Abstract
Mycobacterial heparin-binding haemagglutinin antigen (HBHA) is a virulence factor that induces apoptosis of macrophages. Endoplasmic reticulum (ER) stress-mediated apoptosis is an important regulatory response that can be utilised to study the pathogenesis of tuberculosis. In the present study, HBHA stimulation induced ER stress sensor molecules in a caspase-dependent manner. Pre-treatment of RAW 264.7 cells with an IκB kinase 2 inhibitor reduced not only C/EBP homology protein expression but also IL-6 and monocyte chemotactic protein-1 (MCP-1) production. BAPTA-AM reduced both ER stress responses and caspase activation and strongly suppressed HBHA-induced IL-6 and MCP-1 production in RAW 264.7 cells. Enhanced reactive oxygen species (ROS) production and elevated cytosolic [Ca2+]i levels were essential for HBHA-induced ER stress responses. Collectively, our data suggest that HBHA induces cytosolic [Ca2+]i, which influences the generation of ROS associated with the production of proinflammatory cytokines. These concerted and complex cellular responses induce ER stress-associated apoptosis during HBHA stimulation in macrophages. These results indicate that the ER stress pathway has an important role in the HBHA-induced apoptosis during mycobacterial infection.
Keywords: ER stress, macrophage, mycobacteria
Tuberculosis (TB) remains a major problem, and its spread has been exacerbated by the development of multidrug-resistant strains of Mycobacteria tuberculosis (Mtb). To address this issue, it is necessary to find a way to kill intracellular mycobacteria in multidrug-resistant TB patients and to develop highly effective therapeutic methods for treating TB patients. TB is one of the oldest re-emerging diseases. In 2010, there were 8.8 million cases of TB globally, 1.1 million deaths from TB among HIV-negative people, and an additional 350 000 deaths from HIV-associated TB.1 However, despite this prevalence, the exact pathogenesis of TB is not completely understood.
Apoptosis is a physiological process that requires the synthesis of a series of proteins to translate an apoptotic signal. Although apoptosis in bacterial infections contributes to organ damage, the induction of apoptosis may be beneficial to the host, as it also contributes to the death of microorganisms.2 Macrophages have an essential role in the defence against mycobacterial infections.3 Mtb is one of the most successful human pathogens because of its ability to survive by manipulating host cells via multiple pathways.4, 5, 6 Recent studies have shown that apoptosis is an innate defence function of macrophages against Mtb;3, 6 virulent Mtb is able to inhibit macrophage apoptosis, and instead trigger necrosis at high intracellular bacterial loads.7 Recently, we have shown that the endoplasmic reticulum (ER) stress response may be related to Mtb-induced apoptosis, and that this process has an important role in controlling the survival of intracellular Mtb.4
The ER has an important role in folding secretory and cellular proteins during their transit, and ER chaperon proteins prevent the toxic accumulation of misfolded secretory proteins. A series of ER chaperons is involved in the regulation of protein synthesis and the induction of cell death.8 Although the host-protective effect of apoptosis is generally considered to be responsible for the modulation of intracellular Mtb survival, the biological relevance is still unclear.
Previously, we reported that ESAT-6 antigen induces ER stress-mediated apoptosis, that Mtb-infected macrophages induce growth arrest and DNA damage-induced gene-153 (GADD153) production, and that siGADD153 treatment increases intracellular bacillary loads.4, 9 Seimon et al.10 showed that ER stress is induced in TB granuloma macrophages in areas where apoptotic cells accumulate. Taken together, these results suggest that ER stress responses have important roles in TB pathogenesis.9, 10
The identification of specific antigens secreted from mycobacteria is important to understand the mechanisms involved in the pathogenesis of mycobacterial diseases. Heparin-binding haemagglutinin antigen (HBHA) is a 28-kDa, methylated, surface-exposed protein that has a role in Mtb infectivity.11 We recently reported that HBHA enters macrophages and induces apoptosis through a loss of mitochondrial transmembrane potential and reactive oxygen species (ROS) generation.12 Although ROS generation and nitric oxide (NO) are closely associated with ER stress responses,13, 14 the mechanism of the interaction is not known. Therefore, in the present study, we investigated the mechanisms by which ER stress leads to cell death in macrophages during HBHA stimulation as well as the underlying mechanisms of ER stress-associated apoptosis induced by HBHA.
Results
HBHA stimulation induces ER stress responses and apoptosis in murine macrophage cells
HBHA is a mycobacterial antigen that induces apoptosis in host cells. To investigate the interplay between macrophage apoptosis and ER stress responses, we first confirmed that HBHA induced apoptosis, based on cell cycle analysis using flow cytometry (Figure 1a). We found that HBHA stimulation caused statistically significant accumulation of cells in the sub-G1 phase in RAW 264.7 cells. The percentage of apoptotic cells increased in a dose-dependent manner after HBHA stimulation. Next, ER stress responses after stimulation with HBHA were examined. Phosphorylation of eukaryotic translation initiation factor 2A (eIF2α) was increased in RAW 264.7 cells following HBHA treatment (Figure 1b). Induction of eIF2α phosphorylation was associated with increased expression of GRP78 and C/EBP homology protein (CHOP), which are indicators of unfolded protein responses. Similarly, each ER stress sensor molecule was also increased following HBHA stimulation in bone marrow-derived macrophage (BMDM) cells. ER stress-mediated apoptosis may also be involved in HBHA-stimulated BMDM cells, as caspase-3 activation is directly correlated with the induction of CHOP (Figure 1c). To examine the association between ER and HBHA, western blotting was performed after subcellular fractionation with each proper antibody in RAW 264.7 cells. We detected HBHA not only in the ER fraction but also in the cytosol fraction (Figure 1d). These results suggest that HBHA interacts with ER and induces ER stress responses, leading to apoptosis.
Figure 1.
HBHA-induced apoptosis is associated with the ER stress response in macrophages. (a) Detection of apoptosis by cell cycle measurement. RAW 264.7 cells were stimulated with HBHA (10 μg/ml) for 24 h. The cells were stained with propidium iodide (PI) solution and the sub-G1 stage was determined by flow cytometry analysis. Exposure to 500 nM staurosporine (STS) for 6 h was used as positive control. (b) Immunoblot analysis of p-eIF2α, eIF2α, CHOP, GRP78, and β-actin in cell lysates of RAW 264.7 cells stimulated with HBHA (10 μg/ml) for the indicated time period. (c) Immunoblot analysis of CHOP, GRP78, p-eIF2α, caspase-3, and β-actin in BMDM cells stimulated with HBHA (10 μg/ml) for the indicated time period. Lysate of cells treated with 2 μg/ml tunicamycin (TM) for 6 h was used as a positive control. Bands corresponding to each protein were quantified, and the intensities of each protein were normalised to the intensity of actin. p-eIF2α was normalised to the intensity of eIF2α. Data are representative of at least three independent experiments with similar results. A significant difference was observed in HBHA-stimulated cells compared with unstimulated control (**P<0.01 and ***P<0.001). (d) Immunoblot analysis of HBHA, GRP78, and α-tubulin in both unstimulated control and HBHA-stimulated (10 μg/ml) cell lysates in RAW 264.7 cells. Subcellular fractionation was performed as described in the Materials and Methods
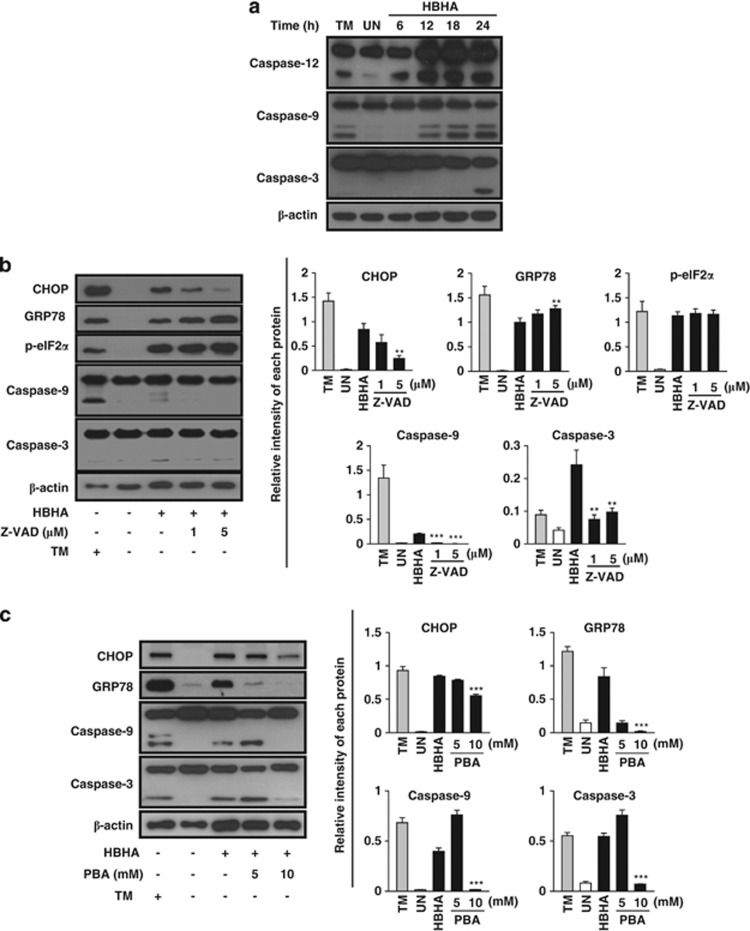
Because ER stress-mediated apoptosis is involved in mycobacterial infection and HBHA induces macrophage apoptosis via caspase activation, we investigated the involvement of the activation of caspases, including the ER stress marker caspase-12. Western blotting showed that HBHA stimulation elicited not only the activation of caspase-9 and caspase-3, but also ER stress resident caspase-12 (Figure 2a). Next, we investigated the relationship between ER stress and caspase-dependent apoptosis after HBHA stimulation in RAW 264.7 cells. Enhanced CHOP expression with HBHA stimulation was significantly attenuated by caspase inhibition by a pan-caspase inhibitor, z-VAD-fmk, in a dose-dependent manner (Figure 2b). In addition, HBHA-induced activation of capsase-9 and caspase-3 was significantly reduced by z-VAD-fmk. However, the levels of GRP78 and eIF2α phosphorylation induced by HBHA were not affected by pre-treatment with z-VAD-fmk. These findings suggest that HBHA-induced CHOP expression is associated with caspase activation, leading to caspase-dependent apoptosis.
Figure 2.
The ER stress response is involved in HBHA-mediated and caspase-dependent apoptosis. (a) RAW 264.7 cells were stimulated with HBHA (10 μg/ml) for the indicated time period. The cell lysates were analysed by western blotting with antibodies for caspase-12, caspase-9, caspase-3, and β-actin. (b) RAW 264.7 cells were stimulated with HBHA for 24 h in the presence or absence of 1 h pretreatment with z-VAD-fmk. Next, total cell lysates were subjected to western blotting for CHOP, GRP78, p-eIF2α, caspase-9, caspase-3, and β-actin analysis. (c) RAW 264.7 cells were stimulated with HBHA for 24 h in the presence or absence of 1 h pretreatment with 4-PBA. Next, total cell lysates were subjected to western blotting for CHOP, GRP78, caspase-9, caspase-3, and β-actin analysis. A cell lysate treated with 2 μg/ml TM for 6 h was used as a positive control to induce ER stress. The blots were also probed for β-actin to control for loading. Bands corresponding to each protein were quantified, and the intensities of each protein were normalised to the intensity of actin. Data are representative of at least three independent experiments with similar results. The asterisks indicate significant differences compared with untreated cells (**P<0.01, ***P<0.001)
To assess the functional significance of ER stress responses in inducing caspase-dependent apoptosis, the chemical chaperone 4-phenyl butyric acid (4-PBA) was pretreated before HBHA stimulation in RAW 264.7 cells. Production of CHOP and GRP78 was significantly attenuated by treatment with 10 mM 4-PBA and the levels of caspase-9 and caspase-3 activation in HBHA-treated RAW 264.7 cells were reduced (Figure 2c). HBHA-mediated apoptosis was reduced by improving ER folding capacity with 4-PBA. Overall, these data provide evidence that HBHA-mediated ER stress responses might be associated with caspase-dependent apoptosis.
HBHA-induced proinflammatory cytokines elicit CHOP production
A functionally impaired ER emits NF-κB activation, which in turn induces production of proinflammatory cytokines.15, 16 Because the promoter regions of monocyte chemotactic protein-1 (MCP-1) and IL-6 contain consensus-binding sites for NF-κB,17, 18 we investigated the relationship between IL-6 and MCP-1 production and the ER stress response in HBHA-stimulated RAW 264.7 cells. In total, 3.61±0.57 ng/ml MCP-1 and 2.13±0.71 ng/ml IL-6 were produced from RAW 264.7 cells after 24-h stimulation with HBHA (Figure 3a). As expected, IκB kinase 2 (IKK2) inhibitor suppressed the release of MCP-1 and IL-6 in a dose-dependent manner (Figure 3a). IKK2 inhibitor strongly inhibited IL-6 production in RAW 264.7 cells in a dose-dependent manner (26.33%±5.54%, 1.43%±1.13%, and 0.31%±0.11% at 1, 5, and 10 μM, respectively). MCP-1 production was also reduced by an IKK2 inhibitor in a dose-dependent manner (96.25%±9.97%, 48.23%±9.22%, and 12.01%±2.32% at 1, 5, and 10 μM, respectively). IKK2 inhibitor treatment reduced the production of CHOP, which was increased by HBHA stimulation in RAW 264.7 cells (Figure 3b). As IKKβ activation has been shown to be closely related to JNK activation, as well as NF-κB,19 we investigated whether inhibition of JNK could suppress HBHA-induced ER stress (Figure 3c). This suggests that HBHA-induced CHOP production was closely associated with the production of IL-6 and MCP-1 through NF-κB activation.
Figure 3.
IL-6 and MCP-1 production with HBHA stimulation might be associated with CHOP expression in RAW 264.7 cells. (a and b) RAW264.7 cells were preincubated for 30 min with the IKK2 inhibitor IKK2 IV and then stimulated with HBHA (10 μg/ml) for 24 h. (a) Effects of IKK2 inhibition on IL-6 and MCP-1 in supernatants 24 h after HBHA stimulation. Measurement of IL-6 and MCP-1 levels from the supernatant was performed using a CBA flow cytometric assay. LPS (100 ng/ml, 24 h) was used as a positive control. (b) The cells were harvested 24 h after HBHA stimulation, and each total cell lysate was analysed by western blotting for CHOP. (c) RAW264.7 cells were preincubated for 1 h with the JNK inhibitor SP600125 and then stimulated with HBHA (10 μg/ml) for 24 h. The cell lysates were analysed by western blotting with antibodies for CHOP, GRP78, p-eIF2α, and β-actin. Cell lysate treated with 2 μg/ml TM for 6 h was used as a positive control to induce ER stress. Data are representative of at least three independent experiments with similar results. A significant difference was observed in HBHA-stimulated cells compared with unstimulated control cells (***P<0.001)
Enhanced intracellular Ca2+ via HBHA stimulation is the major source of ER stress response
Disturbance of Ca2+ homeostasis is a causal factor of ER stress, therefore we explored the possibility that HBHA-induced Ca2+ release causes ER stress in RAW 264.7 cells. Intracellular Ca2+ in RAW 264.7 cells was measured fluorometrically using the Ca2+ indicator Fura-2. As reflected by the representative traces depicted in Figure 4a, there were transient increases in [Ca2+]i after treatment with HBHA. The application of HBHA (10 μg/ml) in bath solution induced a significant increment in [Ca2+]i with a latent period of 20–25 min (Figure 4a). The effect of HBHA on the increase in Ca2+ was also assessed in the absence of extracellular Ca2+. The rate of increase in [Ca2+]i was not high compared with cells stimulated in the presence of Ca2+. We performed direct measurements of ER [Ca2+] in permeabilised cells using the low-affinity Ca2+ indicator Mag-Fura. This indicator's propensity to become trapped in organelles allowed the evaluation of changes in free [Ca2+] in the lumen of InsP3-sensitive Ca2+ stores (i.e., the ER; Figure 4b). Application of a supramaximal dose of InsP3 (1 μM) caused a reversible drop in the 340 and 380 nm Mag-Fura ratio by 63.33%±11.90% in all tested β-escin-permeabilised cells, demonstrating that the permeabilisation procedure was successful, because InsP3 is membrane impermeant. Subsequent HBHA (10 μg/ml) application elicited a significant decrease in the Mag-Fura ratio by 38.40%±2.77% in 61.41%±9.08% β-escin-permeabilised cells because of the release of Ca2+ from internal stores. These results suggest that HBHA was able to directly release Ca2+ from InsP3-sensitive stores (Figure 4b). Therefore, HBHA stimulation hypothetically resulted in extracellular Ca2+ influx and the release of cellular Ca2+ stores. Next, we tested whether mycobacteria increased [Ca2+]i levels in intact cells (Figure 4c). Live mycobacteria increased [Ca2+]i levels in RAW 264.7 cells, whereas dead mycobacteria induced only minimal changes in [Ca2+]i. The [Ca2+]i increase was observed within 40 min after application of live mycobacteria, and in most cases did not return to basal levels. These results suggest that HBHA antigen secreted from mycobacteria may have a role in calcium release from the ER. Next, we investigated the link between ER stress and HBHA-induced Ca2+ release in RAW 264.7 cells. An intracellular Ca2+ chelator, BAPTA-AM, was used to investigate the role of cytosolic Ca2+ increases in inducing ER stress responses. BAPTA-AM reduced not only CHOP production but also eIF2α phosphorylation in HBHA-stimulated RAW 264.7 cells (Figure 4d). Activation of caspase-9 and caspase-3 was also consistently suppressed by BAPTA-AM in a dose-dependent manner. These results showed that HBHA stimulated RAW 264.7 cells in the presence of BAPTA-AM, which chelated HBHA-induced cytosolic calcium, and inhibited the induction of ER stress. As BAPTA-AM can also inactivate IKK,20, 21 the production of IL-6 and MCP-1 was analysed. BAPTA-AM strongly suppressed HBHA-induced IL-6 production in RAW 264.7 cells in a dose-dependent manner (73.39%±0.37%, 22.20%±9.25%, and 3.81%±4.36% at 10, 20, and 30 μM, respectively) and reduced MCP-1 production in a dose-dependent manner (66.20%±16.00%, 44.34%±17.12%, and 7.32%±4.92% at 10, 20, and 30 μM, respectively; Figure 4e).
Figure 4.
HBHA induces intracellular Ca2+ release in RAW 264.7 cells. RAW 264.7 cells were cultured overnight in 12-well plates over a glass coverslip. (a) Intracellular Ca2+ measurements were performed with the fluorescent Ca2+ indicator Fura-2/AM (5 μM), using the Diaphot inverted fluorescence microscope as described in the Materials and Methods section. Next, HBHA (10 μg/ml) was treated in the presence (normal line) and absence of Ca2+ (dashed line) with HBSS solution. Representative recordings of HBHA-evoked cytosolic Ca2+ transients were recorded according to the Fura-2 ratio (F340/F380). The colour of the stable cells was green or blue and the colour of cells releasing Ca2+ because of HBHA stimulation changed to orange and red. (b) Representative traces of HBHA-induced Ca2+ release from ER in RAW 264.7 cells. Cells were incubated with Mag-Fura-2/AM (10 μM) for 1 h and then permeabilised with β-escin (20 μM) for 80–100 s. Representative changes in Ca2+ release were recorded according to the Mag-Fura ratio (F340/F380) in RAW 264.7 cells treated with HBHA (10 μg/ml) and PBS using the TILL Photonics imaging system (Universal Imaging, West Chester, PA, USA). (c) Representative traces of mycobacteria-induced Ca2+ release from ER in RAW 264.7 cells. Cells in a nominally Ca2+-free HBSS solution were infected with live and 5% formaldehyde-fixed bacteria (MOI, 0.5). Cells were incubated with Fura-2/AM (5 μM), and cytosolic Ca2+ transients were recorded according to the Fura-2 ratio as in a. (d) Intracellular Ca2+ chelation with BAPTA-AM modulates HBHA-induced ER stress responses and caspase activation. To clarify the effects of BAPTA-AM on HBHA-induced ER stress sensor molecules and caspase activation, RAW 264.7 cells were preincubated with BAPTA-AM (10, 20, 30 μM) for 30 min and then stimulated with HBHA (10 μg/ml) for 24 h. Total cell lysate was analysed with western blotting using specific antibodies for each target protein. A cell lysate treated with 2 μg/ml tunicamycin for 6 h was used as a positive control to induce ER stress. The blots were also probed for β-actin to control for loading. Bands corresponding to each protein were quantified, and the intensities of each protein were normalised to the intensity of actin. (e) Intracellular Ca2+ chelation with BAPTA-AM modulates HBHA-induced IL-6 and MCP-1 production. Measurement of IL-6 and MCP-1 levels was performed using the CBA flow cytometric assay. Data are representative of at least three independent experiments with similar results. A significant difference was observed in HBHA-stimulated cells compared with unstimulated control cells (*P<0.05, **P<0.01, ***P<0.001)
Generation of ROS and NO is involved in HBHA-induced ER stress responses
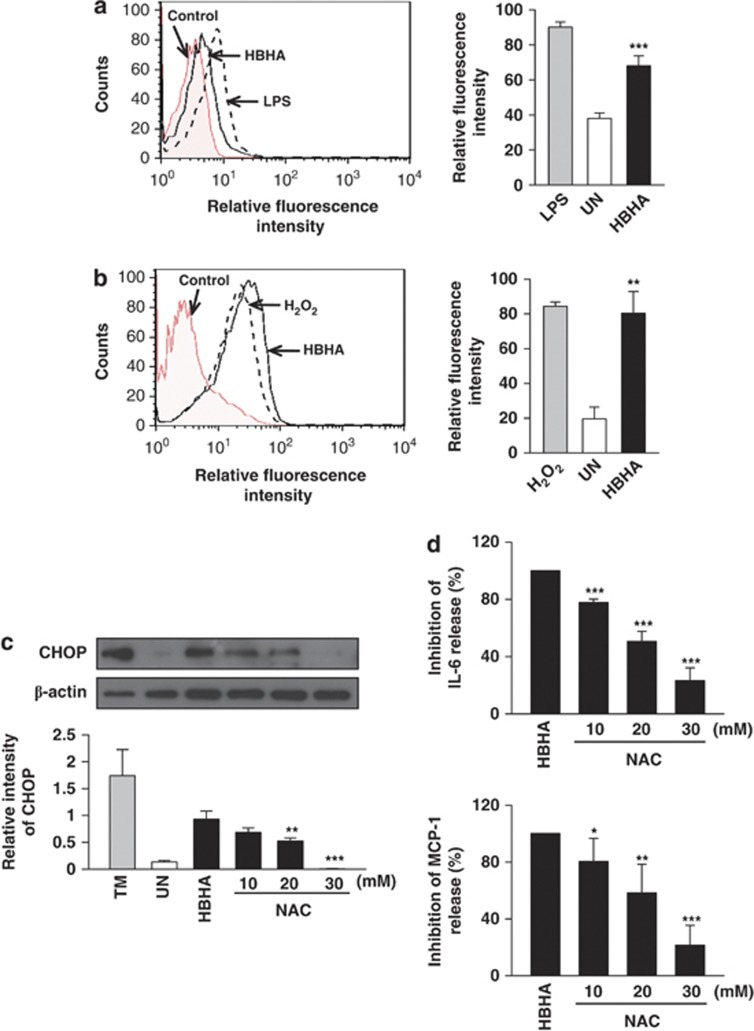
ROS has been implicated in mycobacterial infection and ER stress.4, 9, 10 ROS can induce proinflammatory cytokine production through MAPK activation.22 Therefore, we investigated the relationships between ROS synthesis, proinflammatory cytokine production, and intracellular Ca2+ release. HBHA induced increased ROS generation in RAW 264.7 cells compared with control (Figure 5a). Treatment of RAW 264.7 cells with HBHA for 18 h resulted in enhanced mitochondrial superoxide production (Figure 5b).
Figure 5.
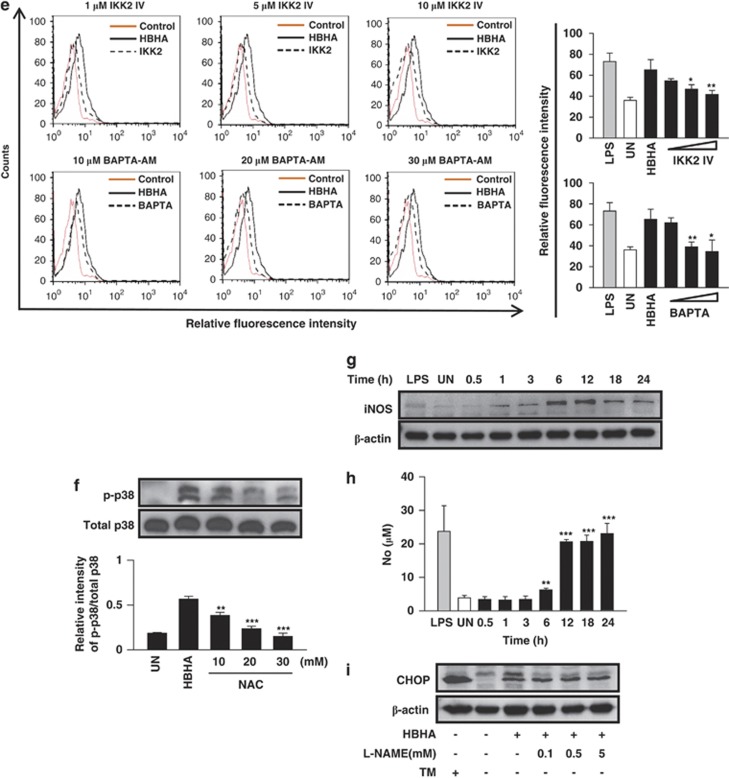
HBHA induces ER stress through ROS generation, which is associated with NF-κB activation and intracellular Ca2+ release. (a) Analysis of superoxide was evaluated after HBHA stimulation (10 μg/ml) for 24 h in RAW 264.7 cells by flow cytometry. LPS (100 ng/ml, 24 h) was used as the positive control for superoxide. (b) Representative histograms of flow cytometry experiments demonstrating increase in mean fluorescent intensity of MitoSOX following HBHA stimulation (10 μg/ml) as indicated. Analysis of mitochondria ROS was evaluated after HBHA stimulation for the indicated time period in RAW 264.7 cells by flow cytometry. H2O2 (5 mM, 30 min) was used as the positive control for superoxide. (c) ROS-scavenging effect of NAC on HBHA-induced CHOP production. RAW264.7 cells were preincubated with NAC (10, 20, 30 mM) for 1 h and then stimulated with HBHA (10 μg/ml) for 24 h. (d) The ROS scavenger NAC modulates HBHA-induced IL-6 and MCP-1 production. Measurement of IL-6 and MCP-1 levels was performed using the CBA flow cytometric assay. (e) Analysis of superoxide was evaluated after HBHA (10 μg/ml) stimulation for 24 h in the presence or absence of 30 min pretreatment with IKK2 IV (1, 5, 10 μM) or BAPTA-AM (10, 20, 30 μM). Cells were stained with dihydroethidium (20 μM) for 20 min at 37 °C. Analysis of superoxide was evaluated by flow cytometry. (f) The effect of NAC on the phosphorylation of p38 MAPK during HBHA (10 μg/ml) stimulation in RAW 264.7 cells. The cell lysates were analysed using western blotting against phospho-p38 MAPK (p-p38) and p38 MAPK (p38). (g) Western blot analysis of iNOS in RAW 264.7 cells stimulated with HBHA (10 μg/ml) for 24 h. (h) Nitrite levels in culture supernatants of RAW 264.7 cells were evaluated by Griess reaction. (i) CHOP expression analysis after HBHA (10 μg/ml) stimulation for 24 h in the presence or absence of 30 min pretreatment with N-nitro-L-arginine methyl ester (L-NAME; 0.1, 0.5, 5 mM). Each value represents the mean+S.E.M. from three independent experiments performed in duplicate (***P<0.001, **P<0.01, *P<0.05, compared with control). Data are representative of at least three independent experiments with similar results
Next, we examined CHOP expression in the presence of the ROS scavenger N-acetyl-L-cysteine (NAC) to investigate the interplay between ER stress and ROS generation. HBHA-mediated CHOP production was dramatically reduced by NAC pre-treatment (Figure 5c). NAC treatment reduced the production of IL-6 and MCP-1 with HBHA stimulation (Figure 5d). These results suggest that ROS, which are induced by HBHA, are associated with the production of CHOP, IL-6, and MCP-1 in RAW 264.7 cells. To determine whether depletion of intracellular Ca2+ affected ROS generation in RAW 264.7 cells, BAPTA-AM was used to chelate intracellular Ca2+ before HBHA stimulation. BAPTA-AM significantly suppressed HBHA-induced ROS production (Figure 5e). In addition, pre-treatment with an IKK2 inhibitor before HBHA stimulation also suppressed ROS production in a dose-dependent manner (Figure 5e). To investigate the role of p38 phosphorylation in HBHA-induced ROS generation, RAW 264.7 cells were pretreated with NAC, and phosphorylated p38 was analysed after HBHA stimulation. HBHA-enhanced phosphorylation of p38 was also reduced by NAC (Figure 5f). Therefore, HBHA hypothetically induced ROS production and increased ROS-induced proinflammatory cytokines, such as IL-6 and MCP-1, through MAPK activation.
As NO disrupts ER calcium pump activity and induces ER stress,14 we tested the role of HBHA on NO synthesis in macrophages. HBHA treatment induced substantial iNOS expression in RAW 264.7 cells, which peaked between 6 and 12 h after stimulation, and decreasing thereafter (Figure 5g). HBHA-stimulated RAW 264.7 cells released significantly greater amounts of nitrite at 24 h (23.1±3.1 μM/2 × 105). Unstimulated RAW 264.7 cells released low levels of nitrite spontaneously after 24 h (3.9±0.7 μM/2 × 105; Figure 5h). Enhanced CHOP production was not inhibited by the addition of N-nitro-L-arginine methylester (L-NAME), indicating that the HBHA-mediated ER stress response is not affected by nitrite synthesis (Figure 5i).
Discussion
Generally, infectious intracellular pathogens tend to inhibit apoptosis of their host cells to minimise immune responses. Gan et al.23 suggested that apoptosis, which results in a ‘cellular corpse' with an impermeable envelope, limits mycobacterial replication and facilitates antigen presentation. Many studies have shown that apoptosis is a defence mechanism of macrophages against Mtb.6, 23, 24 We previously showed that HBHA leads to the collapse of mitochondrial membrane potential and eventually results in apoptosis.12 However, the exact molecular regulatory mechanisms of HBHA-mediated apoptosis are unclear. In the present study, we found that HBHA was localised in the ER of HBHA-treated RAW 264.7 cells and induced apoptosis by modulating the ER stress pathway (Figure 1). The data suggest that localisation of HBHA in the ER may contribute to the induction of ER stress with HBHA stimulation even though there is no evidence that HBHA is translocated into the ER from the cytosol.
HBHA, which is involved in the attachment of mycobacteria to epithelial cells, is a surface-associated, secreted Mtb protein.25 Recently, HBHA was demonstrated to have the capacity to bind to macrophages and induce caspase-dependent apoptosis.12 In the present study, we found that HBHA-mediated apoptosis was associated not only with the caspase-dependent pathway but also with the ER stress pathway. HBHA-induced CHOP production was entirely caspase dependent. Caspase inhibition abolishes the ER stress-induced activation of caspase-3 and apoptotic cell death,26 and a pan-caspase inhibitor delays the onset of CHOP induced by Japanese encephalitis virus.27 Which caspases function upstream and are responsible for the regulation of CHOP induction during HBHA stimulation remains unclear.
The results presented here suggest that unfolded protein response may utilise ER-associated caspases to transduce the CHOP apoptotic signal from the ER. Caspase activation can occur downstream of eIF2α phosphorylation,28, 29 and can also activate PKR kinase in mammalian cells.30 It is therefore likely that eIF2α phosphorylation is both a cause and consequence of caspase activation during HBHA stimulation.
HBHA stimulation induces caspase-dependent CHOP production and is closely associated with apoptosis in murine macrophages. Moreover, reduction of ER stress using 4-PBA results in reduced CHOP production and caspase-3 activation. One interpretation of these results is that that 4-PBA might help to stabilise proteins, thereby improving the folding capacity of the ER.31 These data suggest that macrophage apoptosis may be caused by ER overload and unresolved ER stress in response to HBHA stimulation.
ROS are known to cause severe damage to DNA, proteins, and lipids.32 ROS accumulation induces ER stress-induced apoptosis during mycobacterial infection.4, 9 In the present study, HBHA stimulation induced ER stress-induced apoptosis, and ROS generated by HBHA were associated with ER stress responses. Elevated [Ca2+]i was observed in RAW 264.7 cells after HBHA stimulation. Because the ER is a major source of Ca2+ elevation, HBHA could result in an increase in Ca2+ concentration from the ER. Because enhanced [Ca2+]i is responsible for the production of ROS,33 we propose that HBHA stimulation leads to an increase in intracellular ROS production via intracellular Ca2+ influx. Intracellular Ca2+ levels have an important role in regulating phagosome maturation during Mtb infection.34 However, the exact role of Ca2+ during Mtb infection remains unclear. HBHA secreted from mycobacteria might be involved in calcium release from the ER. Our data suggest that intracellular Ca2+ levels induced by HBHA stimulation are critical for the stimulatory effects of ROS generation, which causes ER stress-mediated apoptosis in RAW 264.7 cells. An intracellular Ca2+ chelator, BAPTA-AM, reduced HBHA-mediated caspase activation and CHOP production. Treatment with BAPTA-AM suppressed HBHA-induced MCP-1 and IL-6 production in RAW 264.7 cells. In a previous study, BAPTA-AM treatment not only induced a decrease in intracellular free Ca2+ but also inactivated PKC and IKK.20, 21 Both IL-6 and MCP-1 can induce ROS production;35, 36, 37 BAPTA-AM may help to suppress ROS production by inhibiting production of IL-6 and MCP-1. Alternatively, BAPTA-AM might reduce protein loads within the ER, leading to an attenuation of ER stress. Together, these data suggest that BAPTA-AM may attenuate ER stress through multiple routes.
In the present study, we showed phosphorylation of p38 was reduced by NAC. ROS are able to inactivate MAPK phosphatases through oxidation of catalytic cysteine residues leading to MAPK activation,22 and proinflammatory cytokine production.38 NAC significantly reduced the expression of IL-6, MCP-1, and CHOP following HBHA stimulation, consistent with previous reports.4, 39, 40 ROS-mediated ER stress may therefore be the result of increased IL-6 and MCP-1 expression following stimulation with HBHA.41
We have also shown that HBHA-induced nitrite production may be not associated with ER stress responses. This result is consistent with our previous report of no association between nitrite production and ER stress responses during Mtb infection.4 Combined, these results suggest that although HBHA-induced nitrites alone are not sufficient to induce ER stress in macrophages, they may contribute to ER stress responses when combined with other stimuli.
Differences between ER client protein load and folding capacity lead to ER stress.42 When cells experience irreversible ER stress, this pathway eliminates damaged cells by apoptosis. Subsequently, overproduction of proinflammatory cytokines may contribute to the induction of ER stress responses in macrophages, which was confirmed in the present study: pre-treatment of RAW 264.7 cells with an IKK2 inhibitor reduced ER stress markers after stimulation with HBHA. Consensus-binding sites for NF-κB are located in the promoter region of MCP-1 and IL-6.17, 18 Our results regarding the reduced production of CHOP by selective inhibition of IKK2 indicate that overproduction of proinflammatory cytokines may be involved in the induction of ER stress. CHOP expression might be involved of HBHA-induced upregulation of IL-6 and MCP-1 production through the activation of NF-κB.18 Therefore, we propose that HBHA stimulation may cause an imbalance between ER protein folding load and capacity, leading to ER stress.
In conclusion, we demonstrated that HBHA stimulation causes ROS generation induced by disruption of intracellular Ca2+ homeostasis, and that ROS stimulate the overproduction of proinflammatory cytokines, which leads to ER stress-mediated apoptosis in macrophages. HBHA can induce not only mitochondrial dysfunction but also ER stress via ROS production caused by calcium release or protein-folding stress in the ER. Although HBHA-induced cell death is regulated by various apoptotic stimuli, the ER stress pathway has a significant role in HBHA-stimulated macrophages. A better understanding of the molecular mechanism of ER stress-induced apoptosis by HBHA may provide insight into the pathogenesis of TB.
Materials and Methods
Animals
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, South Korea (Permit Number: 2010-2-32). All animal experiments were performed in accordance with Korean Food and Drug Administration guidelines.
Preparation of HBHA antigen and reagents
Recombinant HBHA protein from Mycobacterium smegmatis was produced and prepared as described previously.12 A chemical chaperone, 4-PBA and L-NAME, was purchased from Sigma (St. Louis, MO, USA). NAC, BAPTA-AM, IκB kinase 2 inhibitor (IKK2 IV), JNK inhibitor II (SP600125), and z-VAD-fmk were purchased from Calbiochem (San Diego, CA, USA).
Cell culture
Murine macrophage cell line RAW 264.7 cells were maintained in Dulbecco's modified Eagle's medium (Lonza, Walkersville, MD, USA) supplemented with 10% FBS, penicillin (100 IU/ml), streptomycin (100 μg/ml), and 1% L-glutamine. After reaching confluence, cells were plated in 12-well dishes at a concentration of 1 × 105 cells per well. BMDMs were isolated from femurs and tibias of C57BL/6 mice (6–8 weeks old) and then differentiated by growth for 3–5 days in medium containing M-CSF (25 μg/ml; R&D, Minneapolis, MN, USA).
Sub-cellular fractionation
RAW 264.7 macrophages were treated with medium and HBHA (10 μg/ml) for 24 h, harvested, and then washed with phosphate-buffered saline (PBS). The cytosolic and ER fractions were purified using the Endoplasmic Reticulum Isolation Kit (Sigma).
Flow cytometric analyses of ROS and cell cycle assay
Cells were harvested with PBS. For ROS staining, dihydroethidium (20 μM; Molecular Probes, Eugene, OR, USA) was added and incubated at 37 °C for 30 min. After washing with PBS, the cells were fixed with 4% paraformaldehyde (PFA). LPS (100 ng/ml; InvivoGen, San Diego, CA, USA) was used as a positive control for ROS production. For mitochondrial ROS staining, RAW 264.7 cells were loaded for 30 min with MitoSOX (10 μM; Molecular Probes), and then washed with PBS. For cell cycle analysis, cells were fixed with 70% ethanol at −20 °C for at least 12 h. Intracellular DNA was labelled with PI (5 μg/ml). Samples were analysed on a FACS Canto II cytometer (BD Biosciences, San Jose, CA, USA) and the data processed using Flow Jo (Tree Star, Ashland, OR, USA).
Mtb culture
Mtb strain H37Ra (ATCC 25177) was grown in Middlebrook 7H9 liquid medium supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase), 5% glycerol, and 0.05% Tween-80 and resuspended in PBS at a concentration of 1 × 108 CFU/ml. Aliquots were frozen at −70 °C until used. In some experiments, Mtb H37Ra was killed by 1 h incubation at 4 °C in PBS-5% PFA. After fixation, PFA was extensively washed off with PBS.
NO synthesis by macrophages
Nitrite production by macrophages was measured by the Griess reaction.4 Equal volumes of cell-free supernatant and Griess reagent (1% sulfanilamine, 0.1% N-(1–naphyl)-ethylene-diamine dihydrochloride, 2.5% H3PO4) were mixed. NaNO2 was used as a standard. Plates were read on a Vmax kinetic microplate reader (Molecular Devices Co., Menlo Park, CA, USA) at 540 nm.
Mouse cytokine analysis
Cell culture supernatants for cytokine profiling were recovered after 24 h stimulation and kept frozen until batch analysis. Proinflammatory cytokines, including IL-6 and MCP-1, were quantified using a mouse inflammatory cytokine cytometric bead array kit (CBA; BD Biosciences, San Diego, CA, USA), a method for capturing a soluble analyte or set of analytes with beads of known size and fluorescence, allowing for detection using a FACS Canto II cytometer (BD Biosciences).
Western blotting analysis
Western blot analysis was performed as described previously.9 Anti-CHOP, anti-caspase-12, anti-caspase-9, anti-caspase-3, and anti-GRP78/BiP were obtained from Cell Signaling (Denvers, MA, USA), anti-phospho (Ser-51)-eIF2α was obtained from Assay Designs, (Farmingdale, NY, USA), anti-iNOS and anti-β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat anti-rabbit-IgG-HRP (Cell Signaling) and rabbit anti-mouse-IgG-HRP (Calbiochem) were use as secondary antibodies. Membranes were developed using a chemiluminescent reagent (ECL; Millipore Corporation, Billerica, MA, USA), and were subsequently exposed to chemiluminescence film to visualise proteins.
Ca2+ measurements
Changes in intracellular calcium concentration ([Ca2+]i) in RAW 264.7 cells were detected as described previously.9 To measure ER calcium content, RAW 264.7 cells adhering to the coverslip at the bottom of the perfusion chamber were incubated with Mag-Fura-2/AM (10 μM; Invitrogen, Carlsbad, CA, USA) for 1 h at 37 °C and then permeabilised for 80–100 s with β-escin (20 μM; Sigma) in intracellular medium (125 mM KCl, 19 mM NaCl, 10 mM HEPES, and 1 mM EGTA, pH 7.3). Next, permeabilised RAW 264.7 cells were washed with intracellular medium for 5 min to remove cytosolic dye, and then were superfused for 1 min with loading buffer (125 mM KCl, 19 mM NaCl, 10 mM HEPES, 1 mM EGTA, 0.65 mM CaCl2 (free Ca2+, 200 nM), 1.4 mM MgCl2, and 3 mM Na2ATP, pH 7.3) to activate SERCA and load Ca2+ stores. After Ca2+ loading, RAW 264.7 cells were superfused with release buffer (125 mM KCl, 19 mM NaCl, 10 mM HEPES, 1 mM EGTA, and 3 mM Na2ATP, pH 7.3) to inactivate SERCA. Inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ release channels were activated by adding IP3 (1 μM; Biomol Research Laboratories, Plymouth, PA, USA) to the release buffer.
Statistical analyses
All experiments were performed independently and repeated at least three times. A P-value <0.05 was considered to be statistically significant in Student's t-tests. Data are presented as the means±95% confidence interval.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010–0025985) and by the NRF grant funded by the Korea government (MSIP) (2007-0054932) and by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A121496).
Glossary
- HBHA
heparin-binding hemagglutinin antigen
- TB
Tuberculosis
- ER
endoplasmic reticulum
- ROS
reactive oxygen species
- CHOP
C/EBP homology protein
- MCP-1
monocyte chemotactic protein-1
- Mtb
Mycobacterium tuberculosis
- NAC
N-acetyl cysteine
- IKK
IκB kinase
- eIF2α
eukaryotic translation initiation factor 2A
- NF-κB
Nuclear factor-κB
- GADD153
growth arrest and DNA damage-induced gene-153
- 4-PBA
4-phenyl butyric acid
- BMDM
bone marrow-derived macrophage
- PBS
phosphate-buffered saline
The authors declare no conflict of interest.
Footnotes
Edited by M Agostini
References
- WHO . WHO global tuberculosis control report 2011. WHO/HTM/TB/201116. World Health Organization; 2011. [Google Scholar]
- Dockrell DH. Apoptotic cell death in the pathogenesis of infectious diseases. J Infect. 2001;42:227–234. doi: 10.1053/jinf.2001.0836. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Kornfeld H. Interactions between naive and infected macrophages reduce Mycobacterium tuberculosis viability. PLoS One. 2011;6:e27972. doi: 10.1371/journal.pone.0027972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, Jo EK, et al. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One. 2011;6:e28531. doi: 10.1371/journal.pone.0028531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, Gan HX, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4:279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50:1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HH, Shin DM, Kang G, Kim KH, Park JB, Hur GM, et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett. 2010;584:2445–2454. doi: 10.1016/j.febslet.2010.04.050. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Kim MJ, Blumenthal A, Koo J, Ehrt S, Wainwright H, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PLoS One. 2010;5:e12772. doi: 10.1371/journal.pone.0012772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra M, Pickett T, Delogu G, Dheenadhayalan V, Debrie AS, Locht C, et al. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect Immun. 2004;72:6799–6805. doi: 10.1128/IAI.72.12.6799-6805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn H, Kim JS, Shin SJ, Kim K, Won CJ, Kim WS, et al. Targeting of Mycobacterium tuberculosis heparin-binding hemagglutinin to mitochondria in macrophages. PLoS Pathog. 2011;7:e1002435. doi: 10.1371/journal.ppat.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- Donadelli R, Abbate M, Zanchi C, Corna D, Tomasoni S, Benigni A, et al. Protein traffic activates NF-kB gene signaling and promotes MCP-1-dependent interstitial inflammation. Am J Kidney Dis. 2000;36:1226–1241. doi: 10.1053/ajkd.2000.19838. [DOI] [PubMed] [Google Scholar]
- Hattori T, Ohoka N, Hayashi H, Onozaki K. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett. 2003;541:33–39. doi: 10.1016/s0014-5793(03)00283-7. [DOI] [PubMed] [Google Scholar]
- Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, et al. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Hong SH, Lee DJ, Park JH, Kim KS, Kim HM. Role of Ca(2+) on TNF-alpha and IL-6 secretion from RBL-2H3 mast cells. Cell Signal. 2002;14:633–639. doi: 10.1016/s0898-6568(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Todisco A, Ramamoorthy S, Pausawasdi N, Tacey K. Carbachol activates IkappaB kinase in isolated canine gastric parietal cells. Biochem Biophys Res Commun. 1999;261:877–884. doi: 10.1006/bbrc.1999.1141. [DOI] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H, Lee J, Ren F, Chen M, Kornfeld H, Remold HG. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol. 2008;9:1189–1197. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi FD, Bischoff R, Fort E, Brennan MJ, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci USA. 1998;95:12625–12630. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Elner VM. Dual involvement of caspase-4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:6006–6014. doi: 10.1167/iovs.09-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002;76:4162–4171. doi: 10.1128/JVI.76.9.4162-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparna G, Bhuyan AK, Sahdev S, Hasnain SE, Kaufman RJ, Ramaiah KV. Stress-induced apoptosis in Spodoptera frugiperda (Sf9) cells: baculovirus p35 mitigates eIF2 alpha phosphorylation. Biochemistry. 2003;42:15352–15360. doi: 10.1021/bi0349423. [DOI] [PubMed] [Google Scholar]
- Aarti I, Rajesh K, Ramaiah KV. Phosphorylation of eIF2 alpha in Sf9 cells: a stress, survival and suicidal signal. Apoptosis. 2010;15:679–692. doi: 10.1007/s10495-010-0474-z. [DOI] [PubMed] [Google Scholar]
- Saelens X, Kalai M, Vandenabeele P. Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem. 2001;276:41620–41628. doi: 10.1074/jbc.M103674200. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, Yoshii M, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Gordeeva AV, Zvyagilskaya RA, Labas YA. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry (Mosc) 2003;68:1077–1080. doi: 10.1023/a:1026398310003. [DOI] [PubMed] [Google Scholar]
- Trimble WS, Grinstein S. TB or not TB: calcium regulation in mycobacterial survival. Cell. 2007;130:12–14. doi: 10.1016/j.cell.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest. 2011;121:1834–1845. doi: 10.1172/JCI44678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Wang K, Niu J, Kim H, Kolattukudy PE. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol. 2011;3:360–368. doi: 10.1093/jmcb/mjr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med. 2000;28 (4 Suppl:N67–N77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–2143. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. N-acetylcysteine reduces chemokine release via inhibition of p38 MAPK in human airway smooth muscle cells. Eur Respir J. 2003;22:43–49. doi: 10.1183/09031936.03.00064803. [DOI] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]