Abstract
The zinc-finger protein A20 is a key player in the negative feedback regulation of the nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-κB) pathway in response to multiple stimuli. Tumor necrosis factor alpha (TNFα), a cytokine with pleiotropic effects on cellular proliferation and differentiation, dramatically increases A20 expression in all tissues. As TNFα inhibits adipocyte differentiation, we have determined the contribution of A20 to the adipogenic capacity of human mesenchymal stromal cells (MSCs). Here we show that A20 is constitutively expressed in MSCs, which previously has been observed only in cells that are either tumor or immune cells (T/B lymphocytes). TNFα stimulation induced a rapid degradation of A20 protein mediated exclusively by the proteasome in MSCs and not by caspases. This degradation is concomitant to the induction of its own mRNA, which suggests that a tight regulation of NF-κB signaling in MSCs is fundamental. On one hand, we demonstrate that the knockdown of A20-mediated transcript dramatically decreases the adipogenic capacity of MSCs, which correlates with the phenotype observed in the presence of TNFα. On the other hand, A20 overexpression blocks NF-κB activation and drives to increased adipogenesis, even in the presence of TNFα treatment. In conclusion, our data demonstrate that the presence of A20 allows MSCs to differentiate into adipocytes by maintaining NF-κB signaling at a basal state.
Keywords: A20 inhibitor, mesenchymal stromal cell, adipogenesis
Tumor necrosis factor alpha (TNFα) is a cytokine involved in several processes in the cell and it has an important role in the regulation of the immune system, apoptosis and inflammation. Dysregulation, and in particular overproduction, of this cytokine has been implicated in a variety of human diseases.1 TNFα can activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway via the TNFα receptor.2 There are two known variants of the NF-κB pathway, the canonical and the non-canonical, although TNFα stimulation is known to trigger mainly the canonical pathway. NF-κB is inactivated in resting cells by binding to any isoform of IκB, a family of proteins that prevents signaling by maintaining the NF-κB dimer in the cytoplasm. In stimulated cells, the IκB kinase (IKK) complex becomes activated leading to phosphorylation of IκB proteins, which are then ubiquitinylated and targeted for degradation by the proteasome. The released transcription factor NF-κB is subsequently translocated to the nucleus where it regulates the transcriptional activity of a large number of genes.3
As for TNFα signaling, accurate regulation of NF-κB activity is crucial to prevent a variety of diseases. There are several mechanisms involved in NF-κB regulation, including the negative feedback control by which NF-κB regulates the transcription of its own inhibitors. Among these inhibitors are the family of IκB and A20, also known as the TNFα-induced protein 3.4 Although A20 is a ubiquitous protein, it is not constitutively expressed in most cell types with the exception of thymocytes, mature T cells and some tumor cells.5, 6 In all cell types, A20 transcription is rapidly induced by a large number of stimuli, including TNFα,7, 8 that triggers the binding of NF-κB to two specific NF-κB-binding sites in the A20 promoter.4 A20, in turn, restricts the duration and intensity of NF-κB signaling. The first function described for A20 was its cytoprotective effect on TNF stimulation of cells, based on the effect of A20 overexpression.8 This was later confirmed genetically as A20−/− murine embryonic fibroblasts and thymocytes were found to be more sensitive to TNF-induced cell death than wild type cells.5 However, the anti-apoptotic function of A20 is not a general feature, as A20 only protects some cell types from specific death inducing agents. The expression, biological activities, and mechanism of action of A20 depend to a large extent on the cellular context. Although a high expression of A20 is often linked to poor prognosis of epithelial malignancies, A20 also functions as a tumor suppressor in several B-cell lymphomas. A20 is composed of two domains, an ovarian tumor (OTU) domain at the N-terminus with deubiquitinase activity and a C-terminal domain built up by seven zinc fingers contributing to its ubiquitin ligase activity. This dual ubiquitin-editing capacity of A20 is involved in the inhibition of the NF-κB pathway.9, 10 In addition, posttranslational modifications of A20 as well as its interaction with ubiquitin binding proteins seem to be critical for its function and activity.11, 12
Adipose tissue has a fundamental role in the energy homeostasis of the body and the differentiation of pre-adipocytes to adipocytes is a tightly regulated process. Mature adipocytes are derived from pluripotent mesenchymal stromal cells (MSCs) that have the capacity to develop not only into this cell type but also into chondrocytes and osteocytes.13, 14, 15 These stem cells reside, among other tissues, in the stroma of the bone marrow and under specific stimuli they undergo a differentiation process in which the progenitor cells become restricted to the adipocyte lineage. Inflammatory cytokines seem to block the adipogenesis of MSCs,16 and in fact adipose tissue dysfunctions such as obesity are frequently correlated with elevated levels of TNFα.17, 18 It has been described that TNFα interferes with the homeostasis of adipose tissue in vivo, affecting both the capacity to incorporate fatty acids and to undergo lipolysis as well as the adipogenic process.18 In 3T3-L1 cells, a mouse embryonic cell line that can differentiate into adipocytes, TNFα has effects on adipocyte gene expression, including suppression of genes essential for insulin responsiveness and selective induction of pre-adipocyte genes. The same study demonstrated that TNFα-stimulated NF-κB activation is required for the repression of key adipocyte genes.19 Additionally, it has been shown that increased IKK activity promotes insulin resistance in obese mice when the kinase is overexpressed.20 Overall, these data clearly demonstrate the relevance of TNFα-induced NF-κB activation in the homeostasis of the adipose tissue.
In this study we show that the TNFα/NF-κB signaling axis regulates adipogenesis in human bone marrow-derived MSCs. Furthermore, we identify A20 as an important factor in adipocyte differentiation. We demonstrate that A20 is constitutively expressed in human MSCs and that its presence facilitates MSCs differentiation into the adipocyte lineage by the continuous inhibition of the NF-κB pathway. We also show that in MSCs, TNFα stimulation induces a temporary A20 degradation by the proteasome and that this process is indispensable for the regulation of adipogenesis.
Results
TNFα inhibits adipogenesis in human MSCs through the activation of the NF-κB pathway
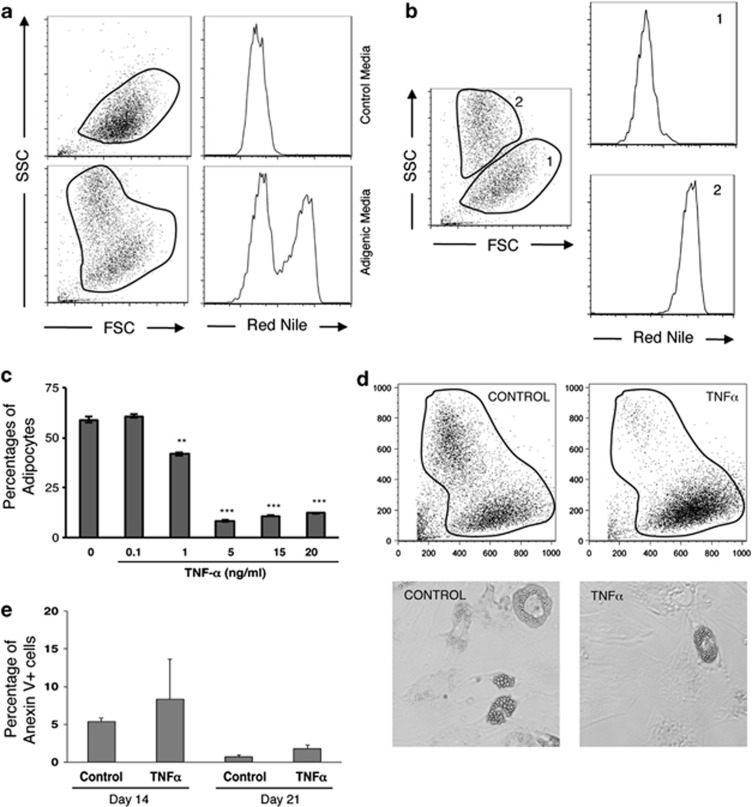
In order to improve our understanding of how TNFα affects adipogenesis via the NF-κB pathway in humans, we studied the influence of this cytokine in a human MSCs culture system. As shown in Figure 1a, the adipogenic process results in the appearance of a cell population characterized by an increased intensity of both Nile Red and side-scatter fluorescence, which exhibit a perfect correlation by flow cytometry analysis (Figure 1b). MSCs cultured in the presence of TNFα concentrations ranging from 0–20 ng/ml, show that TNFα reduces the proportion of adipocytes in a dose-dependent manner (Figure 1c). Indeed, the percentage of adipocytes in culture at the end of the differentiation process decreases from 50–60% in conditions with no or low concentrations of TNFα, to <10% with 5–20 ng/ml TNFα. Mature adipocytes cultured in the presence or absence of TNFα could not be distinguished in terms of morphology (light microscopy) or complexity of the lipid droplets (side-scatter) (Figure 1d). Analysis of cell death using Annexin V staining revealed no significant differences on TNFα stimulation, thus excluding differences in apoptotic rates as a cause for the decreased adipogenesis (Figure 1e). These data taken together with our previous study, where we demonstrated that differentiation of MSCs into adipocytes requires inhibition of cell proliferation,21 indicate that TNFα signaling interferes with the adipogenic capacity of MSCs.
Figure 1.
TNFα inhibits adipocytes differentiation from MSCs. (a) Representative staining of lipid droplets by Nile Red. MSCs were induced to differentiate in the absence (control media) or presence of adipogenic medium during 21 days. (b) Side-scatter fluorescence increase correlates with specific fluorescence Nile Red staining (region 1 and 2). (c) MSCs cultured in the presence of increasing concentrations of TNFα. Percentages of mature adipocytes were determined by flow cytometry. Results show the mean±S.D. of triplicate samples. **P<0.01, ***P<0.001. (d) Representative staining of side-scatter fluorescence in the presence/absence of TNFα. Lipid droplet morphology is also shown (400 × magnification). (e) Analysis of apoptosis in TNFα-treated cells induced to differentiate into adipocytes. The percentage of apoptotic cells (Annexin V positive) was measured at 14 and 21-days post-induction by flow cytometry. Results show the means±S.D. of triplicate samples
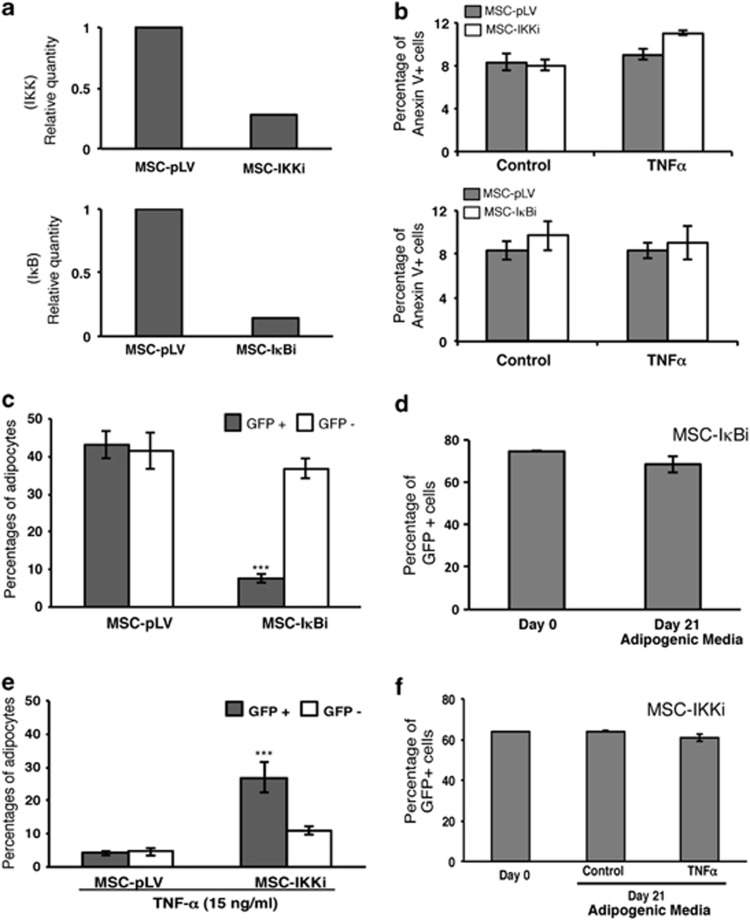
Next, we wanted to see how modulating the NF-κB pathway affects the inhibitory effects of TNFα on adipogenesis. This was done by performing the differentiation process in the presence or absence of TNFα in MSCs in which inhibitor of kappa B alpha (IκBα) or IKKβ transcripts were silenced. A GFP-expressing lentiviral vector (pLVTHM) was used to transduce human MSCs at various multiplicities of infection with a stable IκBα or IKKβ-specific shRNA. As shown in Figure 1b, shRNA-transduced MSCs (MSC-IκBi or MSC-IKKβi) resulted in efficient reduction of the IκBα and IKKβ expression, respectively (Figure 2a). It is important to mention that the absence of either transcripts did not correlate with increased cell death, and this effect is independent of TNFα treatment (Figure 2b). We observed that knocking down of IκBα resulted in almost fivefold fewer adipocytes in transduced (GFP+) MSCs compared with empty GFP+ control cells after induction of the differentiation process (Figure 2c). Thus, silencing IκBα showed similar effects as those observed in presence of TNFα. In contrast, no significant effects are observed in cells knocked down for IKKβ in the absence of TNFα (data not shown). However, the addition of TNFα to the MSCs-IKKβi culture results in a decrease of adipogenesis in GFP− cells similar to the control cells, whereas the adipogenesis in GFP+ cells is only partially affected (Figure 2e). Importantly, MSCs infected with the IκBi or IKKβi (±TNFα) vector maintain the same ratio between GFP+ and GFP− cells during the whole differentiation process (21 days), suggesting that the knockdown of both transcripts has no effect on cell death (Figures 2d and f, respectively). In conclusion, these results show that the effect of TNFα on the adipogenic potential of human MSCs is mainly due to the activation of the NF-κB pathway by this cytokine.
Figure 2.
NF-κB is important for TNFα induced inhibition of adipogenesis in MSCs. (a) IKKβ and IκBα expression levels by real-time qPCR in transduced cells. IKK and IκBα transcripts were ‘knocked down' by specific shRNA with more than 80% efficiency. Relative transcript expression levels were normalized to IKKβ and IκBα transcript expression levels from pLV-empty vector infected MSCs, respectively. (b) Analysis of apoptotic cells in IKKβ (upper panel) and IκBα knockdown cells in the presence/absence of TNFα. The percentage of apoptotic cells (Annexin V positive) was measured at 6-days post-infection by flow cytometry. Results show the means±S.D. of triplicate samples. (c) IκBα knockdown inhibits adipogenesis capacity in MSCs, which is consistent with the phenotype observed in the presence of TNFα, ***P<0.001. (d) Percentage of GFP expression in IκBα transduced cells before (Day 0) and after (Day 21) adipogenesis differentiation period was analyzed by flow cytometry. (e) IKKβ knockdown rescues adipogenesis from the inhibition causes by TNFα. ***P<0.001. (f) Percentage of GFP expression in IKKβ-transduced cells before (Day 0) and after (Day 21) adipogenesis differentiation period in the presence/absence of 15 ng/ml of TNFα was analyzed by flow cytometry). Results show the means±S.D. of triplicate samples
A20 is constitutively expressed in MSCs
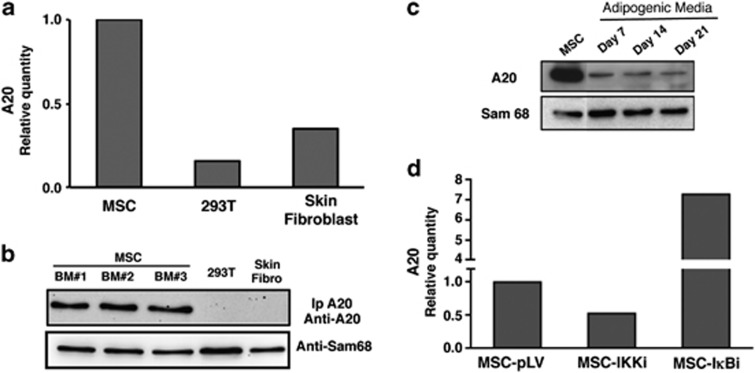
As A20 is one of the main inhibitors of NF-κB and modulation of this pathway significantly affects the adipogenesis, we checked A20 expression in MSCs by RT qPCR and western blot. Contrary to what is found in most cell types, the expression analysis by RT qPCR reveals that A20 is highly expressed in MSCs. Only low levels of A20 could be observed in 293Ts and human skin fibroblasts (Figures 3a and b). We checked this unexpected result by measuring protein levels in MSCs from three different healthy donors by western blot. Figure 3b shows that A20 protein was only detectable in those samples from MSCs, whereas very low or undetectable protein levels were found in 293 T and fibroblast. So far, constitutive expression of A20 has only been described in certain cells of the immune system and some tumor cell lines.11 The adipogenic stimulation gave rise to an important reduction of A20 expression, although we could still detect its expression during the whole culture period (day 7, 14 and 21, Figure 3c). Next, we wanted to know if the constitutive expression of A20 was due to the activity of NF-κB. We used the ‘knock down' of IKKβ and IκBα in MSCs to find out if modulation of the NF-κB pathway activity influenced the expression of A20. A20 expression was again quantified using RT qPCR. In MSCs where IKKβ had been silenced (MSC-IKKβi), and thus the NF-κB activity is abolished, a reduction of the A20 mRNA expression could be observed (Figure 3d). On the contrary, MSCs transduced with the hairpin targeting IκBα, displayed an increased A20 expression (Figure 3d). As there are no changes in A20 expression in MSCs transduced with the empty vector (MSC-pLV), this data demonstrate that the constant expression of A20 in these cells is dependent on the NF-κB activity.
Figure 3.
A20 protein is expressed constitutively in human MSCs. (a) A20 expression levels by real-time qPCR in MSCs, fibroblast and 293 T cells. Relative transcript expression levels were normalized to A20 transcript expression levels to human MSCs. (b) A20 protein analysis in three independent human bone marrow MSCs. Samples were compared with primary fibroblast from human skin and 293 T cell line. A20 protein expression level was measured by western blot using extracts where A20 was immunoprecipitated. (c) A20 expression during the differentiation process. A20 expression levels were measured by western blot in total cell extracts using the antibody for A20. Sam68 was used as a loading control (d) A20 expression is dependent of NF-κB activation. IKKβ (MSC-IKKi) and IκBα (MSC- IκBi) transcripts were ‘knocked down' by specific shRNA. Six-days post-infection MSCs were analyzed for A20 expression levels by real-time qPCR. Relative transcript expression levels were normalized to MSCs transduced with the empty vector (MSC-pLV)
TNFα induces degradation of A20 by the proteasome in MSCs
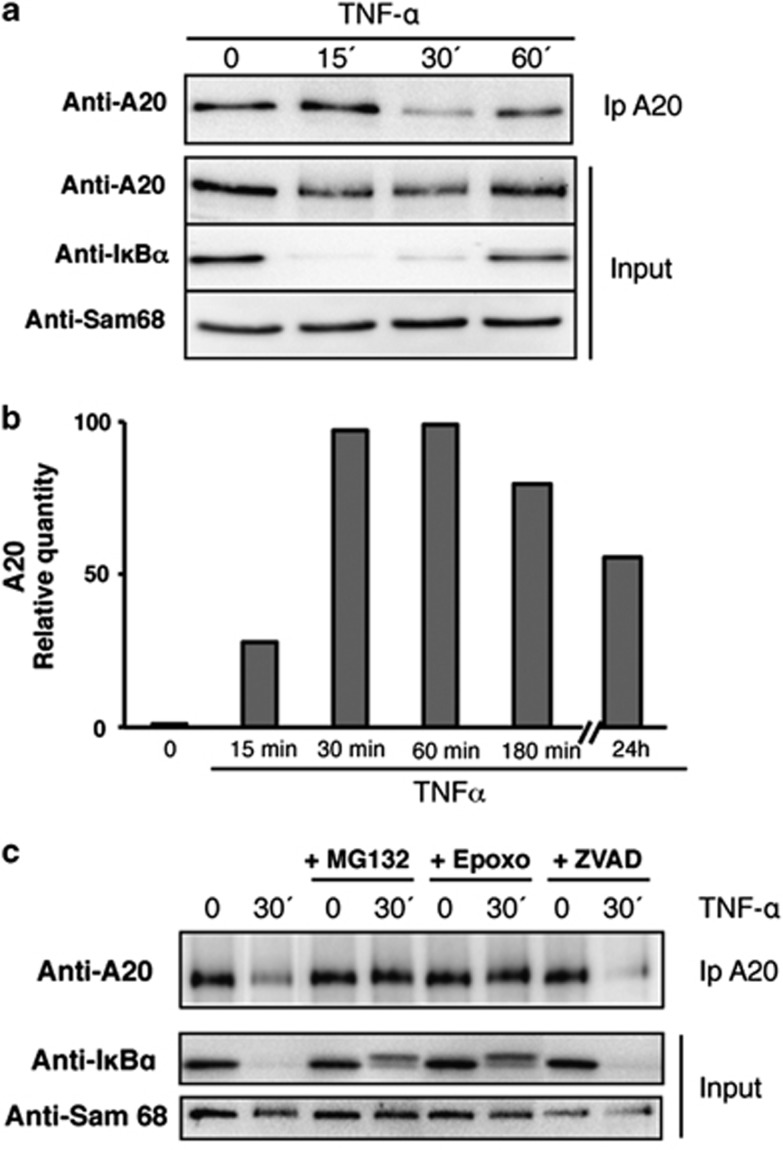
Intrigued by the finding that A20 is constitutively expressed in MSCs, we investigated how the expression of this protein was influenced by TNFα in these cells. Therefore, a time course experiment quantifying A20 protein and mRNA levels in MSCs treated with TNFα was performed. To our surprise, the protein analysis revealed that the stimulation with TNFα does not induce an increase of A20 expression as in most cells but rather a rapid and temporal decrease of the A20 level is observed (Figure 4a). Besides, the quantification of mRNA showed that TNFα rapidly induces an increase in the A20 mRNA expression and maintains the levels during the whole time of the experiment (Figure 4b). A similar decrease of A20 levels can also be observed on T-cell receptor (TCR) stimulation with CD3/CD28 in T cells. This decrease depends mainly on the paracaspase mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT-1), which induces a cleavage of A20 and therefore an activation of NF-κB pathway.22 Other studies suggest that the implication of the proteasome as well in the reduction of A20 induced after TCR stimulation.23 In order to determine the mechanism involved in the decrease of A20 in MSCs, the cells were treated with proteasome inhibitors (MG-132 or epoxomycin) or a generic caspase inhibitor (Benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethyl ketone, ZVAD) before TNFα stimulation. The levels of A20 quantified by western blot show that inhibition of the proteasome, but not of caspases, impedes A20 degradation (Figure 4c). These results indicate that stimulation of MSCs with TNFα provokes the temporal degradation of A20 by the proteasome at the same time as it induces de novo transcription through the activation of the NF-κB pathway. Interestingly, the regulation of A20 expression after TNFα stimulation in MSCs clearly resembles the one observed for IκBα, although with different kinetics.
Figure 4.
TNFα induced A20 degradation is dependent of the proteasome in MSCs. (a) TNFα stimulation induced a degradation of A20 in MSCs. Cells were stimulated with TNFα for the indicated time, and lysates were submitted to A20 immunoprecipitation to detect the level of the protein by western blot. (b) A20 expression levels by real-time qPCR in MSCs stimulated with TNFα for the indicated time. Relative transcript expression levels were normalized to A20 transcript expression levels to non TNFα-treated human MSCs. (c) TNFα-induced A20 degradation is dependent of the proteasome. Before stimulation with TNFα for the indicated time, cells were pretreated with inhibitors of the proteasome (MG132 or epoxomicin) or inhibitor of caspase (ZVAD). Lysates were submitted to an A20 immunoprecipitation to check the level of A20 in each condition
Modification of A20 expression in MSCs affects adipogenesis
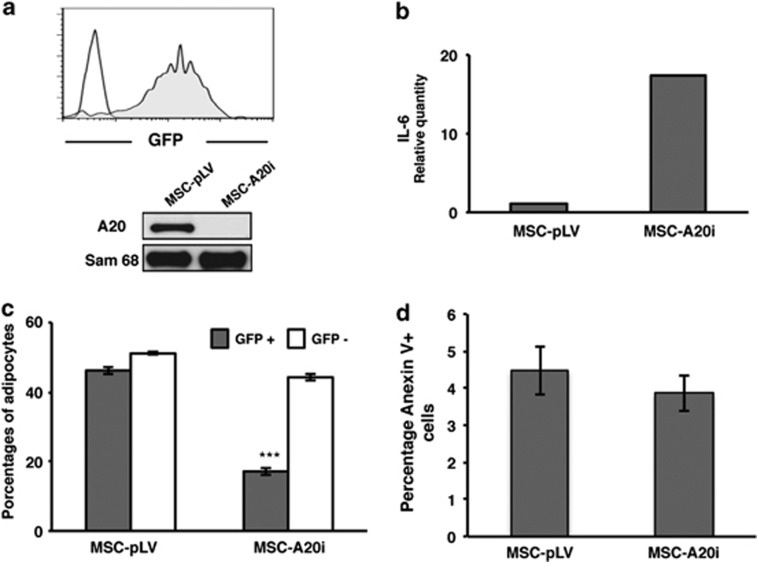
As the level of NF-κB activity influences adipocyte differentiation and human MSCs show a constitutive expression of A20, we next determined the role of A20 in this process. We tested MSCs differentiation in adipogenic conditions after modulating A20 levels with vectors carrying either A20 cDNA (MSC-pRRL-A20) or A20 specific shRNA (MSC-pLV-A20i). As shown in Figure 5a, A20 is clearly reduced as verified by western blot when MSCs are transduced (>95% efficiency) with pLV-A20i vector. To provide further evidence of A20 implication, we measured the induction of IL-6 expression as an indicator of NF-κB activation.24 The reduction in A20 expression led to an expected increase of NF-κB activation and resulted in a marked upregulation of IL-6 expression in MSCs transduced with pLV-A20i vector (Figure 5b). Subsequently, the effect of reduced A20 levels on adipogenesis was investigated by analysis of GFP+ and GFP− in MSCs transduced (∼50%) with the empty or A20i vectors. Results show that the percentage of adipocytes in GFP+ MSCs, in which A20 has been silenced, is significantly lower than in GFP− cells, whereas cells transduced with the empty vector maintained a comparable level of adipogenesis as non-transduced control cells in the same cultures (Figure 5c).
Figure 5.
A20 is essential for the adipogenic capacity of human MSCs. (a) A20 specific gene silencing by lentivirus-mediated shRNA. Four-days transduced MSCs were collected and GFP expression was analyzed by flow cytometry (gray). Negative control: non-infected MSCs (white). Western blot analysis of A20 expression after specific knock down (MSC-A20i). Protein expression levels were analyzed in total cell extracts. MSCs transduced with the empty vector were used to compare the efficiency from the specific shRNA (MSC-pLV). Sam68 was used as a loading control. (b) Quantification by real-time qPCR of IL-6 after 6 days of culture in MSCs transduced with the empty vector or with the specific A20 shRNA. (c) A20 knockdown induced an inhibition of adipogenesis in MSCs. MSCs showing ∼50% infection efficiency with either empty vector (MSC-pLV) or pLV-specific shRNA (MSC-A20i) containing vectors were induced to adipogenic differentiation. Percentages of adipocytes were determined by flow cytometry. Results show the means±S.D. of triplicate samples. ***P<0.001. (d) Analysis of apoptotis in cells where A20 expression was knocked down. The percentage of apoptotic cells (annexin V positive) was measured at 7-days post-infection by flow cytometry (GFP positive). Necrotic cells were discarded by specific TO-PRO-3 staining. Results show the means of triplicate samples
In contrast to its well established role in repressing NF-κB activation, the anti-apoptotic activity of A20 remains controversial and appears to be specific to cell type and stimulus.7, 25, 26, 27 For this reason, we decided to check if the A20 silencing could induce apoptosis in MSCs. As shown in Figure 5d, the inhibition of A20 expression did not correlate with increased percentages of apoptotic cells (MSC-A20i) compared with MSCs transduced with the empty vector (MSC-pLV). This result demonstrates that the impairment of adipogenesis after A20 silencing is not due to increased apoptosis, thus suggesting that it is a result of direct interference with the adipogenic process.
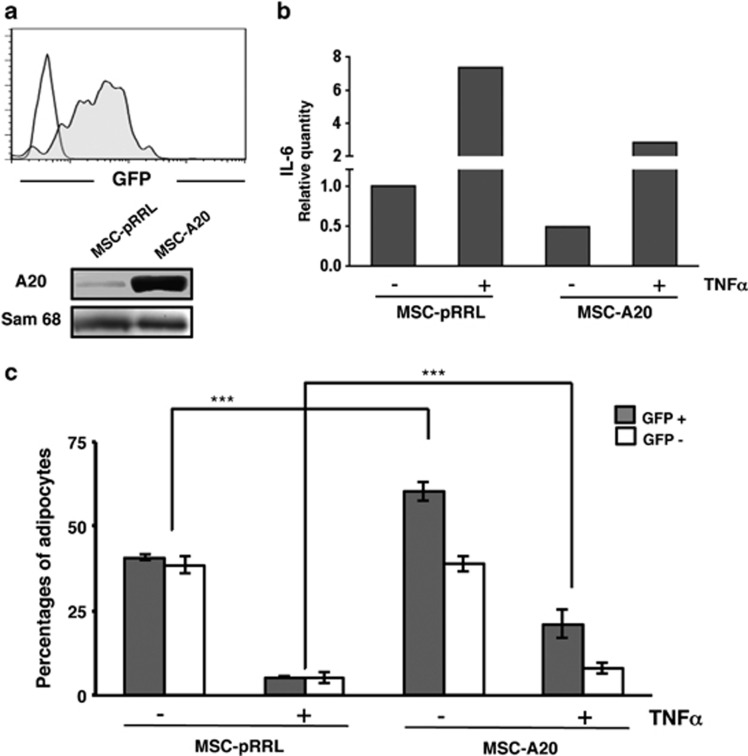
On the other hand, pRRL-A20-transduced cells overexpressed A20 protein (Figure 6a), correlated with a decrease in IL-6 mRNA levels even in the presence of TNFα when compared with cells transduced with the empty vector (Figure 6b). These data clearly demonstrate the reduction of NF-κB activity in MSCs overexpressing A20. To determine how A20 overexpression influences adipocyte differentiation in human MSCs we compared non-infected cells (GFP−) with cell transduced with either the empty or A20 expression vector (GFP+) in the presence or absence of TNFα. As shown in Figure 6c, cells transduced with the empty vector (GFP+) generated the same percentages of adipocytes than non-infected cells (GFP−) indicating no effect from the viral integrations. More important, there was a significant increase in adipocytes from cell overexpressing A20 (GFP+) compared with cells transduced with the empty vector (>20%). This shows that the overexpression of A20 results in a more efficient adipogenesis. Interestingly, the presence of TNFα that inhibited the adipogenesis in cell transduced with the empty vector was partially but significantly reverted in cells overexpressing A20 (Figure 6c), demonstrating that A20 counteracts the activation of NF-κB induced by TNFα. Taken together, these results clearly show that the A20 has an important role in the regulation of the adipogenic capacity in human MSCs.
Figure 6.
A20 overexpression stimulates the adipogenic capacity of human MSCs. (a) MSCs were transduced with the empty bicistronic expression vector pRRL (MSC-pRRL) or containing the A20 cDNA (MSC-A20). Transduction efficiency was analyzed by flow cytometry (GFP+ cells). A20 overexpression was determined by western blot in total cell extracts. Sam68 was used as a loading control. (b) Quantification by real-time qPCR of IL-6. MSCs overexpressing A20 transcript for 4 days were cultured for additional 24 h in the presence or absence of 15 ng/ml TNFα. Relative transcript IL-6 expression levels were normalized to MSCs transduced with the empty vector cultured in the absence of TNFα. (c) MSCs differentiation capacity is influenced by A20 overexpression. MSCs showing ∼50% infection efficiency were induced to differentiate into adipocytes in the presence or absence of TNFα. Percentages of mature adipocytes from GFP− (white bars) and GFP+ (gray bars) cells (with the empty vector or A20-cDNA expressing vector, respectively) after the induction period are represented. Results show the mean±S.D. of triplicates samples. ***P<0.001
Discussion
In this report, we have studied the effect of TNFα on adipogenesis in human MSCs and the mechanisms behind. Adipogenesis is a tightly regulated process initiated by the sequential activation of CCAAT/enhancer binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ). C/EBPβ and C/EBPδ are transiently induced by cAMP and glucocorticoids and lead to the expression of the main adipogenic transcription regulators C/EBPα and PPARγ. These two transcription factors then orchestrate a large number of genes involved in the progression of the differentiation process. TNFα is known to interfere with the adipogenesis via inhibition of C/EBP and PPARγ activity, which will negatively influence the adipogenesis. A number of signals downstream of TNFα have been linked to the suppression of the activity of both transcription factors. These include activation of ERK, JNK, IKKβ and ceramide synthesis.18, 28, 29, 30 However, in this study we demonstrate that TNFα also interferes with adipogenesis via the influence of NF-κB activation, and in particular of A20 activity. The lack of an inhibitor-dependent regulatory circuit (A20 knockdown) leads to prolonged NF-κB signaling in MSCs, which drives to limited adipogenic capacity. Vice versa, permanent interruption of the NF-κB signaling pathway by A20 overexpression produces an increase in the generation of adipocytes. These data clearly demonstrate that the presence of A20 exerts a pro-adipogenic effect in human MSCs. This previously unknown mechanism provides another factor in the complex process of adipogenesis. However, further analysis is necessary in order to elucidate the relationship between A20 and the main transcription factors (PPAR and c/EBP) involved in this differentiation pathway in MSCs.
Surprisingly, we have found that A20, an important negative regulator of NF-κB signaling, is constitutively expressed in MSCs. To our knowledge, this is the first time that constitutive expression of A20 is observed in cells that are neither tumoral nor related to the immune system. In most cell types A20 is absent or expressed at very low basal levels; multiple NF-κB activating stimuli quickly induce A20 expression via NF-κB sites in the A20 promoter. It is believed that A20 acts as a negative regulator that balances the strength and duration of NF-κB activation. In T cells, on the other hand, A20 is highly expressed at basal state and is reduced on stimulation of the TCR. The constitutive expression of A20 in lymphocytes can be understood as a security system to avoid the severe consequences of an uncontrolled NF-κB activation and where A20 is employed as a brake to prevent unspecific activation of the pathway.23 In short, TCR stimulation triggers the cleavage of A20 by MALT-1, which impairs the inhibitory function of A20 and adjust the NF-κB signaling in the T cell.22 An analogous regulation of NF-κB signaling seems to exist in B cells.31 The constitutive expression of A20 in MSCs is obviously regulated in a different manner than in lymphocytes. Our results demonstrate that the stimulation with TNFα induces proteasomal degradation of A20 in MSCs, a regulatory mechanism of A20 that has not been observed previously. We could not observe any effect on the efficiency of TNFα-induced A20 degradation after addition of caspase inhibitor, although inhibition of the proteasome prevented the decrease in A20 levels. Thus, in contrary to what is observed in lymphocytes after TCR stimulation where A20 expression is regulated mainly by MALT-1, and to a lesser extent by the proteasome, the degradation in MSCs is induced by TNFα and performed by the proteasome. A20 degradation observed after TNFα stimulation is accompanied by induction of transcription of its own mRNA due to NF-κB activation, which suggests that a tight regulation of NF-κB signaling in MSCs is fundamental. A complete molecular study of A20 in MSCs could help to understand all the functions and regulation mechanism of this protein, since at least its expression and degradation in those cells are different.
The consequences of NF-κB activity are diverse, depending both on the cell type and on the stimuli received by the cell. A complex network of regulatory mechanisms acts on several levels of the pathway in order to achieve the correct response. In the same way, A20 depends on various adaptor and effector molecules to inhibit NF-κB, and thus functions differently depending on the cell type or received stimuli. Clearly, the inhibitory function of A20 on NF-κB signaling is different in MSCs than in most other cells, as A20 is constitutively expressed and its level after stimulation is differently regulated. In this sense, this study demonstrates for the first time that A20 exerts a protective role on MSCs by influencing the adipogenic differentiation in the presence of inflammatory cytokines.
Materials and Methods
Cell culture
Human bone marrow-derived MSCs were obtained from the Inbiobank Stem Cell Bank (www.inbiobank.org) as previously described.21 Briefly, cadaveric bone marrow was collected from brain-dead donors after informed consent and under the Spanish National Organization of Transplant supervision (Organización Nacional de Transplantes, ONT). Generated MSCs display a typical CD29+, CD73+, CD90+, CD105+, CD166+, CD146+, CD34−, CD45−, CD14−, CD19−, and CD31− phenotype— a fibroblast-like morphology— and at least trilineage potential, including osteocyte, chondrocyte, and adipocyte generation.21 MSCs were cultured in low-glucose DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all from Sigma-Aldrich, St Louis, MO, USA). On reaching confluence, MSCs were trypsinized and seeded at a density of 1 × 103 cells/cm2. Cells were obtained at passage three from the Stem Cell Bank. All experiments were carried out with cultures of low passage (passage number four to eight). 293 T cells were obtained from ATCC (www.atcc.org) and cultured in high-glucose DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml and 0.1 mg/ml streptomycin. Fibroblasts were obtained from the Inbiobank Stem Cell Bank (www.inbiobank.org), and cultured in high-glucose DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml and 0.1 mg/ml streptomycin.
Gene silencing
shRNA expression vectors were constructed using standard cloning procedures. The shRNA sequences have been published previously and were purchased from Sigma-Genosys (St. Louis, MO, USA); A20i, AGTTGGATGAAGCTAACTTAC (The RNAi Consortium, www.broadinstitute.org/rnai/trc); IKKβi, GGAAGTACCTGAACCAGTTTG; IκBi, GAGTCAGAGTTCACGGAGTTC. Oligonucleotides were annealed and cloned into pSUPER plasmid carrying a H1 promoter using BglII-HindIII sites. The H1-shRNA expression cassette was then excised and cloned into pLVTHM (Addgene plasmid 12247, www.addgene.org) using EcoRI-ClaI sites. Viral particles were produced as previously described by the Viral Vector Platform at the Inbiomed Foundation.21 MSCs transduction was carried out at a multiplicity of infection of four in order to achieve 50% infection and ten in order to achieve 100% infection.
Gene overexpression
A20 was amplified from cDNA of purified T cells using the following primers 5′ ACAAACGAATTCATGGCTGAAGTCCTTC3′ and 5′GCCGAGGAATTCTTAGGGGCA-GTTGGGCGTTTC3′ and cloned into pcDNA3 to obtain pcDNA3-A20. A20 was then subcloned into lentiviral vector pRRL.
RT qPCR
Total RNA was extracted using the RNAeasy extraction kit (Qiagen, Valencia, CA, USA). cDNA was obtained using the GeneAmp RNA PCR Core kit (Applied Biosystems, Carlsbad, CA, USA, N8080143G), following the manufacturer's instructions. Quantitative PCRs were performed on these cDNAs using Power SYBR Green PCR Master Mix (Applied Biosystems, 4367659). A20, IL-6, IκBα and GAPDH were amplified using the following oligonucleotide pairs: A20, GTCCGGAAGCTTGTGGCGCT and CCAAGTCTGTGTCCTGAACGCCC (97 bp); IL-6, CCAGGAGCCCAGCTATGAAC and GAGCAGCCCCAGGGAGAA (71 bp); IκBα, GATCCGCCAGGTGAAGGG and GCAATTTCTGGCTGGTTGG (102 bp); and GAPDH, TGCACCACCAACTGCTTAGC and GGCATGGACTGTGGTCATGAG (87 bp, Vandesompele et al., 2002). Reactions were carried out in a Thermocycler Step One Plus (Applied Biosystems). Data were compared using the ΔΔCT method. GAPDH was used as a housekeeping gene control.
Adipocyte differentiation
MSCs were seeded at a density of 25 000 cells per cm2 in low-glucose DMEM medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all from Sigma-Aldrich). Once the cells had adhered (after 18–24 h) the medium was exchanged for fresh medium supplemented with 1 μM dexamethasone (Sigma-Aldrich, D4902), 200 μM indomethacin (Sigma-Aldrich, I7378), 500 μM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich, I5879) and when indicated with TNFα (R&D, Minneapolis, MN, USA, 210-TA). The differentiation medium was refreshed every 2–3 days until the end of the experiment at day 21. Once the differentiation was completed the percentage of adipocytes in the culture was determined by flow cytometry. Briefly, cells were trypsinized and washed with PBS. After fixation (0.5% paraformaldehyde) for 15 min, cells were stained with 1 mg/ml Nile Red (Sigma-Aldrich) for 30 min on ice.32
Protein analysis
For western blots, 105 cells were lysed for 30 min on ice in 50 mM sodium fluoride, 5 mM tetra-sodium pyrophosphate, 10 mM beta-glyceropyrophosphate, 1% Igepal CA-630, 2 mM EDTA, 20 mM Na2HPO4, 20 mM NaH2PO4 and 1.2 mg/ml complete protease inhibitor cocktail (Roche, Indianapolis, IN, USA). When indicated, cells were treated with MG132 (Sigma-Aldrich, 20 μM), Epoxomicin (Enzo Life Sciences, Farmingdale, NY, USA, 50 μM) or ZVAD (Enzo Life Sciences, 100 μM) before TNFα (R&D, 15 ng/ml) stimulation, and lysed as previously described.
Immunoprecipitation experiments were performed using Protein-G cross-linked with the anti-A20 antibody. Western blot analysis was performed with the following primary antibodies: mouse monoclonal anti-human IκBα (Cell Signalling Technology, Beverly, MA, USA) and anti-human A20 (Calbiochem, La Jolla, CA, USA) antibodies, and rabbit polyclonal anti-human Sam68 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Acknowledgments
We would like to thank Pilar Olaizola of Hospital Donostia for Technical support in Inbiobank. This work was supported by the ‘Obra Social KUTXA', Ministerio de Economía y Competitividad (FIS PI12/01982), the Basque Country Government and the Diputación Foral de Guipúzcoa. Valerie Lang was supported by the Ramón y Cajal Program, Ministerio de Economía y Competitividad grant BFU2006-12991.
Glossary
- MSCs
mesenchymal stromal cells
- TNFα
tumor necrosis factor alpha
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- TNFR
TNFα receptor
- A20 or TNFAIP-3
tumor necrosis factor alpha-induced protein 3
- IκBα
inhibitor of kappa B alpha
- IKKβ
IκB kinase beta
The authors declare no conflict of interests.
Footnotes
Edited by R De Maria
References
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Silke J. The regulation of TNF signalling: what a tangled web we weave. Curr Opin Immunol. 2011;23:620–626. doi: 10.1016/j.coi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Wolf FW, Seldin MF, O'Shea KS, Dixit VM, Turka LA. Lymphoid expression and regulation of A20, an inhibitor of programmed cell death. J Immunol. 1995;154:1699–1706. [PubMed] [Google Scholar]
- Opipari AW, Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- Dixit VM, Green S, Sarma V, Holzman LB, Wolf FW, O'Rourke K, et al. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990;265:2973–2978. [PubMed] [Google Scholar]
- Opipari AW, Jr, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC, Beyaert R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3) Biochem Pharmacol. 2010;80:2009–2020. doi: 10.1016/j.bcp.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Okada A, Yamasaki S, Koga T, Kawashiri SY, Tamai M, Origuchi T, et al. Adipogenesis of the mesenchymal stromal cells and bone oedema in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30:332–337. [PubMed] [Google Scholar]
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Carcamo-Orive I, Gaztelumendi A, Delgado J, Tejados N, Dorronsoro A, Fernandez-Rueda J, et al. Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res. 2010;25:2115–2125. doi: 10.1002/jbmr.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- Duwel M, Welteke V, Oeckinghaus A, Baens M, Kloo B, Ferch U, et al. A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains. J Immunol. 2009;182:7718–7728. doi: 10.4049/jimmunol.0803313. [DOI] [PubMed] [Google Scholar]
- Novotny NM, Markel TA, Crisostomo PR, Meldrum DR. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NFkB. Cytokine. 2008;43:215–219. doi: 10.1016/j.cyto.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Hess S, Gottfried E, Smola H, Grunwald U, Schuchmann M, Engelmann H. CD40 induces resistance to TNF-mediated apoptosis in a fibroblast cell line. Eur J Immunol. 1998;28:3594–3604. doi: 10.1002/(SICI)1521-4141(199811)28:11<3594::AID-IMMU3594>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Lee FH, Porter AG. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor alpha in tumor cells. Mol Cell Biol. 1994;14:5661–5670. doi: 10.1128/mcb.14.9.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, et al. Nuclear factor kB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem. 1998;273:31262–31272. doi: 10.1074/jbc.273.47.31262. [DOI] [PubMed] [Google Scholar]
- Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- Chae GN, Kwak SJ. NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression. Exp Mol Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- Tominaga S, Yamaguchi T, Takahashi S, Hirose F, Osumi T. Negative regulation of adipogenesis from human mesenchymal stem cells by Jun N-terminal kinase. Biochem Biophys Res Commun. 2005;326:499–504. doi: 10.1016/j.bbrc.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-kappaB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo-Orive I, Tejados N, Delgado J, Gaztelumendi A, Otaegui D, Lang V, et al. ERK2 protein regulates the proliferation of human mesenchymal stem cells without affecting their mobilization and differentiation potential. Exp Cell Res. 2008;314:1777–1788. doi: 10.1016/j.yexcr.2008.01.020. [DOI] [PubMed] [Google Scholar]