Abstract
Exogenous administration of nitric oxide (NO) markedly decreases neointimal hyperplasia following arterial injury in several animal models. However, the effect of NO on neointimal hyperplasia in hypertension remains unknown. Here, we employ the spontaneously hypertensive rat (SHR) strain, inbred from Wistar Kyoto (WKY) rats, and the carotid artery balloon injury model to assess the effects of NO on neointimal hyperplasia development. Two weeks after arterial injury, we showed that both rat strains developed similar levels of neointimal hyperplasia, but local administration of NO was less effective at inhibiting neointimal hyperplasia in the SHR compared to WKY rats (58% vs. 79%, P<0.001). Interestingly, local administration of NO did not affect systemic blood pressure in either rat strain. Compared to WKY, the SHR displayed more proliferation in the media and adventitia following balloon injury, as measured by BrdU incorporation. The SHR also showed more inflammation in the adventitia after injury, as well as more vasa vasorum, than WKY rats. NO treatment reduced the vasa vasorum in the SHR but not WKY rats. Finally, while NO decreased both injury-induced proliferation and inflammation in the SHR, it did not return these parameters to levels seen in WKY rats. We conclude that NO is less effective at inhibiting neointimal hyperplasia in the SHR than WKY rats. This may be due to increased scavenging of NO in the SHR, leading to diminished bioavailability of NO. These data will help to develop novel NO-based therapies that will be equally effective in both normotensive and hypertensive patient populations.
Keywords: balloon injury, endothelial dysfunction, hypertension, neointimal hyperplasia, nitric oxide, SHR
1. INTRODUCTION
Neointimal hyperplasia limits the long-term efficacy of cardiovascular interventions by leading to restenosis and ultimate occlusion of the artery or vein. Patients undergoing cardiovascular interventions typically have many co-morbidities, with one of the most common being hypertension. Hypertension is associated with endothelial dysfunction and vascular inflammation, both of which promote the development of medial wall thickening and perivascular fibrosis [1]. Previous studies have shown that hypertension is associated with increased development of neointimal hyperplasia in both vein and arterial bypass grafts [2; 3]. In addition, a study of normotensive Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHR) showed an increase in wall thickness of arterial graft in the SHR compared to WKY rats [4]. Thus, hypertension appears to be an important contributor to the development of neointimal hyperplasia following cardiovascular interventions.
We have previously shown that local delivery of nitric oxide (NO) effectively inhibits neointimal hyperplasia following arterial injury in different animal models [5–9]. However, little is known about the effect of supplemental NO on the arterial wall in the setting of hypertension. Given that the overarching goal of our lab is to develop a clinically applicable NO-based therapy for patients with vascular disease, and that many patients with vascular disease have hypertension, the aim of the current paper is to understand the effects of NO on neointimal hyperplasia in the context of hypertension. To conduct this study, we used the SHR and its control, the WKY rat strain. The SHR becomes hypertensive at 4–6 weeks of age without any external intervention [10]. The adult SHR exhibits endothelial dysfunction and low bioavailability of NO, despite having high levels of endothelial nitric oxide synthase (eNOS) [11]. This finding may be due to the fact that the SHR also has high levels of superoxide [12], which can scavenge NO and limit its bioavailability. Given these findings of high superoxide levels and low NO bioavailability, we hypothesized that supplemental administration of NO will not be as effective in preventing neointimal hyperplasia in the SHR compared to WKY rats.
2. MATERIALS AND METHODS
2.1 Diazeniumdiolated proline (PROLI/NO)
The NO donor (PROLI/NO) used in this study was kindly provided by Drs. Joseph Hrabie and Larry Keefer of NCI-Frederick. PROLI/NO was chosen based on its NO release rate (t1/2 = 1.8 s) [13], safety profile, and superior efficacy in the prevention of neointimal hyperplasia when compared to other diazeniumdiolates [5; 6].
2.2 Blood pressure and heart rate measurements
Blood pressure and heart rate measurements were collected using the CODA™ non-invasive blood pressure system (Kent Scientific; Torrington, CT). Measurements were taken prior to and following induction of anesthesia, and at 5 and 10 minutes following balloon injury (see below). For measurements taken prior to induction, animals were restrained in a Broome rat holder and blood pressure and heart rate measured with a large rat tail cuff. For each time point, a maximum of 20 measurement cycles were taken at 5 second intervals and all valid measurements were subsequently included in analysis. All animals were pre-conditioned for the Broome holder daily for several days prior to surgery. Results are reported as an average of the mean blood pressure and heart rate per animal in each treatment group.
2.3 Rat carotid artery injury model
All animal procedures were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication National Academy press, 1996) and approved by the Northwestern University Animal Care and Use Committee. Male, 10-week-old SHR or WKY rats (Charles River; Wilmington, MA) weighing 235–300 g were anesthetized with inhaled isoflurane (1.0 – 5.0%). Treatment groups for each rat strain were as follows: uninjured control (n=3), injury (n=8 for WKY and n=9 for SHR), and injury + NO (10 mg PROLI/NO, n=7 for WKY and n=8 for SHR). Prior to the procedure, atropine (0.1 mg/kg) and carprofen (Rimadyl, 0.15 mg/kg) were administered subcutaneously to decrease airway secretions and to control pain, respectively. Sterile lubricant (Equaline; Boise, ID) was applied to the animal’s eyes. Following a midline neck incision, the left common, internal, and external carotid arteries were identified. After distal ligation of the external carotid artery, the internal and common carotid arteries were occluded with atraumatic clamps. A No. 2 French arterial embolectomy catheter (Edwards Lifesciences; Irvine, CA) was inserted through an arteriotomy into the external carotid artery and advanced into the common carotid artery. Uniform injury was created by inflating the balloon to 5 atmospheres of pressure for 5 minutes. After removal of the balloon, the external carotid artery was ligated and blood flow restored. If applicable, powdered PROLI/NO was applied evenly to the external surface of the injured common carotid artery as we have previously described [6; 8; 9]. Briefly, the external surface of the artery was gently dried with sterile gauze, and an aliquot of PROLI/NO powder was sprinkled on the injured area. Forceps were used to ensure even coverage of the artery with PROLI/NO around its entire circumference, including underneath. The neck incision was closed in two layers. Rats were sacrificed at 2 weeks for morphometric, immunohistochemical, and immunofluorescence analysis.
2.4 Tissue processing
Carotid arteries were harvested following in situ perfusion-fixation with phosphate buffered saline (PBS) and 2% paraformaldehyde (w/v) in PBS. Vessels were placed in 2% paraformaldehyde at 4°C for one hour, then overnight in 30% sucrose (w/v) in PBS at 4°C for cryoprotection. The tissue was quick-frozen in Tissue-Tek® Optimal Cutting Temperature compound (Sakura Finetek USA; Torrance, CA) and 5-μm sections were cut throughout the entire injured segment of the common carotid artery using a Microm HM 550 cryostat (Fisher Scientific; Pittsburgh, PA).
2.5 Morphometric analysis
Carotid arteries harvested at two weeks were examined histologically for evidence of neointimal hyperplasia using routine hematoxylin and eosin (H&E) staining. Digital images were collected with light microscopy using an Olympus BHT microscope (Melville, NY) with 4X, 10X and 40X objectives. Six evenly-spaced sections through each injured carotid artery were analyzed. ImageJ software (NIH; Bethesda, MD) was used to obtain area measurements of the lumen, intima, and media (mm2), and all analysis was done by a single individual.
2.6 Immunohistochemistry
From each animal, three evenly-spaced carotid sections from the area of injury underwent immunohistochemical staining. To assess proliferation, rats received an intraperitoneal injection of bromodeoxyuridine (BrdU, 100 mg/kg) at 24 and 1 hour prior to sacrifice. Frozen sections were fixed in acetone for 5 minutes, and rinsed in PBS-Tween 20 for 2 minutes. Sections were then blocked with horse serum (Sigma; St. Louis, MO) diluted 1:20 in 0.5% bovine serum albumin (BSA) for 30 minutes. Primary antibody against BrdU (ab8955, Abcam; Cambridge, MA) was diluted 1:200 in 0.5% BSA and applied for 1 hour. Following two rinses in PBS-Tween 20, biotinylated anti-mouse IgG secondary antibody diluted 1:500 in BSA was applied for 30 minutes (Vector Labs; Burlingame, CA). The sections were then incubated in Vectastain ABC reagent for 30 minutes, and chromagen/substrate (DAB peroxidase substrate kit, Vector Labs) for 2 minutes. Following counterstaining with Gill’s hematoxylin solution (Fisher Scientific) and dehydration, sections were coverslipped with Permount (Fisher Scientific). For negative controls, PBS was substituted for the primary antibody. Brightfield images were digitally acquired using an Olympus BHT microscope (Melville, NY) and SPOT Basic software (Diagnostic Instruments, Inc.; Sterling Heights, MI). Positively stained cells were counted by a blinded investigator in four high-power fields per arterial section and expressed as an average.
To assess damage caused by reactive oxygen species, sections were stained for nitrotyrosine. Frozen sections were fixed in acetone for 5 minutes, rinsed in PBS-Tween 20 for 2 minutes, and rinsed in PBS for 2 minutes. Endogenous peroxidases were blocked by incubation in a solution containing 2% H2O2 and 60% methanol for 30 minutes. Following rinses in PBS-Tween 20 and PBS, primary antibody raised against nitrotyrosine (ab7048, Abcam) was diluted 1:200 in IHC-Tek Antibody Diluent (1W-1000, IHC World; Woodstock, MD), which also acts as a blocking agent, and applied to sections for 1 hour. Following three rinses in PBS, biotinylated anti-mouse IgG secondary antibody diluted 1:500 in PBS was applied for 30 minutes (Vector Labs), and the rest of the procedure was completed as above. For negative controls, PBS was substituted for the primary antibody. Brightfield images were digitally acquired using a Zeiss Imager.A2 microscope (Hallbergmoos, Germany) and the 5X objective, and staining was quantified by 3 blinded investigators using a scale of 0–3.
2.7 Immunofluorescence
To assess eNOS, monocyte/macrophage, and soluble guanylyl cyclase (sGC) staining, sections were fixed in paraformaldehyde (2%) for 20 minutes at 4°C, and then permeabilized in Triton X-100 (0.3 % in PBS) for 10 minutes at room temperature. After blocking in goat serum diluted 1:20 in 0.5% BSA for 30 minutes at room temperature, primary antibody against NOS3 or ED1 (sc-654 or sc-59103, Santa Cruz Biotechnology; Santa Cruz, CA) was diluted 1:100 or 1:50 or in 0.5% BSA and applied for 1 hour. For sGC staining, primary antibody raised against subunit beta 1 (ab24824, Abcam) was diluted 1:2000 in IHC-Tek Antibody Diluent (IHC World), and applied for 1 hour. Negative controls were incubated without primary antibody. Following a 30 minute incubation with goat anti-rabbit or goat anti-mouse AlexaFluor 555 antibody (1:500 in PBS, Invitrogen), 60 nmol/L DAPI was used to stain nuclei for 1 minute. Slides were coverslipped using ProLong Gold Antifade Reagent (Invitrogen), and digital images were acquired using a Zeiss Imager.A2 microscope and the 5X objective. Stains were quantified by up to 3 blinded investigators using either a scale of 0–3 or 0–4.
2.8 Statistical analysis
All results are given as mean ± the standard error of the mean. SigmaStat (SPSS; Chicago, IL) was used to determine differences between multiple groups by performing one-way analysis of variance and using the Student-Newman-Keuls post hoc test for all pair-wise comparisons. Statistical significance was assumed when P<0.05.
3. RESULTS
3.1 Local NO administration does not affect blood pressure
To determine the effect of local NO administration on blood pressure in the SHR and WKY rats, mean systolic blood pressure measurements were obtained using a tail vein cuff system. As shown in the Table, the SHR had higher blood pressure than the WKY rats prior to balloon injury, and this was maintained throughout the course of the study. Neither balloon injury nor administration of NO had any effect on mean systolic blood pressure.
Table.
Mean blood pressure was not affected by NO administration.
| Mean systolic BP (mm Hg) | Pre-procedure | POD1 | Sacrifice |
|---|---|---|---|
| WKY Injury | 107 ± 2 | 105 ± 1 | 104 ± 2 |
| WKY Injury + NO | -- | 94 ± 1 | 113 ± 1 |
| SHR Injury | 147 ± 4* | 140 ± 2* | 155 ± 2* |
| SHR Injury + NO | -- | 136 ± 1* | 162 ± 1* |
P<0.05 vs. WKY
3.2 NO inhibits neointimal hyperplasia less effectively in hypertensive rats
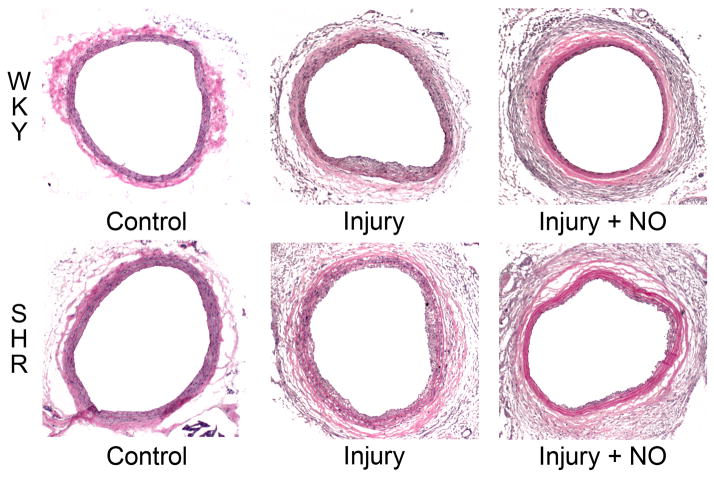
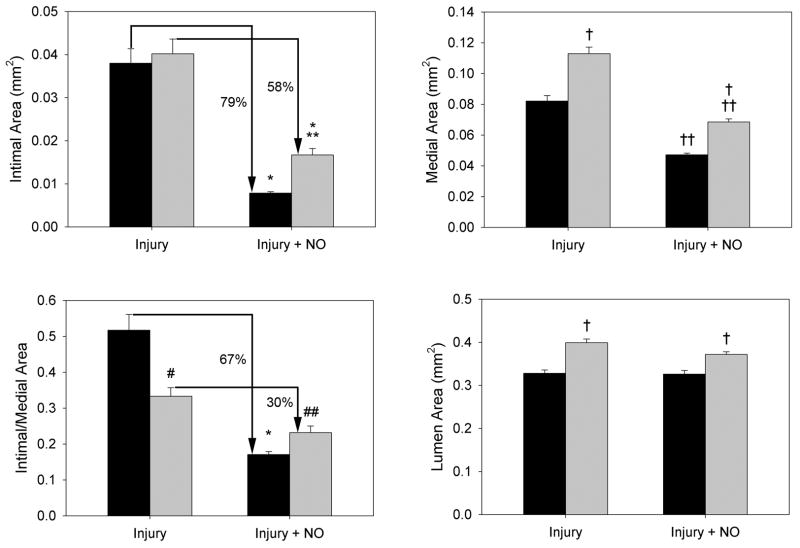
To ascertain the effect of balloon injury and NO on neointimal hyperplasia in the setting of hypertension, H&E-stained carotid artery sections were subjected to morphometric analysis. As shown in Figure 1, NO was more effective at inhibiting neointima formation in WKY rats (79% inhibition) than in the SHR (58% inhibition). Interestingly, while balloon injury induced similar amounts of neointima in the two strains of rats (intimal area 0.038 vs. 0.040 mm2, P=NS), the SHR displayed increased medial hypertrophy (0.113 vs. 0.082 mm2, P<0.001) and lumen area (0.399 vs. 0.328 mm2, P<0.001) compared to the WKY control rats. Although NO inhibited medial area similarly in the SHR and WKY rats, the medial hypertrophy and outward remodeling seen in the SHR in response to injury resulted in greater lumen area. Combined with the greater effect of NO on intimal area in WKY rats, this resulted in a greater effect on the intima/media area ratio in the WKY rats (67% inhibition) compared to the SHR (30% inhibition).
Figure 1. Nitric oxide inhibits neointimal hyperplasia less effectively in hypertensive rats.
(A) Sections of carotid arteries harvested 2 weeks after injury from WKY rats and the SHR were stained with hematoxylin and eosin. Nitric oxide (NO) was more effective at inhibiting neointimal hyperplasia in WKY rats vs. the SHR. Note the outward remodeling displayed in the SHR strain. Treatment groups included control, injury, and injury + NO (n = 7–9/treatment group). (B) Quantitation of intima, media, and lumen area (mm2) was performed using ImageJ. The graphs show that the SHR (gray bars) and WKY rats (black bars) have similar intima formation after injury, but NO was more effective in WKY rats. Quantitation of the media showed that the SHR experience hypertrophy compared to WKY rats, but NO decreased this in both strains. Because of this outward remodeling, the lumen area in the SHR rat injury + NO group was not significantly different from the injury group, and was increased relative to WKY rats. *P<0.001 vs. injury; **P=0.025 WKY vs. SHR, injury+NO; †P<0.001 WKY vs. SHR; ††P<0.001 injury vs. injury+NO; #P<0.001 WKY vs. SHR, injury; ##P=0.009 vs. injury.
3.3 Hypertensive rats display increased proliferation following arterial injury, which is unaffected by NO
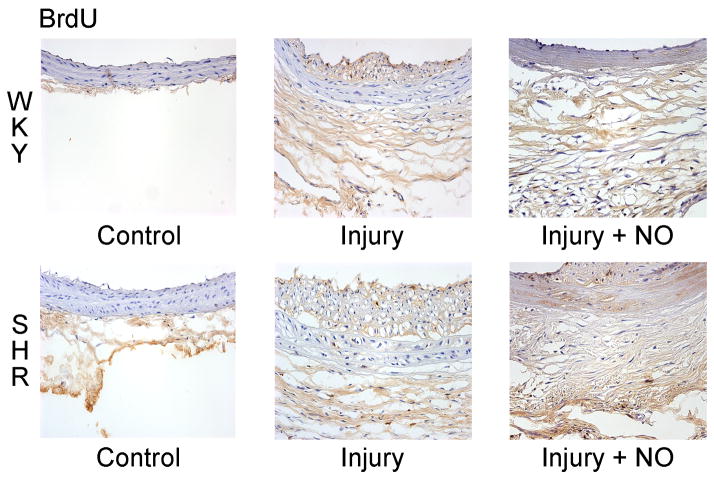
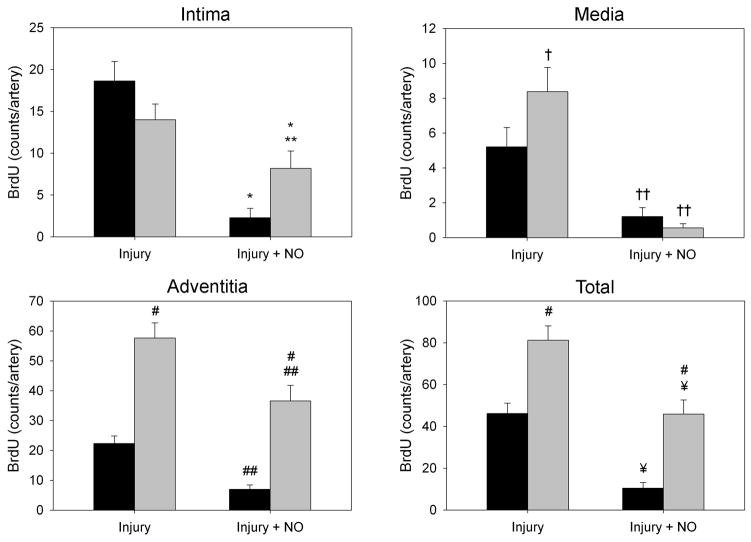
To assess proliferation quantitatively in response to balloon injury in the milieu of hypertension, carotid artery sections were stained for BrdU incorporation. The injured SHR showed a greater level of total BrdU incorporation relative to WKY rats, even in the presence of NO (Figure 2). This was especially evident in the media and adventitia of the injured groups (Figure 2B). While injury caused similar levels of BrdU incorporation in the intima (14.0 vs. 18.6 positive nuclei, P=NS) of both the SHR and WKY rats, NO caused a greater reduction in BrdU incorporation in the intima of WKY rats (2.2 vs. 8.2 positive nuclei, P=0.024, Figure 2B). In the media, injury increased incorporation of BrdU in the SHR (8.4 nuclei) relative to WKY rats (5.2 nuclei), and both rat strains showed significant decreases in medial BrdU incorporation after treatment with NO (1.2 vs. 0.6 positive nuclei, P<0.022), with the SHR strain exhibiting a greater drop (Figure 2B). Most strikingly, in the adventitia, balloon injury caused a 2.5-fold increase in BrdU incorporation in the SHR relative to WKY rats (57.6 vs. 22.3 positive nuclei, P<0.001, Figure 2B). Though treatment with NO caused a significant reduction in BrdU incorporation in the adventitia in both strains, incorporation in the SHR was still 5-fold higher than in WKY controls (36.6 vs. 7.0 positive nuclei, P<0.001, Figure 2B). Total BrdU incorporation assessed across all 3 layers of the vessel wall was almost 2-fold higher in the SHR injury group (81.2 vs. 46.2 positive nuclei, P<0.001), and over 4-fold higher in the injured SHR treated with NO (45.9 vs. 10.5 positive nuclei, P<0.001), relative to WKY rats (Figure 2B).
Figure 2. Hypertensive rats display increased proliferation following arterial injury, which is unaffected by NO.
(A) Carotid sections harvested 2 weeks after injury were stained with antibody against BrdU to assess proliferation. SHR showed more overall staining than WKY rats. (B) Quantitation of staining by counting positive nuclei showed increased proliferation in the intima, media, and adventitia of the SHR (gray bars) following balloon injury. After addition of NO, the SHR showed a significantly higher level of proliferation than WKY rats (black bars), especially in the intima and adventitia. *P<0.03 vs. injury; **P=0.024 WKY vs. SHR, injury+NO; †P<0.022 WKY vs. SHR, injury; ††P<0.01 vs. injury; #P<0.001 WKY vs. SHR; ##P<0.02 vs. injury; ¥P<0.001 vs. injury.
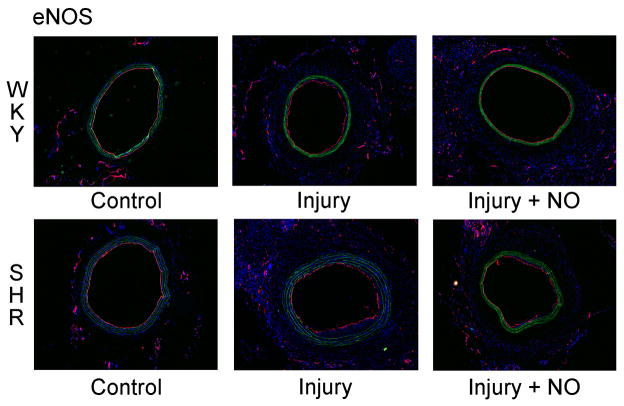
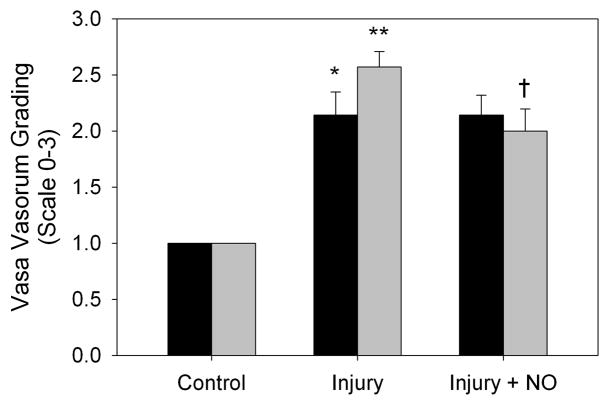
3.4 NO decreases vasa vasorum staining in the adventitia of hypertensive rats
Given the dramatic finding of increased proliferation noted in the adventitia of the SHR following balloon arterial injury, we explored the effect of injury and NO on the vasa vasorum in both animal strains by staining carotid artery sections with an antibody to endothelial nitric oxide synthase (eNOS). Of note, eNOS staining of the lumen was unchanged between uninjured, injury, and injury + NO groups in both the WKY rats and the SHR, indicating complete re-endothelialization (Figure 3A). This was not surprising given that the samples were harvested and evaluated 2 weeks following injury. With respect to the adventitia, compared to uninjured controls, there was an increase in eNOS staining in the adventitia of both WKY rats and the SHR (1.0 vs. 2.0 and 2.5 respectively, Figure 3B). There was a trend toward increased staining following injury in the SHR, but this did not reach statistical significance. Of note, while NO had no effect on eNOS staining in the adventitia of WKY rats, NO decreased eNOS staining by 22% in the SHR (2.6 vs. 2.0, P=0.018, Figure 3B).
Figure 3. NO decreases vasa vasorum staining in the adventitia of hypertensive rats.
(A) Carotid artery sections harvested 2 weeks after injury were stained for eNOS (red) and DAPI (nuclei, blue). Green is autofluorescence of the internal elastic lamina. Note that the entire lumen was re-endothelialized at the 2-week time point in both rat strains. B) Quantitation of vasa vasorum in the adventitia was performed by blinded grading (scale 0–3). An increase in vasa vasorum staining was noted following injury in both strains (black bars = WKY, *P=0.039 vs. control; **P<0.001 vs. control). Addition of NO to the SHR strain (gray bars) decreased vasa vasorum staining (†P=0.018).
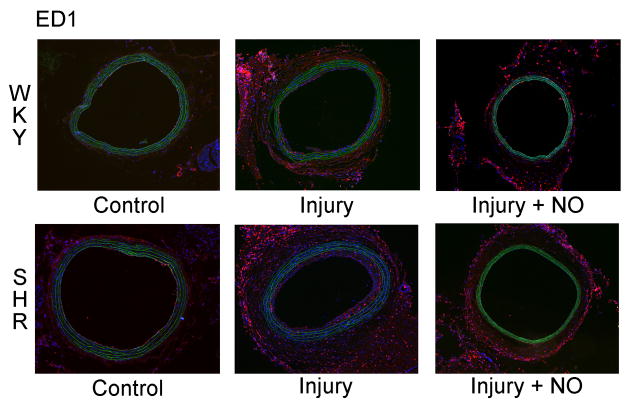
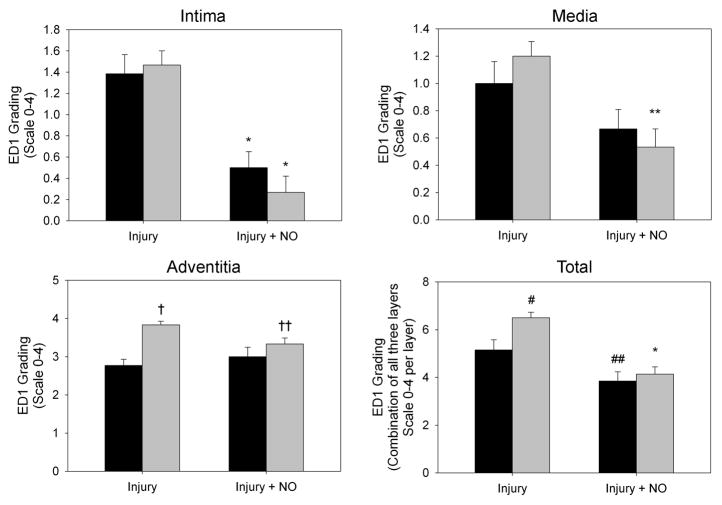
3.5 NO reduces arterial injury-induced macrophage infiltration in hypertensive rats
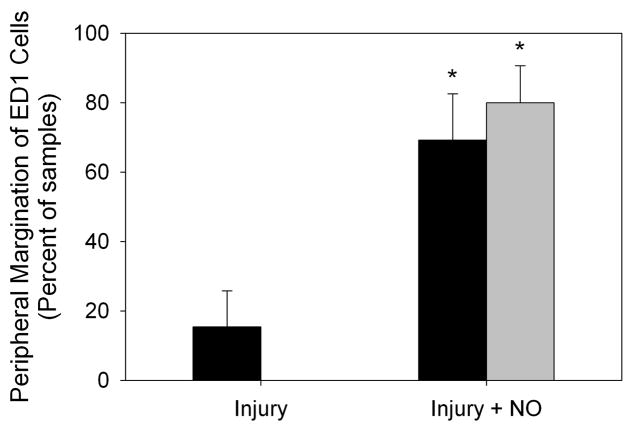
To examine the effects of NO and balloon injury on inflammation in the SHR and WKY rats, immunofluorescent staining for the monocyte/macrophage marker ED1 was performed on carotid artery sections. As seen in Figure 4A, injury caused an overall increase in ED1 staining in the SHR relative to WKY rats. This increase was mainly due to elevated ED1 staining in the adventitia of the SHR following injury, and was reduced back to levels seen in WKY rats with the administration of NO. Blinded grading of sections confirmed that balloon injury caused similar levels of inflammation in the intima of the SHR and WKY rats (1.5 vs. 1.4, P=NS, Figure 4B), with addition of NO significantly inhibiting intimal inflammation in both strains (0.3 and 0.5, respectively, P<0.001 vs. injury, Figure 4B). No difference between the rat strains was seen in medial inflammation after injury, though the SHR had significantly decreased ED1 staining after administration of NO (1.2 vs. 0.5, P<0.004, Figure 4B). There was also a significant reduction in inflammatory infiltrate in the adventitia of the SHR injury + NO group compared to the SHR injury group (3.8 vs. 3.3, P=0.031, Figure 4B). This effect was noticeably absent in the WKY rats (Figure 4B). Finally, and interestingly, both the WKY (15.4% vs. 69.2% of cells) and the SHR (0% vs. 80% of cells) showed a marked increase in peripheral margination of ED1 cells after administration of NO (P<0.001, Figure 4A and 4C).
Figure 4. NO reduces arterial injury-induced macrophage infiltration in hypertensive rats.
(A) To assess macrophage infiltration, carotid artery sections harvested 2 weeks after injury were stained for ED1 (red) and DAPI (blue). Green is autofluorescence of the internal elastic lamina. (B) Quantitation of ED1 staining by blinded grading (scale 0–4) showed that, relative to WKY rats (black bars), a significant increase in macrophage infiltrate was seen in the adventitia of the SHR injury group (gray bars). Throughout the arterial wall of the SHR, administration of NO caused a decrease in macrophage infiltration, most dramatically in the intima and media. *P<0.001 vs. injury; **P<0.004 vs. injury; †P<0.001 WKY vs. SHR; ††P=0.031 vs. injury; #P=0.007 WKY vs. SHR; ##P=0.028 vs. injury. (C) Assessment of the percentage of macrophages displaying peripheral margination indicated significantly higher levels of peripheral margination in the injury + NO group for both rat strains (black bars = WKY, gray bars = SHR). *P<0.001 vs. injury.
3.6 NO decreases levels of soluble guanylyl cyclase (sGC) in normotensive rats
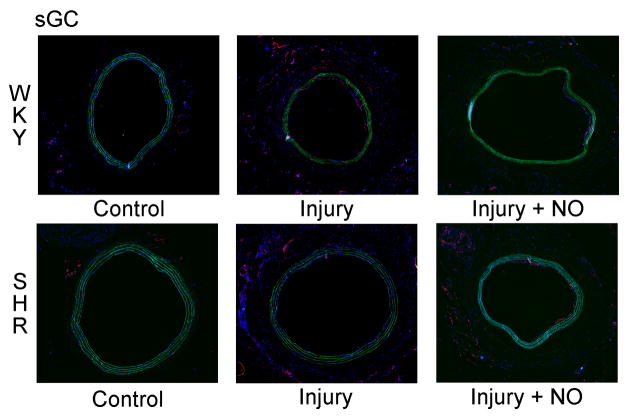
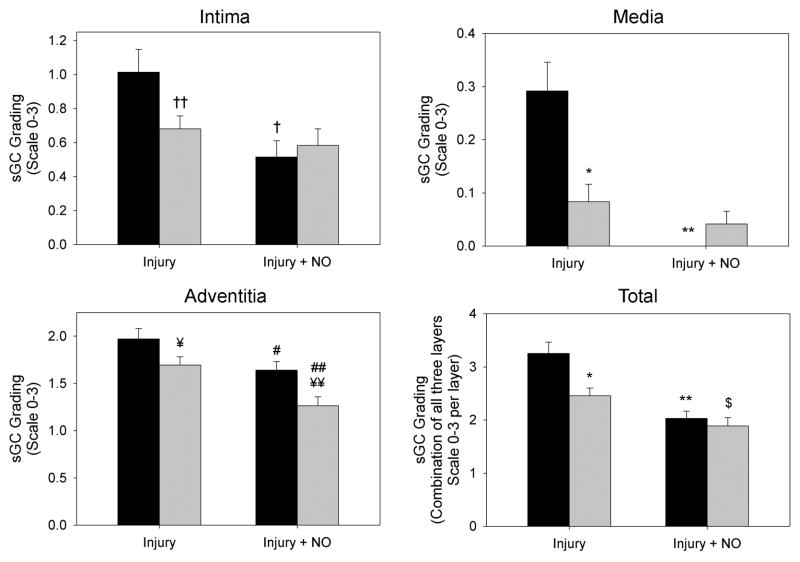
In order to ascertain the effects of NO and injury on the levels of soluble guanylyl cyclase, immunofluorescent staining for the beta 1 subunit was performed on carotid artery sections. Blinded grading of sections showed that balloon injury caused a significant decrease in sGC staining in all 3 layers of the artery in the SHR group relative to WKY rats, but especially in the media (71% decrease, *P<0.001 vs. WKY; Figure 5B). Addition of NO significantly reduced sGC in the adventitia of the SHR (by 25%, ##P=0.004 vs. injury), but not in the intima or media (Figure 5B). While there was a 17% decrease in sGC staining in the adventitia of the SHR injury+NO group (¥¥P=0.006), the total staining score showed no difference with the WKY injury+NO group (P=NS). Interestingly, the WKY injury+NO group showed dramatically decreased levels of sGC, particularly in the intima (49% decrease, †P=0.004 vs. injury) and media (100% decrease, **P<0.001 vs. injury) where levels were below those of the SHR. Total staining scores also showed that SHR had 24% less staining than WKY in the injury group (*P<0.001), and the SHR injury+NO group had 23% less sGC staining vs. injury ($P=0.037), but these differences were normalized by the addition of NO. A stain performed for nitrotyrosine showed no differences in any of the treatment groups (data not shown).
Figure 5. NO decreases levels of soluble guanylyl cyclase (sGC) in normotensive rats.
(A) To assess levels of soluble guanylyl cyclase (sGC), carotid artery sections harvested 2 weeks after injury were stained for sGC (red) and DAPI (blue). Green is autofluorescence of the internal elastic lamina. (B) Quantitation of sGC staining by blinded grading (scale 0–3) showed that, relative to WKY rats (black bars), a significant decrease in sGC staining was seen in all 3 layers of the artery in the SHR injury group (gray bars). Addition of NO significantly reduced sGC staining in the adventitia of the SHR, but not in the intima or media. In WKY, addition of NO significantly reduced sGC staining in all 3 layers, particularly in the media, where it was below levels seen in the SHR. *P<0.001 WKY vs. SHR; **P<0.001 vs. injury; †P=0.004 vs. injury; ††P=0.021 WKY vs. SHR; ¥P=0.038 WKY vs. SHR; ¥¥P=0.006 WKY vs. SHR; #P=0.043 vs. injury; ##P=0.004 vs. injury; $P=0.037 vs. injury.
4. DISCUSSION
Here we show that while balloon injury induced similar amounts of neointimal hyperplasia in the SHR and WKY rat strains, NO was less effective at inhibiting neointimal hyperplasia following arterial injury in the SHR, as assessed by both histology and BrdU incorporation. Furthermore, the SHR was noted to have increased proliferation in the media and adventitia and this corresponded with more medial hypertrophy and outward remodeling following injury compared to the WKY rat. Other interesting observations centered on the adventitia. NO decreased the amount of vasa vasorum present in the adventitia of the SHR compared to WKY rats. Additionally, while balloon injury increased inflammation in both the SHR and WKY rats, the SHR displayed markedly increased inflammation in the adventitia. This inflammation was reduced to the levels observed in WKY rats with the addition of NO. Lastly, while macrophages were increased in the adventitia of the SHR, and to a lesser extent WKY rats, NO induced dramatic margination of the macrophages to the periphery of the adventitia in both strains. Thus, the adventitia appears to have a significant role in the development of neointimal hyperplasia in the hypertensive environment.
Since the development of the SHR from WKY rats [14], much research has been published regarding the characteristics of the strain. While the etiology of hypertension is multifactorial, research has shown that the endothelial dysfunction appears to be secondary to increased production of superoxide from overactive NADH/NADPH [12]. This excess superoxide most likely quenches the NO produced by endothelial cells, thereby limiting the bioavailability of NO and causing hypertension [15]. In fact, studies have elegantly shown that the vasculature from the SHR actually produces approximately 2-fold higher NO levels from eNOS, most likely as a compensatory response to overcome low NO bioavailability [11]. Thus, taking into account the pathophysiology of the SHR, the results of our study make sense. The excess NO we supplied to the arterial wall may also be quenched by superoxide, limiting its bioavailability, and hence limiting the effect on neointimal hyperplasia. Furthermore, superoxide is known to form peroxynitrite upon reaction with NO. It is possible that peroxynitrite formation counters the beneficial effect of NO on the vascular wall.
What remains unclear is where the heightened superoxide is being produced. While vascular smooth muscle cells are likely a source, it is not clear if cells from the adventitia produce excess superoxide in the SHR. Most of the studies examining this pathway were conducted over a decade ago, before the role of the adventitia in the arterial injury response was appreciated. We now know that adventitial fibroblasts, stem cells, pericytes, and inflammatory cells play an important role in the development of neointimal hyperplasia following arterial injury [16]. Knowing this, it is interesting that we did observe key differences in the adventitia between the SHR and WKY rat. In particular, there was a striking increase in BrdU incorporation in the adventitia of the SHR following injury compared to the WKY rats. Even after exposure to NO, BrdU incorporation remained high. We also observed more macrophages in the adventitia of the SHR after injury, and exposure to NO, compared to WKY rats. Both of these findings are curious, given the pathophysiology of the SHR, with high superoxide production and quenching of NO, as well as the known antiproliferative and anti-inflammatory properties of NO. It may be that the adventitia is a greater source of superoxide than the intima and media in the SHR, quenching NO even further, and thereby leading to heightened proliferation and inflammation. This remains to be determined, but is possible given the results we found.
With respect to the development of neointimal hyperplasia following arterial injury, our results differ slightly from published work showing that the SHR develops less neointimal hyperplasia than WKY, Brown Norway, and Sprague Dawley rats [17]. While we found that the intima/media area ratio was less in the SHR compared to WKY rats, intimal area was similar in the two strains following injury. This difference from the literature could be due to the model of arterial injury used. Our model exposes the rat carotid artery to a 2-French balloon catheter inflated to 5 atmospheres of pressure for 5 minutes. This is a very reproducible model, and takes into account the size of the target vessel by using a defined pressure. Previously published work used the iliac artery injury model and created injury by passing an inflated balloon from the aorta to the common femoral artery three times [17]. However, as the balloon was inflated until resistance was encountered, and not to a consistent and reliable pressure, there is some variability with this model.
While we found similar levels of lumenal eNOS staining in the SHR and WKY rats at the 2-week time point, we did encounter an interesting finding in the vasa vasorum. Staining for eNOS was more prominent following arterial injury in the SHR compared to WKY rats. This is consistent with the heightened proliferation, as assessed by BrdU incorporation, we observed in the adventitia. However, exposure to NO reduced the vasa vasorum in the SHR but had no effect on the vasa vasorum in WKY rats. The increase in the vasa vasorum in the SHR could be due to the vascular remodeling observed in the media of the SHR following injury. Furthermore, studies have shown an inverse correlation between the percentage of the wall area that consists of vasa vasorum and the development of neointimal hyperplasia [18]. These later studies have implicated hypoxia from a diminutive vasa vasorum driving proliferation in the development of the heightened neointimal hyperplasia [18; 19]. Thus, we do find it interesting that the SHR developed more vasa vasorum in response to injury. This area of research could benefit from further investigation.
While staining for soluble guanylyl cyclase (sGC) showed that balloon injury caused a significant decrease in sGC staining in all 3 layers of the artery in the SHR group relative to WKY rats, and addition of NO significantly reduced sGC in the adventitia of the SHR, total staining showed no difference with the WKY injury+NO group. Interestingly, the WKY injury+NO group had dramatically decreased levels of sGC, particularly in the intima and media, where levels were below those of the SHR. Since it is known that the SHR have reduced levels of soluble guanylyl cyclase activity, [20; 21] the finding of low sGC in the SHR was not unexpected, but does not explain our finding of decreased neointimal formation in this strain. Given that our SHR were hypertensive throughout the study, even in the presence of NO, perhaps the endothelial dysfunction in this genetically hypertensive strain does not allow for proper response to the stimulatory effects on NO on the sGC pathway. This interesting vein merits further study.
As with any study, ours is not without limitations. First, while the SHR is a model for hypertension, it does not accurately represent the pathophysiology that accounts for human hypertension. Thus, it is unclear if these results will translate to the clinical arena. Second, while our model of external application of a NO donor is simple and reproducible for the study of NO in the vasculature in animal models, it has limited translation to human clinical use. We do envision the use of a NO-releasing perivascular wrap for open vascular and cardiovascular procedures. Our laboratory is working to develop such a therapy; however, this has yet to become a reality and remains in the research realm. Third, we studied the 2-week time point. We felt this was the most appropriate time point to study, as we are interested in the end biological response after arterial injury, namely neointimal hyperplasia. Studies of earlier time points could provide insights into the proliferative and inflammatory response of the arterial wall in the SHR. Yet, we did not feel this justified the use of additional animal lives, given that our overall interest is in the end biological effect of NO on neointimal hyperplasia. Along those same lines, we did not feel it would be fruitful to investigate the effect of proline, a major breakdown product of PROLI/NO, since it is a naturally occurring amino acid. Lastly, since we have previously investigated the effects of the breakdown products of NO (nitrite and nitrate) in the vasculature [22], we did not replicate those experiments here.
Finally, with regards to the amount of NO generated by the short half-life donor PROLI/NO in vivo, we have previously shown that PROLI/NO is more effective at inhibiting neointimal hyperplasia than a donor with a much longer half-life (PAN/NO, t1/2 = >80 days) [6]. We have also recently shown that using a solution of 300 mM PROLI/NO to inflate the balloon catheter for only 5 minutes was effective at inhibiting neointimal hyperplasia two weeks after injury [23]. Our balloon catheter is 5 mm long and the average diameter of the carotid artery is 0.71 mm for WKY rats and 0.98 mm for the SHR [24]. Therefore, the expected injured areas are 11 or 15 mm2, respectively. At rest, the endothelium in the vasculature constitutively releases ~4 pmol/min/mm2 of NO, which equals 5.8 nmol/24hr/mm2 of NO [25]. Ten mg of PROLI/NO can release up to 80 μmoles of NO after being dissolved by the interstitial fluid, yielding 7.2 μmole/mm2 for the WKY rats and 5.2 μmole/mm2 for the SHR. Our previous work showed that 20–80 nmoles of NO diffused through the balloon [23], a thousand fold less than the 80 μmoles PROLI/NO could possibly produce, and this amount was still effective at 2 weeks. However, we must include the caveat that we have not directly measured the amount of NO in vivo, and these calculations are merely a best guess.
In conclusion, we showed that NO inhibited development of neointimal hyperplasia in both the SHR and WKY rats; however, NO was significantly less effective in the SHR. We also found that the SHR developed much more cellular proliferation, inflammation, and vasa vasorum in the adventitia in response to injury, compared to WKY rats. Overall, these data provide insight into the interplay between hypertension and neointimal hyperplasia, NO, inflammation, and vascular remodeling. These data will help us to develop novel NO-based therapies that will be more effective in the hypertensive patient population.
Highlights.
Locally delivered NO does not affect blood pressure in SHR or WKY rats.
NO inhibits injury-induced neointimal hyperplasia less effectively in SHR.
SHR display increased proliferation after injury, which is unaffected by NO.
NO decreases vasa vasorum staining in the adventitia of SHR.
SHR display increased inflammation after injury, which is reduced by NO.
Acknowledgments
The authors would like to express their thanks to Lynnette Dangerfield for her administrative support, and to Edwards Lifesciences for providing the Fogarty balloon catheters.
Sources of Funding: This work was supported in part by funding from the National Institutes of Health (K08HL084203 and T32HL094293), the Department of Veterans Affairs, VA Merit Review Grant I01 BX000409, the Society for Vascular Surgery Foundation, and by the generosity of Mrs. Hilda Rosenbloom and Mrs. Eleanor Baldwin.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomita H, Egashira K, Kubo-Inoue M, Usui M, Koyanagi M, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Inhibition of NO synthesis induces inflammatory changes and monocyte chemoattractant protein-1 expression in rat hearts and vessels. Arterioscler Thromb Vasc Biol. 1998;18:1456–1464. doi: 10.1161/01.atv.18.9.1456. [DOI] [PubMed] [Google Scholar]
- 2.Campeau L, Enjalbert M, Lesperance J, Bourassa MG, Kwiterovich P, Jr, Wacholder S, Sniderman A. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med. 1984;311:1329–1332. doi: 10.1056/NEJM198411223112101. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson JB, Forman MB, Vaughn WK, Robinowitz M, McAllister HA, Virmani R. Morphologic changes in long-term saphenous vein bypass grafts. Chest. 1985;88:341–348. doi: 10.1378/chest.88.3.341. [DOI] [PubMed] [Google Scholar]
- 4.Redwood AJ, Moore S, Sayadelmi L, Tennant M. Autogenous artery grafts in hypertensive (SHR) rats do not have increased smooth muscle cell hyperplasia in the graft neointima, compared with grafts in normotensive rats. J Anat. 1999;195(Pt 3):407–412. doi: 10.1046/j.1469-7580.1999.19530407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, Aalami OO, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Kibbe MR. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med. 2008;44:73–81. doi: 10.1016/j.freeradbiomed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, Jiang Q, Saavedra JE, Keefer LK, Hrabie JA, Kibbe MR. Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;295:H2388–H2398. doi: 10.1152/ajpheart.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varu VN, Ahanchi SS, Hogg ME, Bhikhapurwala HA, Chen A, Popowich DA, Vavra AK, Martinez J, Jiang Q, Saavedra JE, Hrabie JA, Keefer LK, Kibbe MR. Insulin enhances the effect of nitric oxide at inhibiting neointimal hyperplasia in a rat model of type 1 diabetes. Am J Physiol Heart Circ Physiol. 2010;299:H772–H779. doi: 10.1152/ajpheart.01234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogg ME, Varu VN, Vavra AK, Popowich DA, Banerjee MN, Martinez J, Jiang Q, Saavedra JE, Keefer LK, Kibbe MR. Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status. Free Radic Biol Med. 2011;50:1065–1074. doi: 10.1016/j.freeradbiomed.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zicha J, Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev. 1999;79:1227–1282. doi: 10.1152/physrev.1999.79.4.1227. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND, Ni Z, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- 12.Zalba G, Beaumont FJ, San JG, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- 13.Saavedra JE, Southan GJ, Davies KM, Lundell A, Markou C, Hanson SR, Adrie C, Hurford WE, Zapol WM, Keefer LK. Localizing antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J Med Chem. 1996;39:4361–4365. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]
- 14.Okamato K, AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 15.Grunfeld S, Hamilton CA, Mesaros S, McClain SW, Dominiczak AF, Bohr DF, Malinski T. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension. 1995;26:854–857. doi: 10.1161/01.hyp.26.6.854. [DOI] [PubMed] [Google Scholar]
- 16.Havelka GE, Kibbe MR. The vascular adventitia: its role in the arterial injury response. Vasc Endovascular Surg. 2011;45:381–390. doi: 10.1177/1538574411407698. [DOI] [PubMed] [Google Scholar]
- 17.Assadnia S, Rapp JP, Nestor AL, Pringle T, Cerilli GJ, Gunning WT, III, Webb TH, Kligman M, Allison DC. Strain differences in neointimal hyperplasia in the rat. Circ Res. 1999;84:1252–1257. doi: 10.1161/01.res.84.11.1252. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, Edwards WD, Holmes DR, Schwartz RS. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 19.Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT, Baskerville PA, Martin JF. The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis. 1994;105:131–144. doi: 10.1016/0021-9150(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- 21.Ruetten H, Zabel U, Linz W, Schmidt HH. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85:534–541. doi: 10.1161/01.res.85.6.534. [DOI] [PubMed] [Google Scholar]
- 22.Vavra AK, Havelka GE, Martinez J, Lee VR, Fu B, Jiang Q, Keefer LK, Kibbe MR. Insights into the effect of nitric oxide and its metabolites nitrite and nitrate at inhibiting neointimal hyperplasia. Nitric Oxide. 2011;25:22–30. doi: 10.1016/j.niox.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havelka GE, Moreira ES, Rodriguez MP, Tsihlis ND, Wang Z, Martinez J, Hrabie JA, Kiefer LK, Kibbe MR. Nitric oxide delivery via a permeable balloon catheter inhibits neointimal growth after arterial injury. J Surg Res. 2013;180:35–42. doi: 10.1016/j.jss.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein O, Safar ME, Poitevin P, Levy BI. Biaxial mechanical properties of carotid arteries from normotensive and hypertensive rats. Hypertension. 1995;26:15–19. doi: 10.1161/01.hyp.26.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]