Abstract
Much effort has been put in the discovery of ways to selectively kill p53-deficient tumor cells and targeting cell cycle checkpoint pathways has revealed promising candidates. Studies in zebrafish and human cell lines suggested that the DNA damage response kinase, checkpoint kinase 1 (Chk1), not only regulates onset of mitosis but also cell death in response to DNA damage in the absence of p53. This effect reportedly relies on ataxia telangiectasia mutated (ATM)-dependent and PIDDosome-mediated activation of Caspase-2. However, we show that genetic ablation of PIDDosome components in mice does not affect cell death in response to γ-irradiation. Furthermore, Chk1 inhibition largely failed to sensitize normal and malignant cells from p53−/− mice toward DNA damaging agents, and p53 status did not affect the death-inducing activity of DNA damage after Chk1 inhibition in human cancer cells. These observations argue against cross-species conservation of a Chk1-controlled cell survival pathway demanding further investigation of the molecular machinery responsible for cell death elicited by forced mitotic entry in the presence of DNA damage in different cell types and model organisms.
Keywords: Caspase-2, PIDDosome, Chk1, p53, DNA damage
The vast majority of human cancers lack functional p53, a key tumor-suppressor protein that regulates various cellular stress responses, most prominently that induced by DNA damage. In many experimental systems, cell survival upon DNA damage in p53-deficient cells critically depends on intact cell cycle checkpoint pathways that operate in parallel to p53. Targeting these pathways, for example, by chemical inhibition of the checkpoint kinases, Chk1 and Chk2, in p53-deficient tumor cells has been shown to be a promising option for cancer treatment.1 These checkpoint kinases not only have important roles in response to exogenous DNA damage but also in an unperturbed cell division cycle by co-ordinating the onset of mitosis with completion of DNA synthesis.2, 3 In particular, Chk1 seems to be indispensable for normal development and repeated or high-dose application of Chk1 inhibitors may lead to undesired side effects in healthy tissues with high mitotic index such as in the gastrointestinal tract4 or in the immune system.5 Inhibition of Chk1 abrogates the G2/M checkpoint and promotes premature entry into mitosis even in the presence of DNA damage, which frequently results in cell death. The cellular consequences are only beginning to be deciphered and are currently summed up by the term ‘mitotic catastrophe'.6, 7 The molecular machinery responsible for cell killing under these conditions is, however, still unclear but identification of cell death mediators appears pivotal for improved anticancer-drug design and optimization of current therapies.
On the basis of studies in p53-mutant zebrafish embryos as well as p53-defective human cervical (HeLa) and isogenic colon cancer cells (HCT116), it was postulated that pharmacological inhibition of Chk1 or siRNA-mediated ablation of protein expression in combination with γ-irradiation (IR) activates a novel cell death pathway that depends on the multiprotein PIDDosome complex preferentially in p53-defective cells.8 This complex contains the p53-induced protein with a death domain (PIDD), the bipartite adapter RAIDD (receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain) and pro-Caspase-2, a cell-death-associated protease with poorly defined properties.9 Upon DNA damage, ataxia telangiectasia mutated (ATM) directly phosphorylates PIDD on Thr788 within the death domain promoting the interaction with RAIDD that leads to Caspase-2 activation. By a poorly understood mechanism PIDDosome assembly and Caspase-2-dependent cell death are inhibited by Chk1.10 In addition, the downstream effectors of this Chk1-suppressed cell death pathway are undefined but were found to be independent of several well-known apoptosis regulators, including Caspases-3, -8 and -9 and B-cell lymphoma 2 (Bcl-2).8, 10 Of note, cells expressing functional p53 do not seem to engage the Chk1-suppressed pathway effectively, opening a window of opportunity in anticancer therapy.8 Together these studies define the PIDDosome as the initiating protein, promoting death of p53-deficient cells. Hence, PIDD activators would constitute interesting alternatives to Chk1 inhibitors without the obvious loss of essential cell cycle checkpoint functions and therefore might have less severe side effects than Chk1 blockers.11, 12
To further explore the general validity of the Chk1-suppressed cell death pathway in more detail in mammalian cells, we investigated its contribution to cell death in wild-type and p53-deficient mice and derived cell lines. Our study did not confirm conservation or general validity of this mechanism in different primary, immortalized or malignant cells derived from p53−/− or p53−/−Casp2−/− (DKO) mice. Interestingly, although we could confirm additive effects of Chk1-inhibiton and IR-damage as well as partial Caspase-2 dependence of this type of cell death in HeLa cervical carcinoma cells, we failed to notice a clear correlation between the p53 status and Caspase-2 dependence of cell death in isogenic HCT116 cells. Thus, our findings call for a reassessment of the molecular machinery responsible for Chk1 inhibition-dependent cell death in mammals in the context of DNA damage and exclude Caspase-2 or PIDD, and hence the PIDDosome, as master regulators of this type of cell death.
Results
Lack of cross-species conservation of the Chk1-suppressed cell death pathway
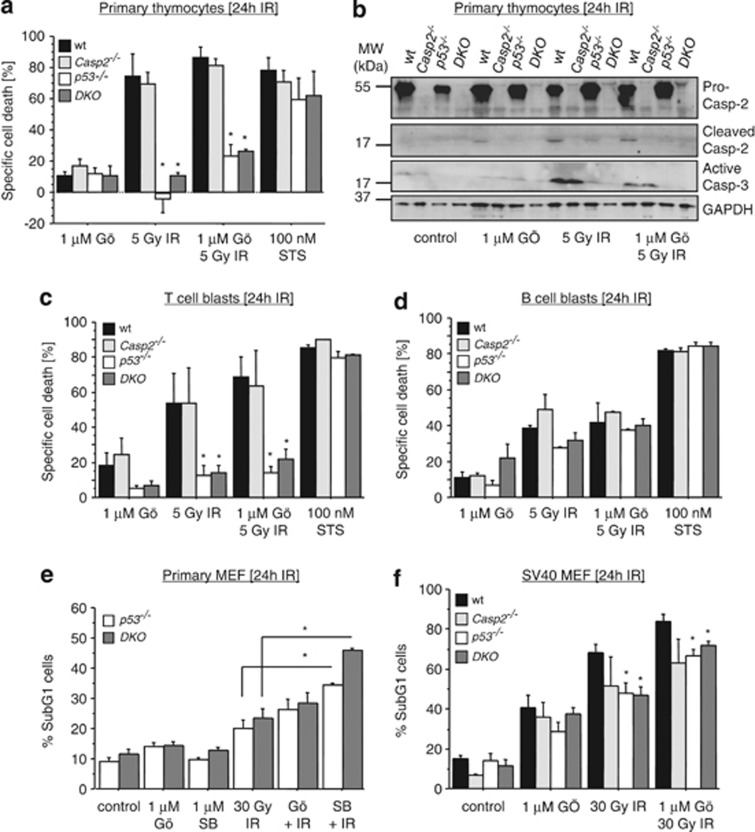
Investigating the Chk1-suppressed pathway in primary thymocytes isolated from wt, Casp2−/−, p53−/− or p53−/−Casp2−/− (DKO) mice revealed high susceptibility to IR-induced cell death in wt and Caspase-2-deficient cells, whereas those lacking p53 or p53 plus Caspase-2 were equally resistant. Blocking Chk1 by the addition of 1 μM Gö6976 had no significant impact on cell death in culture but also failed to significantly sensitize thymocytes to 1.25, 2.5 (not shown) or 5 Gy of IR (Figure 1a; Supplementary Figure S1a). Cleavage of Caspase-2 was best detectable by immunoblotting in wild-type cells exposed to Gö6976, IR or both. Cells lacking p53 also showed some processing of Caspase-2 after IR (Figure 1b), documenting that it is not required for IR killing as these cells are highly radiation resistant.
Figure 1.
Chk1 inhibition fails to sensitize p53-deficient lymphocytes or MEF to DNA damage. Primary thymocytes (a), stimulated T- (c) or B-cell blasts (d) were treated with Gö6976 and/or exposed to IR or both. As a control, cells were treated with staurosporine (STS). Cell viability was assessed after 24 h using AnnexinV/PI staining and flow cytometric analysis. To account for spontaneous cell death of lymphocytes in culture, specific cell death was calculated throughout using the equation (induced apoptosis−spontaneous cell death)/(100−spontaneous cell death). In parallel, thymocytes were harvested and protein lysates were separated by SDS-PAGE to monitor cleavage of Caspase-2 and Caspase-3. Membranes were reprobed with an anti-GAPDH antibody to control protein loading (b). Viability of primary (e) and SV40 immortalized MEFs (f) derived from E14.5 embryos of the indicated genotypes were assessed using sub-G1 staining and flow cytometric analysis. Bars represent means±S.E. of ≥3 independent experiments per genotype and treatment. *Indicates significant differences between wt or Casp2−/− cells and p53−/− or DKO cells (P<0.05) after IR or Gö+IR treatment. No significant differences were noted in (a, c and d) between Gö and Gö+IR treatment
As only a subfraction of thymocytes is actively cycling and hence may be poorly susceptible to the effects of Chk1 inhibition, we next generated spleen-derived T-cell and B-cell blasts by mitogenic stimulation to test the effects of Chk1 inhibition on cell death in the absence of p53. The results from T-cell blasts mirrored our findings in thymocytes, demonstrating lack of substantial cell death in the absence of p53 and a failure of Chk1 inhibition to sensitize these cells to IR. In contrast, stimulation with the pan-kinase inhibitor staurosporine (STS) induced cell death independently of the genotype confirming general cell death susceptibility in the absence of p53 or p53 and Caspase-2 (Figures 1a and c). Similar findings were made in B-cell blasts, albeit these cells were less radio-resistant in the absence of p53 when compared with their cycling T-cell counterparts (Figure 1d). Similarly, using doxorubicin as an inducer of DNA damage in thymocytes (Supplementary Figure S1b) or FACS-sorted mature T or B cells from the spleen (Supplementary Figures S1c and d) yielded the same results, that is, substantial cell death resistance in the absence of p53 but lack of sensitization by Chk1 inhibition. Finally, reducing expression levels of Chk1 to half by removing one allele of the Chk1 locus on a p53−/− background also failed to increase cell death susceptibility of T- or B-cell blasts after IR (Supplementary Figure S1e).
Taken together, these data show that primary mouse lymphocytes lacking p53 respond poorly to G2/M checkpoint inhibition by Gö6976 are not sensitized to IR killing and that Caspase-2 does not contribute to their IR-driven cell death.
To extend our findings to non-hemopoietic cells, we investigated the Chk1-suppressed cell death pathway in early passage primary and immortalized mouse embryonic fibroblasts (MEF) generated from E14.5 embryos. As expected, primary MEF derived from p53−/− or DKO mice showed only moderate cell death, even upon high-dose IR of 30 Gy, as these cells preferentially undergo cell cycle arrest. Treatment of cells with Gö6976 before irradiation failed to substantially sensitize these cells to death, whereas a different Chk1 inhibitor, SB218078, stimulated some cell death in p53-deficient cells when combined with IR. However, this cell death was independent of Caspase-2. If anything, loss of Casp2 rather increased cell death under these conditions (Figure 1e; Supplementary Figure S2a). This finding suggested that Gö6976 might not even target Chk1 in mouse cells. However, it clearly prevented the arrest of primary MEF in G2/M after IR (Supplementary Figure S2b) and both inhibitors prevented the accumulation of inactive CDK1, phosphorylated on tyrosine 15 (Y15), a Chk1-dependent effect after IR, confirming that these compounds were indeed blocking Chk1 activity also in mouse cells (Supplementary Figure S2c).
To sensitize cells to IR-induced apoptosis and blunt p53-responses by other means, we transduced MEF with a retrovirus encoding SV40 large T (LT) antigen and subjected them to IR in the presence or absence of Gö6976. As can be seen in Figure1f, immortalization sensitized these cells to cell death by IR (when compared with primary MEF), although again rather high doses of 30 Gy had to be applied, as 10 Gy of IR proved rather ineffective to induce cell death (not shown). However, Chk1 inhibition per se triggered some death in these cells. Despite the fact that the combined treatment proved additive, all genotypes tested, also those lacking Caspase-2, p53 or both, died at similar rates (Figure 1f; Supplementary Figure S2d).
So far all cell types tested were either primary or immortalized. To test whether the Chk1-suppressed pathway was only active in transformed cells, we also transduced MEF with retroviruses encoding the oncogenes c-Myc and Ha-RasV12 that leads to full cellular transformation.13 These cells were also radiosensitive and showed some cell death in response to Chk1 inhibition but the combined treatment only marginally increased cell death when compared with cells exposed to IR alone. The cell death observed, however, was again independent of Caspase-2 (Supplementary Figure S3a). SV40 MEF or E1A/Ha-RasV12 transformed MEF lacking PIDD were also found equally susceptible to IR and Chk1 inhibition, as Caspase-2-deficient or wt cells excluding PIDDosome formation as a rate-limiting step in this cell death paradigm in MEF (Supplementary Figures S3c–e). In all cases analyzed, cell death was clearly associated with Caspase-3 activation and Caspase-2 processing (indicated by decreased levels of its pro-form) but independent of Caspase-2 function (Supplementary Figures S2e and S3b).
To assess long-term consequences on clonal survival, colony formation assays were performed using early passage primary MEF (passage 2–3) or immortalized/transformed MEF generated from at least two independent embryos per genotype. Exposure of MEF to 10 Gy of IR prevented colony formation irrespective of the mode of transformation and absence or presence of p53, Caspase-2 or both (Supplementary Figure S4a). In contrast, exposure to 3 Gy of IR allowed clonal survival of cells from all genotypes and SV40 MEF lacking Caspase-2 alone, or Caspase-2 and p53, seemed to display superior clonal survival, suggesting for the first time a role of Caspase-2 under these conditions. Such marked differences, however, were not noted in MEF immortalized with c-Myc plus Ha-RasV12 or in primary MEF (Supplementary Figure S4a). Testing independent batches of MEF, however, failed to confirm increased clonal survival in the absence of Caspase-2 after exposure to 3 Gy of IR±Chk1 inhibition. Notably, SV40 MEF lacking PIDD also behaved like wt cells (Supplementary Figure S4b). E1A plus Ha-RasV12-transformed MEF were highly sensitive to the effects of Gö6976 and did not show long-term clonal survival upon Chk1 inhibition (Supplementary Figure S4c). On the basis of these observations, we conclude that Caspase-2 on its own, or recruited in the PIDDosome, is not rate-limiting for the clonal survival of MEF exposed to Chk1 inhibition and IR, neither in the absence nor in the presence of p53.
Combined loss of p53 and Caspase-2 fails to allow postnatal development in the absence of Chk1
Chk1-deficiency leads to early embryonic lethality shortly after implantation before E6.5 post conception. Notably, lack of p53 cannot restore embryonic development of Chk1-deficient embryos.3 We reasoned that under such conditions, basal levels of DNA damage in the embryo might then trigger Caspase-2-dependent cell death curtailing development. Therefore, we investigated whether combined loss of Caspase-2 and p53 might allow postnatal development in the absence of Chk1. Inter-crossing of p53−/−Casp2+/−Chk1+/− male mice with p53+/−Casp2−/−Chk1+/− females (expected frequency of triple mutants 1:16; observed 0/54 offspring) or p53−/−Casp2−/−Chk1+/− males with p53+/−Casp2−/−Chk1+/− females (expected frequency 1:8; observed 0/46) failed to give rise to viable animals that lacked all three alleles, as monitored by genotyping PCR analysis on tail DNA at the time of weaning, 4 weeks after birth (not shown). Together, this demonstrates that combined loss of p53 and Caspase-2 is insufficient to restore normal development in the absence of Chk1.
Lack of Caspase-2 does not affect the tumor-suppressor function of p53
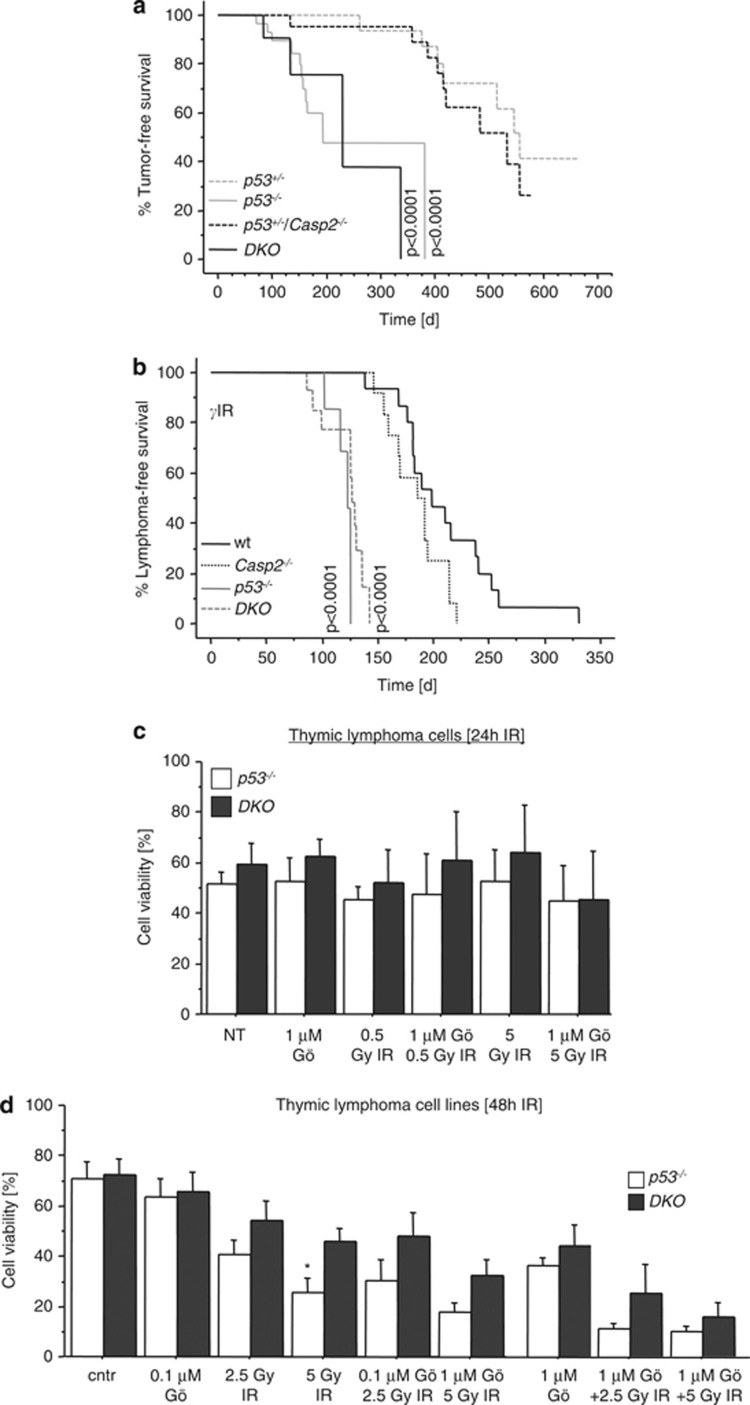
To investigate the potential role of Caspase-2 in p53-dependent tumor suppression and to test cell death sensitivity of p53-deficient tumor cells exposed to DNA damage under conditions of Chk1 inhibition, we followed mice lacking one or two alleles of p53 on a Caspase-2 deficient or proficient background. Lack of Caspase-2 did not have an impact on spontaneous tumor latency (Figure 2a) or tumor type in these mice (not shown). Similarly, lack of Caspase-2 had no influence on thymic lymphomagenesis caused by DNA damage using the well-established fractionated irradiation protocol (Figure 2b). Freshly isolated lymphoma cells derived from these mice were highly susceptible to spontaneous death in culture. Hence, additional Chk1 inhibition or exposure to IR did not further accelerate cell death ex vivo (Figure 2c). Therefore, we also generated lymphoma cell lines from these primary tumors. These cell lines responded with increased cell death upon IR in culture, in line with findings reported earlier.14 Notably, loss of Caspase-2 provided some degree of protection that even achieved statistical significance at higher doses of IR. However, these lymphoma cell lines were also highly vulnerable to the effects of 1 μM Gö6976 in the absence of DNA damage, reducing cell viability to below 40%. Reducing the concentration of the Chk1 inhibitor to more tolerable doses of 0.1 μM, however, failed to significantly increase cell death rates caused by IR in both genotypes tested. Regardless, under conditions of Chk1 inhibition followed by IR, loss of Caspase-2 did not provide significant protection from cell death (Figure 2d).
Figure 2.
Lack of Caspase-2 has no effect on spontaneous or IR-driven thymic lymphomagenesis in p53-null mice. (a) Kaplan–Meier analysis comparing tumor-free survival of p53+/− (median survival 492 days, n=16), p53−/− (median survival 158 days, n=28), p53+/−Casp2−/− (median survival 387 days, n=23) and DKO (median survival 129 days, n=8). (b) Kaplan–Meier analysis of tumor-free survival of wt (median survival 204 days, n=14), Casp2−/− (median survival 185 days, n=13), p53−/− (median survival 117 days, n=8) and DKO (median survival 116 days, n=16) mice after fractionated irradiation. Indicated P-values in (a and b) refer to differences compared with wt mice. (c) Freshly isolated thymic lymphoma cells from p53−/− and DKO mice were put in culture and treated with Gö6976 and/or exposed to IR. Cell survival was assessed after 24 h by AnnexinV/PI staining. (d) Thymic lymphoma cell lines derived from the indicated genotypes were treated with 0.1 μM or 1.0 μM Gö6976 alone, or in combination with increasing doses of IR. Bars represent means±S.E. of four independent experiments. *indicates significant differences (P<0.05) compared between p53−/− and DKO cells
In summary, this documents that Chk1 inhibition per se can kill immortalized or transformed mouse cells in a p53-independent manner and can increase cell death rates caused by IR in some mouse cells lacking p53, such as primary and transformed MEF or thymic lymphoma cells (Figures 1e and f), but Caspase-2 is not required for cell death in these settings.
Caspase-2 contributes to IR-driven cell death of human cancer cells upon Chk1 inhibition
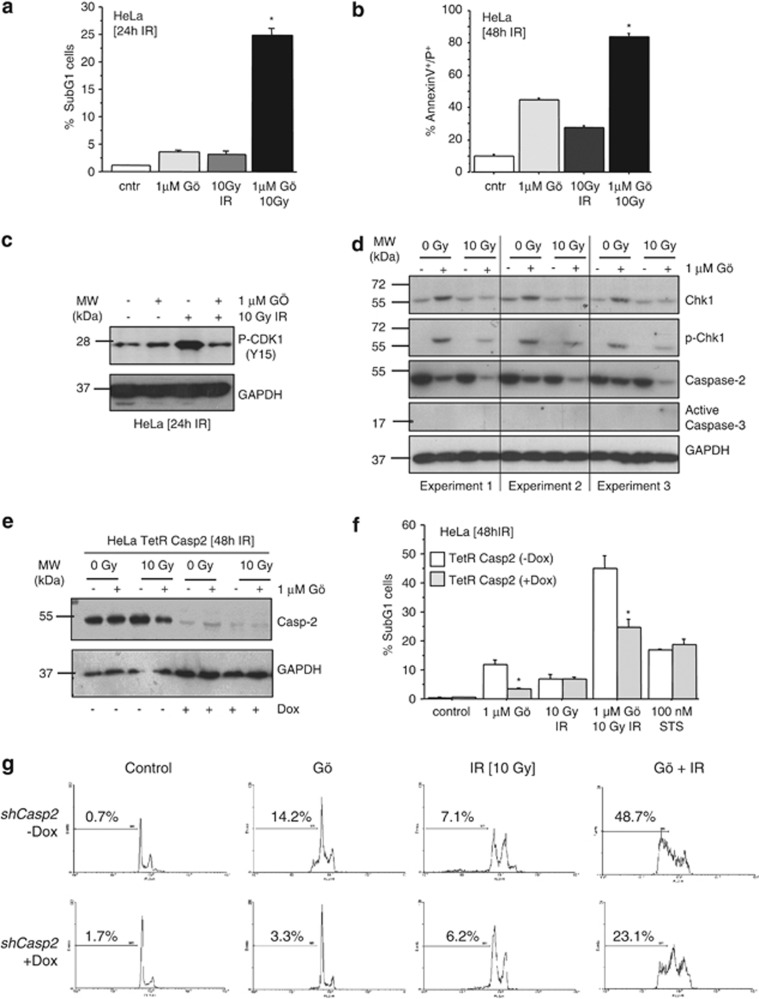
The above experiments suggested that the Chk1-suppressed pathway is not conserved in mice. To interrogate the reported requirement of Caspase-2 in human carcinoma cells, we treated HeLa cells with 1 μM of Gö6976, 10 Gy IR or a combination of both and measured cell death 24 or 48 h later. Although the combination treatment triggered significant cell death already at 24 h, all treatments induced some cell death after 48 h. However, the strongest effect was observed after treating cells with the Chk1 inhibitor plus IR (Figures 3a and b). Interestingly, Gö6976 treatment alone caused the phosphorylation of Chk1 at S345, suggesting ATR activation, most likely due to DNA damage accumulating upon Chk1 inhibition and G2/M checkpoint override. When Gö6976 was combined with IR treatment, the induction of inactivating Tyr15 phosphorylation of CDK1 was prevented, suggesting that the kinase inhibitor did inhibit Chk1 (Figures 3c and d). The induction of cell death by Gö6976 alone or in combination with IR was clearly correlated with Caspase-2 processing, as indicated by a quantitative reduction of its pro-form at 24 h, where indeed no activation of Caspase-3 was detectable by western blotting (Figure 3d). This confirms that Gö6976 treatment can activate Capase-2 in HeLa cells, an effect that is enhanced by IR, which suggests that under these conditions Caspase-2 might be involved in checkpoint signaling and/or acts as an upstream regulator of cell death (Figure 3d). Consistent with a role for Caspase-2 in this cell death paradigm, conditional ablation of Caspase-2 expression by short hairpin RNA (shRNA)-mediated knockdown (Figure 3e) significantly reduced cell death upon IR and Gö6976 treatment (Figures 3f and g). Of note, cell death induced by staurosporine was not affected by Caspase-2 knockdown (Figure 3f).
Figure 3.
Chk1-suppressed cell death pathway is active in HeLa cells. Cells were treated with Gö6796 and/or IR or STS. Cell death was assessed in parallel after 24 h and 48 h of treatment by sub-G1 (a) or AnnexinV/PI staining (b). After 24 h, cells were harvested and lysed for western blot analysis to assess Chk1 inhibition (c). Activation of Chk1, Caspase-2 and Caspase-3 was monitored in parallel by western blotting (d). HeLa cells with regulated knockdown of Caspase-2 were put on doxycycline for 24 h. Impact of Caspase-2 knockdown (e) on cell death induced by Chk1 inhibition and/or IR in HeLa cells, monitored by sub-G1 analysis, is depicted in (f). Representative histograms from sub-G1 analysis are shown in (g). Bars represent means±S.E. of ≥3 independent experiments. *Indicates significant differences (P<0.05) between Casp2 proficient and deficient cells
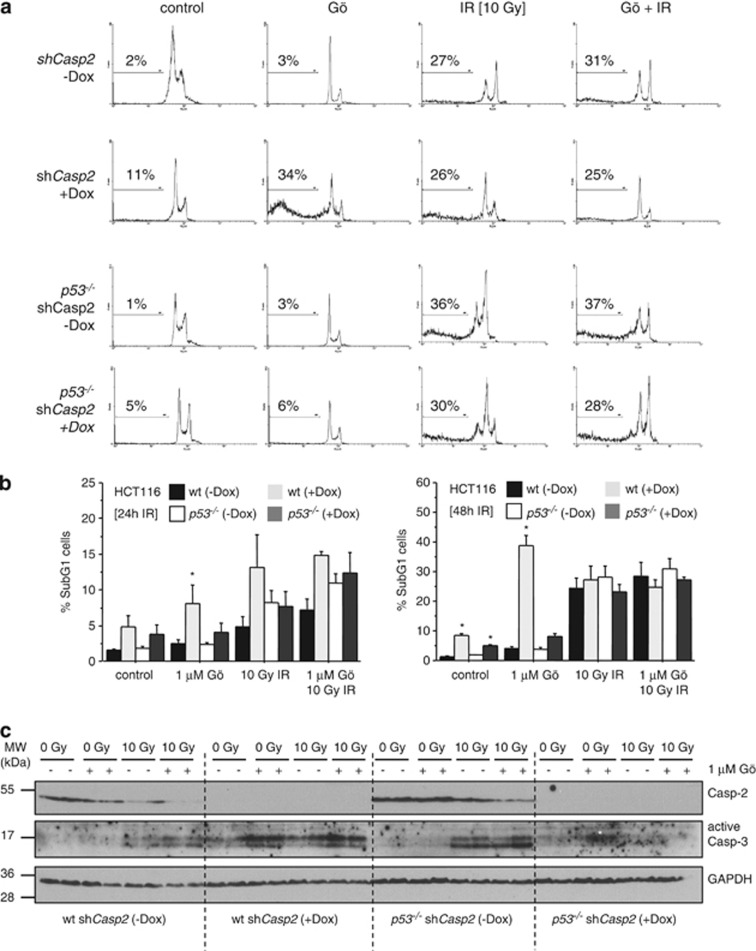
To further investigate whether Caspase-2 knockdown can also rescue other cell types from cell death upon IR and Gö6976, we next turned to isogenic HCT116 cells lacking or expressing p53 and engineered those to conditionally express Caspase-2 RNAi. HCT116 cells underwent apoptosis upon IR treatment but this effect was independent of Caspase-2 and could not be enhanced significantly by additional treatment with Gö6976 (Figures 4a and b). In p53+/+ HCT116 cells, IR treatment triggered the activation of Caspase-3 and this effect was independent of Caspase-2 expression. Interestingly, however, Gö6976 treatment alone caused a significant induction of cell death upon Caspase-2 knockdown, but this was only noted in p53+/+ HCT116 cells (Figures 4a and b). This phenomenon remains currently unexplained but is in line with previously proposed cell cycle checkpoint functions of this protease,15, 16 that when absent may sensitize cells to apoptosis. Finally, although HCT116 p53−/− cells died at similar rates than p53+/+ cells under these conditions, activation of Caspase-3 was no longer detectable after Caspase-2 ablation in the absence of p53 (Figure 4c), suggesting an involvement of alternative non-apoptotic cell death modalities upon DNA damage.6 Notably, ‘sub-G1' cells can also accumulate in response to non-apoptotic cell death inducers.17
Figure 4.
Cell death caused by Chk1 inhibition does not correlate with p53 status. Isogenic HCT116 cells lacking or expressing functional p53 and an shRNA targeting Caspase-2 were treated with Gö6796 and/or exposed to IR. Representative histograms from sub-G1 analysis performed after 48 h are shown in (a). Percentage of cell death was assessed by sub-G1-staining and flow cytometric analysis after 24 h and 48 h (b). Bars represent means±S.E. of ≥3 independent experiments per genotype and treatment. *Indicates significant differences (P<0.05) between controls and Dox-treated cells. (c) Caspase-2 expression was ablated by the addition of Doxycycline for 24 h prior exposure of cells to Gö or Gö+IR. Lysates for western blotting were harvested after additional 24 h. (*) In the image marks non-specific precipitates that leave the impression that IR triggers Caspase-3 activation. Higher magnification of the image will reveal the lack of the p17 fragment of active Caspase-3
Discussion
In this study, we investigated the relevance of the Chk1-suppressed cell death pathway in primary and transformed mouse cells and human cancer cell lines. Our experiments suggest that primary lymphocytes lacking p53 function cannot be sensitized effectively to cell death upon IR-inflicted DNA damage by Gö6976-mediated Chk1 inhibition (Figure 1). MEF and thymic lymphoma cell lines derived from p53-deficient mice showed trends of increased cell death upon Chk1 inhibition and IR exposure (Figures 1 and 2). However, these effects were rather minor and, for example, in the case of p53-deficient primary MEF only noted with one of the two different Chk1 inhibitors used (Figure 1e). More importantly, lack of Caspase-2 did never protect from cell death under conditions of Chk1 inhibition and IR exposure (Figures 1 and 2; Supplementary Figures S1 and S3). Given the vast body of evidence from human cancer cell lines, demonstrating increased cell death sensitivity of p53-defective cells upon G2/M checkpoint override,11, 12, 18 we believe that these observations are rather surprising, as they question the general validity of this concept, strongly pointing toward species and/or cell type-specific differences. One simple explanation for our findings may be lack of efficient Chk1 inhibition by Gö6976 in mouse cells. However, p53-deficient MEF clearly failed to arrest in G2 upon Gö6976 treatment (Supplementary Figure S2b) and the compound prevented the accumulation of Tyr15 phosphorylated CDK1 (Supplementary Figure S2c), that serves as a read out for Chk1 activity.8 It is worth mentioning here that Gö6976, an indolocarbazol, has been identified as a kinase inhibitor that targets most selectively classical Ca++-dependent PKC isoforms (αβ)19 and its inhibitory action on Chk1 was only noted a decade later.20 Hence, Gö6976 at the concentrations used here and in related studies8, 10 may cause cell death not only by inhibition of Chk1 and checkpoint override. Of note, classical PKC isoforms are generally considered as pro-survival kinases21 and inhibition of PKC isozymes, activated in response to DNA damage, for example, by the bisindolymaleimide Gö6850 sensitizes human fibroblasts to the effects of IR.22
To increase confidence in our observations, we also used a different Chk1 inhibitor, that is, SB218087, in some of our experiments, yielding essentially identical results as those obtained using Gö6976 (Figure 1e; Supplementary Figure S3b). Both compounds trigger some death on their own and this was found most pronounced in combination with IR, but lack of Caspase-2 failed to provide protection from killing (Figure 1e; Supplementary Figure S3e). Notably, ablation of one allele of Chk1 also failed to sensitize primary lymphocytes to IR (Supplementary Figure S1e), suggesting that mouse cells can deal better than human cells with DNA damage in the presence of reduced Chk1 activity. It also remains possible that in mice G2/M checkpoint fidelity in the absence of p53 may rely more on alternative control mechanisms, such as the recently reported MAPKAP kinase 2 (MK2)–p38 MAPK network that putatively may engage additional regulators of mitotic entry next to Cdc25c.23, 24 However, the early lethality of Chk1-deficient mice and severe checkpoint deficits in derived ES cell cultures argues against a significant degree of redundancy between these two checkpoint pathways in vivo.2, 3 Furthermore, in contrast to Chk1 RNAi, knockdown of MK2 also failed to cause premature G2/M transition in the presence of DNA damage in human U2OS cells engineered to lack p53, a finding in support of species and/or cell type-dependent differences.25
It remains formally possible that mouse lymphocytes simply lack the capacity to activate cell death signaling emanating from premature G2/M transition in the presence of DNA damage, as none of the normal lymphocyte populations tested here showed increased cell death rates after Chk1 inhibition (Figure 1; Supplementary Figure S1). In contrast, in MEF expressing different oncogenes, Chk1 inhibition per se caused already some cell death, and combination with IR was most effective in cell killing (Supplementary Figures S3 and S4), similar to findings made in HeLa cells where DNA damage and Chk1 inhibition were shown to integrate on the formation of the PIDDosome and Caspase-2-dependent cell death.10 We also found that DNA damage activates Caspase-2 before any active Caspase-3 becomes detectable by western blotting, suggesting that it might be an upstream regulator of cell death and/or checkpoint control under these conditions, at least in HeLa cells (Figure 3). However, our mouse and HCT116 data also show that Caspase-2 is not strictly required for cell death under these conditions, which is consistent with earlier studies in mice and human cancer cells that failed to support a proapoptotic role of the postulated PIDDosome complex after DNA damage.26, 27, 28 Admittedly, none of these studies tested the consequences of PIDD loss or knockdown in response to Chk1 inhibition plus DNA damage but our studies in HCT116 cells (Figure 4) and previous studies suggesting a dominant role for PIDD in DNA damage-induced nuclear factor kappa-light-chain enhancer (NF-kB) activation29 argue against a general Caspase-2- or PIDDosome dependence of this type of cell death. Thus, although Gö6976 can sensitize HeLa cells to DNA damage-induced killing the exact role of this kinase inhibitor and the molecular wiring of these events are still unclear.
The tumor-suppressor p53 contributes to the strength of the G2/M checkpoint by regulating p21 and 14-3-3sigma and loss of p53 facilitates premature G2/M transition upon Chk1 inhibition in human cancer cells. Our data (Figure 4) and a previous study show, however, that p53 status is not strongly correlated with cell death initiation.25 As neither p53, PIDD, Caspase-2 nor BCL-2 seem to be commonly involved in the regulation of cell death following G2/M checkpoint override the molecular players in ‘mitotic catastrophe' remain to be defined on a (tumor) cell type-specific basis.
In summary, these observations argue against a general cross-species conservation of the Chk1-suppressed cell death pathway in vertebrates and cell type-dependent differences also in human cancer cells, demanding reassessment of the molecular machinery responsible for tumor cell death elicited upon forced mitotic entry in the presence of DNA damage.
Materials and Methods
Mice
All animal experiments were performed in accordance with the Austrian legislation (BGBl. Nr. 501/1988 i.d.F. 162/2005). The generation and genotyping of the Casp2−/−,30 p53−/− mice31 and Chk1f/fmice5 have been described. Mice harboring a floxed allele were mated with Ubi-Cre deleter strain to generate Chk1+/− mice. To induce thymic lymphomas by IR, mice were exposed to whole body irradiation (4 × 1.75 Gy) at the age of 4 weeks32 in weekly intervals in a linear accelerator. All mice were maintained on C57BL/6 genetic background.
Cell lines, tissue culture and cell sorting
Freshly isolated thymocytes, splenocytes or thymic lymphoma cells33 and MEF28 were isolated and cultured as described. For survival analysis and colony formation assays using MEF, at least two independent batches from individual embryo preparations were tested and the FACS data were pooled. Chk1 inhibitors were commonly applied 1 h prior irradiation.
Generation of Caspase-2-knockdown lines
Caspase-2 targeting shRNA encoding oligonucleotides was cloned into HindIII-BglII digested pENTR-THT-III, a GATEWAY cloning compatible ENTR vector harboring a tetracycline-regulatable H1-RNA gene promoter. After sequence verification, the shRNA expression cassette was shuttled into a selectable puromycin resistance conferring as well as a TetR-GFP-expressing lentiviral vector. Target cells (HeLa and HCT116) were transduced with lentiviral particles generated by transient transfection of lentiviral constructs with packaging and pseudotyping plasmids as described previously.34 Transduced target cells were selected using 2.5 μg/ml puromycin and induced using 1 μg/ml doxycycline. The selected target site in human Caspase-2 mRNA was: 5′-GCCCAAGCCTACAGAACAA-3′.
Quantification of cell death and cell cycle distribution
Lymphocytes and myeloid progenitors were cultured at a density of 5 × 105/ml and after 24 and 48 h post-irradiation cells were stained with AnnexinV-FITC (eBioscience, Vienna, Austria) plus 5 μg/ml propidium iodide (Sigma, Vienna, Austria) in AnnexinV staining buffer and analysed using FACS. MEF, HeLa or HCT116 cells were plated in six-well plates (100 000 cell/dish) and stimulated the following morning. Cells were then washed once in PBS, spun down at 1500 r.p.m. at 4 °C and subsequently fixed in pre-chilled 70% ethanol/PBS overnight at 4 °C. For sub-G1 analysis, cells were washed twice in PBS and after RNase A (Sigma) digestion (100 μg/ml in PBS, 30 min at 37 °C) cells were stained with propidium iodide (40 μg/ml) and the percentages of sub-G1, G1, S and G2/M cells were monitored by flow cytometry.
Colony forming assays
For colony formation assays early passage MEF (P2) or transduced MEF selected for 1 week were seeded at a density of 2000, 4000, 8000 or 16 000 cells in 3.5 cm tissue culture dishes. The next morning cells were pretreated with 0.1 μM Gö6976 for 1 h before IR (3 or 10 Gy). Cells were fixed after 9 or 12 days by removal of media and a wash in PBS by the addition of crystal violet staining solution (0.2% crystal violet in 50% methanol). Experiments were performed independently at least three times in triplicates.
Immunoblotting
Cells were lysed in lysis-buffer (Buffer: 10 mM HEPES, 1.5 mM MgCl2, 300 mM sucrose, 10 mM KCl, 0.5% NP40, Roche Complete Protease Inhibitor Cocktail (Roche, Vienna, Austria), pH adjusted to 7.0, 5 mM DTT added freshly from a 1 M stock) for at least 1 h on ice. Protein was separated by SDS/PAGE, transferred to a nitrocellulose membrane and incubated with the relevant antibodies Caspase-2 (11B4) Alexis (Vienna, Austria); cleaved Caspase-3 (Asp175) (5A1), pCDK1 (Y15), Chk1 and p-Chk1 (S345; 133D3) all from Cell Signalling (New England Biolabs, Frankfurt, Germany); GAPDH (71.1) Sigma. Immune-reactivity was visualized using an enhanced chemiluminescence detection system (ECL) (GE Healthcare, Freiburg, Germany).
Statistics
Statistical analysis was performed using unpaired Student t-test and ANOVA as indicated and for Kaplan–Meier analysis the Logrank (Mantel–Cox) test was used, applying Stat-view 4.1 software program. P-values of <0.05 were considered to be statistically different.
Acknowledgments
We are grateful to K Rossi, V Rauch and I Gaggl for technical assistance and animal care; P Lukas and his team (LINAC1-4) from the Department of Radio-oncology for enabling irradiation experiments; P Jost for help with establishing thymic lymphoma cell lines. We thank D Vaux, M Serrano and T Mak for Casp2−/−, p53−/− and Chk1f/f mice, respectively. This work was supported by grants from the Austrian Science Fund–FWF (SFB021 and the Doctoral College, MCBO) to AV and SG; the EU-FP06 Marie Curie Research Training Network ‘Apoptrain' to AV & FB; the Austrian Cancer Society Branch Tirol (Tiroler Krebshilfe) to FB, GK, CM and LF; and the Medical University Innsbruck intramural funding program MUI-START to CM. LP is recipient of a Doc-Fellowship, sponsored by the Austrian Academy of Science (ÖAW). LF is a recipient of an EMBO-LT fellowship.
Glossary
- PIDD
p53-induced protein with a death domain
- RAIDD
receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain
- Chk1
checkpoint kinase 1
- NF-kB
nuclear factor kappa-light-chain enhancer
- Nemo/IKKg
NF-kB essential modulator/nuclear factor kappa-B kinase subunit gamma
- ATM
ataxia telangiectasia mutated
- Bcl-2
B-cell lymphoma 2
- DISC
death-inducing signaling complex
- MEF
mouse embryonic fibroblast
- Mdm2
murine double minute
- RIP-1
receptor-interacting serine/threonine-protein kinase 1
- IR
γ-irradiation
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Melino
Supplementary Material
References
- Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21:245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1-/- mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Greenow KR, Clarke AR, Jones RH. Chk1 deficiency in the mouse small intestine results in p53-independent crypt death and subsequent intestinal compensation. Oncogene. 2009;28:1443–1453. doi: 10.1038/onc.2008.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg K, Su YW, Reilly PT, Moolani Y, Cheung CC, Hakem R, et al. Cross-talk between Chk1 and Chk2 in double-mutant thymocytes. Proc Natl Acad Sci USA. 2007;104:3805–3810. doi: 10.1073/pnas.0611584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- Janssen A, Medema RH. Mitosis as an anti-cancer target. Oncogene. 2011;30:2799–2809. doi: 10.1038/onc.2011.30. [DOI] [PubMed] [Google Scholar]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock FJ, Peintner L, Tanzer M, Manzl C, Villunger A. P53-induced protein with a death domain (PIDD): master of puppets. Oncogene. 2012;31:4733–4739. doi: 10.1038/onc.2011.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Kernan JL, Liu PH, Sanda T, Logette E, Tschopp J, et al. PIDD Death-domain phosphorylation by ATM controls prodeath versus prosurvival PIDDosome signaling. Mol Cell. 2012;47:681–693. doi: 10.1016/j.molcel.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2011;17:88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts M, Linardopoulos S, Turner NC. Tumour selective targeting of cell cycle kinases for cancer treatment. Curr Opin Pharmacol. 2013;13:529–535. doi: 10.1016/j.coph.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Sohn D, Budach W, Janicke RU. Caspase-2 is required for DNA damage-induced expression of the CDK inhibitor p21(WAF1/CIP1) Cell Death Differ. 2011;18:1664–1674. doi: 10.1038/cdd.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA. 2009;106:5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner D, Manzl C, Soratroi C, Villunger A, Krumschnabel G. Necrosis-like death can engage multiple pro-apoptotic Bcl-2 protein family members. Apoptosis. 2012;17:1197–1209. doi: 10.1007/s10495-012-0756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Fernandez-Capetillo O. Targeting ATR and Chk1 kinases for cancer treatment: a new model for new (and old) drugs. Mol Oncol. 2011;5:368–373. doi: 10.1016/j.molonc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Kohn EA, Yoo CJ, Eastman A. The protein kinase C inhibitor Go6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res. 2003;63:31–35. [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluwstein A, Kumar N, Leger K, Traenkle J, Oostrum J, Rehrauer H, et al. PKC signaling prevents irradiation-induced apoptosis of primary human fibroblasts. Cell Death Dis. 2013;4:e498. doi: 10.1038/cddis.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Hasskamp P, Schmedding I, Morandell S, van Vugt MA, Wang X, et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol Cell. 2010;40:34–49. doi: 10.1016/j.molcel.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenvirt S, Kravchenko-Balasha N, Levitzki A. Status of p53 in human cancer cells does not predict efficacy of CHK1 kinase inhibitors combined with chemotherapeutic agents. Oncogene. 2010;29:6149–6159. doi: 10.1038/onc.2010.343. [DOI] [PubMed] [Google Scholar]
- Vakifahmetoglu H, Olsson M, Orrenius S, Zhivotovsky B. Functional connection between p53 and caspase-2 is essential for apoptosis induced by DNA damage. Oncogene. 2006;25:5683–5692. doi: 10.1038/sj.onc.1209569. [DOI] [PubMed] [Google Scholar]
- Kim IR, Murakami K, Chen NJ, Saibil SD, Matysiak-Zablocki E, Elford AR, et al. DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis. 2009;14:1039–1049. doi: 10.1007/s10495-009-0375-1. [DOI] [PubMed] [Google Scholar]
- Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F, et al. Caspase-2 activation in the absence of PIDDosome formation. J Cell Biol. 2009;185:291–303. doi: 10.1083/jcb.200811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock FJ, Krumschnabel G, Manzl C, Peintner L, Tanzer MC, Hermann-Kleiter N, et al. Loss of PIDD limits NF-kappaB activation and cytokine production but not cell survival or transformation after DNA damage. Cell Death Differ. 2013;20:546–557. doi: 10.1038/cdd.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Ekert P, Harvey N, Marsden V, Cullen L, Vaux DL, et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ. 2002;9:832–841. doi: 10.1038/sj.cdd.4401033. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kaplan HS, Brown MB. A quantitative dose-response study of lymphoid-tumor development in irradiated C57 black mice. J Natl Cancer Inst. 1952;13:185–208. [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O'Reilly L, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22:370–377. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.