Abstract
Object
Low-grade gliomas (LGGs) frequently infiltrate highly functional or “eloquent” brain areas. Given the lack of long-term survival data, the prognostic significance of eloquent brain tumor location and the role of functional mapping during resective surgery in presumed eloquent brain regions are unknown.
Methods
We performed a retrospective analysis of 281 cases involving adults who underwent resection of a supratentorial LGG at a brain tumor referral center. Preoperative MR images were evaluated blindly for involvement of eloquent brain areas, including the sensorimotor and language cortices, and specific subcortical structures. For high-risk tumors located in presumed eloquent brain areas, long-term survival estimates were evaluated for patients who underwent intraoperative functional mapping with electrocortical stimulation and for those who did not.
Results
One hundred and seventy-four patients (62%) had high-risk LGGs that were located in presumed eloquent areas. Adjusting for other known prognostic factors, patients with tumors in areas presumed to be eloquent had worse overall and progression-free survival (OS, hazard ratio [HR] 6.1, 95% CI 2.6–14.1; PFS, HR 1.9, 95% CI 1.2–2.9; Cox proportional hazards). Confirmation of tumor overlapping functional areas during intraoperative mapping was strongly associated with shorter survival (OS, HR 9.6, 95% CI 3.6–25.9). In contrast, when mapping revealed that tumor spared true eloquent areas, patients had significantly longer survival, nearly comparable to patients with tumors that clearly involved only noneloquent areas, as demonstrated by preoperative imaging (OS, HR 2.9, 95% CI 1.0–8.5).
Conclusions
Presumed eloquent location of LGGs is an important but modifiable risk factor predicting disease progression and death. Delineation of true functional and nonfunctional areas by intraoperative mapping in high-risk patients to maximize tumor resection can dramatically improve long-term survival.
Keywords: low-grade glioma, eloquence, functional mapping, prognosis, survival
Low-grade gliomas are infiltrative cerebral neoplasms characterized by insidious progression, frequently invading functionally critical or “eloquent” brain regions. Eloquent brain areas in the cerebral hemispheres are defined practically as the essential areas for carrying out basic neurological functions and include the sensorimotor cortex, language cortex, and subcortical structures such as the basal ganglia and internal capsule. Unlike malignant gliomas, LGGs often spare neural function in the process of slow infiltration or instead induce cortical reorganization.8
Although many investigations have addressed risk factors for survival, almost all have overlooked eloquence as one of the most important and unique aspects related to cerebral neoplasms.1,6,7,12,13,15,19,26,31 Few studies have been conducted with sufficient long-term follow-up after the widespread application of MR imaging, the current standard for detection of LGGs.6,7
Current surgical decision making relies upon weighing impressions about the benefit of resection on long-term survival versus the perceived risks of surgery. This issue is especially critical for patients with LGGs in eloquent areas, as the risks of surgery are potentially greater than for patients with tumors in noneloquent locations. Recent studies have demonstrated that greater extent of resection is associated with improved long-term survival.3,14,28 However, injury of eloquent brain regions can result in significant neurological impairments that can have devastating consequences on quality of life and survival.25
One approach to minimizing these potential risks of surgery, while aiming for a maximal resection, is the use of intraoperative functional brain mapping. Mapping is usually conducted by applying direct electrical stimulation to successive neighboring cortical or subcortical areas to evoke either movement of the face, arms, or legs (motor mapping) or the interruption of speech (language mapping). Those areas deemed to be essential for motor or language function are carefully preserved during resection. While this technique has been shown to greatly minimize morbidity, its adoption has been limited by the fact that no study to date has demonstrated that its application can affect long-term survival.
We conducted a comprehensive review of a large cohort of 281 patients with LGGs that were treated surgically at our tertiary brain tumor referral center. The specific aims of this study were as follows: 1) to determine PFS and OS for patients with LGGs that infiltrate eloquent brain areas; 2) to determine if intraoperative functional mapping modifies the risk of progression or modifies duration of survival in patients with LGGs presumed to involve eloquent cortex based on preoperative imaging; and 3) to determine if functional mapping increases the extent of resection of these lesions.
Methods
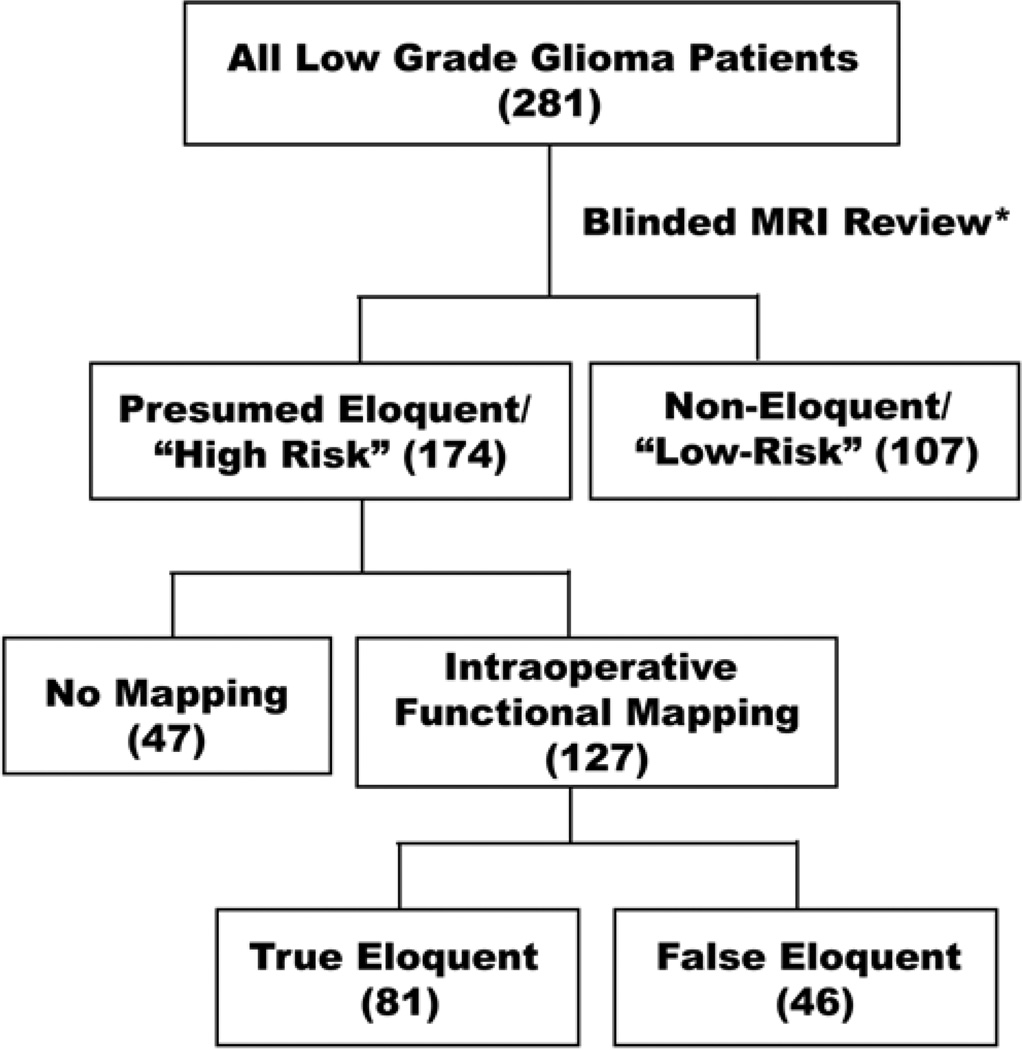
We conducted a retrospective review with long-term follow-up of 281 consecutive cases involving adult patients with hemispheric infiltrative LGGs that were treated surgically at the UCSF between January 1989 and June 2005. All included patients had newly diagnosed, histologically confirmed WHO Grade II low-grade infiltrative gliomas. Patients with pilocytic or gemistocytic astrocytomas were excluded. All research activities were approved by the UCSF institutional review board for human research. Figure 1 shows the algorithm for analysis and grouping by treatment in this study.
Fig. 1.
Algorithm for analysis and grouping by treatment in this study. Preoperative MR images from 281 patients with LGGs were blindly reviewed for tumor location that occupied presumed eloquent locations, based upon anatomical criteria. Those patients with tumors in presumed eloquent locations were considered to be “high-risk” and were further stratified by whether they underwent intraoperative functional mapping. Cases in which tumors were confirmed to be in an eloquent location were designated as “true eloquent” compared with those that were determined by mapping to be adjacent but not directly overlapping eloquent brain (“false eloquent”).
All demographic and radiographic features were derived from hospital records. Clinical variables including age, sex, and KPS score were recorded. The KPS score was classified as follows: 100 (asymptomatic, incidental findings), 90 (minor symptoms, such as a presenting seizure or persistent headaches), 80 (normal activity with effort, with some signs or symptoms of disease).
Blinded MR Imaging Review
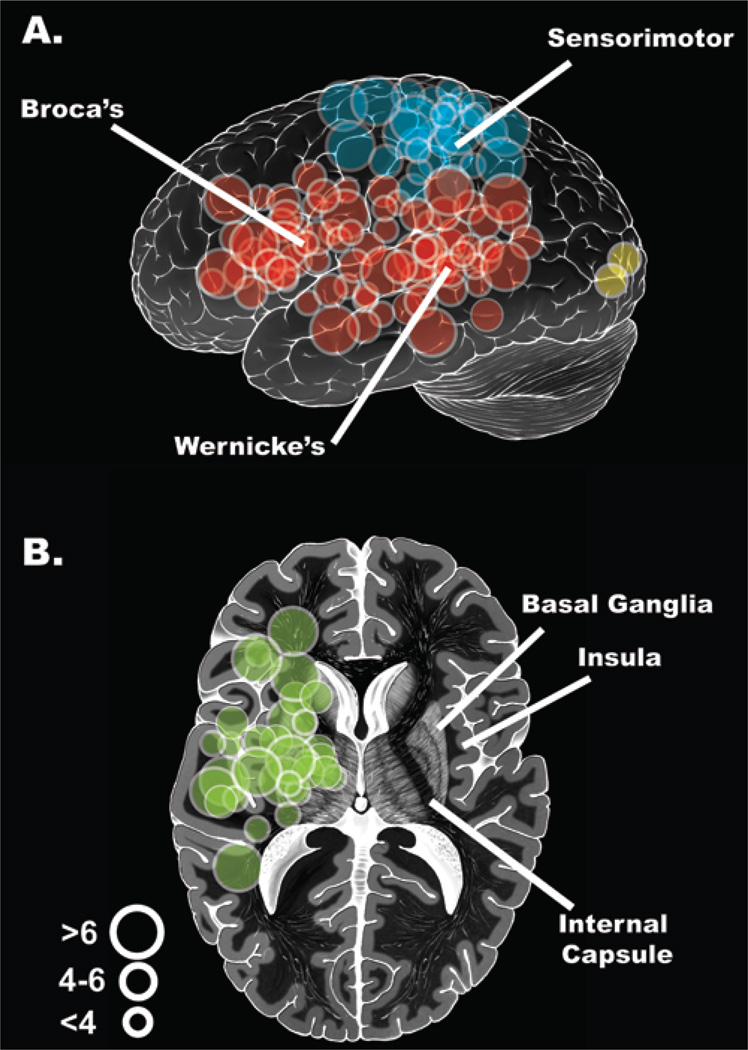
Preoperative MR images were independently reviewed by 2 investigators (E.C. and M.W.M.), who were blinded to patient outcome to control for observer bias and surgeon operative preference. Tumor location and anatomical characteristics were carefully documented, including whether tumors had infiltrated brain areas presumed to be eloquent (Fig. 2). The eloquent areas consisted of the sensorimotor strip (precentral and postcentral gyri), dominant hemisphere perisylvian language areas (superior temporal, inferior frontal, and inferior parietal areas), the basal ganglia/internal capsule, the thalamus, and the calcarine visual cortex. It is worth emphasizing that this designation of “presumed” eloquence is based upon preoperative diagnostic anatomical MR imaging, not functional imaging or intraoperative functional mapping, which will be addressed below. Using blinded evaluation allowed us to apply an unbiased differentiation of cases into high and low surgical risk categories; patients were considered to have high-risk lesions if there was involvement of eloquent areas and low-risk lesions if there was no involvement of areas presumed to be eloquent.
Fig. 2.
Representative schematic illustrations of eloquent tumor locations in left lateral view for cortical sites (A) and axial transection for deeper subcortical sites (B). Red signifies language areas; blue, sensorimotor cortex; green, deep subcortical structures, including the thalamus, basal ganglia, and internal capsule. The 3 circle sizes designate tumor diameter in centimeters.
Surgical Intervention
During the study period, 8 neurosurgeons employed a variety of surgical approaches in treating these lesions. The surgical approaches included in this study population ranged from minimal resection to maximal tolerated resection sparing functional brain areas. Intraoperative mapping techniques using direct electrocortical stimulation (Ojemann Cortical Stimulator, Integra LifeSciences Corp.) included language and/or motor mapping and were applied if they were part of the surgeon’s routine practice. Language mapping surgery is facilitated by local anesthesia so patients are fully awake during intraoperative testing. Sites where stimulation induces speech arrest or errors in naming or reading are designated as eloquent language areas. Motor mapping can be done with the patient under general anesthesia or awake. Sites where stimulation evokes visually or electromyographically detected movements of the face or limbs are designated as eloquent motor areas. Details of these procedures have been described previously.4,16 To further evaluate the contribution of eloquence and mapping to prognosis, cases in which tumors were found to invade eloquent brain were categorized as “true eloquent,” and those in which tumors spared eloquent brain were categorized as “false eloquent” (that is, cases in which the tumor was found to be near or adjacent to eloquent brain, but not directly overlapping it).
Outcome Measures
The main outcome measures were OS and PFS. Progression was defined as an unequivocal increase in the FLAIR/T2 signal abnormality and/or newly detected areas of contrast enhancement on follow-up MR imaging compared with the baseline postoperative MR images obtained within 3 days following surgery. Survival and progression data were available for 281 patients (100%).
A secondary outcome measure was volumetric extent of resection. Manual segmentation was performed with region-of-interest analysis to measure preoperative and postoperative tumor volumes (in cm3) based on FLAIR axial slices (5-mm thickness, no gap) as previously described. Comparisons were made between pre- and postoperative FLAIR imaging as well as postoperative T1-weighted MR imaging to determine areas of residual tumor. Extent of resection was calculated as (preoperative tumor volume – postoperative tumor volume)/preoperative tumor volume × 100%. Preoperative contrast-enhanced T1-weighted images were reviewed for each case, and when enhancing tumor was present, its volume was also measured. Determination of volumes was made without knowledge of clinical outcome. Data on extent of resection were available for 224 patients (80%).
Follow-up information was obtained primarily by extensive chart review, telephone interview, and through the National Death Index archives. Telephone follow-up was stopped on October 19, 2008. The follow-up period for OS analyses was defined as the time interval between date of initial surgery and the date of death or date that the patient was last known to be alive. Duration of PFS was defined as the time interval between the date of surgery and the date of the MR imaging study on which the first progression event was detected or the date of the last known MR imaging study with no evidence of disease progression (whichever was first).
Statistical Considerations
For categorical variables, the Fisher exact test was used when analyzing differences in demographic characteristics between patients with tumors in presumed eloquent areas and those with tumors in presumed noneloquent areas. The Student t-test was used to test the difference in means for continuous variables. Kaplan-Meier estimates were generated to illustrate the time-to-event curves. The log-rank test was used to compare differences in OS and PFS. Multivariate analysis by Cox proportional hazards modeling was used to adjust for the known prognostic factors: age at diagnosis, KPS (as continuous variable), histological tumor subtype (astrocytoma, oligodendroglioma, and oligoastrocytoma), and maximum tumor diameter. Adjustment for multiple comparisons was made by setting the threshold for statistical significance at 0.01. Analysis of variance was used to compare differences in extent of resection between the different mapping groups. The Tukey HSD test was used for post hoc comparison of individual differences.
Results
Baseline Characteristics
The baseline characteristics of the study population and tumors are shown in Table 1. Patients with tumors in presumed eloquent areas did not differ from those with tumors in noneloquent areas with respect to age at diagnosis and histological tumor subtype. However, patients with tumors in eloquent areas had lower KPS scores, larger tumors, and a greater proportion of left-sided tumor locations. The majority of presumed eloquent-area tumors were located in the sensorimotor (41%), language (48%), and/or internal capsule/basal ganglia (47%). Less than 10% were located in visual cortex, the hypothalamus, or the thalamus. Most of the presumed eloquent-area tumors (63%) involved 1 eloquent area, while involvement of 2 eloquent areas was less frequent (32%) and involvement of 3 was rare (3.4%).
TABLE 1.
Summary of clinicopathological differences between study patients with LGGs in presumed eloquent and noneloquent areas
| Characteristic | Eloquent | Noneloquent | p Value |
|---|---|---|---|
| no. of cases | 174 | 107 | |
| patient age | |||
| median | 38 | 38 | 0.78* |
| range | 15–68 | 20–72 | |
| KPS score | |||
| median | 90 | 90 | <0.001* |
| range | 80–100 | 90–100 | |
| KPS = 100 (%) | 19 (11) | 38 (35.5) | |
| KPS = 90 (%) | 146 (84) | 69 (64.5) | |
| KPS = 80 (%) | 7 (4) | 0 (0) | |
| pathology (%) | |||
| astrocytoma | 53 (31) | 28 (26) | 0.72† |
| oligodendroglioma | 59 (34) | 42 (39) | |
| oligoastrocytoma | 62 (36) | 37 (35) | |
| side of tumor (%) | |||
| left | 102 (59) | 48 (45) | 0.04† |
| light | 72 (41) | 58 (54) | |
| location (%) | |||
| frontal | 77 (44) | 75 (70) | <0.001† |
| parietal | 15 (9) | 9 (8) | |
| temporal | 25 (14) | 17 (16) | |
| insula | 56 (32) | 5 (5) | |
| basal ganglia | 1 (1) | 0 (0) | |
| resection (%) | |||
| gross total | 24 (14) | 69 (65) | <0.001† |
| subtotal | 150 (86) | 38 (36) | |
| extent of resection (%) | |||
| median | 63.6 | 9 3 .1 | <0.001* |
| range | 5–100 | 5–100 | |
| maximum diameter in cm | <0.001* | ||
| median | 5.3 | 3.8 | |
| range | 1. 4–11 | 1.4–9 | |
| eloquent locations (%) | |||
| sensorimotor | 72 (41.4) | ||
| language | 83 (47.7) | ||
| visual | 2 (1.1) | ||
| hypothalamus | 7 (4.0) | ||
| thalamus | 6 (3.4) | ||
| internal capsule/basal ganglia | 82 (47.1) | ||
| no. of eloquent locations (%) | |||
| 1 | 110 (63.2) | ||
| 2 | 55 (31.6) | ||
| 3 | 6 (3.4) |
Student t-test.
Fisher exact test.
Clinical Outcome
The median duration of follow-up for those patients still alive at the time of last follow-up was 62.4 months (range 3–152 months). The overall estimated 5-year OS was 86%, with 214 patients who were censored. The overall estimated 5-year PFS was 62%, with 143 patients who were censored. In 174 patients (62%) LGGs were found to have infiltrated “high-risk” presumed eloquent locations.
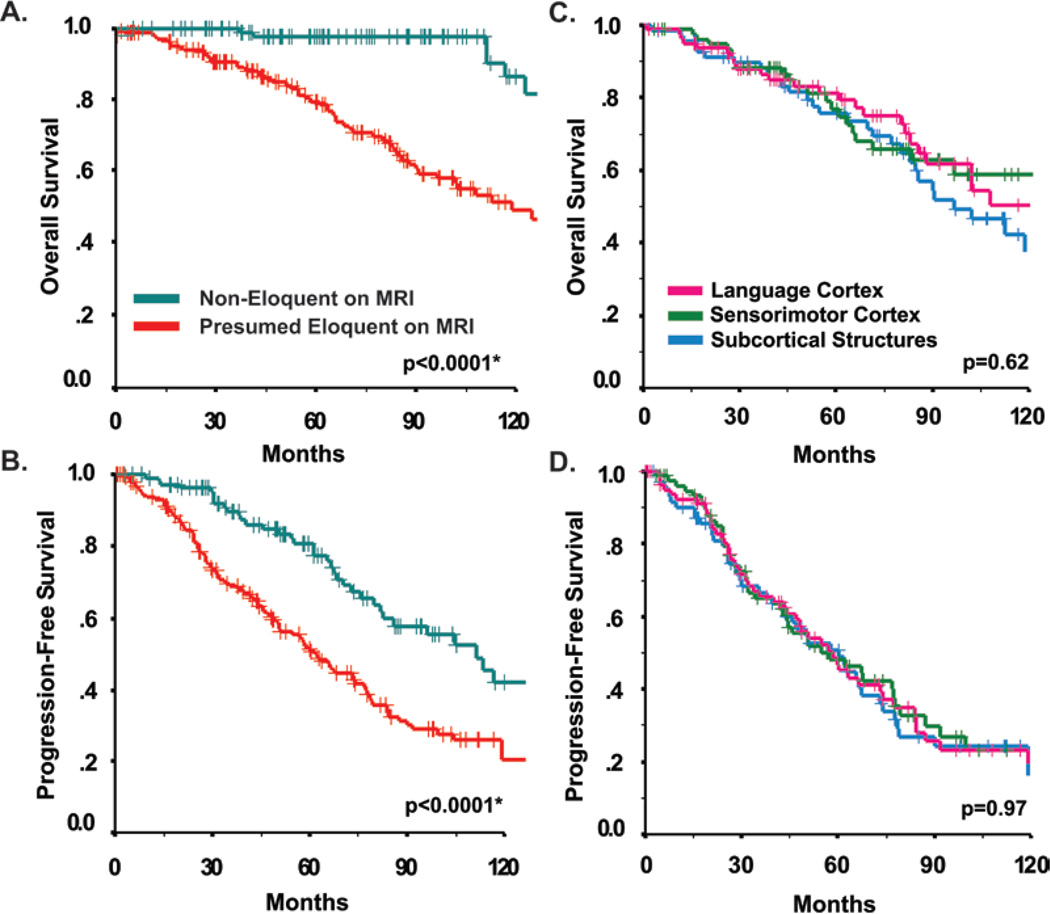
Patients with tumors in “high-risk” presumed eloquent locations had significantly shorter estimated OS (p < 0.0001, log-rank test; estimated 5-year OS probability: high-risk = 0.79, low-risk = 0.98; Fig. 3A). A similar association was found for PFS (5-year PFS, high-risk = 50%, low-risk = 80%) (p < 0.0001, log-rank test; Fig. 3B). After adjustment for other prognostic factors such as age, performance status, tumor histological type, and tumor size, we found that patients with eloquent-area LGGs demonstrated an increased hazard for shorter OS and PFS (OS, p < 0.001, HR 6.1, 95% CI 2.6–14.1; PFS, p = 0.003, HR 1.9, 95% CI 1.2–2.9; Cox proportional hazards). Of note, only 8 death events were observed among 107 patients with low-risk tumors located outside of presumed eloquent areas.
Fig. 3.
A and B: Kaplan-Meier survival estimates of OS (A) and PFS (B) stratified by “presumed eloquence” on MR images. C and D: Kaplan-Meier survival estimates of OS (C) and PFS (D) stratified by specific eloquent regions. (Probability values based on log-rank test.)
Since eloquent location was so strongly predictive of all outcome measures, a subanalysis was carried out to determine if the effect was additive by comparing outcomes of patients with tumors involving 1 versus 2 or 3 known eloquent areas. Involvement of multiple additional eloquent areas did not appear to confer significantly higher risk of death (HR 0.60, 95% CI 0.52–1.6). Within the group of patients with LGGs in presumed eloquent areas, no significant difference among different eloquent regions was observed for OS or progression estimates (Fig. 3C and D).
Intraoperative Mapping and Clinical Outcome
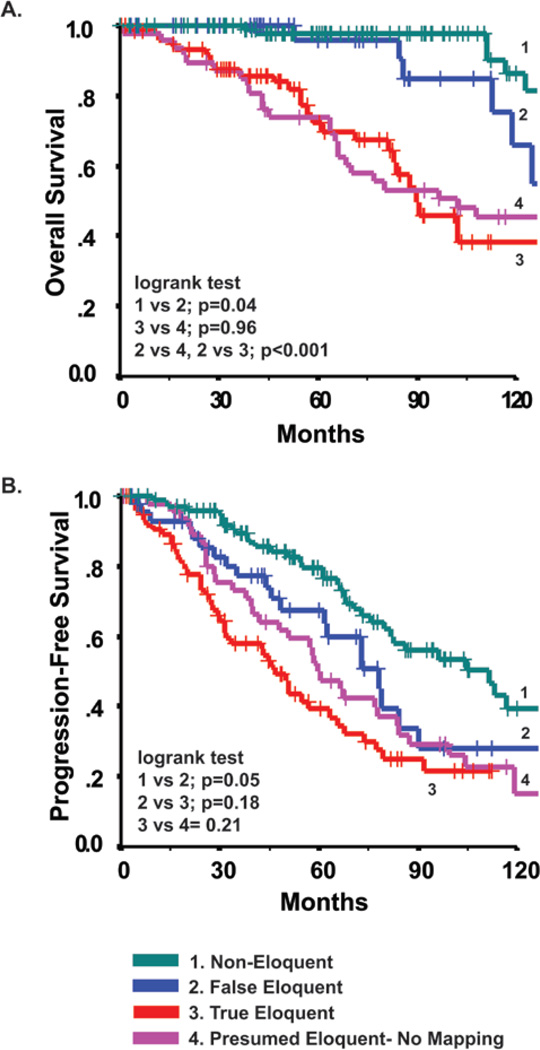
To determine the role of intraoperative functional mapping, patients with tumors in presumed eloquent locations were then stratified according to whether they underwent mapping (“mapping yes” [127 patients]/”mapping no” [47 patients]). Patients who underwent mapping were also grouped by whether functional brain areas were directly involved with or spared from tumor infiltration (“True-Eloquent” [81 patients]/“False-Eloquent” [46 patients]). No significant difference in OS was observed between those patients who underwent mapping and those who did not (p = 0.21, log-rank test).
However, analysis of the intraoperative mapping findings revealed that OS was far shorter in the True-Eloquent Group than in the False-Eloquent Group (Fig. 4A, p < 0.001, log-rank test; see Table 2 for adjusted HR). Furthermore, patients in the True-Eloquent Group had survival estimates similar to patients with tumors in areas presumed to be eloquent who did not undergo mapping (p = 0.96, log-rank test). Likewise, patients in the False-Eloquent Group did not have significantly different survival from those who were harboring tumors in low-risk noneloquent areas, despite a trend that suggested that those in the False-Eloquent Group fared slightly worse (p = 0.04, log-rank test). Therefore, when functional mapping delineated safe boundaries between tumor and eloquent brain regions, OS was greatly improved.
Fig. 4.
Kaplan-Meier survival estimates of OS (A) and KPS (B) stratified by subgroups. “False Eloquent Mapping” refers to cases in which the brain region was preoperatively presumed to be eloquent based on anatomical imaging, but was actually not eloquent based on intraoperative functional mapping. “True Eloquent Mapping” refers to those cases in which intraoperative functional mapping confirmed the anatomical imaging determination of eloquence. (Probability values based on log-rank test.)
TABLE 2.
Multivariate analysis of mapping groups, PFS, and OS—adjusted for age, KPS score, tumor diameter, and histological type*
| OS |
PFS |
||||||
|---|---|---|---|---|---|---|---|
| Group† | No. of Pts | No. of Events | Median FU for Censored (yrs) |
HR (95% CI) | No. of Events | Median FU for Censored (yrs) |
HR (95% CI) |
| Noneloquent | 107 | 8 | 5.6 | 1.0 | 37 | 4.3 | 1.0 |
| No Mapping | 47 | 28 | 10 | 9.0 (3.6–22.3) | 35 | 6.9 | 1.9 (1.2–3.2) |
| False-Eloquent | 46 | 7 | 4.2 | 2.9 (1.0–8.5) | 20 | 3.8 | 1.5 (0.9–2.7) |
| True-Eloquent | 81 | 25 | 4.0 | 9.6 (3.6–25.9) | 46 | 3.4 | 2.5 (1.5–4.3) |
FU = follow-up.
Groups were defined as follows: The Noneloquent Group included all patients with tumors determined to involve only noneloquent areas on the basis of MR imaging. The No Mapping Group included all patients with tumors presumed to involve eloquent areas on the basis of MR imaging who did not undergo intraoperative mapping. The False-Eloquent Group included all those whose tumors were presumed to involve eloquent areas but which were found to spare eloquent brain based on intraoperative mapping. The True-Eloquent Group included all those in whom the presumption of eloquent brain involvement was confirmed through intraoperative mapping.
Similar analysis was performed for the end point of PFS (Fig. 4B). Despite a similar pattern of separation as seen in OS, the benefit due to functional mapping is not as apparent in terms of PFS. Specifically, the difference between the True-Eloquent and False-Eloquent Groups did not reach statistical significance (p = 0.18, log-rank test). One plausible explanation would be the lack of reliability in the assessment of tumor progression based on the standard MR imaging technique.
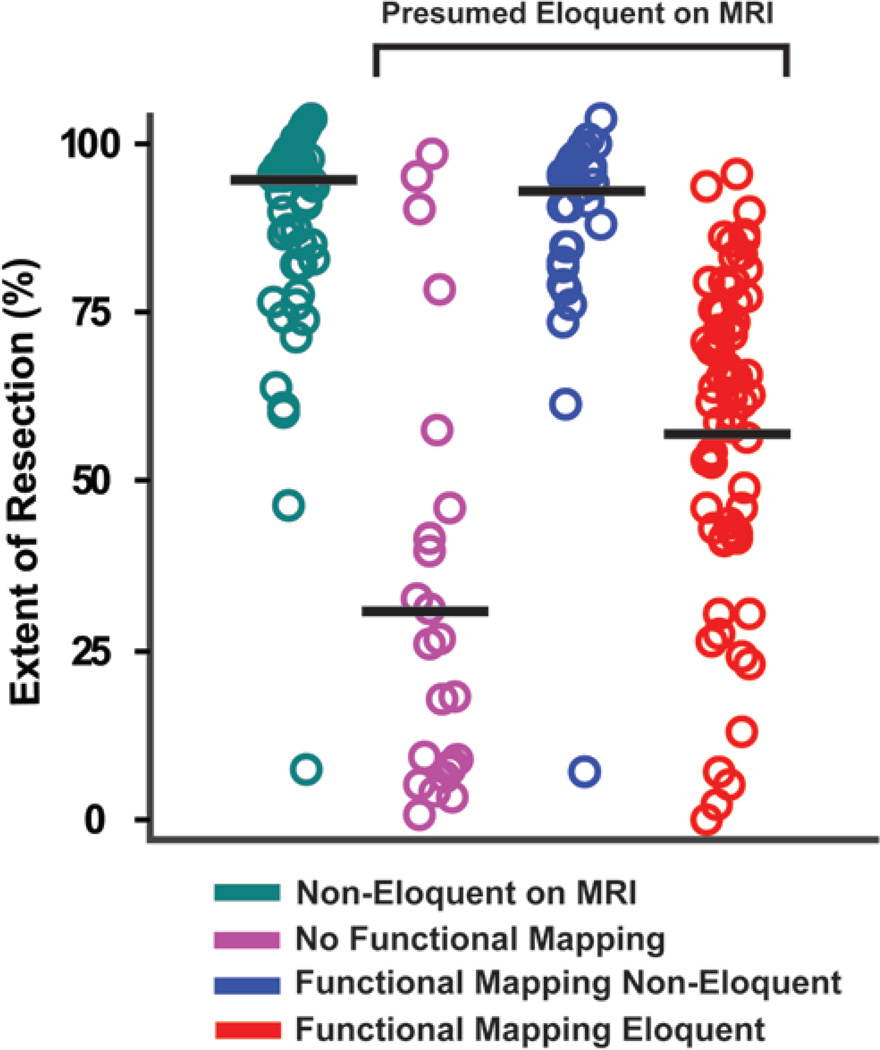
To determine how intraoperative mapping influenced resection, extent of tumor resection was analyzed for the following 4 groups (Fig. 5): 1) patients whose tumors were considered to involve only noneloquent areas based on MR imaging (Noneloquent Group); 2) patients with tumors in areas presumed to be eloquent based on who did not undergo intraoperative mapping (No Mapping Group); 3) patients whose tumors were presumed to involve eloquent areas based on MR imaging but were found through intraoperative mapping to involve only noneloquent areas (False-Eloquent Group); and 4) patients whose tumors were presumed based on MR imaging and confirmed through intraoperative mapping to involve eloquent areas (True-Eloquent Group). In accordance with the survival data, extent of resection was greater in patients in the Noneloquent Group and the False-Eloquent Group (93.1% ± 14.1% and 91.6% ± 16.5%, respectively [mean ± SD]), whereas the mean extent of resection in the No Mapping Group was 32.4% ± 32.2% and the mean extent of resection in the True-Eloquent Group was 59.2 ± 22.8% (p < 0.001, ANOVA). Post hoc analysis demonstrated no statistically significant difference in extent of resection between the Noneloquent Group and the False-Eloquent Group (p = 0.98, Tukey HSD). These results suggest that the long-term survival benefits associated with functional mapping are likely facilitated through increased extent of resection by definitively eliminating risk of injury to eloquent brain in patients with tumors that involve these areas.
Fig. 5.
Surgical extent of resection stratified by mapping subgroups. The black line corresponds to the median value for each subgroup data distribution (circles).
Discussion
Intraoperative mapping in the surgical treatment of brain tumors located in eloquent brain areas requires validation with outcome measures. We evaluated a large series of patients with extensive follow-up and showed that tumor location in eloquent brain areas was associated with shorter OS and PFS. Of the high-risk tumors located in presumed eloquent brain areas, however, we found that the use of intraoperative functional mapping played a critical role in delineating those tumors truly involved eloquent areas versus those that did not. A major finding was that patients in the False-Eloquent Group had excellent survival outcomes, which suggests that mapping can drastically change the long-term prognosis for these patients.
The role of eloquence as a prognostic factor has been largely ignored in several studies published about the long-term prognosis associated with LGGs.1,20 However, LGGs are commonly located in eloquent areas of the brain.9 In our series, more than half of patients harbored tumors that directly involved areas that were located in or adjacent to presumed eloquent areas, which underscores the importance of tumor location for treatment planning and prognosis. Tumor location in eloquent areas of the brain can influence survival through neurological impairment due to malignant transformation and/or by precluding complete debulking of tumor burden. Subtotal resection is associated with recurrence of gliomas of a higher grade.3,29 Sawaya et al.23 examined 327 patients with both primary and secondary brain tumors, including 40 LGGs, and found that eloquent location was associated with increased deficits in the immediate postoperative period, but the effect on long-term survival was not assessed.
The current study demonstrates that intraoperative functional mapping can improve long-term survival for patients with LGGs. The proportion of cases that resulted in a gross-total resection increased, as did the percentage extent of tumor resection. Because extent of resection has previously been shown to be an important predictor of long-term survival,13,17,28 it is not surprising to find that functional mapping also plays an important role in survival, as it is the principal method by which resection can be maximized while being carried out safely. Surgeon skill and experience has an important role when considering aggressive resection of LGGs with ill-defined borders. Increased extent of resection prolongs survival in part by decreasing the risk of transformation into a higher-grade tumor.19
Intraoperative functional mapping can improve long-term survival associated with LGGs located in eloquent brain regions. Magnetic resonance imaging can usually clearly demonstrate the location of the pre- and postcentral gyrus in the normal setting, but tumors can distort the normal anatomy of this area, necessitating intraoperative localization of these eloquent areas. If tumors are mapped, gross-total or subtotal resection of LGGs located in somatosensory cortex can be accomplished with minimal deficits.10 Tumors located in supplementary motor areas can be fully resected with transient postoperative deficits that recover with time.21 Similarly, language cortex can be located outside of the Broca and Wernicke areas; thus, intraoperative mapping is essential in preventing aphasia.11,22 When mapping is employed, tumors located in presumed speech areas can be completely resected without permanent deficits.2 Smaller intraoperative mapping studies have demonstrated functional tissue within the confines of tumor tissue in both low- and high-grade glial tumors.18,27 A recent study using magnetic source imaging has confirmed functional activity within LGGs.25 In patients with functional tissue located within the tumor who underwent gross-total resection, all had new neurological deficits postoperatively.
The role of other mapping methods, such as functional MR imaging, magnetic source imaging, or diffusion tensor tractography, was not directly evaluated in our analyses. While these methods have demonstrated utility in identifying regions involved in a given neurological function, they do not evaluate whether those areas are critical for carrying out that function. This distinction is important since resection of some functional involved areas can be tolerated with minimal observable effects, because either the function is widely distributed across the cortical network or because of intrinsic redundant circuitry. At our own institution, these other mapping methods are primarily used for preoperative planning, and sometimes to help guide, but they never replace electrocortical stimulation.
Currently, intraoperative cortical mapping is used at selected centers and is not universally employed during the resection of LGGs located in eloquent cortex. Intraoperative mapping has previously been shown to be safe.5,24 Recent studies have also shown it to be cost-effective as patients require shorter periods of hospitalization and less time in the intensive care unit.30 Based on the results of the present study, we recommend the routine use of intraoperative mapping during the resection of LGGs located in presumed eloquent cortex to provide the best possible long-term survival benefit.
Conclusions
Presumed eloquent location of LGGs is an important predictor of OS and PFS. This is the first demonstration that the use of functional stimulation mapping can significantly modify long-term survival as it relates to presumed eloquent tumor location. These effects are likely to be mediated through maximization of the extent of resection and reduction of tumor burden. Therefore, the standard of care for patients with LGGs in presumed eloquent locations necessitates intraoperative mapping; to do otherwise deprives patients of precious survival time.
Abbreviations used in this paper
- HR
hazard ratio
- HSD
honestly significant difference
- KPS
Karnofsky Performance Scale
- LGG
low-grade glioma
- OS
overall survival
- PFS
progression-free survival
- UCSF
University of California, San Francisco
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: EF Chang. Acquisition of data: EF Chang, Clark, Smith, SM Chang, Barbaro, Parsa, McDermott. Analysis and interpretation of data: EF Chang, Clark, Smith, Polley, SM Chang, Barbaro, McDermott. Drafting the article: EF Chang, Clark, Polley, SM Chang, Barbaro, McDermott. Critically revising the article: EF Chang, Clark, Smith, Polley, SM Chang, Barbaro, Berger. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: EF Chang, Polley, SM Chang. Administrative/technical/material support: EF Chang, SM Chang, McDermott, Berger. Study supervision: EF Chang, Berger.
References
- 1.Bauman G, Lote K, Larson D, Stalpers L, Leighton C, Fisher B, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45:923–929. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 2.Benzagmout M, Gatignol P, Duffau H. Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations. Neurosurgery. 2007;61:741–753. doi: 10.1227/01.NEU.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- 3.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Berger MS, Rostomily RC. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34:85–101. doi: 10.1023/a:1005715405413. [DOI] [PubMed] [Google Scholar]
- 5.Cedzich C, Taniguchi M, Schäfer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962–970. doi: 10.1097/00006123-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 7.Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, et al. Preoperative prognostic classification system for adult hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817–824. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 8.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 9.Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622–2626. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 10.Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Lopes M, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Janny P, Cure H, Mohr M, Heldt N, Kwiatkowski F, Lemaire JJ, et al. Low grade supratentorial astrocytomas. Management and prognostic factors. Cancer. 1994;73:1937–1945. doi: 10.1002/1097-0142(19940401)73:7<1937::aid-cncr2820730727>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 14.Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735–745. doi: 10.3171/jns.2001.95.5.0735. [DOI] [PubMed] [Google Scholar]
- 15.Lote K, Egeland T, Hager B, Stenwig B, Skullerud K, Berg-Johnsen J, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 16.Matz PG, Cobbs C, Berger MS. Intraoperative cortical mapping as a guide to the surgical resection of gliomas. J Neurooncol. 1999;42:233–245. doi: 10.1023/a:1006122122404. [DOI] [PubMed] [Google Scholar]
- 17.McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–708. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 18.Ojemann JG, Miller JW, Silbergeld DL. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39:253–259. doi: 10.1097/00006123-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Piepmeier J, Christopher S, Spencer D, Byrne T, Kim J, Knisel JP, et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996;38:872–879. doi: 10.1097/00006123-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 21.Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 23.Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1056. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 24.Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TP. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg. 2002;97:1333–1342. doi: 10.3171/jns.2002.97.6.1333. [DOI] [PubMed] [Google Scholar]
- 25.Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TP. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;49:1313–1321. doi: 10.1097/00006123-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R. et al: Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 27.Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678–685. [PubMed] [Google Scholar]
- 28.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 29.Talos IF, Zou KH, Ohno-Machado L, Bhagwat JG, Kikinis R, Black PM, et al. Supratentorial low-grade glioma resectability: statistical predictive analysis based on anatomic MR features and tumor characteristics. Radiology. 2006;239:506–513. doi: 10.1148/radiol.2392050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 31.van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. et al: Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]