Abstract
Osteoarthritis (OA) of the knee is often characterized by joint space narrowing on X-ray, knee pain, and a loss of joint function through progressive cartilage degradation and intermittent synovial inflammation. The objective of this work was to develop an in vitro model in a clinically relevant system. Normal human synovial fibroblasts were cultured with U937 cells for 3 days then combined with a chondrogenic stem cell pellet for another 4 days. This culture system mimicked many of the aspects of early stage OA including production of cytokines and degradative enzymes, MMP-1 and MMP-3, resulting in a conditioned medium profile similar to OA synovial fluid. This catabolic environment resulted in the release of glycosaminoglycan (GAG) from the pellet. In a similar manner to early stage OA, the pellet had increased aggrecan and collagen II expression. All of these effects are hallmarks of early stage OA. This relatively simple tissue model containing a 3D cartilage component interacting with synoviocytes and macrophages could be useful to understand early causes and progression of OA. It can be scaled easily thus useful for high throughput screening of disease modifying drugs in a clinically relevant system.
Keywords: Arthritis, Cartilage tissue engineering, Modeling, Macrophage, Fibroblast
1. Introduction
Osteoarthritis (OA) of the knee, characterized most prominently by knee pain and eventual loss of joint function [1] occurs with increasing frequency and severity with age and remains a signifi-cant unmet medical need worldwide. It is the most common form of arthritis in the United States, an estimated 27 million adults had OA in 2005 [2]. Treatment of both pain and structural damage progression is challenging, with current pain therapies providing inconsistent benefit and treatments to halt or slow loss of structural integrity nonexistent [3,4].
Although the facets of OA are not well understood, it is clear that the disease represents a complex combination of metabolic processes affecting the cartilage, underlying bone, synovium, and surrounding tissues [5,6] of articular joints. This complexity makes it difficult to develop accurate models. There are animal models that are used for studying OA including surgical instability models, such as anterior cruciate ligament (ACL) transection, genetic models such as the STR/ort mouse model, and spontaneous models, such as the Duncan Hartley guinea pig [7–9]. Given the cost, time, and complexity associated with performing in vivo studies, preliminary study of disease biology as well as identification, validation and testing of potential therapeutic targets can benefit greatly from studies initially performed in vitro in well designed models ideally translatable to both in vivo research and clinical settings.
In vitro, OA is most often studied through monolayer cultured primary cells exposed to high concentrations of cytokines or chemokines [10]. In contrast to complex in vivo models, cell culture can provide a simplified, cost-effective and focused analysis. However, this approach can produce results that are overly simplified or less relevant to the in vivo situation. With the analysis focused on a limited number and/or types of cells, the signals from key cells necessary to duplicate clinical outcomes may be missing in these cultures.
We previously reported the utility in combining tissue engineered cartilage with conditioned medium from macrophages as compared to monolayer cultures with supra-physiological concentrations of cytokines [11]. Although this work was done with very high concentrations of cytokines not matching physiological levels present in OA, this result showed that the inclusion of macrophages, cells present in OA joints [12], created a more realistic in vitro model of the disease. Macrophages and fibroblasts communicate via soluble autocrine, paracrine and juxtacrine signals associated with direct cell–cell contacts [13–15]. Hence, both chemical and physical cues exchanged between macrophages and fibroblasts can be important in OA. Further, it can be beneficial to use a 3D human tissue system, as cell–cell and cell–extracellular matrix interactions are important for the study of cartilage, and these conditions are poorly reflected by conventional two-dimensional (2D) cell culture systems [16].
Our hypothesis was that a 3D cartilage component interacting with synoviocytes as well as macrophages would simulate a disease environment similar to that present in developing OA. Further, having all human-derived cells would allow this in vitro OA model to be useful to understand the formation and progression of OA as well as screening for disease modifying drugs in a clinically relevant system. The development of this system as a model of early stage OA was judged based on three criteria: the production of cytokines, including IL-8 and MCP-1, and degradative enzymes, MMP-1 and MMP-3, resulting in a conditioned medium profile similar to OA synovial fluid, the release of glycosaminoglycan (GAG) from the cartilage component, and an early anabolic response as measured by increased aggrecan and collagen II expression.
2. Materials and methods
2.1. Cell preparation and expansion culture
Human synovium from the knee (donor age – 73) was obtained from Articular Engineering (Northbrook, IL). This donor was identified as “normal,” having had no prior documented history of osteoarthritis or knee pain. Cells were isolated from this tissue by overnight treatment at 37 °C with 1.0 mg/mL collagenase (Sigma – St. Louis, MO). The cells were expanded through 3 passages in standard culture medium, High Glucose Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) obtained from Invitrogen (Carlsbad, CA). The cells were passaged at 80–90% confluency. The three passages effectively eliminated all synovial macrophages from the synovial fibroblasts, as three passages are sufficient to enrich synovial fibroblasts to >95% of the cells [17]. THP-1 and U937 cells were obtained from ATCC (Manassas, VA). Cells were expanded in RPMI-1640 with 10% FBS (Invitrogen). Cells were expanded between 200,000 and 1,000,000 cells/mL with full medium change every 2–3 days. Human mesenchymal stem cells (MSC) were obtained from Lonza (Walkersville, MD). Cells were expanded two passages in standard culture medium, Low Glucose DMEM with 10% FBS and 10 ng/mL of basic fibroblast growth factor (bFGF) from R&D Systems (Minneapolis, MN). The cells were passaged at 80–90% confluency.
2.2. Pellet cultures
MSC pellets were formed in a similar manner as described by Penick et al. [18]. Briefly, cells are resuspended in defined chondrogenic medium containing high glucose DMEM with Penicillin (10,000 U/mL – Invitrogen) and streptomycin (10,000 μg/mL – Invitrogen) supplemented with 1% ITS+ (BD Biosciences – Bedford, MA), 50 μg/mL ascorbic acid (Sigma), 10–7 M dexamethasone (Sigma) and 5 ng/mL TGF-b2 (R&D Systems). The cells were adjusted to 1.25 × 106 cells/mL. Two hundred microliter aliquots were dispensed into a sterile 96-well polypropylene microplate (BD Biosciences). The plate was spun for 5 min at 500× g and incubated at 37 °C. Twenty four hours after seeding, pellets were released from the bottom of the well by gently removing and expelling the medium back into each well. Media was replaced every 2–3 days with a fresh 200 μL of the chondrogenic medium. The pellets were cultured for 2 weeks when they were then used in coculture experiments with synovial fibroblasts, U937 cells, or both.
2.3. Coculture
Cocultures were plated in a similar to manner to Chen et al. [19]. First, synovial fibroblasts were plated in 3 micron transwell inserts (BD Biosciences) at 25,000 cells/cm2 in DMEM with 10% FBS and allowed to adhere overnight. Monocytes (THP-1 or U937) were resuspended in DMEM with 10% FBS and seeded on top of the synoviocytes at 100,000 cells/cm2. These cells were cultured together for 3 days. After the 3 days, the media was removed and the cells (synovial fibroblasts and monocytes) were washed with phosphate buffered saline (PBS). At this point, the media was switched to serum free DMEM with or without the addition of a stem cell pellet, as described above, added to the well outside the transwell insert. After 4 days, the cultures were terminated with the media and cells collected further analysis. At times, it was necessary to analyze the monocyte cells separately from the synovial fibroblasts. These two cell types were separated by treating the cocultured synovial fibroblasts and monocytes with ice cold 2 mM ethylenediaminetetraacetic acid (EDTA – Invitrogen) for 5 min. This released the monocytes, while leaving the synovial fibroblasts adhered to the inserts.
2.4. Macrophage marker gene expression in THP-1 and U937 cells
In order to explore the activation of the two monocyte cell lines, THP-1 and U937 cells were assayed for macrophage marker gene expression. CD14 and CD80 expression levels were investigated in conditions of monocytes cultured alone or combined in a coculture with synoviocytes as described above. When necessary, monocytes were separated from synoviocytes using EDTA. Further, U937 cells were cultured under four conditions to test for potential inducers of CD80 gene expression up-regulation. In order to investigate whether cell–cell contact might be important, plates were coated with recombinant N-Cadherin (R&D Systems-cell culture grade, free of endotoxin), 10 μg/mL, 100 μL/well overnight. At the same time, human synoviocytes were plated in transwell inserts at 25,000 cells/cm2 in a similar manner as above and allowed to adhere overnight. The next day, all media was removed and plates/transwell inserts were washed with PBS. U937 cells were then plated at 100,000 cells/cm2 in each of four conditions in DMEM. U937 alone wells contained just the U937 cells, U937/Syn Together wells contained U937 cells seeded upon the synoviocytes in the inserts in a similar manner as above, U937/Syn Separate wells contained U937 cells seeded outside the transwell inserts containing synoviocytes such that they could interact but not directly contact the synoviocytes, U937 + N-Cadherin wells were seeded upon the N-Cadherin coated wells. After 3 days, U937 cells were harvested and RNA isolated for message expression analysis.
2.5. Protein analysis
Conditioned media from cultures of U937/synoviocytes and THP-1/synoviocytes in the absence of a cartilage component, i.e. the stem cell pellet, were analyzed for proteins commonly found in OA synovial fluid, including IL-8 [20,21], MCP-1 [22,23], MIP-1α [24,25], RANTES [25,26], VEGF [27,28], MMP-1 [29], and MMP-3 [29,30]. The cell types were then compared to each other to select one to move forward with in the coculture system. In order to place the values in context, concentrations of these proteins found in OA synovial fluid as reported in the literature were included in the comparison. Later, conditioned media from the full coculture system was analyzed for these same proteins. To confirm these protein concentrations in the culture medium, ELISA kits (R&D Systems) were used following the protocols provided. Briefly, conditioned medium was taken from the culture wells and added into antibody pre-coated microplates. The media was incubated for 2 h, then enzyme-linked polyclonal antibody specific for target molecules was added to the well, followed by a substrate solution for color development. At each step, unbound material was washed away. Color intensity was measured at 450 nm with a wavelength correction set to 540 nm.
2.6. GAG and DNA analyses
Cell pellets were digested overnight at 60° in Proteinase K (Sigma) at 0.5 mg/mL. This digest was then evaluated for Glycosaminoglycan (GAG) and DNA content. GAG content was used as a measure of the chondrogenic pellet integrity and was determined by evaluating the binding of 1,9 dimethylmethylene blue (Sigma), a cationic blue dye, to sulfated GAG in solution as described by Farnadale et al. [31]. When bound, the dye absorbs at 520 nm, and can be detected using a spectrophotometer. The absorbance was then compared to a standard curve of chondroitin sulfate from shark cartilage (Sigma). In order to normalize the pellets, DNA content, as a measure of cell number, was evaluated using the Quant-iT™ PicoGreen® dsDNA assay kit (Invitrogen) following the manufacturer’s instructions. Briefly, Proteinase K digests were combined with Quant-iT™ PicoGreen® dsDNA reagent and excited at 480 nm. The fluorescence emission intensity was measured at 520 nm using a spectrofluorometer. These values were compared to a standard curve of dsDNA supplied in the kit.
2.7. RNA isolation and gene expression analysis
Total RNA from pellets or macrophages was isolated and purified using the Promega SV Total RNA Isolation System according to the manufacturer’s directions (Promega – Madison, WI). cDNA was reverse transcribed from RNA using the qScript™ cDNA SuperMix (Quanta BioSciences – Gaithersburg, MD) according to the manufacturer’s directions. TaqMan Gene Expression assay kits (Applied Biosystems) were used to analyze transcription levels of the matrix-related genes: aggrecan (ACAN NM_013227.2) and collagen type II (Col2 NM_001844) or macrophage markers CD14 (NM_000591.3) and CD80 (NM_005191.3). Gene expression was normalized to GAPDH (NM_002046.3).
2.8. Statistical analysis
Data from all samples within each group were combined with means ± S.E.M. determined. Data were averaged from three independent experiments each containing at least 4 replicates. Comparisons were made using a t test or one-way ANOVA. Where significant differences among groups were detected, an all-pair wise multiple comparison procedure (Tukey test) was performed to determine which groups were significantly different from one another. Significance was set at p < 0.05.
3. Results
3.1. Cocultures of U937s compared to THP-1s are more similar to OA synovial fluid
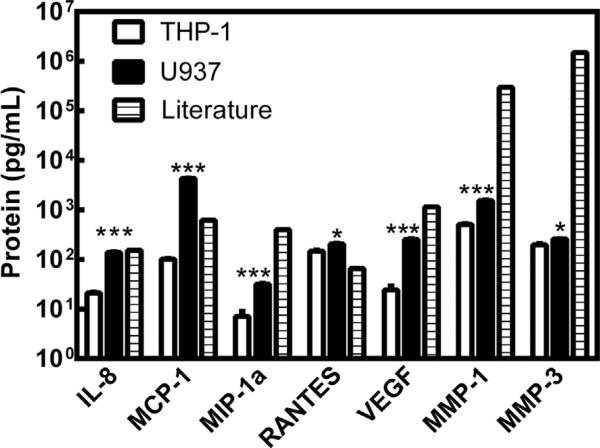
As shown in Fig. 1, both coculture systems produced these proteins to varying degrees. In both cases IL-1β and TNF-α were not detected in the conditioned media (data not shown). For each protein, U937 cocultures produced significantly more of the cytokine or degradative enzyme. The U937 cultures appeared to be more similar to published data for OA synovial fluid, thus further analysis focused on this cell type.
Fig. 1.
Protein production in coculture model systems compared to OA synovial fluid [20–30]. White bars represent synoviocytes cultured with THP-1 cells. Black bars represent synoviocytes cultured with U937 cells. Hatched bars represent measured values of the proteins in OA synovial fluid as reported in the literature. Statistical comparisons are between THP-1 cocultures and U937 cocultures for each protein. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
3.2. U937 cocultures increase CD80 expression possibly due to cell–cell contact
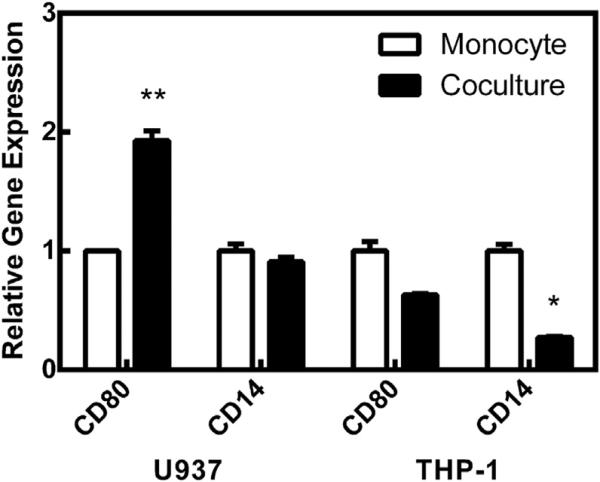
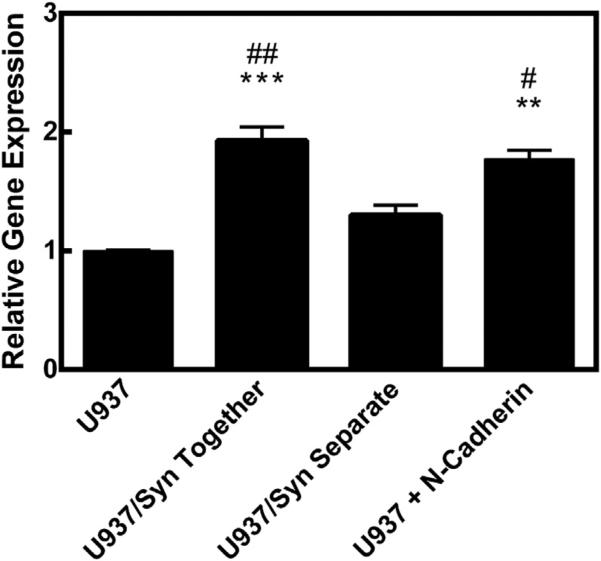
As described above, monocytes were separated from the synoviocytes to be analyzed for gene expression of common macrophage markers, CD80 and CD14. As shown in Fig. 2, U937 cells cultured with synoviocytes increased CD80 expression compared to U937s alone. There was no change in CD14 expression for these cells. In contrast, THP-1 cells did not significantly change expression of CD80, but there was a significant down-regulation in CD14 expression. In this figure, the expression was normalized to the specific monocyte alone. This up-regulation of CD80 expression could be explained by cell–cell contact. U937 cells cultured in the presence of recombinant N-Cadherin significantly increased CD80 expression in a similar manner to U937 cocultures with synoviocytes (Fig. 3).
Fig. 2.
Relative gene expression of monocyte cells. White bars represent the monocytes cultured alone. Black bars represent monocytes cultured with synoviocytes. Statistical comparisons are between coculture and monocyte alone groups for each gene. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
Fig. 3.
CD80 relative gene expression. ** = p < 0.01, *** = p < 0.001 when compared to U937 (alone) group. # = p < 0.05, ## = p < 0.01 when compared to U937/Syn Separate group.
3.3. Cocultures significantly increase OA protein production
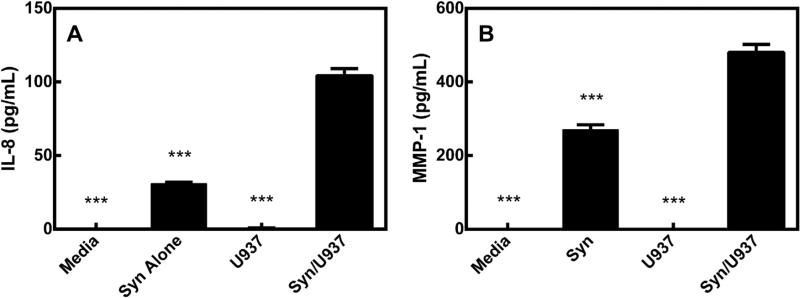
The OA-associated proteins assayed were produced in the pellet-containing systems at similar levels to the cocultures alone. Further, these proteins were significantly increased in the synoviocyte/U937 coculture system as compared to the other groups (Table 1). IL-8 and MMP-1 are representative proteins from this group (Fig. 4). Conditioned media from pellets alone or pellets cultured with U937 cells did not contain appreciable concentrations of most of these proteins. Media from pellets in the presence of synoviocytes contained most of these proteins, but media from the full coculture (pellets, synoviocytes and U937 cells) produced the highest levels.
Table 1.
Production of OA proteins in pellet-containing culture systems.
| IL-8 | MCP-1 | MIP-1α | RANTES | VEGF | MMP-1 | MMP-3 | |
|---|---|---|---|---|---|---|---|
| Media | ND*** | 7.1 ± 0.5*** | 11.3 ± 2.7*** | ND*** | 10.2 ± 4.2*** | ND*** | 175 ± 43.1*** |
| Syn | 30.7 ± 1.0*** | 407 ± 18.1*** | 11.1 ± 2.0*** | 9.3 ± 0.6*** | 47.1 ± 3.1*** | 270 ± 13.6*** | 554 ± 39.7 NS |
| U937 | ND*** | 7.5 ± 0.8*** | 10.6 ± 1.7*** | ND*** | 12.2 ± 4.0*** | ND*** | 87.6 ± 21.8*** |
| Syn/U937 | 104 ± 4.8 | 3530 ± 330 | 24.4 ± 1.8 | 132 ± 7.2 | 208 ± 5.3 | 480 ± 22.1 | 524 ± 51.5 |
Each row represents a different pellet-containing culture system. Media = media alone, Syn = synoviocytes alone, U937 = U937 cells alone, Syn/U937 = full coculture. The actual protein concentrations ± SEM (pg/mL) for each group are shown in that corresponding row. Each group is compared to the Syn/U937 group with
*= p < 0.05
**= p < 0.01
= p < 0.001 when compared to Syn/U937 group. ND = not detected. NS = Not significant. IL-1β and TNF-α were not detected in any group.
Fig. 4.
A) IL-8 in conditioned media from pellet-containing cultures. B) MMP-1 in conditioned media from pellet-containing cultures. Each bar represents a group containing pellets cultured with the corresponding title. Media = media alone, Syn = synoviocytes alone, U937 = U937 cells alone, Syn/U937 = full coculture. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 when compared to Syn/U937 group.
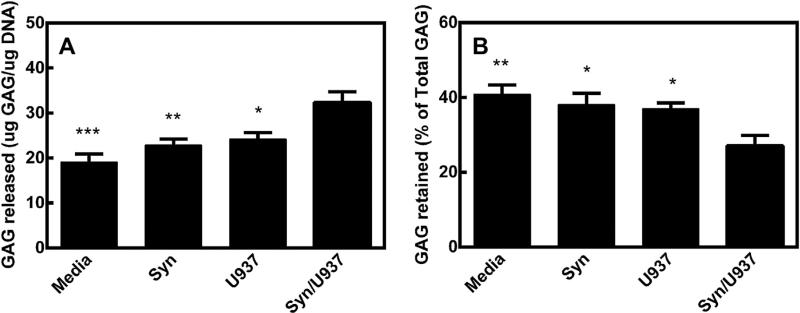
3.4. U937 cocultures cause pellets to release GAG
As shown in Fig. 5, there was a significant increase in GAG released when comparing the pellets with the coculture to pellets alone, pellets with synoviocytes alone, and pellets with U937 cells alone. In a similar manner, the pellets with the cocultures retained a significantly lower amount of GAG in the pellet as compared to the other groups. There were no significant differences in the DNA content of the pellets and each contained within 10% of the same amount of DNA (data not shown), indicating similar numbers of cells within each pellet.
Fig. 5.
A) GAG released to the conditioned media relative to DNA content of pellets. B) GAG retained in the pellet relative to total GAG (retained + released). Each bar represents a group containing pellets cultured with the corresponding title. Media = media alone, Syn = synoviocytes alone, U937 = U937 cells alone, Syn/U937 = full coculture. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 when compared to Syn/U937 group.
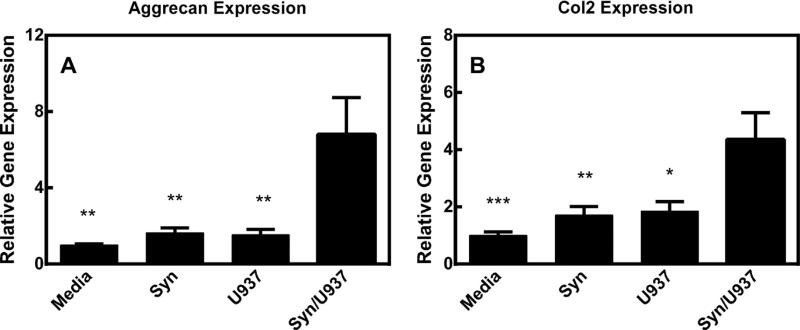
3.5. Pellets with coculture increase aggrecan and collagen II gene expression
As shown in Fig. 6, pellets cultured in the presence of the synoviocyte/U937 coculture significantly increased gene expression of matrix proteins, aggrecan and collagen II. This change is significantly increased with regard to pellets alone, pellets with synoviocytes or pellets with U937s.
Fig. 6.
A) Aggrecan relative gene expression in pellet cultures. B) Collagen II relative gene expression in pellet cultures. Each bar represents the gene expression from pellets cultured with the corresponding title. Media = media alone, Syn = synoviocytes alone, U937 = U937 cells alone, Syn/U937 = full coculture. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 when compared to Syn/U937 group.
4. Discussion
Synovial and cartilage destruction are hallmarks of OA. By understanding the mechanism of this destruction, we can develop more effective therapies to treat this disease. In this study, we examined an in vitro model of human synoviocytes, U937 cells, a human monocytic cell line, and human mesenchymal stem cell pellets. Our results show that when synoviocytes and macrophages are cultured together, they can cause the neocartilage to release glycosaminoglycan (GAG). Further, these cultures mirror many of the hallmarks of early-stage OA and as such could represent an in vitro screening tool with predictive outcomes of clinical OA treatment with candidate therapeutics.
Early stage OA is a complex combination of both a catabolic and anabolic environment marked by degradation of cartilage and increases in degradative enzymes and proinflammatory cytokines. Matrix metalloproteinases play a large role in the destruction of the osteoarthritic joint. MMP-1, a collagenase, and MMP-3, an aggrecanase, are present in high levels in OA synovial fluid (Fig. 1). Osteoarthritic cartilage has been shown to increase gene expression of these MMPS as well [32,33]. The cartilage then undergoes a loss of GAG and collagen II content [34], likely due to this increase in aggrecanase and collagenase activity. In response to this GAG and collagen loss, the cartilage responds by increasing aggrecan and collagen II gene expression [35,36].
In our studies, we found our system responded in a very similar manner compared to early stage OA. Both MMP-1 and MMP-3 production in the coculture were increased (Table 1 and Fig. 4). In this way, the aggrecanase and collagenase activity was present similarly to OA. In this catabolic environment, the cartilage component, the pellet, released GAG over the 4 day culture (Fig. 5). The pellet had increased expression of aggrecan and collagen II (Fig. 6). This increase in aggrecan expression did not replace the GAG lost to the media as evidenced by the decrease in GAG retained over time. It should be noted that synoviocyte/U937 cultures in the absence of a pellet did not release detectable GAG to the medium. Further, this model offers many additional advantages. It could be scaled to screen potential disease modifying targets. Besides producing many of the hallmarks of early stage OA, the culture includes the interaction between synovium and cartilage, two crucial aspects of the joint. Finally, the model does not need a supra-physiological dose of IL-1β or TNF-α. The concentration of IL-1β and TNF-α in OA synovial fluid is extremely low and in most cases undetectable [37,38].
An interesting result of these studies was the comparison of THP-1 and U937 cells. Both cell lines are long established [39,40] and have been used as monocytic cells that can be activated by the addition of different chemicals [41]. In our hands, when these cells were cultured upon synovial fibroblasts, both cocultures produced many proteins common to OA synovial fluid. It appeared that U937 cocultures were able to produce an environment closer to OA synovial fluid (Fig. 1) and thus were used in the resulting complete coculture studies. Although, the reason for this difference was beyond the scope of these studies, expression of CD80, a macrophage activation marker [42], suggests U937 cells became activated when cultured with the synovial fibroblasts unlike the THP-1 cells (Fig. 2). The data suggests cell–cell contact is important for this activation and that this could be mediated through N-Cadherin (Fig. 3). N-Cadherin, but not E-Cadherin, is found on activated macrophages [43]. N-Cadherin is also on synovial fibro-blasts and in OA synovium [44]. Given that cell adhesion is mediated by the homodimerization of cadherins, N-Cadherin exists on both the synoviocytes and macrophage cells, and that exogenous N-Cadherin increased CD80 expression in a similar manner as the contact coculture, it’s possible that this activation was through the N-Cadherin interaction. More work would have to be done to further elucidate this mechanism.
The coculture model presented here includes three relevant cell types in OA, synovial fibroblasts, macrophages, and a cartilage component. In previous work, contact between the synovium and cartilage components was necessary to initiate GAG loss [45]. A similar coculture model was shown to degrade a cartilage explant in the presence of the catabolic stimulant, fibronectin fragment [46]. In that model, the cocultured synovial fibroblasts and U937 cells were separated from the explants and did not degrade the cartilage significantly without the addition of fibronectin fragment. It should be noted that explants with fibronectin fragment alone lost significant GAG content with no significant difference with the addition of the coculture. Many of the OA proteins and specifically MMP concentrations were substantially higher with fibronectin fragment than in the current model shown above. In the current model, degradation occurred without contact of the two components or an additional catabolic stimulant. It remains to be seen if this is due to using a stem cell pellet, i.e. neocartilage, in contrast to a cartilage explant. Stem cells have been shown to be able to be expanded extensively and retain the ability to differentiate, thus the reason used in this study [47]. However, expanded chondrocytes or a cartilage explant could be explored as well. A cartilage explant contains more matrix and would seem to be more robust and thus less likely to degrade. The advantage to keeping the components separated is to maintain the ability to analyze each component independently. In this way, the contribution of each type can be at least partially determined.
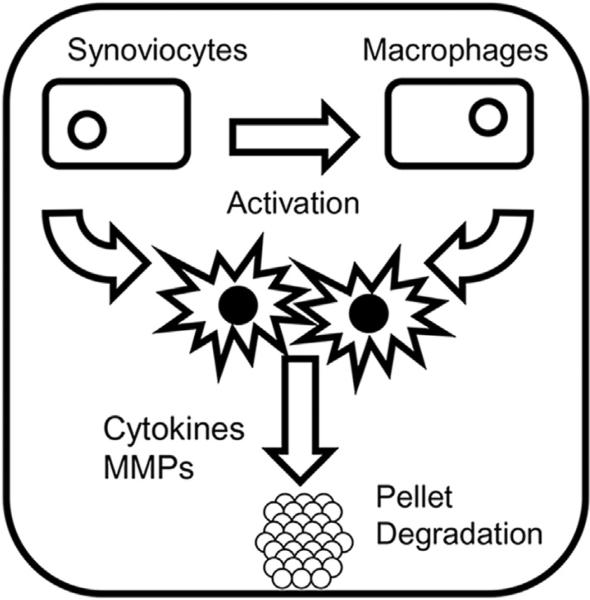
The synoviocytes increased production of OA proteins, but not to the level of the synoviocyte/U937 cultures. Synoviocytes cultured with pellets also did not result in GAG loss. As described above, our lab showed the importance of activated macrophages in mimicking early stage OA. Although in the two models the activation was accomplished in a different manner, activation via the addition of cytokines and activation by culture with synoviocytes, the result is similar nonetheless. It appears the synoviocytes activate the macrophages, which in turn also activate the synoviocytes. Together, these cells release cytokines and MMPs that degrade the pellet (Fig. 7). Our cultures were designed to be short term, but perhaps longer term cultures would be more instructive to study chronic effects versus acute ones. OA is a chronic disease with a long onset, thus more work could be done exploring these long term effects by using extended timelines in this system. It should be noted that for the synovial fibroblasts and stem cells, one donor was used in the present study. It has been previously established that stem cell pellets will form a chondrogenic pellet with extracellular matrix containing aggrecan and collagen type II within two weeks [48]. It has also been shown by Chen et al. [19], Scott et al. [45], and DiBenedetto et al. [46] that the culture of synovial fibroblasts with U937 cells will produce proinflammatory cytokines and MMPs. This it can be hypothesized that similar results to those shown here could be attained using multiple donors for the stem cells and synovial fibroblasts. This can be addressed in future studies. Currently, we developed a human cell based model of OA containing a 3D cartilage component interacting with synoviocytes and macrophages. This model appears to mimic many of the facets of early stage OA and could be useful to understand the progression of OA. It also can be useful for screening of disease modifying drugs in a clinically relevant system.
Fig. 7.
Scheme for the current model system. Synoviocytes activate macrophages and then the combined cells release cytokines and MMPs capable of degrading the chondrogenic pellets.
5. Conclusions
A human cell based model of early stage osteoarthritis containing a 3D cartilage component interacting with synoviocytes and macrophages was developed. The interaction of these components simulated a disease environment similar to that present in developing OA. This included the production of cytokines, such as IL-8 and MCP-1, and degradative enzymes, MMP-1 and MMP-3, resulting in a conditioned medium profile similar to OA synovial fluid, the release of glycosaminoglycan from the cartilage component, and an early anabolic response as measured by increased aggrecan and collagen II expression. Further, this disease state was produced in the absence of a catabolic stimulant not present in OA, i.e. a supra-physiological dose of IL-1β or TNF-α. Finally, the coculture model presented here included three relevant cell types in OA that could easily be separated maintaining the ability to analyze each component independently. In this way, the contribution of each type can be at least partially determined. This coculture appears to model many of the facets of early stage OA and could be useful to understand the progression of OA. It also can be useful for screening of disease modifying drugs in a clinically relevant system.
Acknowledgments
The authors would like to thank Michael DiMicco and Peter DiBenedetto at Genzyme for their intellectual and technical input. The authors thank the NIH for support through the Tissue Engineering Resource Center (P41 EB002520, R01 AR005593 and R01 AR061988).
References
- 1.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recommendations for the medical management of osteoarthritis of the hip and knee. 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Dieppe P, Brandt KD, Lohmander S, Felson DT. Detecting and measuring disease modification in osteoarthritis. The need for standardized methodology. J Rheumatol. 1995;22:201–3. [PubMed] [Google Scholar]
- 6.Kuettner KE, Goldberg VM, editors. Osteoarthritic disorders. American Academy of Orthopaedic Surgeons Symposium; Rosemont, Illinois: 1995. [Google Scholar]
- 7.Appleton CT, McErlain DD, Pitelka V, Schwartz N, Bernier SM, Henry JL, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9:R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts MJ, Adams SB, Jr, Patel NA, Stamper DL, Westmore MS, Martin SD, et al. A new approach for assessing early osteoarthritis in the rat. Anal Bioanal Chem. 2003;377:1003–6. doi: 10.1007/s00216-003-2225-2. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci. 1997;47:598–601. [PubMed] [Google Scholar]
- 10.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of MMP-13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Wang X, Kaplan DL. A 3D cartilage - inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials. 2011;32:5581–9. doi: 10.1016/j.biomaterials.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–7. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 13.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9:39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Li J, Zhang J. STAT3-decoy ODN inhibits cytokine autocrine of murine tumor cells. Cell Mol Immunol. 2007;4:309–13. [PubMed] [Google Scholar]
- 15.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch T. Cell-cell and cell-matrix interactions during development and pathogenesis. Curr Opin Orthop. 2006;17:387–9. [Google Scholar]
- 17.Rosengren S, Boyle DL, Firestein GS. Acquisition, culture, and phenotyping of synovial fibroblasts. Methods Mol Med. 2007;135:365–75. doi: 10.1007/978-1-59745-401-8_24. [DOI] [PubMed] [Google Scholar]
- 18.Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Bio-techniques. 2005;39:687–91. doi: 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen V, Croft D, Purkis P, Kramer IM. Co-culture of synovial fibroblasts and differentiated U937 cells is sufficient for high interleukin-6 but not interleukin-1b or tumour necrosis factor-a release. Br J Rheumatol. 1998;37:148–56. doi: 10.1093/rheumatology/37.2.148. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–9. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 21.Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, et al. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147:2187–95. [PubMed] [Google Scholar]
- 22.Stankovic A, Slavic V, Stamenkovic B, Kamenov B, Bojanovic M, Mitrovic DR. Serum and synovial fluid concentrations of CCL2 (MCP-1) chemokines in patients suffering rheumatoid arthritis and osteoarthritis reflect disease activity. Bratisl Lek Listy. 2009;110:641–6. [PubMed] [Google Scholar]
- 23.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–9. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, et al. Macrophage inflammatory protein-1a. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 26.Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 27.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–4. [PubMed] [Google Scholar]
- 28.Koch AE, Harlow LA, Haines GK, Unemori EN, Wong WL, Pope RM, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–56. [PubMed] [Google Scholar]
- 29.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–61. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribbens C, Andre B, Kaye O, Kaiser MJ, Bonnet V, Jaspar JM, et al. Synovial fluid matrix metalloproteinase-3 levels are increased in inflammatory arthritides whether erosive or not. Rheumatology. 2000;39:1357–65. doi: 10.1093/rheumatology/39.12.1357. [DOI] [PubMed] [Google Scholar]
- 31.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 32.Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–9. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–89. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007:213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 35.Eid K, Thornhill TS, Glowacki J. Chondrocyte gene expression in osteoarthritis: correlation with disease severity. J Orthop Res. 2006;24:1062–8. doi: 10.1002/jor.20137. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther. 2005;7:R156–65. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagura SMC, Thomas SG, Woodhouse LJ, Ezzat die S, Marks P. Circulating and synovial levels of IGF-I, cytokines, physical function and anthropometry differ in women awaiting total knee arthroplasty when compared to men. J Orthop Res. 2005;23:397–405. doi: 10.1016/j.orthres.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of Interleukin-6, Interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 39.Sundström C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976;17:565–77. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26:171–6. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 41.Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983;62:709–72. [PubMed] [Google Scholar]
- 42.Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996;89:592–8. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon CA, Johnson JL, Williams H, Sala-Newby GB, George SJ. Soluble N-cadherin overexpression reduces features of atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2009;29:195–201. doi: 10.1161/ATVBAHA.108.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal SK, Lee DM, Kiener HP, Brenner MB. Coexpression of two mesenchymal cadherins, cadherin 11 and N-cadherin, on murine fibroblast-like synoviocytes. Arthritis Rheum. 2008;58:1044–54. doi: 10.1002/art.23369. [DOI] [PubMed] [Google Scholar]
- 45.Scott BB, Weisbrot LM, Greenwood JD, Bogoch ER, Paige CJ, Keystone EC. Rheumatoid arthritis synovial fibroblast and U937 macrophage/monocyte cell line interaction in cartilage degradation. Arthritis Rheum. 1997;40:490–8. doi: 10.1002/art.1780400315. [DOI] [PubMed] [Google Scholar]
- 46.DiBenedetto P, Chen F, Yu Q, DiMicco M, Matthews G. Generation of osteoarthritis-like cytokine profiles using an in vitro coculture model.. Poster session presented at: World Congress on Osteoarthritis; Rome, Italy. 2008 Sep 18-21. [Google Scholar]
- 47.Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells:choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–66. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 48.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]