Abstract
Background
The metabolic pathways associated with colonic motility are unknown. To identify potential metabolic targets for treatment of constipation, we examined the metabolic profile before and after a meal challenge in a cohort of children with constipation and determined its relationship with postprandial colon motility patterns.
Methods
In this prospective study, 187 metabolites were measured by liquid chromatography–mass spectrometry at multiple time points before and after a standardized meal in constipated children undergoing a colon manometry. Postprandial metabolite levels were compared with baseline and also correlated with multiple manometric measurements, including the number, frequency, and amplitude of pressure peaks as well as the motility index (MI).
Key Results
A total of 20 subjects were included (mean age 13.1 ± 3.4 years). No significant metabolite changes were observed at 10 min after the meal, whereas 16 amino acid and 22 lipid metabolites had significant (P < 0.005) postprandial changes, including decreases in methylhistamine, histamine, and GABA, by 60 min. Correlations were observed between normal and abnormal postprandial motility patterns and changes in specific metabolites, including glycerol, carnosine, alanine, asparagine, cytosine, choline, phosphocholine, thyroxine, and triiodothyronine. Interestingly, subjects without the normal postprandial increase in area under the curve (AUC), had markedly increased levels of kynurenic acid and adenosyl-homocysteine.
Conclusions & Inferences
This is the first study to examine postprandial metabolic changes in children and also to correlate changes in specific metabolites with colonic motility. The results suggest possible metabolic pathways associated with motility and identify potential targets for the treatment of constipation.
Keywords: Children, Colon motility, Constipation, Metabolomic profile
INTRODUCTION
Recent advances in metabolite profiling are allowing simultaneous quantitative detection of large numbers of circulating metabolites in response to specific stimuli, such as glucose challenge,1 exercise,2 or planned myocardial infarction.3 In each of these cases, the results have revealed complex responses and provided insights into metabolic interactions, thereby yielding important information underlying normal physiology and disease pathogenesis. Exercise, for example, was found to induce greater increases in glycerol in fit than in unfit individuals.2 Together with other metabolic profiles identified during exercise, these findings may have predictive value for cardiovascular morbidity. Like exercise, ingestion of food has substantial metabolic effects, including major effects on diverse physiologic functions, including sleep, mood, and colonic motility.4,5Understanding how these effects are mediated at the metabolic level would provide important knowledge on these fundamental physiologic processes and may yield insight into associated disorders. Colon motility is known to be regulated by food ingestion, with the gastrocolic response representing a normal increase in colonic motility in response to food intake. However, the mechanism of this response is not well understood, and the metabolic changes associated with it are entirely unknown. Given the high prevalence of constipation, understanding the factors associated with normal colonic motility and identifying the metabolic abnormalities associated with constipation, could have important implications for designing targeted therapies. The goal of this study was twofold: (1) to examine the metabolic profile in children with constipation following ingestion of a standardized meal containing fixed proportions of fat, carbohydrate, and protein, and (2) to determine whether any relationships exist between those metabolic changes and postprandial colonic motility.
METHODS
To systematically characterize the biochemical response to a standardized meal in children, we obtained plasma samples from a cohort of pediatric subjects with functional constipation scheduled for clinically indicated colonic motility testing. A total of 187 metabolites were quantitatively measured before, and at three time points after, a meal. Metabolite changes identified were then correlated with colon motility measurements.
Patient selection
Children 8–18 years of age scheduled for a clinically indicated colon manometry study at the Gastrointestinal Motility Program at Boston Children’s Hospital for management of intractable constipation were included in this study after obtaining institutional approval and informed consent and assent. All subjects had functional constipation, as defined by Rome III criteria,6,7 and intractable constipation, with failure to respond to conventional therapy, as defined by the North American Society of Pediatric Gastroenterology and Nutrition.8 Subjects with Hirschsprung’s disease, anorectal malformations, previous gastrointestinal surgery, clotting disorders, diabetes, and intolerance to bisacodyl were excluded.
Colonic motility
Colonic motility was performed as previously described.9 Subjects were admitted for IV catheter placement and bowel cleanout with polyethylene glycol solution via nasogastric tube until stools were clear, as per standard protocol for colonic motility studies at our institution. The following morning, all subjects were taken to the endoscopy suite, general anesthesia induced, and a colonoscopy performed for placement of a colon motility catheter.9,10 Subjects were recovered from anesthesia and returned to their room. The following day, following an overnight fast, colonic motility testing was performed.9
Baseline colonic motility was recorded for 1 h (fasting period). Children were then given a standardized meal over a maximum of 10 min. The meal composition was as follows: 1 plain bagel, 8 oz. low-fat fruit yogurt, 1 packet peanut butter, and 8 oz. of water. The total caloric content is 475 kilocalories (77% carbohydrate, 16% protein, and 7% fat). Colon motility was recorded for 1 h after the meal (postprandial period).
Metabolite profiling
As shown in Fig. 1, blood sample #1 was drawn just prior to beginning the meal, and samples #2, #3, and #4 were drawn at 10, 30, and 60 min, respectively, after starting the meal. At each time point, 5 mL of blood was drawn into EDTA-coated tubes, spun, and the plasma immediately frozen at −80 °C. The timing of sample collection was based on previous studies evaluating the gastrocolic response to a meal.10
Figure 1.

Experimental protocol describing the timing of blood sampling.
Metabolomics was performed at Massachusetts General Hospital. We profiled 187 amino acids, biogenic amines, lipids, and other plasma metabolites by liquid chromatography–mass spectrometry (LC-MS) and analyzed as previously described.11,12 Reagents included formic acid, ammonium acetate, LC-MS–grade solvents, valine-d8 (Sigma-Aldrich, St. Louis, MO, USA), and phenylalanine-d8 (Cambridge Isotope Laboratories, Andover, MA, USA). Plasma and media samples were prepared for LC-MS analyses via protein precipitation with the addition of nine volumes of 74.9 : 24.9 : 0.2 vol/vol/vol acetonitrile/methanol/formic acid containing two additional stable isotope-labeled internal standards for valine-d8 and phenylalanine-d8. The samples were centrifuged (15 000 g for 10 min) and the supernatants were injected directly. Metabolic profiling of complex lipids including triacylglycerols, diacylglycerols, and phospholipids was performed as previously described.13
We performed LC-MS metabolic profiling at baseline and at multiple time points for 1 h following the standardized meal. The metabolite platform comprised two distinct methods. The first used targeted analysis to measure 73 distinct metabolite species. Metabolite classes measured included hydrophilic molecules such as amino acids, biogenic amines, purines, and pyrimidines. The second method employed a semitargeted approach to analyze 114 definite complex lipids. Species surveyed included phospholipids, such as phosphatidylethanolamines (PE), phosphatidylcholines (PC) and sphingomyelins (SM), lysophospholipids including lysophosphatidylethanolamines (LPE) and lysophosphatidylcholine (LPC), and the glycerides, diacylglycerols (DAG) and triacylglycerols (TAG). De-identified data were obtained as median (range) metabolite integrated peak areas and entered into an IBM SPSS Statistics database.
Colonic motility measurements
Colonic motility was measured throughout the baseline and postprandial periods and its relationship to metabolite changes characterized. The following five manometry measurements were obtained: (i) number of pressure peaks, (ii) peaks per minute, (iii) mean amplitude of the pressure peaks (mmHg), (iv) area under curve (AUC) of pressure waves, and (v) motility index (MI = Ln (number of contractions × sum amplitudes + 1). An ‘increase’ in any of these parameters was defined as a postprandial change of at least 15% above baseline, reflecting the presence of a colonic motor response to a meal.14,15 Each subject was classified as exhibiting either a postprandial increase or lack of increase for each of these five parameters separately.
Statistical analyses
The median value for each metabolite was calculated at every time point and the percent change from baseline was tested for statistical significance using the non-parametric test Mann–Whitney. For metabolomic analysis, a nominal P-value < 0.005 was considered significant to account for multiple hypothesis testing.11 For colon motility measurements, the median values for each amino acid metabolite were compared between subjects with and without an increase for each of the five motility parameters. Differences with P < 0.05 were considered significant. IBM SPSS Statistics version 19 was used for statistical analyses.
We performed two separate analyses: firstly, we evaluated the changes from baseline of the 187 metabolites at three different points of time after a meal challenge and we also correlated metabolite levels with colon motility measurements.
RESULTS
Postprandial metabolomic profile
A total of 25 subjects were enrolled and 20 completed the study (two patients ate prior to the baseline blood draw, two patients lost IV access during the study, and one patient’s samples clotted). The mean age of the 20 subjects was 13.1 ± 3.4 years and nine (45%) were female.
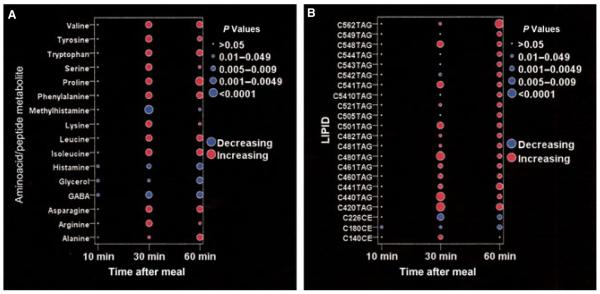
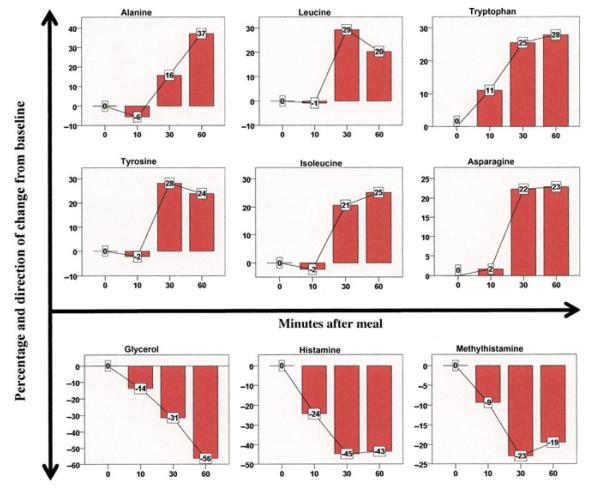
We found no significant metabolite changes at 10 min following the start of the meal. We observed that creatinine and glucose did not change significantly throughout the course of the study. Compared with baseline, 16 aminoacids changed significantly within 60 min of the meal challenge (Fig. 2A). Of those, 11 amino acids increased significantly within 30 min and one (alanine) increasing by 60 min. The remaining four metabolites decreased following the meal. Methylhistamine, histamine, and GABA decreased significantly by 30 min, whereas glycerol showed a significant decrease at 60 min. Figure 3 shows the changes over time in those amino acid metabolites that demonstrated at least a 20% postprandial change compared with baseline.
Figure 2.
Amino acid (A) and lipid (B) metabolites with significant postprandial changes. The metabolites shown exhibited a significant (P < 0.005) change in median concentration at any of the postprandial time points compared with baseline. The figure depicts the level of the metabolite as either increasing (red) or decreasing (blue), with the size of the dot reflecting the level of significance. CE: cholesterol ester; TAG: triacylglycerol; C56:2 refer to a lipid with 56 carbon molecules and 2 double bonds.
Figure 3.
Metabolic changes in amino acids or their metabolites following meal ingestion. Nine metabolites demonstrated a significant postprandial change in plasma level of at least 20% compared with baseline. Data represent the entire cohort of 20 patients. Bars denote percentage change from baseline, lines connect the percentage changes, and the boxes denote the actual percentage change and its direction.
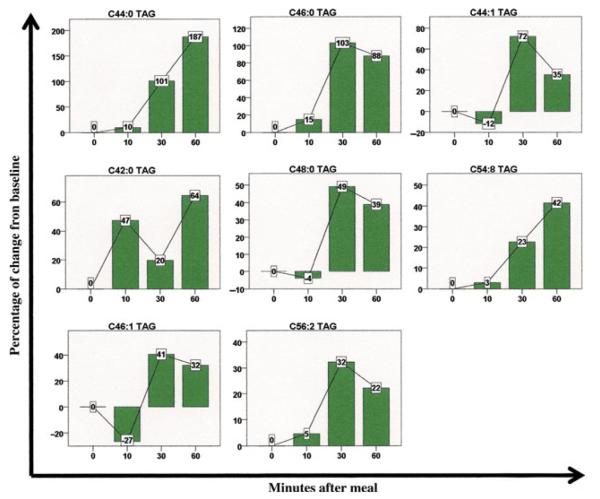
Among the 114 lipids and lipid metabolites analyzed, 22 demonstrated significant postprandial changes compared with baseline (Fig. 2B). Among these, 19 metabolites were triacylglycerols (TAG), all of which increased significantly by the 30- or 60-min time points. Several cholesterol esters also achieved statistically significant changes, with two decreasing and one increasing in concentration (Fig. 2B). Interestingly, many classes of lipids, including DAGs, LPEs, LPCs, PEs, and SM did not demonstrate any significant changes throughout the postprandial period. In Fig. 4, lipid metabolites with at least a 25% postprandial change from baseline are depicted graphically.
Figure 4.
Metabolic changes in lipids after meal ingestion. Eight lipids exhibited a significant postprandial change in plasma level of at least 25% compared with baseline. Data represent the entire cohort of 20 patients. Bars denote percentage change from baseline, lines connect the percentage changes, and the boxes denote the actual percentage change and its direction. TAG, triacylglycerol. C44:1 refers to a lipid chain with 44 carbon molecules and 1 double bond.
Relationship between postprandial colon motility and metabolic changes
A significant postprandial increase in MI was observed in 13 patients. This MI increase occurred at a median of 8.0 ± 5.2 min (range 3–22 min) after starting the meal. We observed no significant association between the median levels of any metabolite and increase in MI. However, those subjects without a postprandial increase in MI (i.e., those with absent gastrocolic response) had significantly higher levels of glycerol at 10 min (P = 0.019) and carnosine at 30 min (P = 0.015), as shown in Table 1.
Table 1.
Relationship between postprandial colon motility parameters and metabolite changes
| Motility parameter (N) | Metabolite | Time point after the meal (min) | Change* (↓ or ↑) | P-value |
|---|---|---|---|---|
| Motility index | ||||
| Increase (13) | None | |||
| No increase (7) | Glycerol | 10 | ↑ | 0.019 |
| Carnosine | 30 | ↑ | 0.015 | |
| Number of peaks | ||||
| Increase (14) | Alanine | 0 | ↑ | 0.03 |
| Alanine | 10 | ↑ | 0.03 | |
| No increase (6) | Glycerol | 10 | ↑ | 0.02 |
| Peak frequency | ||||
| Increase (13) | Alanine | 0 | ↑ | 0.03 |
| Asparagine | 60 | ↑ | 0.04 | |
| Cytosine | 0 | ↑ | 0.015 | |
| Cytosine | 10 | ↑ | 0.01 | |
| Cytosine | 60 | ↑ | 0.015 | |
| No increase (7) | None | |||
| Peak amplitude | ||||
| Increase (5) | Cytosine | 10 | ↑ | 0.03 |
| Choline | 0 | ↑ | 0.03 | |
| Phosphocholine | 30 | ↑ | 0.03 | |
| Thyroxine | 10 | ↑ | 0.03 | |
| Triiodothyronine | 10 | ↑ | 0.03 | |
| No increase (15) | None | |||
| AUC | ||||
| Increase (9) | None | |||
| No increase (11) | 5HIAA | 60 | ↑ | 0.015 |
| Adenosyl-homocysteine | 0 | ↑ | 0.006 | |
| Adenosyl-homocysteine | 10 | ↑ | 0.001 | |
| Adenosyl-homocysteine | 60 | ↑ | 0.04 | |
| Deoxyadenosine | 0 | ↑ | 0.04 | |
| GABA | 30 | ↑ | 0.03 | |
| Histamine | 0 | ↑ | 0.04 | |
| Isoleucine | 30 | ↑ | 0.02 | |
| Kynurenic acid | 0 | ↑ | 0.003 | |
| Kynurenic acid | 10 | ↑ | 0.01 | |
| Kynurenic acid | 30 | ↑ | 0.01 | |
| Kynurenic acid | 60 | ↑ | 0.01 | |
| Triiodothyronine | 10 | ↑ | 0.02 | |
| Valine | 30 | ↑ | 0.02 |
Comparison between patient groups with an ‘increase’ or ‘no increase’ in each of the motility parameters listed. Time point 0 corresponds to the baseline, before meal was ingested.
In addition to MI, we characterized the number, frequency, and amplitude of colonic pressure peaks detected during the baseline and postprandial periods for each subject. We observed a significant increase in number, frequency, and amplitude of colonic pressure peaks in 14, 13, and 5 subjects, respectively. Subjects were separated into those with a normal postprandial increase in these parameters and those with no change. Metabolite levels in each group were compared to identify significant correlations. The results are summarized on Table 1. Interestingly, subjects with the expected postprandial increase in the frequency and amplitude of colon pressure peaks exhibited significant increases in several metabolites, whereas those with no change postprandially had no significant associated metabolic features.
We observed a postprandial increase in AUC in nine patients (Table 1). The only metabolite associated with an increase in AUC was a higher level of valine 30 min after the meal. Subjects without a postprandial AUC increase had significantly higher median levels of nine metabolites at one or more time points (Fig. 5). Kynurenic acid was significantly increased at all time points, and adenosyl-homocysteine was increased at baseline, 10, and 60 min.
Figure 5.
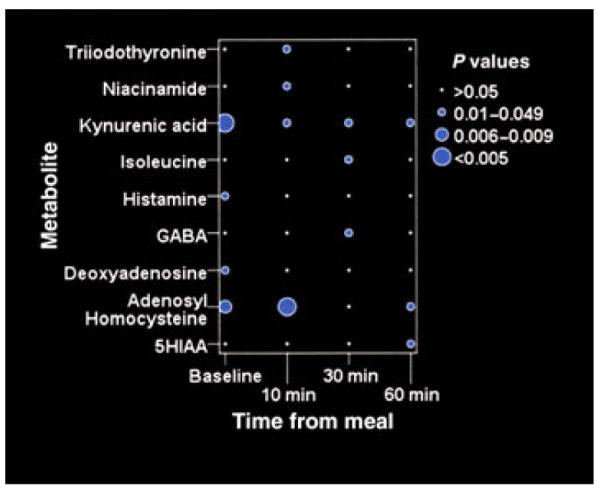
Metabolic changes associated with abnormal postprandial AUC. The area under the curve of the pressure waves (AUC) was calculated by colonic manometry. Subjects who did not exhibit a normal increase in postprandial AUC had significantly higher median levels of the nine metabolites shown. Kynurenic acid and adenosylhomocysteine levels are significantly higher in all samples except for adenosyl-homocysteine at 30 min.
DISCUSSION
Using a mass spectrometry-based metabolite profiling platform, we identified a panel of amino acids, peptides, and lipids that change significantly following a standardized meal. Postprandial metabolomics have previously been characterized following a variety of foods,16–20 and it is clear that different diets elicit different metabolic responses. We were specifically interested in characterizing the metabolic changes associated with this standardized meal composition in children as it is routinely administered at our institution during pediatric colonic manometry studies. These results represent the first characterization of the postprandial metabolic response in children, and also this is the only study to date that characterizes the association between postprandial metabolic changes and colonic motor activity.
The timing and magnitude of postprandial metabolic changes depend on many factors, including gastrointestinal motility, luminal digestion, mucosal absorption, tissue uptake, and metabolite breakdown. In our amino acid analysis, no metabolite demonstrated a significant change in concentration at 10 min, whereas 13 metabolites changed significantly by 30 min. This timing is consistent with recent studies in adults showing that the earliest increases in plasma amino acid levels following a glucose challenge occurred at 15 min,20 or at 30 min after eating bread.16 Ashley et al.18 analyzed the amino acid response to a 20% protein meal, similar to our standardized diet, and found that certain essential amino acids rose rapidly within 1 h, including leucine, isoleucine, valine, phenylalanine, and tryptophan. We also found significant increases within 1 h in these amino acids, as well as the basic amino acids, arginine and lysine. Among the non-essential amino acids, others have described alanine as exhibiting the greatest postprandial increase,18 a finding consistent with our results showing a 37% increase in alanine at 1 h.
Dietary tryptophan may be an important amino acid with respect to intestinal function as it is the precursor of serotonin, which mediates effects not only on sleep and appetite but also on neural reflexes that regulate gut motility, secretion, and sensation.21 Tryptophan increased significantly at 30 and 60 min following the meal, although its metabolites, 5-HIAA and serotonin, did not.
The significant decreases in postprandial histamine, methylhistamine, and GABA are intriguing. Histamine regulates multiple intestinal functions, including gastrointestinal motility and mucosal secretion, and its receptors are expressed throughout the bowel, in the epithelium, muscle, enteric nervous system, and immune cells.22 Dysregulated histamine activity, with significantly elevated receptor levels, has been identified in patients with food allergy and irritable bowel syndrome.22 Therefore, the normal postprandial decrease in histamine and its major metabolite, methylhistamine, may serve to regulate diet-induced effects on motility, secretion, or immune function, but the functional implications need to be further studied. Importantly, histamine is rapidly metabolized after its release, and therefore our findings may not reflect local histamine levels and should be interpreted cautiously. GABA, a major inhibitory neurotransmitter in the brain, is known to mediate gastrointestinal functions relevant following a meal, including regulating relaxation of the lower esophageal sphincter (LES), gastric accommodation, and duodenal tone.23 Animal studies have shown that GABA stimulation results in decreased colonic motor activity,24 whereas GABA blockade results in increased colonic motility,25 although whether this affects propagating activity remains unknown.26 The postprandial decrease in GABA may therefore play a role in regulating colonic motor activity after a meal. Although we did not observe an association between GABA levels and MI, we did observe a higher level of GABA in subjects lacking an increase in AUC after a meal, supporting this potential role. Functional studies are needed to define further the role of GABA following food intake.
The analysis of metabolic changes and their relationship to colonic motor function yielded interesting and unexpected results. Importantly, the gastrocolic response, defined as a postprandial increase of at least 15% in the MI, occurred at a median of 8.0 ± 5.2 min (range 3–22 min) after starting the meal. This is interesting in light of the fact that we did not observe a significant change of any metabolite in the first 10 min after the meal. This may suggest that the initiation of the gastrocolic response may not be metabolically mediated, but rather neurally controlled, perhaps by the vagus nerve, consistent with the observation that electrical stimulation of the vagus in rats elicits contractions throughout the colon.27 However, we cannot exclude the possibility that local metabolite levels may have changed more quickly than serum levels, or that other metabolites or neuropeptides not analyzed may be responsible for the reflex. Analysis of plasma metabolomic profiles in this study is therefore not able to reveal the mechanisms responsible for initiating the gastrocolic response.
Children that showed no significant change between baseline and postprandial AUC exhibited multiple metabolic differences when compared with those children that had an increased postprandial AUC. The AUC is a useful indicator of colon motor activity, as it corresponds to the sum of the area of all pressure waves during the period of measurement. Absence of a postprandial increase in AUC is indicative of abnormal colon motility. The children with no increase in postprandial AUC had significantly higher levels of triiodothyronine, niacinamide, kynurenic acid, leucine, histamine, GABA, deoxyadenosine, adenosylhomocysteine, and 5HIAA, leading us to hypothesize that these metabolites represent potential candidates for mediating postprandial colonic motility. Kynurenic acid levels were significantly increased at all time points in subjects without a postprandial AUC increase. Kynurenic acid, a metabolite of tryptophan, is an antagonist of N-methyl-D-aspartate (NMDA) glutamate receptors and has been shown to decrease colonic motility in rats28 and dogs.29 Elevated levels of this metabolite in subjects with manometric evidence of colon dysmotility suggest a potential causative role that merits further investigation.
This study represents the first analysis of postprandial metabolomics in children and also the first attempt to correlate postprandial metabolic changes with colonic motor function. Our results are consistent with several prior studies in both the timing and pattern of amino acid changes following a meal. Our results also reveal new and potentially important changes in the postprandial metabolomic profile. The significant decreases in histamine and GABA may represent important postprandial functions for these mediators worthy of further study. We also identified multiple metabolites whose postprandial levels were markedly different in children with normal and abnormal colonic motility. Our study is limited by the small number of subjects and by the specific metabolites analyzed. Future work needs to include a larger number of subjects with normal and abnormal motility, and also a larger variety of metabolites, including intestinal neuropeptides and neurotransmitters. Results from these studies will yield important information on the mechanisms underlying colonic motor activity and could have a major impact on the management of patients with colonic dysmotility.
Acknowledgments
FUNDING This study was funded by a Harvard Clinical and Translational Center Award (UL1 RR025758-01).
REFERENCES
- 1.Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33–37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis GD, Wei R, Liu E, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–12. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snape WJ, Jr, Wright SH, Battle WM, Cohen S. The gastrocolic response: evidence for a neural mechanism. Gastroenterology. 1979;77:1235–40. [PubMed] [Google Scholar]
- 5.Wright SH, Snape WJ, Jr, Battle W, Cohen S, London RL. Effect of dietary components on gastrocolonic response. Am J Physiol. 1980;238:G228–32. doi: 10.1152/ajpgi.1980.238.3.G228. [DOI] [PubMed] [Google Scholar]
- 6.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130:1519–26. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 7.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–37. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker S, Liptak G, Colletti R, et al. Evaluation and treatment of constipation in infants and children recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43:e1–13. doi: 10.1097/01.mpg.0000233159.97667.c3. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo C, Flores AF, Reddy SN, Hyman PE. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr. 1992;120:690–5. doi: 10.1016/s0022-3476(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 10.Hamid SA, Di Lorenzo C, Reddy SN, Flores AF, Hyman PE. Bisacodyl and high-amplitude-propagating colonic contractions in children. J Pediatr Gastroenterol Nutr. 1998;27:398–402. doi: 10.1097/00005176-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted metabolomics. Curr Protoc Mol Biol. 2012 doi: 10.1002/0471142727.mb3002s98. Chapter 30: Unit 30.2.1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–11. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von der Ohe MR, Hanson RB, Camilleri M. Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut. 1994;35:536–41. doi: 10.1136/gut.35.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camilleri M, Bharucha AE, di Lore-nzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–82. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 16.Bondia-Pons I, Nordlund E, Mattila I, et al. Postprandial differences in the plasma metabolome of healthy Finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr J. 2011;10:116. doi: 10.1186/1475-2891-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellis L, van Erk MJ, van Ommen B, et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics. 2012;8:347–59. doi: 10.1007/s11306-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashley DV, Barclay DV, Chauffard FA, Moennoz D, Leathwood PD. Plasma amino acid responses in humans to evening meals of differing nutritional composition. Am J Clin Nutr. 1982;36:143–53. doi: 10.1093/ajcn/36.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Tessari P, Kiwanuka E, Zanetti M, Barazzoni R. Postprandial body protein synthesis and amino acid catabolism measured with leucine and phenylalanine-tyrosine tracers. Am J Physiol Endocrinol Metab. 2003;284:E1037–42. doi: 10.1152/ajpendo.00416.2002. [DOI] [PubMed] [Google Scholar]
- 20.Skurk T, Rubio-Aliaga I, Stamfort A, Hauner H, Daniel H. New metabolic interdependencies revealed by plasma metabolite profiling after two dietary challenges. Metabolomics. 2011;7:388–99. [Google Scholar]
- 21.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–88. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 22.Sander LE, Lorentz A, Sellge G, et al. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504. doi: 10.1136/gut.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smid SD, Young RL, Cooper NJ, Blackshaw LA. GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1494–501. doi: 10.1152/ajpgi.2001.281.6.G1494. [DOI] [PubMed] [Google Scholar]
- 24.Ishizawa M. Effects of GABA and homotaurine on the colonic motility of the guinea pig. Nihon Heikatsukin Gakkai Zasshi. 1987;23:441–7. doi: 10.1540/jsmr1965.23.441. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood B, DiMicco JA. Activation of the hypothalamic dorsomedial nucleus stimulates intestinal motility in rats. Am J Physiol. 1995;268(3 Pt 1):G514–21. doi: 10.1152/ajpgi.1995.268.3.G514. [DOI] [PubMed] [Google Scholar]
- 26.Tonini M, Crema A, Frigo GM, et al. An in vitro study of the relationship between GABA receptor function and propulsive motility in the distal colon of the rabbit. Br J Pharmacol. 1989;98:1109–18. doi: 10.1111/j.1476-5381.1989.tb12654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil. 2010;22:688–93. doi: 10.1111/j.1365-2982.2009.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga G, Erces D, Fazekas B, et al. N-Methyl-D-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol Motil. 2010;22:217–25. e268. doi: 10.1111/j.1365-2982.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaszaki J, Palasthy Z, Erczes D, et al. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol Motil. 2008;20:53–62. doi: 10.1111/j.1365-2982.2007.00989.x. [DOI] [PubMed] [Google Scholar]