Abstract
Background
Antegrade continence enema (ACE) has become an important therapeutic modality in the treatment of intractable constipation and fecal incontinence. There are little data available on the long-term performance of the ACE procedure in children.
Methods
A retrospective review of patients who underwent the ACE procedure was conducted. Irrigation characteristics and complications were noted. Outcome was assessed for individual encounters based on frequency of bowel movements, incontinence, pain, and predictability.
Results
One hundred seventeen patients underwent an ACE. One hundred five patients had at least 6 months of follow-up, and were included in the analysis. Diagnoses included myelodysplasia (39%), functional intractable constipation (26%), anorectal malformations (21%), nonrelaxing internal anal sphincter (7%), cerebral palsy (3%), and other diagnoses (4%). The average follow-up was 68 months (range 7–178 months). At the last follow-up, 69% of patients had successful bowel management. Of the 31% of patients who did not have successful bowel management, 20% were using the ACE despite suboptimal results, 10% required surgical removal, and 2% were not using the ACE because of behavioral opposition to it. Patients were started on normal saline, but were switched to GoLYTELY (PEG-3350 and electrolyte solution) if there was an inadequate response (61% at final encounter). Additives were needed in 34% of patients. The average irrigation dose was 23 ± 0.7mL/kg. The average toilet sitting time was 51.7 ± 3.5minutes, with infusions running for 12.1 ± 1.2minutes. Stomal complications occurred in 63% (infection, leakage, and stenosis) of patients, 33% required surgical revision and 6% eventually required diverting ostomies.
Conclusions
Long-term use of the ACE gives successful results in 69% of patients, whereas 63% had a stoma-related complication and 33% required surgical revision of the stoma.
Keywords: anorectal malformation, antegrade continence enema, constipation, defecation disorder, fecal incontinence, myelodysplasia, nonrelaxing internal anal sphincter
The antegrade continence enema (ACE) is a surgically constructed conduit into the colon that allows the administration of antegrade irrigations. The ACE has become a mainstay in the treatment of refractory constipation and fecal incontinence in many centers since it was first described by Malone et al in 1990 (1). The ACE appeals to patients because it may allow them to avoid colostomy, lead to more predictability in fecal continence, and increase patient autonomy in their bowel management. Previous studies have shown that the ACE procedure is safe and effective in the treatment of children with defecation abnormalities (2–6). There is limited information about the complications related to the ACE (6–9), quality of life in patients who have received an ACE (2,4,5,10,11), and functional outcomes of patients after the ACE (6,11–14). The long-term outcomes are rarely described, and most studies do not include a wide variety of patients.
The aim of the present study was to present our center's long-term multidisciplinary experience with the use of an ACE in a diverse group of children with bowel movement difficulties.
Methods
The present study was approved by the institutional review board at Children's Hospital Boston. We reviewed the charts of 117 patients who had undergone the ACE at Children's Hospital Boston, or received their care there, since 1995. Characteristics of patients before the ACE placement, including the bowel management program, were obtained. The indication for the ACE and characteristics of the surgical procedure were recorded.
Each follow-up visit in the electronic medical record (EMR) of our hospital was reviewed. These visits included clinical encounters from multiple specialties, in particular, gastroenterology, urology, and surgery. In our institution, the medical management of the ACE is done by the gastroenterology department that gives support to the surgical departments. Of the encounters identified, 1169 were related to ACE management. Data on functional outcome, irrigation characteristics, and complications were analyzed for each encounter of each patient. Of these, 880 (75%) encounters had enough data to assign ACE performance grades to them.
Outcome analysis was done with only those 105 patients who had more than 6 months of follow-up. In the analysis of complications, we included all 117 patients to accurately capture the perioperative events.
Functional Outcomes
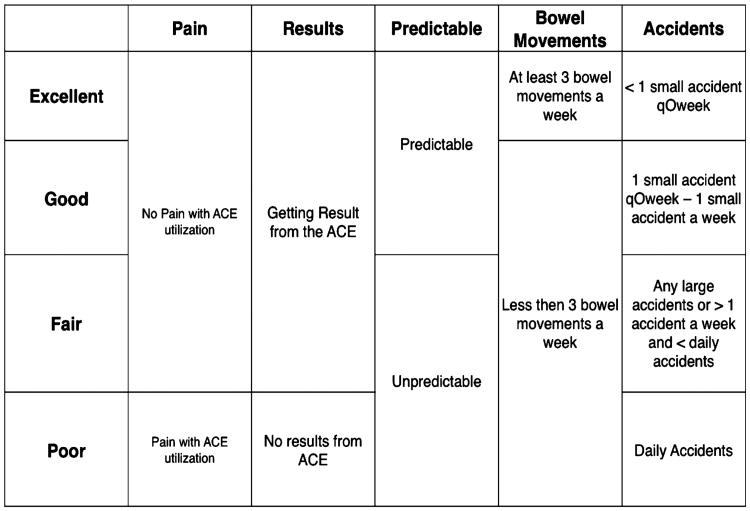
ACE performance was graded for each encounter, based on a classification scheme that was devised a priori by the whole research team (excellent, good, fair, and poor) (Fig. 1). We considered bowel management successful if it was graded as “excellent” or “good” and unsuccessful if it was graded as “fair” or “poor.” In addition, patients no longer using the ACE while doing clinically well were considered successful. Those who had the ACE closed or were no longer using it while doing poorly were considered unsuccessful.
Figure 1.
ACE assessment scheme. Each domain is assessed from left to right. For example, a patient with pain cannot be better than “poor.” A patient who is unpredictable cannot be better than “fair.” If the patients had stoma closure because of lack of response, then they were considered in the “poor” category. ACE = antegrade continence enema.
We considered patients as having experienced their first relapse when an encounter was graded as fair or poor after having achieved a previous assessment of good or excellent.
Irrigation Characteristics
We analyzed the type of solution, irrigation dose, use of additives, frequency of use, and the duration of the infusion and of toilet sitting (time needed for complete defecation). Comparison of success rates between groups was done to compensate for any bias encountered by the uneven frequency of visits among diagnostic groups.
Complications
All 117 patients were included in our analysis of complications. We focused on 7 domains: perioperative complications, the need for revision, stomal infection, stomal stenosis (tightening), stomal leakage, peritonitis, and stomal closure. Overall rates were analyzed by underlying diagnosis and type of ACE performed.
Statistical Analysis
Data are reported as mean ± standard error of the mean, except for patient characteristics that are reported as standard deviations. Functional outcomes are reported at the latest follow-up visit for all of the patients. When available, functional outcomes were also performed at baseline and compared with the last follow-up. Analysis of baseline and final assessments were done with McNemar test when comparing the rates of successful bowel management, and with paired t test when comparing the degree of change. Other analyses were done using the χ2 test, Student t test, or Wilcoxon rank sum test, depending on whether the variables were parametric or nonparametric. A P value of <0.05 was considered statistically significant. All of the analyses were performed using SPSS version 16.0 (SPSS, Inc, Chicago, IL).
Results
Patient Characteristics
An ACE was performed in 117 patients with the following diagnoses: myelomeningocele in 46 patients, functional intractable constipation in 30 (those patients with constipation without an underlying organic pathology and who were refractory to at least 3 months of intensive medical management based on the administration of oral osmotic and stimulant laxatives and rectal interventions in our tertiary center), anorectal malformations in 24 (22 with high malformations and 2 with low malformations), nonrelaxing internal anal sphincter (IAS) in 8 (internal anal sphincter achalasia in 5 and postoperative Hirschsprung disease in 3), cerebral palsy in 4, and other diagnoses in 5 (cervical spinal cord injury in 3, cystic fibrosis in 1, and mitochondrial disease in 1).
Table 1 shows the main characteristics of only those 105 patients who had more than 6 months of follow-up. The mean follow-up time was 68 months (range 7–178 months, median 61 months).
Table 1. Characteristics of patients with more than 6 months of follow-up (n = 105).
| Sex | |
| Male | 46 |
| Female | 59 |
| Age at placement | |
| Mean | 11.2 ± 5 years |
| Median | 11.1 |
| Diagnosis | |
| Myelodysplasia | 40 |
| Functional intractable constipation | 27 |
| Anorectal malformations | 22 |
| Nonrelaxing IAS | 7 |
| Cerebral palsy | 4 |
| Other | 5 |
| Presenting symptom | |
| Constipation | 38 |
| Fecal incontinence | 21 |
| Mixed constipation and incontinence | 46 |
| Department | |
| General surgery | 62 |
| Urology | 29 |
| Gastroenterology | 10 |
| Outside hospital | 4 |
| Procedure | |
| Appendicostomy | 90 |
| Retubularized intestine ACE | 3 |
| Surgical cecostomy | 1 |
| Laparoscopic appendicostomy | 1 |
| Percutaneous | 10 |
| Vesicostomy + CE | |
| Appendicostomy + appendicovesicostomy | 10 |
| Appendicostomy + Monti Vesicostomy | 6 |
| Appendicostomy + other Vesicostomy | 2 |
| Retubularized ACE + appendicovesicostomy | 1 |
| Lap appendicostomy + Monti Vesicostomy | 1 |
ACE = antegrade continence enema; IAS = internal anal sphincter.
As can be seen in Table 1, most of the procedures were performed by the general surgery department, followed by the urology and gastroenterology departments. Most procedures were performed by the authors. SF performed 68% of the procedures done by general surgery, SB 76% of those done by urology, and SN 100% of those performed by gastroenterology.
Functional Outcomes
Successful bowel management was achieved in 72 of 105 (69%) patients at their latest follow-up. Six (6%) of these patients no longer needed their stomas and had normal bowel movements. Of these patients, 3 had functional intractable constipation, 2 had a nonrelaxing IAS, and 1 had cerebral palsy.
Thirty-three of 105 (31%) patients were considered unsuccessful. Of these patients, 21 (20%) were still using the ACE unsuccessfully at the last follow-up, 10 (10%) had stomal closure due to poor outcome, and 2 (2%) were not using the stoma because of behavioral opposition to it. Of the 10 patients who had the stoma closed, 6 (6% of 105) were transitioned to ostomies (4 to ileostomies and 2 to colostomies). Four (4% of 105) did not like the results of the ACE and requested its removal, and they continued to have bowel management problems.
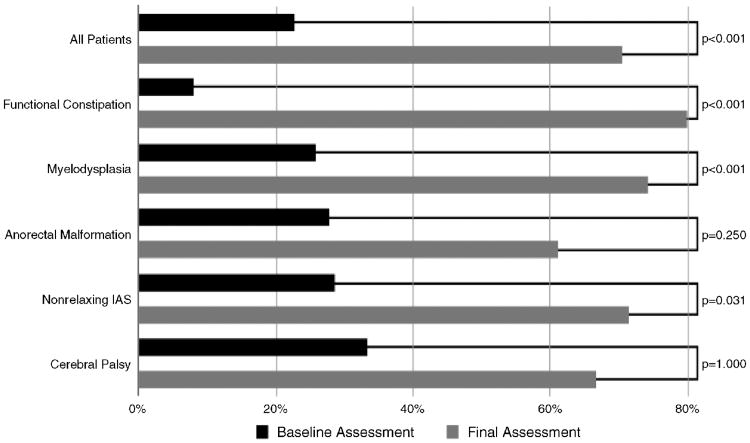
The success rates among the different diagnostic categories can be seen in Figure 2. There was no difference found when comparing the outcome among groups (P = 0.165). In addition, there was no difference in the rates of success when comparing age, sex, or procedure type.
Figure 2.
Percentage of successful management before and after the antegrade continence enema. There was significant improvement in the overall population, the functional intractable constipation, myelodysplasia, nonrelaxing IAS, and anorectal malformations group. IAS = internal anal sphincter.
Comparison Between Baseline and Final Assessment
Figure 2 shows a comparison between baseline and latest assessment for those 88 patients for whom both a baseline and final assessment could be determined. Before the ACE placement, 20 (23%) patients were successful and 68 (77%) were unsuccessful with their bowel management program (P < 0.05). At the last follow-up, 62 (71%) were successful and 26 (29%) were unsuccessful (P < 0.05).
Figure 2 also shows the response based on etiology. There was a significant difference comparing baseline versus final assessment in those with functional intractable constipation, myelodysplasia, and nonrelaxing IAS (P < 0.05).
Successful continence with medical therapy before the ACE placement was a predictor of success with ACE irrigations. The ACE was performed in 20 children who were successful with their bowel management program before their ACE. The indication for placement was to increase patient autonomy and independence. Of these, 16 (80%) were successful with bowel management at the final follow-up. Of the 68 with unsuccessful bowel management programs before the ACE placement, 46 (68%) were successful at the last follow-up (P < 0.001).
Irrigation Characteristics
The main irrigation characteristics are shown in Table 2.
Table 2. Irrigation characteristics.
| Irrigation characteristic | All patients (n = 87) | Successful (n = 66) | Unsuccessful (n = 21) | Significance |
|---|---|---|---|---|
| Solution | GoLYTELY: 53 (61%) | GoLYTELY: 40 (61%) | GoLYTELY: 13 (62%) | P = 0.078 |
| NS: 27 (31%) | NS: 22 (33%) | NS: 5 (24%) | ||
| Other: 7 (8%) | Other: 4 (6%) | Other: 3 (15%) | ||
| Irrigation volume | 847 ± 55 mL | 845 ± 68 mL | 852 ± 82 mL | P = 0.951 |
| 23 ± 0.7 mL/kg | 25 ± 0.8 mL/kg | 23.2 ± 1.4 mL/kg | P = 0.257 | |
| Irrigation frequency | 5 ± 0.3 d/wk | 5 ± 0.3 d/wk | 4 ± 0.7 d/wk | P = 0.208 |
| Infusion time | 12.1 ± 1.2 min | 10.6 ± 0.8 min | 16.1 ± 4.0 min | P = 0.045 |
| Total toilet time | 51.7 ± 3.5 min | 47.8 ± 3.5 min | 63.0 ± 9.3 min | P = 0.063 |
| Additives used | None: 57 (66%) | None: 42 (64%) | None: 15 (71%) | P = 0.562 |
| Bisacodyl: 24 (28%) | Bisacodyl: 20 (30%) | Bisacodyl: 4 (19%) | ||
| Other: 6 (7%) | Other: 4 (6%) | Other: 2 (10%) |
NS = normal saline.
Irrigation Composition
Irrigations were started with normal saline (NS) and then transitioned to GoLYTELY (PEG-3350 and electrolyte solution, Braintree Laboratories, Braintree, MA) if unsuccessful. Of the 87 patients who were still using the ACE on the final assessment, 53 (61%) were taking GoLYTELY and 27 (31%) were taking NS. An additional 5 patients (6%) were using tap water and 2 (2%) were using other solutions. GoLYTELY produced a successful response in 40 of the 53 patients (75%) who were transitioned to it after the NS failed.
When comparing solutions used among groups, we found that proportionately fewer myelodysplasia patients had been transitioned to GoLYTELY at the latest follow-up (P = 0.022). Fifteen of 37 myelodysplasia patients (41%) were taking GoLYTELY. This compares with 17 of 20 (85%) with functional intractable constipation, 13 of 20 (65%) in the anorectal malformations group, 3 of 3 (100%) in the nonrelaxing IAS group, 2 of 3 (67%) in the cerebral palsy group, and 3 of 4 (75%) in the “miscellaneous” group.
Additives were used only when the patients' bowel management was unsuccessful. Thirty (34%) patients used additives at the final assessment, 24 (28%) used bisacodyl, 4 (5%) glycerin, 1(1%) phosphosoda, and 1 (1%) magnesium citrate. Twenty-four of the 30 patients (80%) taking additives were being managed successfully.
Infusion Dynamics
At the final follow-up visit, the mean irrigation dose was 847±55mL (23 ±0.7 mL/kg). Mean irrigation frequency was 5 ± 0.3 days/week. Once-daily irrigations were most common, performed by 31 patients (36%), whereas 20 patients (23%) received irrigations every other day. Two patients (2%) did twice-daily irrigation, 5 (6%) were getting irrigations every third day, 6 (7%) were using daily irrigations with 1 or 2 days off per week, 14 (16%) were instilling irrigations with 3 to 6 days off per week, and 9 (10%) were using irrigations less than once per week. Mean infusion time was 12.1 ± 1.2 minutes (range 1–90 min), and mean toilet sitting time was 51.7 ± 3.5 minutes (range 10–180 min). Patients with successful ACE regimens had no statistical difference when compared with unsuccessful patients in terms of dose and frequency. Successful patients had significantly shorter infusion times than unsuccessful patients, and although not statistically significant, a similar trend was noted in total toilet time (Table 2).
Time to First Relapse
There were 98 patients (93%) who achieved initial success during their follow-up. Sixty-seven patients had a subsequent relapse (64%).
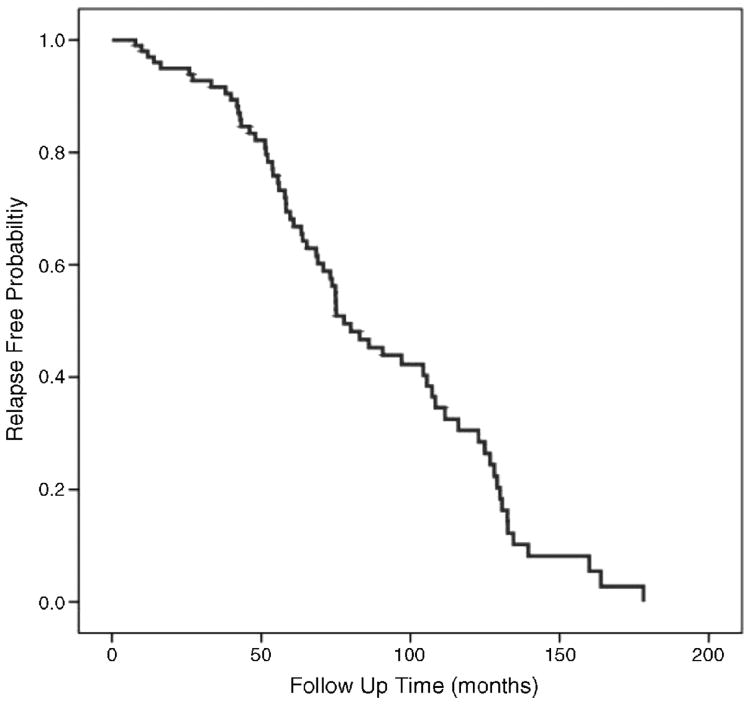
Mean time to first relapse was 88.4 ± 4.9 months. Figure 3 demonstrates the time to first relapse in the entire cohort. Stratifying by diagnosis, the mean time to relapse was 59.4 ± 7.7 months in the functional intractable constipation group, 98.8 ± 8.3 months in the myelodysplasia group, 91.1 ± 8.9 months in the anorectal malformation group, 121.9 ± 29.7 months in the cerebral palsy group, and 72.2 ± 6.3 months in the nonrelaxing IAS group. The difference in mean time to first relapse among diagnosis groups was a significant finding (P = 0.016).
Figure 3.
Kaplan-Meier curve for relapse-free probability. The mean time for the first relapse was 88.4 ± 4.9 months.
Complications
In the entire cohort of 117 patients, 74 (63%) experienced complications related to the ACE, although most of them were minor. Several experienced more than 1 complication.
Immediate complications were seen in a small group of patients. Three (3%) patients had peritonitis, and all of them had a percutaneously created ACE. Three other (3%) patients had perioperative complications that were not peritonitis. Two of these patients had early catheter occlusion and 1 experienced a temporary nerve injury secondary to positioning in the operating room.
Stomal narrowing was seen in 44 (38%) patients; it developed within a mean of 8 months (range 20 days–74 months) after surgery. Stomal leakage developed in 41 (35%) patients within a mean of 19 months (range 7 days–89 months) after initial surgery. Stomal infection was seen in 29 (25%) patients within a mean of 14 months (range 10 days–121 months) after surgery.
Thirty-nine (33%) patients required some form of revision of their stoma. The mean time to stomal revision was 21 months (range 1–89 months) after the initial surgery. Revisions were generally done for stomal leakage or stomal stenosis. Eleven (9%) patients required >1 revision. Eleven (9%) of the entire cohort of 117 patients had closure of the stoma due to a poor outcome. Four (3%) of these patients continued to do poorly with medical management and 7 (6%) were transitioned to a colostomy or ileostomy. Two (2%) patients were not using the stoma due to behavioral opposition to it and continued to have significant problems with bowel management.
In addition, there were 5 patients who transitioned from a surgically created ACE to a percutaneous ACE due to complications. Three with an appendicostomy had severe stomal stenosis and had a percutaneous endoscopic cecostomy (PEC) placed. One had an abscess at the appendicostomy site and was transitioned to a percutaneous endoscopic sigmoidostomy (PES). One patient with a PEC had an unacceptable amount of leaking from PEC site and was changed to PES.
Table 3 shows the complications stratified by the type of procedure performed. There was a statistically significant increase in the rates of total complications, peritonitis, stomal infection, and stomal closure with a poor outcome in the percutaneous ACE group. After excluding patients whose ACE was created percutaneously, 64 of the 106 (60%) patients with a surgically created ACE had some form of complication. In this group, there was a statistically significant difference in the total complication rates and the rates of stomal infection between the various procedures performed.
Table 3. Complications by procedure type.
| Complication | Appendicostomy (n = 96) | Retubularized (n = 7) | CecoStomy button (n = 2) | Percutaneous (n = 11) | Laparoscopic appendicostomy (n = 1) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All complications | 61 | 63.5% | 1 | 14.3% | 2 | 100% | 10 | 90.9% | 0 | 0% | 0.008 |
| Perioperative | 3 | 3.1% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0.955 |
| Peritonitis | 0 | 0% | 0 | 0% | 0 | 0% | 3 | 27.2% | 0 | 0% | <0.001 |
| Stomal revision | 32 | 33.3% | 1 | 14.3% | 0 | 0% | 6 | 54.5% | 0 | 0% | 0.210 |
| >1 revision | 11 | 11.6% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0.753 |
| Stomal infection | 18 | 18.8% | 0 | 0% | 2 | 100% | 9 | 81.9% | 0 | 0% | <0.001 |
| Stomal stenosis | 40 | 41.7% | 1 | 14.3% | 0 | 0% | 3 | 27.3% | 0 | 0% | 0.072 |
| Stomal leakage | 34 | 35.4% | 1 | 14.3% | 2 | 100% | 4 | 36.4% | 0 | 0% | 0.328 |
| Closure of stoma (poor outcome) | 6 | 6.3% | 0 | 0% | 1 | 50% | 4 | 36.4% | 0 | 0% | 0.002 |
Death
Seven (6%) patients died during the course of follow-up after the ACE placement. These patients died of cardiorespiratory failure secondary to the natural history of their primary illnesses at a mean of 58 ± 17 months after creation of the ACE. One patient died within 1 month of its formation. He was 14 years old, with myelodysplasia who had previously undergone a Nissen fundoplication. A laparotomy was performed to fashion an appendicostomy and to augment his bladder with small bowel. On postoperative day 20, he presented with massive abdominal distention and an acute abdomen. On performing emergency exploratory laparotomy, he was found to have a gastric perforation and extensive peritoneal adhesions. The perforation was corrected and a loop ileostomy was performed; however, he died the next day of respiratory failure.
Discussion
We found that 69% of patients with the ACE procedure had successful long-term management of fecal incontinence and intractable constipation secondary to a variety of medical diagnoses.
One of the strengths of our study was the inclusion of children with a variety of etiologies. Although previous studies have reported successful bowel management with ACE irrigations (2,3,5,13–17), many of them have focused on only 1 diagnostic group.
Despite that our reported rate of successful long-term ACE management falls within the range of outcomes that have been described in the literature (59%–97%) (2,5,13,15,17–19), several centers have reported continence rates of >90% (3,13,19). Our lower rate of a long-term successful outcome may be related to several factors. One possibility is the fact that our criteria for success are relatively strict. By contrast, in some studies the criteria for continence are not discussed. In others, the rate of success is based on a single questionnaire reflecting a high degree of recollection bias. In some studies, only selected patients who were successfully managed with rectal irrigations receive an ACE. In addition, in other studies, there is a predominance of a single type of patient, for example, myelodysplasia, in whom an ACE may be more efficacious.
The most likely explanation, however, is that lower rates of success are a function of the length of follow-up time. A similar deterioration in long-term ACE performance has been shown recently in a report by Yardley et al (18). They found that only 59% of patients continued to use the ACE after a mean follow-up of 11 years. This compares with the previous finding of the same group that 72% of patients continued to use the ACE at the follow-up of 5 years (6). Many of their patients cited complications or inconsistent results as the reason for their lack of compliance (18). Their report also suggests that ACE management generally becomes less optimal over time.
After patients achieve a successful outcome with the ACE, the overall relapse rate is high. Thus, reports with short-term follow-up may demonstrate higher success rates. Furthermore, this typical fluctuating course of relapses and recovery is not well documented in the literature. This phenomenon of relapse and subsequent response to adjustments in the irrigations has several implications: it highlights the fluctuating nature of the response after the ACE, it points to the need for close follow-up in these patients, and it underscores the importance of a smooth transition from pediatric to adult care.
The number of patients who were unsuccessful in our cohort is similar to those reported in the literature. Curry et al reported failure in 39% of patients in their early series (15). In our experience, patients with functional intractable constipation had the lowest incidence of unsuccessful bowel management, and similar rates have been shown previously in the literature for this population. Cascio et al reported failure in 16% (20) and Curry et al reported a failure rate of 29%. The reason for the lower rates of unsuccessful management among the functional intractable constipation group is unclear. Van den Berg et al previously showed that the presence of high amplitude propagating contractions (HAPCs) or the response to bisacodyl on colonic manometry was a predictor of success with ACE irrigations (21). Perhaps the group that does not fare well among those with functional intractable constipation has an underlying colonic dysmotility.
The best irrigation regimen has not been well defined. The literature is replete with a wide variety of possible regimens ranging from tap water (22) to saline (23) or GoLYTELY (12). There are no prospective controlled studies to determine which irrigation solution is ideal. In our practice, patients are started on normal saline because of ease of availability and lower cost, and then transitioned to GoLYTELY if irrigations are unsuccessful. We do not prefer tap water because of the risk of electrolyte imbalances (22). At the final follow-up, two-thirds of patients were successfully managed with GoLYTELY, which indicates that most patients failed with NS. In 34% of our patients with problems, additives were added to the irrigation and their use helped us achieve success in 80% of patients in whom they were used.
When analyzing irrigation characteristics obtained at every clinical encounter, there did seem to be an inverse correlation between ACE success and the toilet sitting time. It is unclear whether this represents a cause or an effect. Previous studies on the quality of life in patients with ACE have, however, shown that longer duration of the irrigation process is a major factor in ACE dissatisfaction and a diminished quality of life (5,24). Yerkes et al reported that several patients expressed frustration over the time required to complete ACE irrigations. In addition, in Toogood's early ACE scoring scheme, the time to complete the irrigation was established as a major component for efficacy (24). Our result reinforces the importance of screening “toilet time” during follow-up visits and proactively attempts to address it. This can be achieved by further elevating the infusion bag, using additives, or inserting a larger sized catheter.
Another important finding in the present study is that a successful bowel management program before the ACE is not a prerequisite for success after the ACE. This finding is important because some centers perform the ACE only in those children who have previously achieved success in their bowel management (25). It has also been recommended that diversion ostomies be performed in those who cannot achieve continence with rectal enemas before surgery (26). Our findings indicate that an ACE should be considered even in those children who have failed preoperative management before an ostomy is considered. The 6% incidence of an ostomy after ACE placement indicates that only a small number of children will require a subsequent diversion. On the contrary, and different from previous reports (27), we found that not all of the children who were successfully continent before the ACE remained so after the surgery. This fact needs to be emphasized to families who are considering the operation.
There was a high incidence of complications. Many of these complications were minor and could be addressed with minimal intervention; for example, leaving the catheter in place for several days when the patients noticed the stoma may have been getting tighter. One-third, however, required some surgical intervention of their stoma. The incidence of stomal narrowing, leakage, and infection are similar to what has been described before. Dey et al reported that 57% of their children had some complications, the most common being stomal stenosis, which affected 47%, with 22% requiring a stomal revision (6). Other authors have reported rates of stomal stenosis that range from 14% to 50% (3,4,15,20) and of leakage that range from 7% to 10% (3,15). The need for surgical revision of the stoma also varies, with reported rates of up to 50% (4,20). These findings suggest there is a significant incidence of stoma-related complications.
We noted that percutaneously placed ACEs had a much higher incidence of peritonitis, stomal infection, stomal leakage, and closure due to a poor outcome. The risk of infection is probably related to the colonoscopy and the possibility of stool leakage during the procedure. All of the patients had received a bowel clean out and perioperative antibiotics. The peritonitis appeared within the first 24 hours postoperatively. All of the patients received intravenous antibiotics and recovered uneventfully, although their hospitalization was lengthened. In their initial publication on the placement of colonoscopically placed PECs, Rivera et al did report that 1 of their 12 patients had fever that resolved in the postoperative period (28). Shandling et al also had a patient in their initial series of fluoroscopically placed PECs who experienced postoperative fevers, but these resolved soon (29). No other published report has, however, looked at the long-term complications of the percutaneous colonoscopically placed ACE.
There are some limitations to the present study. Our analysis is retrospective, and as such is subject to observer bias. We attempted to account for this by creating a grading scheme a priori against which all follow-up clinic visits were weighed. The assessments were done by 1 investigator who was not involved in the clinical care of the patients. It is also possible that the retrospective nature of the review may bias our results against successful encounters, because it is more likely that patients return to the clinic upon having problems.
Our functional outcome assessment is limited in its inability to fully capture the time-related variability of patients' experiences. We know that patients have fluctuations in their medical course with relapses after achieving success, and then later respond to irrigation adjustments. Comparing 2 discrete time points may overlook the patients' aggregate success with the ACE procedure. Despite these limitations, we feel this large diverse patient experience can add to the collective knowledge about the ACE procedure.
In conclusion, we have shown that patients generally experience improvement in bowel management after an ACE procedure. Relapse of incontinence should be expected, but it usually responds to adjustment in the irrigation. Failure of a preoperative bowel management program is not a contraindication for the ACE. Patients with an ACE have a high incidence of minor complications, and there is a frequent need for surgical intervention.
Acknowledgments
We thank all of the surgical and nursing staff who participated in the care of the patients. In particular, we thank Jennifer Skowron, Sandy Quigley, Ellen O'Donnell, Fiona Paul, Patricia Bianchi, Rosemary Grant, and Mary Dunleavy. We also thank Dr Laurie Fishman for helpful suggestions.
Supported by NIH K24DK082792A.
Footnotes
The authors report no conflicts of interest.
References
- 1.Malone PS, Ransley PG, Kiely EM. Preliminary report: the antegrade continence enema. Lancet. 1990;336:1217–8. doi: 10.1016/0140-6736(90)92834-5. [DOI] [PubMed] [Google Scholar]
- 2.King SK, Sutcliffe JR, Southwell BR, et al. The antegrade continence enema successfully treats idiopathic slow-transit constipation. J Pediatr Surg. 2005;40:1935–40. doi: 10.1016/j.jpedsurg.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Bani-Hani AH, Cain MP, Kaefer M, et al. The Malone antegrade continence enema: single institutional review. J Urol. 2008;180:1106–10. doi: 10.1016/j.juro.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 4.Mattix KD, Novotny NM, Shelley AA, et al. Malone antegrade continence enema (MACE) for fecal incontinence in imperforate anus improves quality of life. Pediatr Surg Int. 2007;23:1175–7. doi: 10.1007/s00383-007-2026-3. [DOI] [PubMed] [Google Scholar]
- 5.Yerkes EB, Cain MP, King S, et al. The Malone antegrade continence enema procedure: quality of life and family perspective. J Urol. 2003;169:320–3. doi: 10.1016/S0022-5347(05)64116-X. [DOI] [PubMed] [Google Scholar]
- 6.Dey R, Ferguson C, Kenny SE, et al. After the honeymoon: mediumterm outcome of antegrade continence enema procedure. J Pediatr Surg. 2003;38:65–8. doi: 10.1053/jpsu.2003.50012. [DOI] [PubMed] [Google Scholar]
- 7.McAndrew HF, Griffiths DM, Pai KP. A new complication of the Malone antegrade continence enema. J Pediatr Surg. 2002;37:1216. doi: 10.1053/jpsu.2002.34481. [DOI] [PubMed] [Google Scholar]
- 8.Soylet Y, Yesildag E, Besik C, et al. Antegrade continence enema: an analysis of 20 children with faecal incontinence. Eur J Pediatr Surg. 2006;16:251–4. doi: 10.1055/s-2006-924337. [DOI] [PubMed] [Google Scholar]
- 9.Koivusalo AI, Pakarinen MP, Pauniaho SL, et al. Antegrade continence enema in the treatment of congenital fecal incontinence beyond childhood. Dis Colon Rectum. 2008;51:1605–10. doi: 10.1007/s10350-008-9327-z. [DOI] [PubMed] [Google Scholar]
- 10.Becmeur F, Demarche M, Lacreuse I, et al. Cecostomy button for antegrade enemas: survey of 29 patients. J Pediatr Surg. 2008;43:1853–7. doi: 10.1016/j.jpedsurg.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Aksnes G, Diseth TH, Helseth A, et al. Appendicostomy for antegrade enema: effects on somatic and psychosocial functioning in children with myelomeningocele. Pediatrics. 2002;109:484–9. doi: 10.1542/peds.109.3.484. [DOI] [PubMed] [Google Scholar]
- 12.Bani-Hani AH, Cain MP, King S, et al. Tap water irrigation and additives to optimize success with the Malone antegrade continence enema: the Indiana University algorithm. J Urol. 2008;180(4 Suppl):1757–60. doi: 10.1016/j.juro.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 13.Sinha CK, Grewal A, Ward HC. Antegrade continence enema (ACE): current practice. Pediatr Surg Int. 2008;24:685–8. doi: 10.1007/s00383-008-2130-z. [DOI] [PubMed] [Google Scholar]
- 14.Worsoe J, Christensen P, Krogh K, et al. Long-term results of antegrade colonic enema in adult patients: assessment of functional results. Dis Colon Rectum. 2008;51:1523–8. doi: 10.1007/s10350-008-9401-6. [DOI] [PubMed] [Google Scholar]
- 15.Curry JI, Osborne A, Malone PS. The MACE procedure: experience in the United Kingdom. J Pediatr Surg. 1999;34:338–40. doi: 10.1016/s0022-3468(99)90204-x. [DOI] [PubMed] [Google Scholar]
- 16.Yang CC, Stiens SA. Antegrade continence enema for the treatment of neurogenic constipation and fecal incontinence after spinal cord injury. Arch Phys Med Rehabil. 2000;81:683–5. doi: 10.1016/s0003-9993(00)90054-6. [DOI] [PubMed] [Google Scholar]
- 17.Marshall J, Hutson JM, Anticich N, et al. Antegrade continence enemas in the treatment of slow-transit constipation. J Pediatr Surg. 2001;36:1227–30. doi: 10.1053/jpsu.2001.25768. [DOI] [PubMed] [Google Scholar]
- 18.Yardley IE, Pauniaho SL, Baillie CT, et al. After the honeymoon comes divorce: long-term use of the antegrade continence enema procedure. J Pediatr Surg. 2009;44:1274–6. doi: 10.1016/j.jpedsurg.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Thomas J, Dietrich M, Trusler L, et al. Continent catheterizable channels and the timing of their complications. J Urol. 2006;176(4 Pt 2):1816–20. doi: 10.1016/S0022-5347(06)00610-0. [DOI] [PubMed] [Google Scholar]
- 20.Cascio S, Flett M, De la Hunt M, et al. MACE or caecostomy button for idiopathic constipation in children: a comparison of complications and outcomes. Pediatr Surg Int. 2004;20:484–7. doi: 10.1007/s00383-004-1220-9. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg M, Hogan M, Caniano D, et al. Colonic manometry as predictor of cecostomy success in children with defecation disorders. J Pediatr Surg. 2006;41:730–6. doi: 10.1016/j.jpedsurg.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Yerkes EB, Rink RC, King S, et al. Tap water and the Malone antegrade continence enema: a safe combination? J Urol. 2001;166:1476–8. [PubMed] [Google Scholar]
- 23.Hyde S, Coulthard M, Jaffray B, et al. Using saline solutions for ACE washouts. Arch Dis Child. 2008;93:149–50. doi: 10.1136/adc.2007.123489. [DOI] [PubMed] [Google Scholar]
- 24.Schell SR, Toogood GJ, Dudley NE. Control of fecal incontinence: continued success with the Malone procedure. Surgery. 1997;122:626–31. doi: 10.1016/s0039-6060(97)90137-9. [DOI] [PubMed] [Google Scholar]
- 25.Levitt M, Peña A. Update on pediatric faecal incontinence. Eur J Pediatr Surg. 2009;19:1–9. doi: 10.1055/s-2008-1039190. [DOI] [PubMed] [Google Scholar]
- 26.Peña A, Guardino K, Tovilla J, et al. Bowel management for fecal incontinence in patients with anorectal malformations. J Pediatr Surg. 1998;33:133–7. doi: 10.1016/s0022-3468(98)90380-3. [DOI] [PubMed] [Google Scholar]
- 27.Levitt MA, Soffer SZ, Pena A. Continent appendicostomy in the bowel management of fecally incontinent children. J Pediatr Surg. 1997;32:1630–3. doi: 10.1016/s0022-3468(97)90470-x. [DOI] [PubMed] [Google Scholar]
- 28.Rivera M, Kugathasan S, Berger W, et al. Percutaneous colonoscopic cecostomy for management of chronic constipation in children. Gastrointest Endosc. 2001;53:225–8. doi: 10.1067/mge.2001.112182. [DOI] [PubMed] [Google Scholar]
- 29.Shandling B, Chait P, Richards H. Percutaneous cecostomy: a new technique in the management of fecal incontinence. J Pediatr Surg. 1996;31:534–7. doi: 10.1016/s0022-3468(96)90490-x. [DOI] [PubMed] [Google Scholar]