Abstract
Alzheimer's disease (AD) is a chronic neurodegenerative disease characterized by progressive neuronal loss and cognitive decline. Oligomeric amyloid β (oAβ) is involved in the pathogenesis of AD by affecting synaptic plasticity and inhibiting long-term potentiation. Although several lines of evidence suggests that microglia, the resident immune cells in the central nervous system (CNS), are neurotoxic in the development of AD, the mechanism whether or how oAβ induces microglial neurotoxicity remains unknown. Here, we show that oAβ promotes the processing of pro-interleukin (IL)-1β into mature IL-1β in microglia, which then enhances microglial neurotoxicity. The processing is induced by an increase in activity of caspase-1 and NOD-like receptor family, pyrin domain containing 3 (NLRP3) via mitochondrial reactive oxygen species (ROS) and partially via NADPH oxidase-induced ROS. The caspase-1 inhibitor Z-YVAD-FMK inhibits the processing of IL-1β, and attenuates microglial neurotoxicity. Our results indicate that microglia can be activated by oAβ to induce neuroinflammation through processing of IL-1β, a pro-inflammatory cytokine, in AD.
Keywords: microglia, oligomer Aβ, NLRP3, IL-1β
Alzheimer's disease (AD) is a chronic neurodegenerative disease characterized by progressive cognitive decline.1, 2, 3 The pathological hallmarks of AD are neuronal loss, neurofibrillary tangles, accumulation of activated glial cells, gliosis, and the deposition of amyloid that forms senile plaques. The imbalance between the production of amyloid β (Aβ), the proteolytic fragment of amyloid precursor protein (APP), and its clearance is considered to be central event in the pathogenesis of AD.4, 5 The Aβ peptide undergoes transition from monomer to oligomer and then forms insoluble fibrillar Aβ (fAβ), which is the major component of senile plaque.6, 7 Although fAβ was thought to be the primary entity responsible for AD, recent evidence suggests that soluble oligomeric amyloid β (oAβ) found in the cortex of AD patients contributes to the pathogenesis of AD.8 Indeed, the level of oAβ in the AD brain or cerebrospinal fluid is directly correlated with the degree of synaptic loss and severity of cognitive decline.9, 10
Inflammatory process initiated by activated microglia is another essential component of AD.11 Accumulation of activated microglia is observed around degenerating neurons. It has been shown that microglial activation precedes cognitive decline and the formation of senile plaque in different APP transgenic mice, animal models of AD.12, 13, 14
Interleukin (IL)-1β, a member of the IL-1 cytokine family, is produced as the inactive precursor pro-IL-1β in the cytoplasm in response to a wide variety of stimuli.15, 16 In order to exert its functions, pro-IL-1β must be processed into its mature active form by the protease caspase-1, which itself is activated by cytosolic multiprotein complexes called inflammasomes.17, 18 NOD-like receptor family, pyrin domain containing 3 (NLRP3) is the most intensively studied inflammasome complex protein and undergoes bipartite activation in macrophage and microglia.17 The first signal, usually microbial toxins-like lipopolysaccharides (LPS), induces NLRP3 and pro-IL-1β expression. The second signal, usually many unrelated entities like urate, extracellular ATP, and fAβ, induces NLRP3 oligomerization with the adapter protein apoptosis-associated-speck-like protein (ASC), which leads to autocatalytic activation of caspase-1. This activation of caspase-1 requires an efflux of potassium (K+).19 Activated caspase-1 then processes pro-IL-1β to mature IL-1β.20, 21, 22, 23 In addition, NLRP3 activation in microglia is reported to contribute to the progression of AD-like pathology in APP/PS1 transgenic mice, and NLRP3 knock out (KO) mice are reported to have decreased disease burden.24 Although oAβ is postulated to activate inflammasomes,25 how oAβ induces NLRP3 activation to process pro-IL-1β to the mature form remains unknown. Here we show that oAβ increases the processing of pro-IL-1β into mature IL-1β in microglia via reactive oxygen species (ROS)-dependent activation of NLRP3.
Results
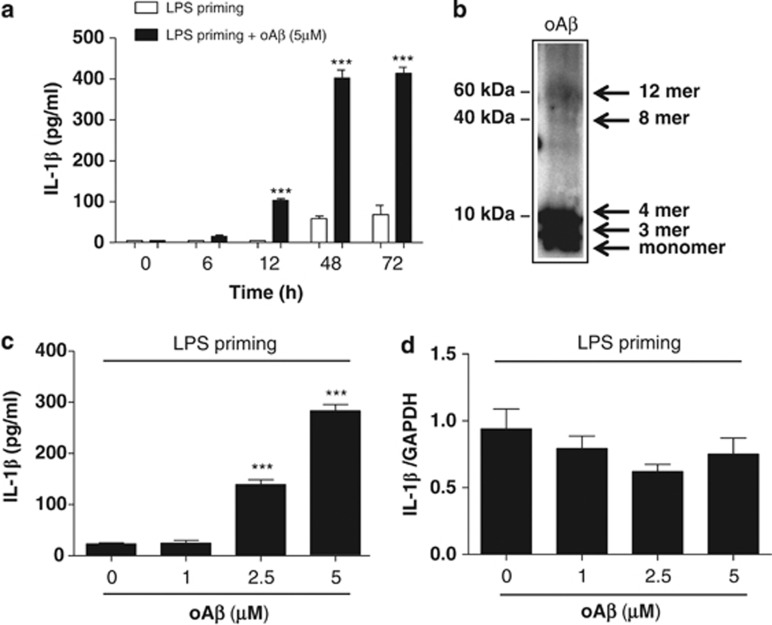
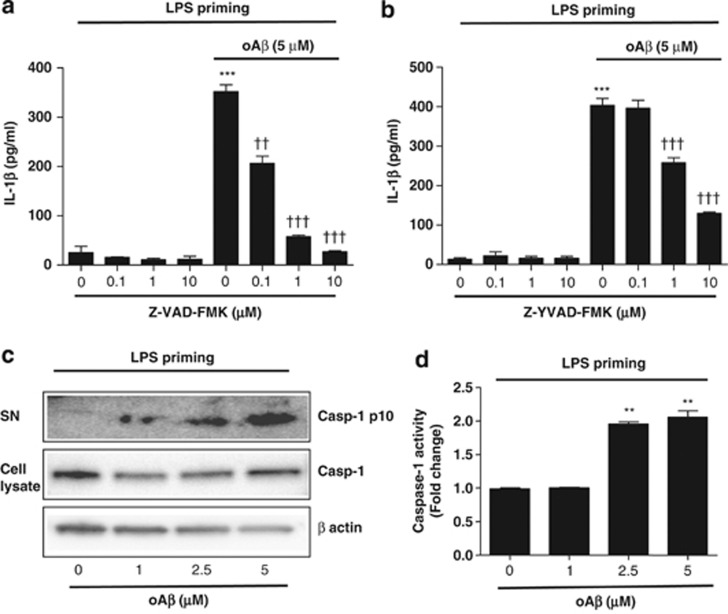
We first assessed whether oAβ induces IL-1β mRNA or processes IL-1β protein in microglia. We found that oAβ alone did not induce IL-1β mRNA and protein in microglia (Supplementary Figures 1a and b). To assess whether oAβ affects the processing of IL-1β, we transiently activated microglia with LPS (1 μg/ml) for 3 h (LPS priming). The cells were then washed twice with ice-cold PBS and further stimulated with oAβ (5 μM) for varying times (0–72 h), and IL-1β concentration in the culture supernatant was measured. We found that oAβ time-dependently increased IL-1β concentration in the culture supernatant when compared with transiently activated microglia with LPS for 3 h, which served as control (Figure 1a). Western blot analysis of oAβ used in the present study was shown in Figure 1b. In addition, oAβ dose-dependently increased IL-1β secretion (Figure 1c). As oAβ alone did not upregulate mRNA levels of IL-1β (Figure 1d), these results indicate that oAβ upregulates processing of IL-1β in LPS-primed microglia. As pro-IL-1β is reported to be processed by a caspase-dependent pathway.15 To determine whether oAβ-induced IL-1β secretion is dependent on caspase, microglia primed with LPS for 3 h were treated with the pan-caspase inhibitor Z-VAD-FMK or caspase-1 inhibitor Z-YVAD-FMK for 30 min before oAβ stimulation. We then measured IL-1β in culture supernatant at 48 h. Both Z-VAD-FMK and Z-YVAD-FMK dose-dependently decreased IL-1β secretion in the culture supernatant (Figures 2a and b). We next assessed the cleaved fraction of caspase-1 (Casp-1 p10) by western blotting and found that oAβ dose-dependently increased the secretion of Casp-1 p10 in the culture supernatant (Figure 2c). Similarly, treatment of oAβ after LPS priming dose-dependently increased caspase-1 activity in microglia (Figure 2d).
Figure 1.
oAβ induces IL-1β release in LPS-primed microglia. Microglia primed with LPS for 3 h were washed with ice-cold PBS and treated with oAβ for varying times; the concentration of IL-1β in the culture supernatant was then measured (a). ***P<0.001, versus 0 h. Western blot analysis of oAβ used in the present study (b). The blot was incubated with mouse anti oAβ monoclonal antibodies (6E10) (1 : 1000, Chemicon). LPS-primed microglia were treated with oAβ for 48 h, and the concentration of IL-1β in the culture supernatant as well as mRNA expression were measured by ELISA (c) and qPCR (d). Data indicate means±S.D. for five independent experiments. ***P<0.001, versus LPS-primed microglia
Figure 2.
oAβ induces IL-1β secretion/release via caspase-1 activation. LPS-primed microglia were treated with Z-VAD-FMK (a) or Z-YVAD-FMK (b) for 30 min before oAβ stimulation, and IL-1β in the culture supernatant was measured at 48 h. Data indicate means±S.D. for four independent experiments. ***P<0.001, versus LPS-primed microglia as control (ctl). ††, or ††† denotes P<0.01, or 0.001, respectively, versus LPS-primed microglia+oAβ. (c) After LPS-priming microglia were treated with oAβ for 48 h, Casp-1 p10 in the culture supernatant as well as caspase-1 and β-actin in the cell lysates were assessed by western blotting. Data are representative of two independent experiments. (d) LPS-primed microglia were treated with oAβ for 48 h, and caspase-1 activity was measured. Data indicate means±S.D. for three independent experiments. **P<0.01, versus LPS-primed microglia without oAβ
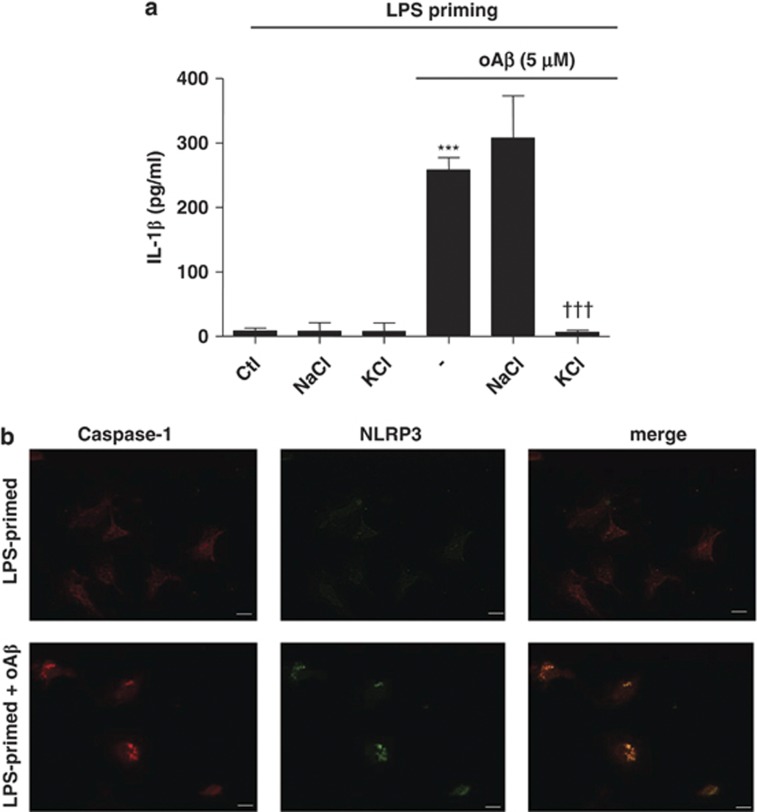
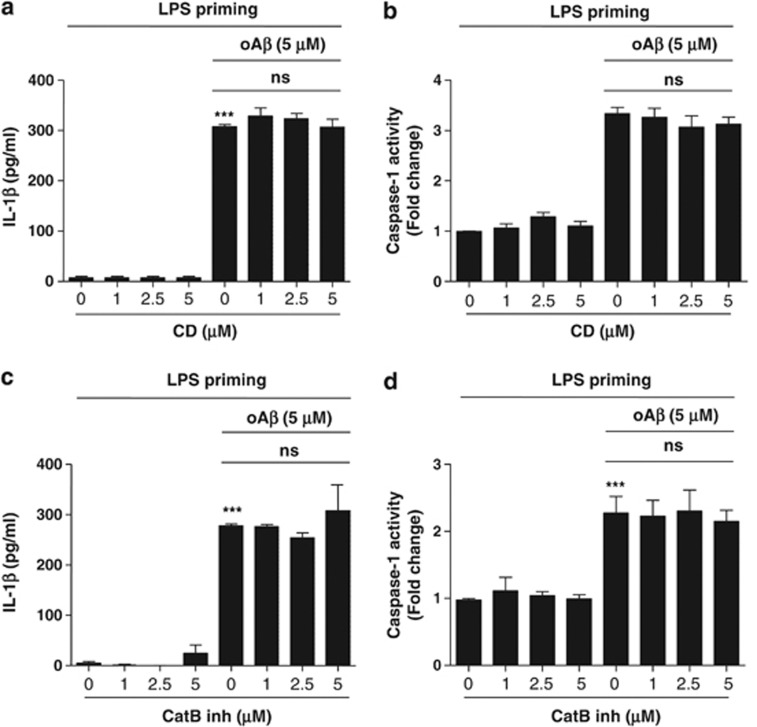
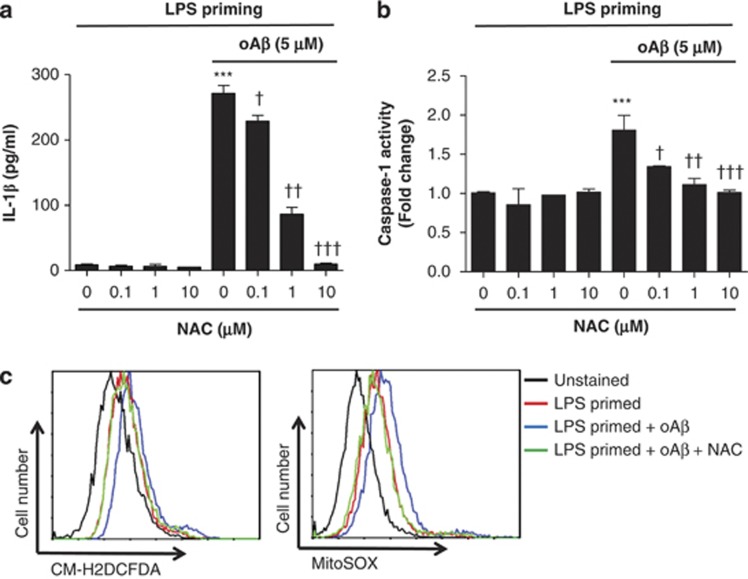
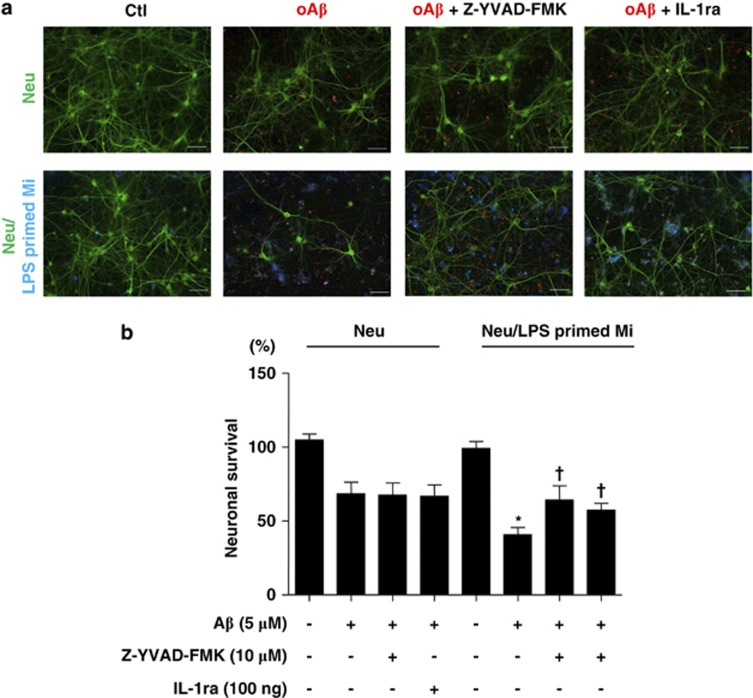
To determine whether oAβ-induced IL-1β processing is dependent on NLRP3, we increased the K+ concentration in the culture medium, which was previously described to inhibit NLRP3.19 We found that the increased K+ concentration, by the addition of KCl, significantly decreased IL-1β release from microglia (Figure 3a). The addition of NaCl did not affect IL-1β release. Furthermore, oAβ stimulation induced the co-localization of caspase-1 with NLRP3 (Figure 3b). NLRP3 is reported to be activated by lysosomal destabilization and release of cathepsin B in response to phagocytosis.22, 23, 26 To evaluate the requirement of phagocytosis and cathepsin B release in oAβ-induced IL-1β secretion, phagocytosis and cathepsin B were pharmacologically inhibited with cytochalasin D and cathepsin B inhibitor, respectively. We found that cytochalasin D inhibited fAβ-induced IL-1β release and caspase-1 activity as previously described22 (Supplementary Figures 2a and b); however, it had no effect on oAβ-induced IL-1β secretion and caspase-1 activity (Figures 4a and b). Similarly, cathepsin B inhibitor decreased fAβ-induced IL-1β secretion and caspase-1 activity as previously described22 (Supplementary Figures 2c and d), it had no effect on oAβ-induced IL-1β secretion and caspase-1 activity (Figures 4c and d). ROS are reported to act as danger signal for NLRP3 inflammasome activation.21, 27, 28 High concentrations of ROS inhibitors are reported to block NF-κB-mediated by priming of NLRP3 inflammasome.29 We treated microglia with N-acetylcysteine (NAC), a potent ROS scavenger, for 30 min after LPS priming and before the addition of oAβ. NAC dose-dependently decreased oAβ-induced IL-1β secretion (Figure 5a). Similarly, NAC also inhibited oAβ-induced caspase-1 activity (Figure 5b). Similarly, gp91ds-tat, an NADPH oxidase (NOX)-specific inhibitor, also dose-dependently decreased oAβ-induced IL-1β secretion as well as caspase-1 activity, but not as potently as NAC (Supplementary Figures 3a and b). These results indicate that oAβ-induced IL-1β secretion is partially dependent on NOX. Mitochondrial ROS are reported to activate NLRP3, so we next determined cellular and mitochondrial ROS production by flow cytometry. Microglia treated with oAβ after LPS priming produced cellular and mitochondrial ROS, which were inhibited by NAC (Figure 5c). We also assessed whether LPS-primed microglia affect neuronal viability. LPS-primed microglia were cocultured with primary cortical neurons and treated with oAβ (5 μM) with or without Z-YVAD-FMK or IL-1ra. Neuronal cultures were also treated with oAβ with or without Z-YVAD-FMK or IL-1ra. We found that treatment of neuronal cultures with oAβ decreased the viability of neurons. The neuronal damage with oAβ was further enhanced in the neurons/LPS-primed microglia cocultures. Although Z-YVAD-FMK or IL-1ra had no effect in the neuronal cultures, it attenuated microglia-induced neurotoxicity in the neuron/LPS-primed microglia cocultures (Figures 6a and b).
Figure 3.
oAβ-induced caspase-1 activation is dependent on NLRP3. (a) LPS-primed microglia were treated with NaCl or KCl before oAβ stimulation, and IL-1β in the culture supernatant was measured at 48 h. Data indicate means±S.D. for four independent experiments. ***P<0.001, versus LPS-primed microglia (ctl). †††P<0.001, versus LPS priming+oAβ+KCl. (b) LPS-primed microglia were treated with oAβ for 48 h, and co-localization of NLRP3 and caspase-1 were assessed by immunocytochemistry. Data are representative of three independent experiments. Scale bar represents 10 μm
Figure 4.
oAβ-induced IL-1β release is independent on phagocytosis or cathepsin B. LPS-primed microglia were treated with cytochalasin D for 30 min before oAβ stimulation, and IL-1β (a) in the culture supernatant as well as caspase-1 activity (b) were measured at 48 h. Data indicate means±S.D. for three independent experiments. ***P<0.001, versus LPS-primed microglia (ctl). ns indicates not significant. LPS-primed microglia were treated with cathepsin B inhibitor for 30 min before oAβ stimulation, and IL-1β (c) in the culture supernatant as well as caspase-1 activity (d) were measured at 48 h. Data indicate means±S.D. for three independent experiments. ***P<0.001, versus LPS-primed microglia (ctl). ns indicates not significant
Figure 5.
oAβ-induced IL-1β release is dependent on ROS. LPS-primed microglia were treated with varying doses of NAC (0.1–10 μM) for 30 min before oAβ stimulation, and IL-1β (a) in the culture supernatant as well as caspase-1 activity (b) were measured at 48 h. Data indicate means±S.D. for three independent experiments. ***P<0.001, versus LPS-primed microglia (ctl). †, ††, or ††† denotes P<0.05, 0.01, or 0.001, respectively, versus LPS priming+oAβ+NAC (c). LPS-primed microglia were treated with the ROS scavenger NAC (10 μM) for 30 min before oAβ stimulation and cellular (CM-H2DCFDA) and mitochondrial (MitoSOX) ROS were assessed by flow cytometry. Data are one representative of three independent experiments
Figure 6.
LPS-primed microglia treated with oAβ induce neuronal cell death via caspase-1, and IL-1β. LPS-primed microglia were cocultured with primary cortical neurons. The cells were then treated with Z-YVAD-FMK or IL-1ra before oAβ treatment. Neurons were stained with anti-MAP2 antibodies (green), microglia were stained with Cy-5-conjugated anti-CD11b antibodies (blue), and oAβ was stained with anti-4G8 antibodies (red). Neuronal viability was assessed by MAP2 staining (a and b). Data indicate means±S.D. for three independent experiments. *P<0.05, versus neurons treated with oAβ, †P<0.05, versus neurons cocultured with LPS-primed microglia treated with oAβ. Scale bar represents 50 μm. Neu indicates neurons and Mi indicates microglia
Discussion
Microglial-mediated neuroinflammation contributes to the pathogenesis of AD. Indeed, microglial activation and subsequent production of neurotoxic pro-inflammatory molecules have a pivotal role in the progression of AD. However, whether Aβ, a main component of misfolded protein in the AD brain, could induce the production of pro-inflammatory cytokines is controversial. It has been reported that oAβ does not induce IL-1β mRNA in microglia.30 However, other reports indicate that oAβ induces various inflammatory mediators such as IL-1β, TNF-α, and NO.31, 32, 33 In this study, we have shown that oAβ alone is not sufficient to induce IL-1β mRNA or increase IL-1β secretion in unstimulated microglia. oAβ induces IL-1β secretion via activation of caspase-1 when microglia are primed with toll-like receptor (TLR) 4 ligand LPS. We also showed that both mitochondrial as well as NOX2-induced ROS contribute to oAβ-induced caspase-1 activation. Furthermore, oAβ has been shown to induce ROS in microglia by activation of NOX and mitochondria damage.34, 35, 36, 37, 38 ROS are reported to activate caspase-1 via NLRP3.27, 28 ROS induce oxidation of K+ channel.39 Similarly, oAβ is reported to induce pore formation in the cell membrane40 and to alter K+ current in neurons.41 Thus, oAβ-induced pore formation or oxidation of K+ channel might lead to K+ efflux activating NLRP3 inflammasome in microglia. We have also shown that inhibition of K+ efflux decreases oAβ-induced IL-1β secretion. K+ efflux is required for NLRP3 activation.19
Our results indicate that the mechanism of oAβ-induced IL-1β secretion is different from that induced by fAβ. oAβ-induced IL-1β secretion by microglia was not dependent on phagocytosis and lysosomal disruption with subsequent release of cathepsin B, because we found that the inhibition of phagocytosis by cytochalasin D and cathepsin B inhibitors had no effect on IL-1β secretion. However, fAβ-induced IL-1β secretion is dependent on phagocytosis with subsequent lysosomal disruption. oAβ and fAβ differentially activate microglia and neurons.30, 42 For instance, oAβ is reported to inhibit phagocytosis, whereas fAβ is reported to stimulate phagocytosis.42 Moreover, oAβ is reported to be more neurotoxic than fAβ.30 We have also shown that oAβ induces far greater secretion of IL-1β than fAβ in LPS-primed microglia. We further showed inflammasome activation in microglia increases oAβ-induced neuronal cell death, which is ameliorated by the inhibition of caspase-1 and IL-1β. Consistent with this observation, genetic deletion of NLRP3 in mice expressing mutant human APP/PS1, an animal model of AD, deceases their disease burden.24
The role of IL-1β in AD pathology is complex. IL-1β transgenic mice expressing mutant human APP/PS1 are reported to have decreased plaque formation, although the total amount of oAβ is unaltered.43 However, IL-1β transgenic mice are reported to have learning and memory impairment.44 IL-1β can also affect synaptic plasticity and inhibit long-term potentiation.45, 46 It has been shown that secreted mature IL-1β induces the phosphorylation of tau protein and mediates the formation of neurofibrillary tangles.47, 48 IL-1β can be elevated before the formation of amyloid plaque in patients with Down syndrome, who invariably develop AD-like pathology.49 Thus, oAβ-induced IL-1β secretion by microglia may augment neuroinflammation, increase neuronal cell death, and contribute to the pathogenesis of AD. Indeed, the infusion of oligomeric human amyloid β in mice lacking IL-1 receptor antagonist (IL-1ra) induces microglial activation and causes neuronal cell death.50
In conclusion, our results indicate that oAβ induces the secretion of active IL-1β via increased activation of caspase-1 in LPS-primed microglia, which is dependent on mitochondrial and NOX2-induced ROS production. Secreted IL-1β is involved in neuronal cell death that is ameliorated by inhibiting caspase-1 activation or by neutralization of IL-1β. Thus, the cascade of oAβ-induced IL-1β secretion in microglia may be a target for treating AD.
Materials and Methods
Reagents
LPS, N-acetyl-L-cysteine (NAC) and cytochalasin D were obtained from Sigma-Aldrich (St. Louis, MO, USA). Z-VAD-FMK (pan-caspase inhibitor), Z-YVAD-FMK (caspase-1 inhibitor), and Ac-Leu-Val-lysinal (cathepsin B inhibitor) were obtained from Calbiochem (Gibbstown, NJ, USA). Anti-cryopyrin (sc-34410) and anti-caspase-1 antibodies (sc-514) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Gp-91 ds tat was from Anaspec (Fremont, CA, USA). IL-1ra was obtained from R&D (Minneapolis, MN, USA).
Cell culture
All animal experiments were conducted under protocols that were approved by the Animal Experiment Committee of Nagoya University. All primary cultures were prepared from C57BL/6 mice (Japan SLC, Hamamatsu, Japan).
Microglia were isolated from primary mixed glial cell cultures prepared from newborn mice on day 14 using the ‘shaking off' method as previously described.51 The purity of the cultures (>99%) was determined by anti-CD11b immunostaining (BD Biosciences, Franklin Lakes, NJ, USA). The cultures were maintained in Dulbecco's modified Eagle's minimum essential medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (SAFC Biosciences, Lenexa, KS, USA), 5 μg/ml bovine insulin (Sigma-Aldrich) and 0.2% glucose.
Primary neuronal cultures were prepared from the cortices of mouse embryos at embryonic day 17 (E17) as described previously.52 Briefly, cortical fragments were dissociated into single cells in dissociation solution (Sumitomo Bakelite, Akita, Japan) and resuspended in nerve culture medium (Sumitomo Bakelite). Neurons were seeded onto 12-mm polyethyleneimine (PEI)-coated glass cover slips (Asahi Techno Glass Corp, Chiba, Japan) at a density of 5 × 104 cells/well in 24-well plates. The purity of the culture was more than 95% as determined by NeuN-specific immunostaining (Merck Millipore, Billerica, MA, USA).
Neuron-microglia cocultures were prepared as follows: 1 × 105 microglia in 100 μl of neuronal medium were added to neuronal cultures (5 × 104 neuronal cells) in 24-well plates on day 14.
Preparation of oAβ and fAβ
oAβ and fAβ were prepared as previously described.30 To form fAβ synthetic human Aβ1-42 (Peptide Institute, Osaka, Japan) was dissolved in 0.02% ammonia solution at a concentration of 250 μmol/l, diluted to 25 μmol/l in PBS, and incubated at 37 °C for 72 h. Briefly, oAβ1-42 was prepared by dissolving Aβ1-42 to 1 mmol/l in 100% 1,1,1,3,3,3-hexafluoro-2-propanol. 1,1,1,3,3,3-Hexafluoro-2-propanol was dried by a vacuum desiccator and resuspended to 5 mmol/l in DMSO. To form oligomers, amyloid peptide was diluted to a final concentration of 100 μmol/l with Ham's F-12, incubated at 4 °C for 24 h, and then immediately added to cultures at a final concentration 5 μmol/l. Formation of oAβ was confirmed by western blotting as previously described.30
Measurement of IL-1β and caspase-1 activity
Microglia, seeded at a density of 1 × 105 cells/well in 24-well plates, were treated with LPS for 3 h. The cells were then washed twice and treated with oAβ. Supernatants were collected and the levels of IL-1β in culture supernatant were determined by ELISA according to the manufacturer's instruction (BD Biosciences). Microglia, seeded at a density of 1 × 107 cells and treated as described above, were measured for caspase-1 activity according to the manufacturer's instruction (Merck Millipore).
RT-PCR
For quantitative PCR, the total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized from total cellular RNA that was denatured for 5 min at 65 °C, followed by a reverse transcription reaction using the SuperScript II (Life Technologies, Carlsbad, CA, USA). The cDNA served as a template to amplify genes in quantitative PCRs with TaqMan Gene Expression assays (Applied Biosystems, Foster City, CA, USA), Universal PCR Master Mix (Applied Biosystems), and Rotor-Gene Q (Qiagen). Expression levels of target genes were calculated using a comparative method and normalization to GAPDH expression levels as previously described.53 The following primers and probes were obtained from Applied Biosystems: IL-1β, Mm00434228_m1; GAPDH, Mm99999915_g1.
Immunocytochemistry
Immunocytochemistry was conducted as previously described. Microglia plated on a glass cover slip were fixed with 4% paraformaldehyde for 10 min. The cells were then permeabilized with 0.05% Triton X-100 for 5 min and blocked with 5% bovine serum albumin for 1 h, followed by incubation with anti-caspase-1 (1 : 500), and anti NLRP3 (1 : 500) antibodies overnight at 4 °C. The cells were then incubated with Alexa 488- or Alexa 568-conjugated secondary antibodies for 1 h. Cells were examined with a deconvolution fluorescence microscope system (Bio Zero, Keyence, Osaka, Japan). Neuronal viability was assessed as previously described.30, 52 To determine the viability of neurons in microglia-neuronal cocultures, microglia were labeled with Cy5 conjugated anti-CD11b (1 : 250) for 30 min before permeabilization with 0.05% Triton X-100 for 5 min, and blocked with 5% goat serum for 1 h, followed by incubation with anti-4 G8 antibodies (Chemicon, Temecula, CA, USA, 1 : 1000), and anti-MAP2 antibodies (Merck Millipore, 1 : 1000) for 2 h at room temperature. Then, the cells were incubated with Alexa 488- or Alexa 568-conjugated secondary antibodies (1 : 1000) for 1 h. Cells were examined with a deconvolution fluorescence microscope system.
Western blotting
Western blotting was done as previously described.22 Cell culture supernatants were precipitated by the addition of an equal volume of methanol and 0.25 volumes of chloroform, followed by vortexing and centrifugation for 10 min at 20 000 × g. The upper phase was discarded and 500 μl methanol was added to the interphase. This mixture was centrifuged for 10 min at 20 000 × g, and the protein pellet was dried and resuspended in Laemmli buffer. The samples were boiled for 5 min at 99 °C. The samples were then separated by SDS-PAGE and transferred onto nitrocellulose membranes. Blots were incubated with rabbit polyclonal anti-mouse caspase-1 antibodies. To determine the caspase-1 level in the cell lysate, microglia were lysed with TNES buffer (1 M Tris-HCL, 20% SDS, and 2.5% glycerol) containing phosphatase (Sigma-Aldrich) and protease inhibitor (Roche, Mannheim, Germany). Fifty micrograms of protein from the total lysate was assayed for caspase-1 and β-actin.
Flow cytometry
Flow cytometry was conducted as previously described.53 Briefly, LPS-primed microglia treated with oAβ or left untreated were stained with 5-(and-6)-chloromethyl–2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) or MitoSOX red superoxide indicator (both from Invitrogen, Carlsbad, CA, USA) for 15 min according to the manufacturer's instruction. After washing twice, cells were analyzed using a Cytomics FC500 (Beckman Coulter, Brea, CA, USA).
Statistical analysis
Statistically significant differences between experimental groups were determined by a one-way ANOVA followed by the Tukey's test for multiple comparisons. Statistical analysis was performed using the software program Prism 4.0 (GraphPad Software, San Diego, CA, USA). P-values <0.05 were considered to be statistically significant.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number 24659430, a Grant from the Advanced Research for Medical Products Mining Programme of the National Institute of Biomedical Innovation (NIBIO), and grants from the Ministry of Health, Labour and Welfare of Japan.
Glossary
- AD
Alzheimer's disease
- oAβ
oligomeric amyloid β
- CNS
central nervous system
- IL-1β
interleukin-1β
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- ROS
reactive oxygen species
- LPS
lipopolysaccharides
- APP
amyloid precursor protein
- ASC
apoptosis-associated-speck-like protein
- NAC
N-acetylcysteine
- NOX
NADPH oxidase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Walsh DM, Teplow DB. Alzheimer's disease and the amyloid beta-protein. Prog Mol Biol Transl Sci. 2012;107:101–124. doi: 10.1016/B978-0-12-385883-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a011460. doi: 10.1101/cshperspect.a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug GM, Losic D, Subasinghe SS, Aguilar MI, Martin LL, Small DH. Beta-amyloid protein oligomers induced by metal ions and acid pH are distinct from those generated by slow spontaneous ageing at neutral pH. Eur J Biochem. 2003;270:4282–4293. doi: 10.1046/j.1432-1033.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- Mastrangelo IA, Ahmed M, Sato T, Liu W, Wang C, Hough P, et al. High-resolution atomic force microscopy of soluble Abeta42 oligomers. J Mol Biol. 2006;358:106–119. doi: 10.1016/j.jmb.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AN, Ewers M, Minthon L, Simm A, Silber RE, Blennow K, et al. Amyloid-beta oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer's disease. J Alzheimers Dis. 2012;29:171–176. doi: 10.3233/JAD-2012-111361. [DOI] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Witton J, Olabarria M, Noristani HN, Verkhratsky A. Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer's disease. Cell Death Dis. 2010;1:e1. doi: 10.1038/cddis.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology. J Neuroinflammation. 2012;9:62. doi: 10.1186/1742-2094-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M.Interleukin-1beta (IL-1beta) processing pathway Sci Signal 20103cm2. [DOI] [PubMed] [Google Scholar]
- Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer's pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Yamada J, Hayashi Y, Wu Z, Uchiyama Y, Peters C, et al. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia. 2010;58:114–124. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y, Mizuno T, Maki Y, Jin S, Mizoguchi H, Ikeyama M, et al. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am J Pathol. 2009;175:2121–2132. doi: 10.2353/ajpath.2009.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathy S, Rajadas J, Ryan H, Vaziri S, Anderson L, Murphy GM., Jr Abeta peptide conformation determines uptake and interleukin-1alpha expression by primary microglial cells. Neurobiol Aging. 2009;30:1792–1804. doi: 10.1016/j.neurobiolaging.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Zimin PI, Wulff H, Jin LW. Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem. 2011;286:3693–3706. doi: 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg C, Selenica ML, Westlind-Danielsson A, Schultzberg M. Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. J Mol Neurosci. 2005;27:1–12. doi: 10.1385/JMN:27:1:001. [DOI] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause KH. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 2006;27:1577–1587. doi: 10.1016/j.neurobiolaging.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F, Liu S, Cai SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration. Trends Cell Biol. 2010;20:45–51. doi: 10.1016/j.tcb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Yu SP, Farhangrazi ZS, Ying HS, Yeh CH, Choi DW. Enhancement of outward potassium current may participate in beta-amyloid peptide-induced cortical neuronal death. Neurobiol Dis. 1998;5:81–88. doi: 10.1006/nbdi.1998.0186. [DOI] [PubMed] [Google Scholar]
- Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, et al. Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: implications for Alzheimer's disease. Mol Neurodegener. 2011;6:45. doi: 10.1186/1750-1326-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O'Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, et al. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Hirsch E, Van Eldik LJ. Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. J Neuroinflammation. 2005;2:15. doi: 10.1186/1742-2094-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Takayanagi T. Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res. 1998;787:139–142. doi: 10.1016/s0006-8993(97)01166-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, Sonobe Y, et al. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-beta neurotoxicity. Am J Pathol. 2011;179:2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, et al. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation. 2012;9:268. doi: 10.1186/1742-2094-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.