar), or PGS plus 1% ethanol. Plates were incubated at 20°C. Viability was tested every 24 h by visual examination. Worms were considered dead if they no longer moved or responded to touch.
RESULTS
Antagonistic and synergistic interactions between yeast and bacteria.
To explore the interactions between yeast and other microbes, we tested natural isolates of S. cerevisiae for their ability to affect bacterial growth on solid media. Thirty yeast strains were tested: 20 strains were from immunosuppressed patients (12), 4 were from cactus plants (36), and 6 were standard laboratory strains (1 produced killer toxin, another was an isogenic killer toxin-defective strain, and the remaining 4 were unrelated killer toxin-defective, well-characterized laboratory strains) (Table 1). These different yeast strains were spotted onto lawns of bacteria, incubated overnight at room temperature, and examined for changes in the growth of the bacterial lawns (Fig. 1A). A total of 61 microbial strains from 15 genera were tested, including strains from Acinetobacter (33 strains), Pseudomonas (6 strains), Staphylococcus (4 strains), Agrobacterium (3 strains), Escherichia (2 strains), Salmonella (2 strains), Enterobacter (2 strains), and others (Table 1).
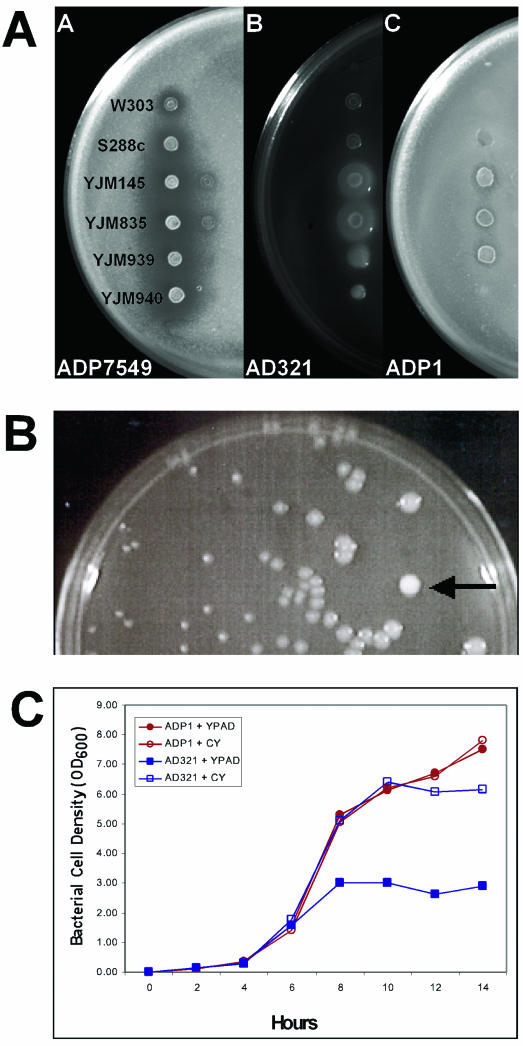
FIG.1.
Antagonism and synergism by a diffusible factor. Standard laboratory yeast strains W303 and S288c and clinical isolates YJM145, YJM835, YJM939, and YJM 940 were spotted onto lawns of Acinetobacter strains ADP7594, AD321, and ADP1. (A) Halos of ADP7594 nongrowth surrounding yeast spots, especially around the clinical strains. AD321 growth was enhanced around spots of YJM145 and YJM835. Strain ADP1 showed neither inhibited nor enhanced growth. (B) AD321 cells were mixed with YJM835 yeast cells, serially diluted, and spread onto plates of YPAD. After 24 h of incubation at room temperature, the bacterial colonies closest to the yeast colony (arrow) were observed to be larger in diameter than those bacterial colonies further away from the yeast spot. (C) Cells were incubated in YPAD at room temperature with shaking. Open icons indicate growth in YPAD. Closed icons indicate growth in YPAD supplemented with 10% conditioned YPAD. Medium was conditioned as described in Materials and Methods. Squares, strain AD321; circles, strain ADP1.
In most cases, neither the yeast nor the bacteria affected the growth of the other. However, several significant responses were observed: three bacteria, Pseudomonas putida, Shigella sonnei, and Acinetobacter strain ADP7594, exhibited reduced growth around the yeast. Surprisingly, in another response nine Acinetobacter strains representing at least five species (A. baumannii [ATCC 17978], A. haemolyticus [ATCC 17906], A. johnsonii [LUH540], A. radioresistens [AZR3517], BWB1, AC423, AD53B, AC223, and AD321) exhibited enhanced growth around the yeast patches. Several of the Acinetobacter strains that showed enhanced growth were natural isolates. AD321 was isolated from soil, AZR3517 was isolated from a hospital pillow, and LUH540 was isolated from waste sludge. The most pronounced effects on bacterial growth were observed when cocultured with the clinical isolates of yeast (YJM145 and its derivatives [26]) and the natural isolates from cactus (91-917.1, 87-2421.1, 83.883.3, and 79-65). The laboratory yeast strains exhibited little to no activity in this assay.
Enhanced bacterial growth is due to a diffusible factor.
The enhanced growth of Acinetobacter in the presence of yeast was not anticipated and therefore was studied further. Coculturing experiments were performed to determine if the yeast-derived factor was diffusible. While several isolates of Acinetobacter displayed the enhanced growth phenotype, AD321 was used exclusively for the determination of the yeast-derived factor, since it displayed the highest level of growth enhancement of all the strains tested. A low number of YJM835 yeast cells were mixed with Acinetobacter strain AD321, serially diluted, and spread onto YPAD plates. In the experiment illustrated in Fig. 1B, a single yeast colony was surrounded by many bacterial colonies. The AD321 colonies closest to the yeast colony grew to a much larger diameter (about 10 times larger) than those that resided farther away. Thus, the yeast-derived factor is diffusible, and direct contact of the two species is not required to enhance bacterial growth.
To test directly whether the growth enhancement of bacteria requires the presence of yeast cells, strains of Acinetobacter were incubated in fresh medium (YPAD) supplemented with filter-sterilized medium prepared from overnight cultures of YJM835 yeast. The conditioned yeast medium (CY) was added to fresh medium to a final concentration of 10%. A time course of Acinetobacter growth was then plotted using the OD600 as a measure of cell growth. We found that Acinetobacter strain AD321 grew to 2.0 ± 0.1 times the cell density (OD600, 6.1 ± 0.7 versus 3.1 ± 0.5) in 10% CY compared to cells grown in YPAD alone (Fig. 1C). Analysis of the growth curves indicated that bacterial cells in CY medium grew at the same rates as controls but to higher cell densities. This increase in optical density of the bacterial cultures after 24 h was always accompanied by a corresponding increase in CFU from these cultures ([4.61 ± 1.3] × 1011 CFU/ml for cells grown in 10% CY, compared to [1.67 ± 0.8] × 1011 CFU/ml for cells grown in YPAD alone). Control strain ADP1 was neither enhanced nor inhibited by CY in either the plate assay or the liquid assay (Fig. 1C). These data suggest that yeast enhance bacterial cell growth not by altering growth rates but instead by affecting the final cell densities to which these cells can grow.
One obvious explanation for this effect on bacterial growth is that YPAD is a suboptimal medium for bacterial growth and the yeast are providing nutrients that are normally present in bacterial growth media. To that end, the liquid assay described above was repeated using the bacterial media LB or LAMM with or without the CY supplement. We found that strain AD321 was enhanced by CY to the same extent when added to either LB or LAMM (40.5% ± 7.0% increase) as it is when grown in YPAD. Moreover, the enhanced growth was not due to limited amino acids or nitrogen; doubling the amount of amino acids or primary nitrogen source in the LB medium compared to LAMM did not enhance the growth of bacteria. Thus, the bacterial growth-enhancing factor is not a nutrient that yeast contribute to the media, but a different yeast-derived compound.
The stimulatory growth factor accumulates in mid-log-phase yeast cultures and is a small molecule.
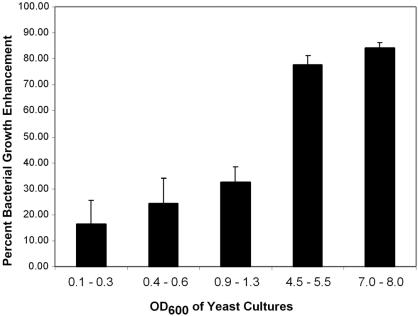
To determine the nature of the production of the yeast-derived bacterial growth-enhancing compound, strains of Acinetobacter were incubated in media (YPAD) supplemented with filter-sterilized medium prepared from pre-log-, early-log-, mid-log-, or late-log-phase yeast cultures. We found that the mid-log- and late-log-phase yeast cultures were best able to enhance the growth of bacteria (Fig. 2). This suggests that the factor responsible for enhancing bacterial growth accumulates preferentially in late-phase yeast cultures.
FIG. 2.
AD321 is further enhanced by older yeast cultures. YJM835 yeast were grown in YPAD at 30°C. The cell densities were determined, and the culture medium was filter sterilized. These media were then added to fresh YPAD at a final concentration of 10% and used as a culture medium for Acinetobacter strain AD321 cells. After overnight growth, the OD600 of the Acinetobacter cultures was determined. Bacterial cell densities are shown as the percent enhancement compared to that of bacterial cells grown in YPAD alone.
To assess whether the secreted factor(s) is a protein, two experiments were performed. First, the bioassay on solid medium was repeated using YPAD plates supplemented with the serine protease pronase E. The growth of strain AD321 was enhanced to the same level in the presence or absence of protease, indicating that pronase E had no effect on the growth-enhancing compound. In contrast, the growth inhibitory factor made by yeast was lost in this assay, suggesting that the growth inhibitory compound is a peptide. In a second assay, CY medium was treated with or without pronase E and added to bacteria. As in the plate assay, bacterial growth was enhanced to the same extent with or without pronase E. Thus, the growth-enhancing factor appears not to be a peptide. The growth-enhancing compound was also unaffected by heating CY to as high as 70°C for 15 min, indicating that it is heat stable. Initial attempts to purify this factor from conditioned medium using molecular weight cutoff filters revealed that it is smaller than 3 kDa in size. Attempts to concentrate this factor proved difficult, because considerable bacterial growth-enhancing ability was lost either following evaporation under vacuum or during lyophilization.
The enhanced growth factor is ethanol.
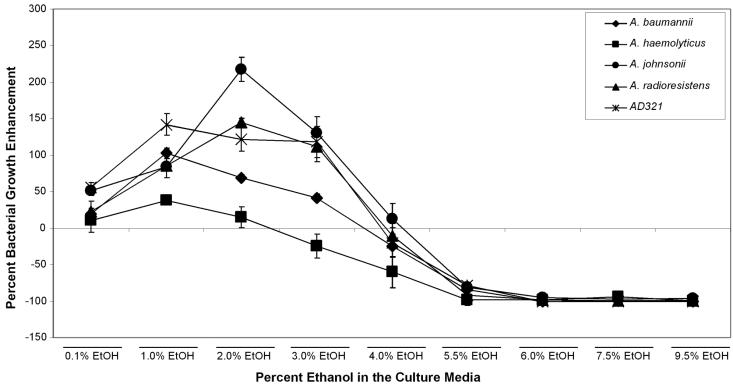
One compound that possesses all of the properties of the growth-enhancing factor (produced by late-log cultures of yeast, small, heat and protease resistant, volatile) is ethanol. Thus, we tested the ability of ethanol to affect the growth of AD321 (Fig. 3). A 95% ethanol solution was diluted in YPAD to various concentrations ranging from 0 to 9.5%. Acinetobacter strain AD321 growth was enhanced by 50% in medium containing low levels of ethanol (0.1%), and the bacterial cell density more than doubled in medium containing between 1 and 4% ethanol; at concentrations of 4.5 and 5% ethanol, these cells grew only as well as in YPAD with no ethanol supplement. We then retested several of the isolates that we recovered in our original screen, and all of the isolates were enhanced by low concentrations of ethanol (up to 2 to 3%). We did observe strain-specific differences in the level of enhancement and tolerance to ethanol. For example, A. baumannii was enhanced maximally by 1% ethanol, while A. johnsonii and A. radioresistens grew best in 2 to 3% ethanol. A. haemolyticus was the most sensitive to ethanol concentration, since it could be enhanced by low levels (0.1 to 1%) but was inhibited at higher concentrations (>3%).
FIG. 3.
Effect of ethanol on AD321 growth. Ethanol concentration was titrated in YPAD from 0 to 9.5% and tested for its ability to enhance the growth of AD321 cells. Bacteria were grown overnight at room temperature, and optical densities were determined. Cell densities are shown as the percent enhancement or inhibition compared to that of cells grown in YPAD alone.
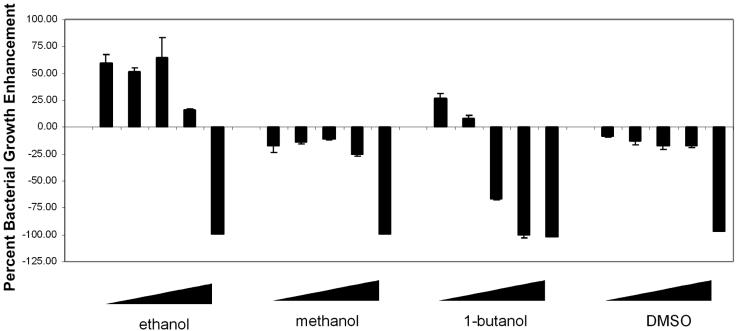
This enhanced-growth effect appears to be specific to ethanol, in that dimethyl sulfoxide or methanol did not enhance bacterial growth. In fact, these latter solvents inhibited bacterial growth by 8.5 to 17.9% at concentrations of up to 5% (Fig. 4). In contrast, 0.1% 1-butanol increased Acinetobacter growth by a modest 26.3%, but at concentrations higher than 0.1% 1-butanol caused a decrease in bacterial growth (Fig. 4). Thus, exogenously added ethanol, but not other similar organics tested, is sufficient to enhance the growth of Acinetobacter strain AD321.
FIG. 4.
Ethanol, but not other organics, enhances Acinetobacter growth. Bacteria were grown in YPAD or YPAD supplemented with ethanol, methanol, butanol, or dimethyl sulfoxide. The concentration of each organic used was 0.1, 0.5, 1.0, 5.0, or 9.5%. Bacteria were grown overnight at room temperature, and cell densities were measured by the OD600. Cell densities are shown as the percent enhancement or inhibition compared to that of cells grown in YPAD alone.
Next, we measured the amount of ethanol that was produced by yeast cells in YPAD. YJM835 produced 0.99% ± 0.2% ethanol when grown to cell densities of 5 × 107 to 7 × 107 cells/ml. Standard lab strains, which enhance AD321 growth poorly, typically produced approximately half as much alcohol: late-log W303 cells typically made 0.52% ± 0.3% ethanol. AD321 cells grown overnight in YPAD plus either exogenously added ethanol or YJM835-conditioned medium were enhanced to the same extent as measured by both OD600 (66.7% ± 13.2% versus 61.5% ± 6.6% growth enhancement) and CFU ([4.55 ± 1.5] × 1011 versus [4.26 ± 1.8] × 1011 CFU/ml). For these assays, the final concentration of ethanol, in either case, was 0.1%. Therefore, ethanol and yeast-conditioned medium containing ethanol enhanced AD321 growth to the same extent.
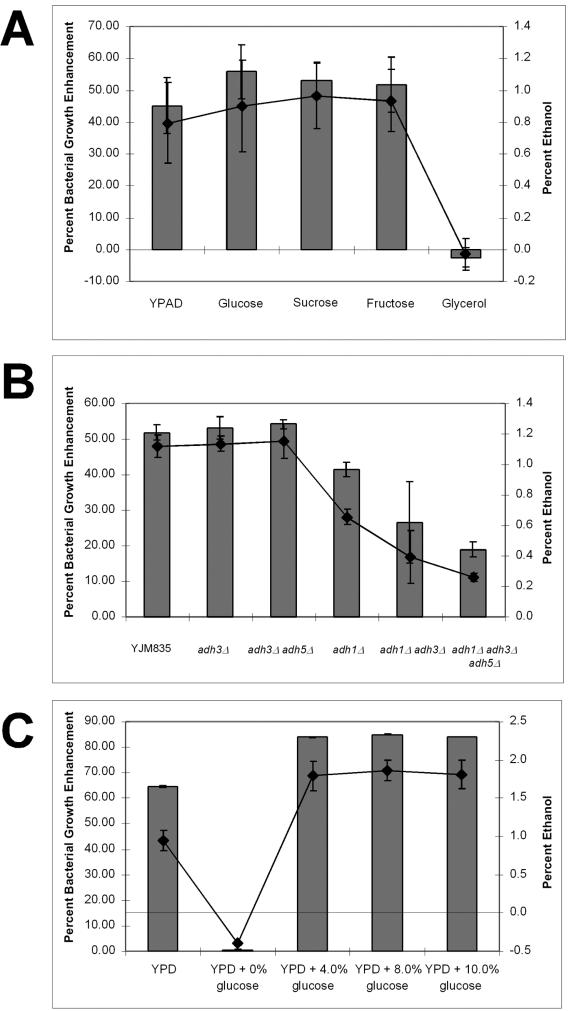
To determine if ethanol production is required for production of bacterial growth enhancement, we grew YJM835 on a variety of carbon sources and tested its ability to affect bacterial growth (Fig. 5A). Glucose is metabolized via glycolysis into pyruvate, which is subsequently converted into either ethanol (fermentation) or carbon dioxide (respiration). Yeasts ferment sugars even when grown aerobically (22, 23). Some carbon sources, such as glycerol, which is nonfermentable, require respiration and, thus, result in no ethanol production. YJM835 grown in the fermentable sugars glucose, fructose, or sucrose produced 0.93% ± 0.2% ethanol and enhanced bacterial growth by 53.6% ± 6.3%. Cells grown in glycerol neither produced significant amounts of ethanol (0.03% ± 0.1% ethanol) nor enhanced bacterial growth (−2.54% ± 2.97% growth enhancement), suggesting that yeast-derived ethanol may be the stimulatory component.
FIG. 5.
Ethanol is required for bacterial growth enhancement. (A) YJM835 cells were grown in YPAD or YP plus each of the carbon sources listed to a cell density of 5 × 107 to 7 × 107 cells/ml. Media were sterilized, and ethanol concentration was determined as described in Materialsand Methods. Bacteria were grown overnight in CY medium at room temperature, and cell densities were measured by the OD600. Cell densities are shown as the percent enhancement or inhibition compared to that of cells grown in YPAD alone. (B) Gene deletions were performed as described in Materials and Methods. Cells were grown in YPAD, conditioned media were sterilized, ethanol concentrations were determined, and bacteria were cultured as described above. (C) YJM835 cells were grown in YPAD or YP plus glucose at the concentrations listed to a cell density of 5× 107 to 7 × 107 cells/ml. Conditioned media were sterilized, ethanol concentrations were determined, and bacteria were cultured as described above. For all panels, columns represent bacterial growth enhancement and lines indicate ethanol concentration.
To determine if the ethanol produced by YJM835 is necessary to enhance the growth of AD321, we disrupted the genes responsible for ethanol production in an attempt to specifically remove ethanol from yeast-conditioned medium. In yeast, there are five alcohol dehydrogenases (ADH1 to ADH5). ADH1 is the cytoplasmic isoform of alcohol dehydrogenase and the major enzyme required for the conversion of acetaldehyde to ethanol (6, 25). ADH2 expression is repressed by growth on glucose and is mainly involved in ethanol consumption, converting ethanol into acetaldehyde (6, 11, 25). ADH3 is a mitochondrial isozyme of alcohol dehydrogenase (42). ADH4 is a formaldehyde dehydrogenase and has no effect on ethanol production (8). Therefore, ADH2 and ADH4 were not disrupted. ADH5 has been sequenced, but its enzymatic function is currently unknown. In our study, deletion of ADH1 resulted in a 50% decrease in the amount of ethanol produced and a reduction in bacterial growth-enhancing capacity (Fig. 5B). Strains lacking both Adh1p and Adh3p or strains lacking Adh1p, Adh3p, and Adh5p produced less ethanol and showed a corresponding decrease in their ability to enhance bacterial growth. The triple deletion strain consistently produced 35% less ethanol than an adh1 adh3 double deletion strain, indicating that Adh5p can produce ethanol. Deletion of adh3 by itself or in combination with adh5 deletion had little effect on either the amount of ethanol produced or the ability to enhance bacterial growth. Thus, deletion of the genes that are required for the production of ethanol results in less ethanol produced as well as a corresponding reduction in bacterial growth enhancement (Fig. 5B).
We reasoned that if ethanol concentration were the primary determinant for enhancement of bacterial growth, then increasing the amount of ethanol produced by yeast should also result in an increase in bacterial growth enhancement up to a certain percentage, as shown in Fig. 3. For these experiments, we grew yeast strain YJM835 overnight in YPAD containing 4, 8, or 10% glucose (standard YPD contains 2% glucose). In all cases, we measured the amount of ethanol produced by the yeast and the level of bacterial growth enhancement. YJM835 yeast produced 0.9% ± 0.19% ethanol from 2% glucose, whereas 2.0% ± 0.18% ethanol was produced from 4, 8, or 10% glucose (Fig. 5C). Clearly, saturation was reached, since these yeast could not produce more than 2% ethanol despite the increasing amounts of glucose provided. Nevertheless, bacterial growth enhancement was ethanol dependent. For example, additional ethanol was produced with between 2 and 4% glucose, and under these conditions bacterial growth was enhanced by 17 to 18% (Fig. 5C). Yeast incubated without glucose failed to grow or produce ethanol and were also ineffective at stimulating bacterial growth. These studies indicate that ethanol is necessary and sufficient to stimulate acinetobacter growth.
Ethanol induces a specific cell tolerance response.
Along with salt and heat, ethanol is a commonly used stimulus to induce the general stress response in many bacteria (14, 30, 32). While the typical ethanol concentrations used for general stress stimulation are considerably higher than the concentrations produced by yeast in YPAD (4 versus 1%), it is possible that low concentrations of ethanol can also elicit a stress response. In low doses, ethanol might serve a signaling role by specifically altering the physiology of the bacterial cells. It has long been known that ethanol can induce thermotolerance in yeast and mammals (24, 31), and we reason that ethanol may similarly induce tolerance of other stresses in Acinetobacter.
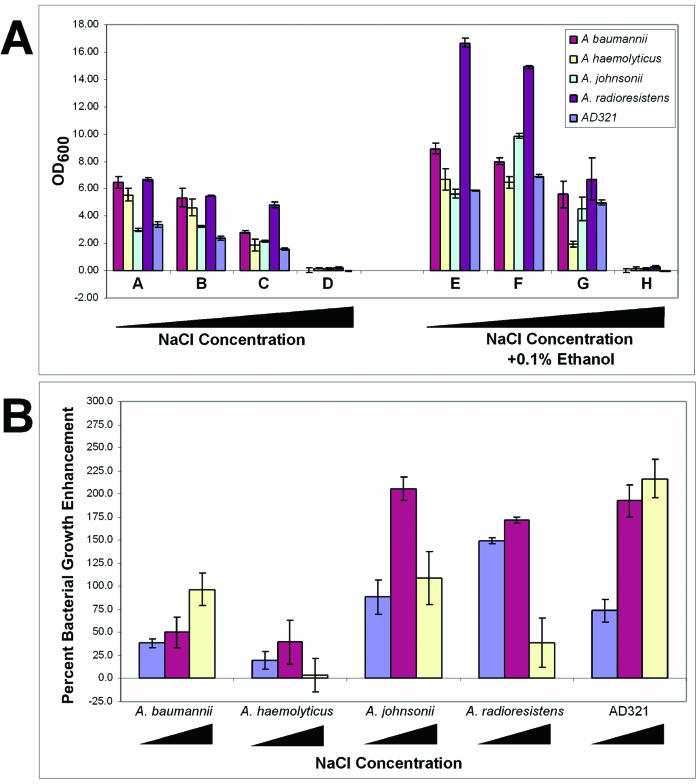
We incubated Acinetobacter cells in high salt, H2O2, or high temperatures in the presence or absence of yeast-conditioned medium or 0.1% ethanol. Addition of NaCl to the culture medium restricted the growth of all strains tested: 1% NaCl inhibited growth 16.8 to 21.1%, 2.5% NaCl inhibited growth by up to 65.9%, and 5% NaCl inhibited growth by more than 94.6% (Fig. 6A). However, addition of either yeast-conditioned medium or exogenously added ethanol protected all bacterial strains from the negative effect of the high salt, as depicted by the optical density and relative increase in growth (Fig. 6A and B). Similar results were obtained when the cells were challenged with KCl or CaCl2 (data not shown). Unlike the protection from high salt, AD321 cells challenged with high temperature or oxidative damage were not protected by conditioned yeast medium or ethanol. Combined, these data suggest that ethanol is not stimulating the general stress response but is instead inducing a specific response, in this case, enabling growth on high salt.
FIG. 6.
Ethanol increases salt tolerance of acinetobacters. Bacteria were grown in YPAD or YPAD supplemented with 0.1% ethanol. (A) Cells were challenged with various concentrations of NaCl as follows: no salt (A and E); 1% (B and F); 2.5% (C and G); and 5% NaCl (D and H). Bacteria were grown overnight at room temperature, and cell densities were measured by the OD600. Cell densities are shown as absolute values. (B) Resulting cell densities, shown as the percent enhancement or inhibition compared to that of cells growth without ethanol. The blue bars represent media containing no salt, red bars represent media containing 1% NaCl, and the yellow bars represent media containing 5% NaCl.
How does growth on ethanol lead to enhanced growth under certain but not all stress conditions? One hypothesis is that ethanol induces a signal transduction cascade that leads to the expression of several proteins, including membrane transporters, kinases, and transcription factors, that lead to osmoadaptation (34) and the ability to grow in high salt. This possibility is reasonable, given that ethanol has been shown to induce heat shock proteins in Acinetobacter (4). An alternative hypothesis is that ethanol may induce changes in the plasma membrane that would enable cells to tolerate high salt. It has been shown that short-chain alcohols can desaturate the plasma membrane-associated fatty acids in Acinetobacter (19). Furthermore, plasma membrane fatty acid desaturation is required for tolerance of high salt by cyanobacteria (33). To test this hypothesis directly, we incubated Acinetobacter cells in the presence of ethanol or butanol and challenged these cells with salt stress. As shown above, bacteria grown in 2.5% NaCl (or KCl) were inhibited from growing by 44.2%. However, medium supplemented with 0.1% ethanol resulted in a 196% increase in bacterial growth. In contrast, addition of 0.01% butanol (a concentration that induces a similar amount of plasma membrane desaturation as 0.1% ethanol in Acinetobacter spp. [19]) resulted in only a 59% increase in growth over cells grown in salt alone. Therefore, while ethanol and butanol can induce comparable levels of plasma membrane desaturation, these two agents cannot stimulate salt resistance to the same extent. Thus, we suggest that ethanol induces a signal transduction cascade as hypothesized above.
Ethanol increases the pathogenicity of A. baumannii.
Chemical stresses are not the only stresses that confront organisms in nature. To survive predation, prey species have evolved a myriad assortment of defense mechanisms. In some cases, prey species have even become parasitic to their predator-host. Several Acinetobacter species are parasitic; in particular, A. baumannii has become a problematic human pathogen worldwide. It is often the case that the extent to which a parasite disables its host is directly correlated with its own reproductive capacity. It is reasonable to suggest, then, that conditions which lead to an increased reproductive capacity of A. baumannii could also lead to an increase in pathogenicity.
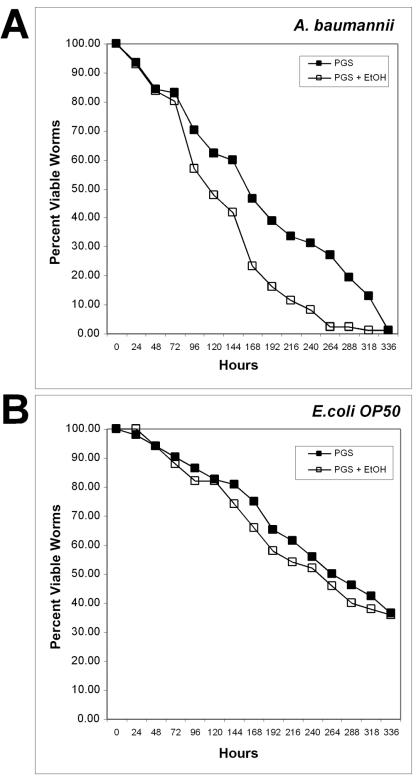
To test this hypothesis, we utilized the free-living nematode C. elegans as predator-host of A. baumannii. C. elegans has been used in the past to study the pathogenicity of several bacteria, including Pseudomonas aeruginosa and Serratia marcescens (20, 21, 39, 40). These studies illustrated not only that bacteria can infect and kill worms, but also that composition of the medium can influence pathogenicity. Rich medium induced the expression of virulence genes which resulted in a “fast killing” phenotype (39, 40). Thus, we tested the effect of ethanol on the ability of A. baumannii to kill worms. Incubation of L4-stage worms on lawns of A. baumannii grown on NGM (minimal medium; see Materials and Methods) resulted in the proliferation of worms at a rate comparable to the growth observed when the worms were fed E. coli control strain OP50. We conclude that C. elegans can consume Acinetobacter spp. cells and use them as a food source. Incubation of L4 worms on lawns of A. baumannii grown on the rich medium PGS resulted in worm lethality. The LT50 (time for half of the worms to die) on A. baumannii grown on PGS was 180 ± 36 h (7.5 days; n = 77) (Fig. 7A). The LT50 for worms that were fed A. baumannii grown on PGS plus 1% ethanol was 126 ± 12 h (5.5 days; n = 86). This represents a 30.0% increase in the rate of death of the worms supplied with ethanol-fed bacteria. In contrast, worms fed E. coli OP50 grown on PGS displayed an LT50 of 264 ± 32 h (11 days; n = 52) in the absence of ethanol and 256 ± 32 h (10.7 days; n = 50) (Fig. 7B) in the presence of ethanol in the media under our experimental conditions. These data indicate that C. elegans can be used as a model system for A. baumannii pathogenesis and that ethanol stimulates an increase in A. baumannii virulence.
FIG. 7.
Ethanol promotes virulence of A. baumannii. Bacteria were grown overnight in LB and spread onto PGS agar plates with (open squares) or without (solid squares) the addition of 1% ethanol. L3/L4-stage worms were placed onto each lawn of bacteria and incubated at 20°C. The plates were then scored for live worms every 24 h. Worms were considered dead when they no longer responded to touch. (A) Worms fed E. coli OP50; (B) worms fed A. baumannii.
DISCUSSION
In this study we have presented a complex interaction between fungi, bacteria, and nematodes. We screened a variety of natural isolates of yeast and bacteria in an effort to uncover yeast-derived factors that might affect bacterial growth. We found that pathogenic isolates of yeast can both inhibit and enhance the growth of bacteria from the genus Acinetobacter. We studied the growth-enhancing phenotype further and found that that the active molecule promoting this interaction is ethanol. We believe that ethanol is the active component based on the following data: (i) older yeast cultures enhance bacterial growth better than younger ones; (ii) the effect on bacteria is correlated with the amount of ethanol in the medium—removal of ethanol results in loss of growth enhancement, whereas an increase in ethanol production results in greater growth enhancement; (iii) the biochemical properties of the enhancing factor are consistent with a small organic molecule, yet other alcohols cannot substitute for ethanol; (iv) exogenously added ethanol is sufficient to enhance bacterial growth; and (v) yeast make and secrete ethanol at concentrations shown to be sufficient for bacterial growth enhancement.
Ethanol stimulates acinetobacters to grow to higher cell density, as measured both by optical density and by counting the number of CFU. Since ethanol is consumed by these bacteria (unpublished observation), it is likely utilized as a carbon source (albeit a poor one, since it is present at low levels). However, the benefit of ethanol to acinetobacters extends beyond increasing cell number. Ethanol stimulates salt tolerance but not thermotolerance or resistance to oxidative damage in acinetobacters. Ethanol-fed acinetobacters can also kill a natural predator more efficiently than bacteria fed other carbon sources. These data indicate that ethanol also induces signaling pathways required for specific stress tolerance and virulence.
Although our studies were confined to the laboratory, we expect them to be pertinent to nature, as they involve organisms that we predict to interact in nature. Standard laboratory strains of yeast were shown to be unable to produce the quantity of ethanol required to enhance bacterial growth. In contrast, natural isolates and, in particular, pathogenic isolates of yeast were able to generate sufficient amounts of ethanol to affect the growth of acinetobacters. S. cerevisiae and Acinetobacter are ubiquitously found in soil, water, and vegetation, they both prefer acidic pH (5.5 to 6.0) environments (2, 3, 13, 37), and they can both be opportunistic human pathogens (5, 10, 26, 41). Thus, we expect them to occupy the same ecological niches and have the potential for direct interactions in the wild.
It is possible that the relationship between the two microbes is proto-commensalistic. That is, in certain environments the bacteria benefit, while in others the yeast benefit. The preferred carbon source for the yeast Saccharomyces is sugar, as its name indicates. Yeast typically ferment sugar into ethanol and carbon dioxide, even in the presence of oxygen, which is curious given the unfavorable energetics of the reaction (7, 22, 23). Acinetobacters are obligate aerobes and are known to consume a wide range of carbon sources, although only a few strains can utilize glucose (3, 16). Thus, many acinetobacters would be dependent on yeast to convert a plentiful sugar source into a more readily catabolized one, namely ethanol. The reciprocal situation may also exist, in which acinetobacters metabolize compounds that yeast cannot and provide yeast with a more suitable metabolite.
Alternatively, but not exclusively, the yeast may benefit indirectly from the bacteria via a form of mutualistic antipredation. Recently, C. elegans has been shown to be able to consume yeast as a food source (27). Since ethanol-fed acinetobacters can kill worms more rapidly than those without ethanol, both microbes stand to gain from the elimination of their common predator.
The deleterious effect of ethanol-fed A. baumannii on worms may be due to expression of bacterial virulence genes that cause physical harm to the worms. Alternatively, the bacteria may be responding to the presence of ethanol by altering their physiology such that they provide the worms with a lesser-quality food source and lead, ultimately, to the decreased worm life span observed. A third possibility is that the presence of ethanol in the medium may affect worms independently of its effect on bacteria. While ethanol in the medium, per se, does not adversely affect worm life span as evidenced by the normal life spans observed with ethanol-fed OP50, ethanol may negatively affect the immune system of the worms. These immunocompromised worms would then be more susceptible to A. baumannii infection. Separating these possibilities will be the subject of future experiments.
Acknowledgments
We thank John McCusker for the pathogenic yeast strains and Andre Lachance for the natural yeast isolates. We also thank Nick Ornston, Arnold Barton, Thomas Burr, and Stefan Pukatzki for bacterial strains. The work involving C. elegans could not have been performed without the assistance of Frank Slack and his lab, in particular, Monica Vella and Stephen Johnson. We also thank William Firshein, Anthony Infante, Dan Nowakowski, Dan Gelperin, Antonio Casamoyer, and Anthony Borneman for their critical reviews of the manuscript. M.G.S. especially thanks Dave Young for strains, suggestions, and invaluable support.
REFERENCES
- 1.Baumann, P. 1968. Isolation of Acinetobacter from soil and water. J. Bacteriol. 96:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann, P., M. Doudoroff, and R. Y. Stanier. 1968. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J. Bacteriol. 95:58-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, P., M. Doudoroff, and R. Y. Stanier. 1968. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J. Bacteriol. 95:1520-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benndorf, D., N. Loffhagen, and W. Babel. 1999. Induction of heat shock proteins in response to primary alcohols in Acinetobacter calcoaceticus. Electrophoresis 20:781-789. [DOI] [PubMed] [Google Scholar]
- 5.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciriacy, M. 1997. Alcohol dehydrogenases, p. 213-223. In K.-D. Entian (ed.), Yeast sugar metabolism. Technomic Publishing Company, Lancaster, Pa.
- 7.De Deken, R. H. 1966. The Crabtree effect: a regulatory system in yeast. J. Gen. Microbiol. 44:149-156. [DOI] [PubMed] [Google Scholar]
- 8.Drewke, C., J. Thielen, and M. Ciriacy. 1990. Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J. Bacteriol. 172:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming, A. 1929. On the antibacterial action of cultures of Penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 10:226-236. [Google Scholar]
- 10.Forster, D. H., and F. D. Daschner. 1998. Acinetobacter species as nosocomial pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 17:73-77. [DOI] [PubMed] [Google Scholar]
- 11.Ganzhorn, A. J., D. W. Green, A. D. Hershey, R. M. Gould, and B. V. Plapp. 1987. Kinetic characterization of yeast alcohol dehydrogenases. Amino acid residue 294 and substrate specificity. J. Biol. Chem. 262:3754-3761. [PubMed] [Google Scholar]
- 12.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie, C. F., and G. Fink (ed.). 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, San Diego, Calif.
- 14.Hecker, M., and U. Volker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 15.Juni, E. 1978. Genetics and physiology of Acinetobacter. Annu. Rev. Microbiol. 32:349-371. [DOI] [PubMed] [Google Scholar]
- 16.Juni, E. 1972. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J. Bacteriol. 112:917-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juni, E. 1974. Simple genetic transformation assay for rapid diagnosis of Moraxella osloensis. Appl. Microbiol. 27:16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juni, E., and A. Janik. 1969. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabelitz, N., P. M. Santos, and H. J. Heipieper. 2003. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 220:223-227. [DOI] [PubMed] [Google Scholar]
- 20.Kurz, C. L., S. Chauvet, E. Andres, M. Aurouze, I. Vallet, G. P. Michel, M. Uh, J. Celli, A. Filloux, S. De Bentzmann, I. Steinmetz, J. A. Hoffmann, B. B. Finlay, J. P. Gorvel, D. Ferrandon, and J. J. Ewbank. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurz, C. L., and J. J. Ewbank. 2000. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 8:142-144. [DOI] [PubMed] [Google Scholar]
- 22.Lagunas, R. 1979. Energetic irrelevance of aerobiosis for S. cerevisiae growing on sugars. Mol. Cell. Biochem. 27:139-146. [DOI] [PubMed] [Google Scholar]
- 23.Lagunas, R. 1986. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast 2:221-228. [DOI] [PubMed] [Google Scholar]
- 24.Li, G. C., and G. M. Hahn. 1978. Ethanol-induced tolerance to heat and to adriamycin. Nature 274:699-701. [DOI] [PubMed] [Google Scholar]
- 25.Lutstorf, U., and R. Megnet. 1968. Multiple forms of alcohol dehydrogenase in Saccharomyces cerevisiae. I. Physiological control of ADH-2 and properties of ADH-2 and ADH-4. Arch. Biochem. Biophys. 126:933-944. [DOI] [PubMed] [Google Scholar]
- 26.McCusker, J. H., K. V. Clemons, D. A. Stevens, and R. W. Davis. 1994. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics 136:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navon-Venezia, S., Z. Zosim, A. Gottlieb, R. Legmann, S. Carmeli, E. Z. Ron, and E. Rosenberg. 1995. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl. Environ. Microbiol. 61:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurmikko, V. 1956. Biochemical factors affecting symbiosis among bacteria. Experientia 12:245-249. [DOI] [PubMed] [Google Scholar]
- 30.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Volker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plesset, J., C. Palm, and C. S. McLaughlin. 1982. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 108:1340-1345. [DOI] [PubMed] [Google Scholar]
- 32.Rince, A., S. Flahaut, and Y. Auffray. 2000. Identification of general stress genes in Enterococcus faecalis. Int. J. Food Microbiol. 55:87-91. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto, T., and N. Murata. 2002. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 5:208-210. [DOI] [PubMed] [Google Scholar]
- 34.Sleator, R. D., and C. Hill. 2001. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 35.Sniegowski, P. D., P. G. Dombrowski, and E. Fingerman. 2002. Saccharomyces cerevisiae and Saccharomyces paradoxus coexist in a natural woodland site in North America and display different levels of reproductive isolation from European conspecifics. FEMS Yeast Res. 1:299-306. [DOI] [PubMed] [Google Scholar]
- 36.Starmer, W. T., P. F. Ganter, V. Aberdeen, M. A. Lachance, and H. J. Phaff. 1987. The ecological role of killer yeasts in natural communities of yeasts. Can. J. Microbiol. 33:783-796. [DOI] [PubMed] [Google Scholar]
- 37.Strathern, J. J., and E. W. Broach, Jr. (ed.). 1981. Life cycle and inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sulston, J., and J. Hodgkin. 1988. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towner, K. J. 1997. Clinical importance and antibiotic resistance of Acinetobacter spp. Proceedings of a symposium held on 4-5 November 1996 at Eilat, Israel. J. Med. Microbiol. 46:721-746. [DOI] [PubMed] [Google Scholar]
- 42.Young, E. T., and D. Pilgrim. 1985. Isolation and DNA sequence of ADH3, a nuclear gene encoding the mitochondrial isozyme of alcohol dehydrogenase in Saccharomyces cerevisiae. Mol. Cell. Biol. 5:3024-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]