Abstract
Plerixafor, given on day 4 of G-CSF treatment is more effective than G-CSF alone in mobilizing hematopoietic progenitor cells. We tested a strategy of preemptive plerixafor use following assessment of the peak mobilization response to 5 days of G-CSF. Patients were eligible for plerixafor if, on day 5 of G-CSF, there were less than 7 circulating CD34+ cells/μl or if <1.3 × 106 CD34+ cells/kg were collected on the first day of apheresis. Plerixafor (0.24 mg/kg subcutaneous) was given on day 5 of G-CSF followed by apheresis on day 6. This was repeated for up to two additional doses of plerixafor. The primary endpoint of the study was the percentage of patients who collected at least 2 × 106 CD34+ cells/kg. Twenty candidates for autologous stem cell transplantation enrolled on the trial. The circulating CD34+ cell level increased a median of 3.1 fold (range 1–8 fold) after the first dose of plerixafor and a median of 1.2 fold (range 0.3–6.5 fold) after the second dose of plerixafor. Fifteen of 20 (75%) patients achieved the primary endpoint. In conclusion, the decision to administer plerixafor can be delayed until after the peak mobilization response to G-CSF has been fully assessed.
Introduction
The use of high dose chemotherapy with autologous peripheral blood stem cell rescue for treatment of high risk malignancies is contingent upon collection of a minimum CD34+ cell dose of approximately 2 × 106/kg. Inadequate mobilization of CD34+ cells results in a failed collection in up to 30% of candidates for autologous stem cell transplantation. 1–5 Plerixafor reversibly inhibits binding of stromal cell-derived factor 1 alpha to the chemokine receptor CXCR4 resulting in mobilization of bone marrow hematopoietic progenitor cells. 6–8 Phase 3 studies demonstrate that when given in the evening of day 4 of G-CSF treatment, plerixafor significantly improves the chance of successful peripheral blood stem cell collection compared to G-CSF alone in patients with multiple myeloma and non-Hodgkin lymphoma. 1, 2 The drug has international regulatory approval for this purpose, and is now widely used.
The peak mobilization response to G-CSF alone occurs on the fifth day of administration. 9, 10 Therefore, the current standard of plerixafor administration on day 4 of G-CSF means that the decision to administer this agent occurs before the peak mobilization response to G-CSF can be assessed. Routine use of plerixafor on day 4 of G-CSF treatment may not be necessary since many patients will mobilize CD34+ cells adequately in response to G-CSF alone. For the patients that fail single agent G-CSF mobilization, a second mobilization session with the addition of plerixafor dosed on day 4 of G-CSF can rescue 63% of patients 11. In the context of a prospective, single center phase 2 study, we tested the hypothesis that plerixafor could be used to rescue poor mobilizers on day 5 of G-CSF thereby avoiding a second apheresis session.
Methods
Protocol Eligibility
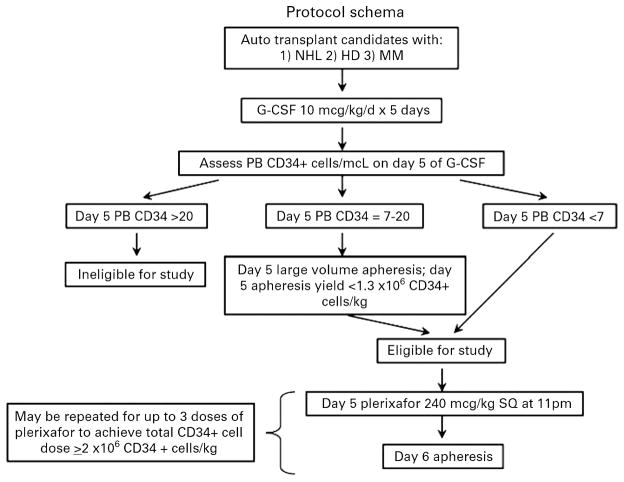
Protocol eligibility included patients aged 18–75 years with a diagnosis of multiple myeloma, non-Hodgkin or Hodgkin lymphoma who were candidates for high dose chemotherapy and autologous stem cell rescue. Patients must have recovered from acute toxic effects of prior chemotherapy, and achieved adequate hematologic recovery defined by an absolute neutrophil count of > 1.0 × 109/L and a platelet count of 75 × 109/L. Patients were excluded if they had failed previous attempts at peripheral blood stem cell collection, or had prior radioimmunotherapy (Ibritumomab Tiuxetan or Tositumomab). Patients were screened for protocol participation prior to, or during G-CSF mobilization. Patients were deemed eligible for protocol participation if, on day 5 of G-CSF treatment, the circulating CD34+ cell level was <7/μl. These patients did not undergo apheresis on day 5 of G-CSF treatment as historically at our center, the poor CD34+ cell yield does not justify the expense of the procedure. In addition, patients with 7 to 20 circulating CD34+ cells/μl on day 5 of G-CSF treatment were also eligible for protocol therapy if <1.3 × 106 CD34+ cells/kg were collected on the first day of apheresis (Figure 1). Justification for targeting patients with 7 to 20 circulating CD34+ cells for preemptive plerixafor therapy comes from review of outcomes from a historical cohort. During a 12 month period, 5 patients (multiple myeloma, n=4, non-Hodgkin lymphoma, n=1) were identified. Two of the 5 patients achieved a transplantable stem cell dose of ≥ 2 × 106 CD34+ cells/kg. Three of the 5 patients failed to collect the minimum cell dose and thus may benefit from an alternative mobilization strategy.
Figure 1.
Protocol Schema
Study Design
The study was approved by the institutional review board of the Duke University School of Medicine. Patients from the Duke Adult Stem Cell Transplant Program were prospectively screened up to 3 days prior to the commencement of G-CSF mobilization for enrollment to the study. The study was open for accrual over a period of 15 months. All patients provided written informed consent prior to participation. The protocol schema is demonstrated in Figure 1. All patients were treated with G-CSF 10μg/kg subcutaneously qAM. Consented patients received dose 1 of plerixafor 0.24mg/kg subcutaneously on day 5 of G-CSF treatment at approximately 22:00. The process of evening dosing with plerixafor followed the next morning by G-CSF and a 3 blood volume (±20%) apheresis procedure on a COBE Spectra (Gambro BCT, Lakewood, CO) was repeated for up to a total of 3 days or until ≥5 × 106 cells/kg were collected. Peripheral blood CD34+ cell counts were determined by flow cytometry at the Duke University Medical Center using the ISHAGE protocol. Toxicities were monitored and recorded from the time of plerixafor administration until completion of apheresis using the WHO common toxicity criteria (CTC) version 3.
Statistical Considerations
This is a phase II observational study. The primary endpoint of the study was the percentage of patients who collected at least 2 × 106 CD34+ cells/kg. The sample size was determined to be of adequate size to justify expanded study of the pharmacoeconomic properties of preemptive plerixafor usage. Although there were no specific sample size requirements for the study, a sample size of 20 was chosen as it provided a 95% confidence interval for the percentage of patients reaching the primary endpoint with a width no wider than ±22.0%, assuming a success rate of 50%.
Results
Patient Characteristics
Patient characteristics are outlined in Table 1. During the 15 month time period of study activation, a total of 38 patients were screened for protocol participation. Eighteen patients with a median age of 58 years (range 24 to 76) and a median of 1 prior regimen (range 1–3 regimens) were screened for study participation but did not meet eligibility due to >20 peripheral blood CD34+ cells or 7–20 peripheral blood CD34+ cells with a day 1 apheresis yield of ≥ 1.3 × 106 CD34+ cells/kg. All 18 patients achieved the minimum CD34+ cell yield of 2 × 106 CD34+ cells/kg. All patients achieved neutrophil (ANC >500) and 17 of 18 achieved platelet (>20,000) engraftment at a median time of 12 and 16 days following transplantation, respectively. The time to neutrophil and platelet engraftment for patients who did not receive plerixafor did not differ from that observed by the study patients (12 vs. 11 days, p=0.6; 18 vs. 19 days, p=0.7, respectively). Twenty patients with a median age of 61 years (range 39 to 70) met eligibility criteria for enrollment in the protocol, and were available for analysis. The patients were candidates for high dose chemotherapy with autologous stem cell rescue for treatment of non-Hodgkins lymphoma (n=10), Hodgkins disease (n=2) or multiple myeloma (n=8). The patients had a median of 2 courses of prior chemotherapy (range 1–3). Six of 8 patients (63%) with multiple myeloma had a median of 5 cycles (range 4–15) of lenalidomide prior to undergoing stem cell mobilization. Eleven patients (55%) met eligibility due to mobilization of < 7 CD34+ cells/μl following 5 doses of G-CSF. The remainder met eligibility due to collection of < 1.3 × 106 CD34+ cells/kg body weight following 5 doses of G-CSF treatment and a 3 blood volume apheresis session. Patients received a median of 2 doses of plerixafor on study (range 1–3).
Table 1.
Patient Characteristics
| UPN | AGE/SEX | DIAGNOSIS | DISEASE STATUS AT TIME OF MOBILIZATION | PRIOR CHEMOTHERAY (Regimens) | CHEMOTHERAPY TO D1 G-CSF (Days) | PRIOR LENALIDOMIDE THERAPY (Months) |
|---|---|---|---|---|---|---|
| 1 | 69/M | NHL | PR | RCHOP, RESHAP | 47 | - |
| 2 | 51/M | MM | CR | DVD, VM | 88 | - |
| 3 | 45/F | NHL | PR | R-CHOP, GCD, RICE | 47 | - |
| 4 | 39/F | HD | PR | ABVD, ICE, GVD | 61 | - |
| 5 | 57/M | NHL | CR | RCHOP, mBEAM | 44 | - |
| 6 | 59/M | MM | PR | VD, MPV, RD | 27 | 5 |
| 7 | 63/F | NHL | CR | RCHOP, RICE | 65 | - |
| 8 | 65/M | NHL | PR | RCHOP, RICE | 28 | - |
| 9 | 68/M | MM | CR | RD | 30 | 4 |
| 10 | 69/F | NHL | PR | RGO, RICE | 34 | - |
| 11 | 43/M | NHL | CR | RCHOP, RICE | 35 | - |
| 12 | 70/M | MM | PR | RD | 34 | 5 |
| 13 | 59/M | NHL | CR | RCHOP, RICE | 40 | - |
| 14 | 66/F | MM | PR | VDC | 27 | - |
| 15 | 60/M | HD | CR | ABVD, ICE | 38 | - |
| 16 | 67/M | MM | PR | MPV, RD | 22 | 15 |
| 17 | 68/M | NHL | CR | RCHOP, RESHAP | 69 | - |
| 18 | 60/M | MM | PR | RD | 14 | 4 |
| 19 | 56/M | MM | PR | RD | 21 | 12 |
| 20 | 67/M | NHL | CR | RCHOP, RICE | 49 | - |
NHL; Non-Hodgkin Lymphoma, HD; Hodgkin Lymphoma, MM; Multiple Myeloma RCHOP; rituximab, cyclosphosphamide doxorubicin prednisone, RESHAP; rituximab etoposide solumedrol cytarabine cisplatin, DVD; liposomal doxorubicin vincristine dexamethasone, VM; bortezomib melphalan, GCD; gemcitabine cisplatin dexamethasone, RICE; rituximab ifosphamide etoposide, ABVD; adriamycin vinblastine bleomycin vincristine dacarbazine, GVD; gemcitabine vinorelbine liposomal doxorubicin, mBEAM; mini carmustine etoposide cytarabine melphalan, VD; bortezomib dexamethasone, MPV; melphalan prednisone bortezomib, RD; lenolidomide dexamethasone, RGO; rituximab gemcitabine oxaliplatin, VDC; bortezomib dexamethasone cyclophosphamide
Mobilization Response to Plerixafor Beginning Day 5 of G-CSF administration
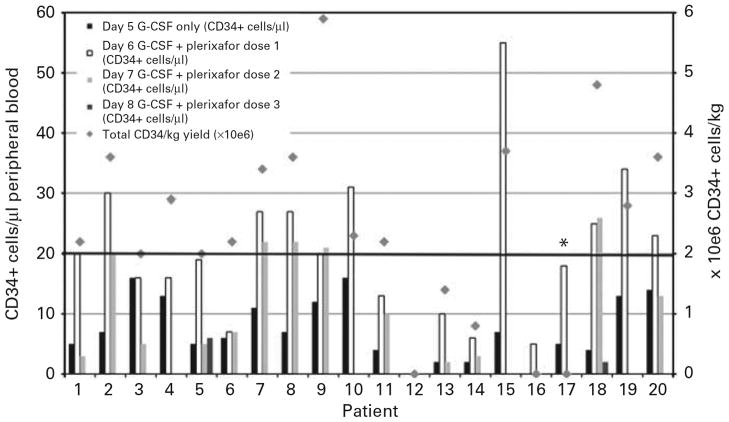
The response to preemptive dosing of plerixafor is demonstrated in figure 2. The median circulating CD34+ cell level on day 5 of G-CSF administration was 6.5/μl (range <1–16/μl). The median circulating CD34+ cell level on day 6 of G-CSF following dose 1 of plerixafor was 19.5/μl (range <1–55/μl), representing a median 3.1 fold (range 1–8 fold) increase in circulating CD34+ cell level between day 5 and day 6 of G-CSF administration. Thirteen patients received a second dose of plerixafor on day 6 of G-CSF administration. The median circulating CD34+ cell level measured the following morning was 10/μl (range 2–26/μl) representing a median 1.2 fold (range 0.3 to 6.5 fold) change in circulating CD34+ cells from the baseline measurement following 5 days of G-CSF administration and a median 0.7 fold change in circulating CD34+ cells from the level measured after the first dose of plerixafor. Two patients received a third dose of plerixafor on day 7 of G-CSF mobilization. The circulating level CD34+ cells measured the following morning was 1 and 1.8 fold different from the baseline measurement following 5 days of G-CSF administration. Neither of these patients achieved a circulating CD34+ cell level high enough to justify apheresis.
Figure 2.
Mobilization response and total CD34+ cells/kg yield following preemptive dosing of plerixafor given on day 5, 6 and 7 of G-CSF treatment. * indicates patient who did not undergo apheresis due to lack of venous access.
CD34+ cell yield
Fifteen of 20 patients (75%) who had a suboptimal mobilization response on day 5 of G-CSF treatment achieved the primary endpoint of the study by providing ≥ 2 × 106 CD34+ cells/kg. An additional patient (patient #17) responded to preemptive plerixafor, going from 5 CD34+ cells/μl on day 5 of G-CSF to 18 CD34+ cells/μl on day 6 of G-CSF following dose 1 of plerixafor. However the patient did not undergo apheresis due to inadequate venous access. Eleven patients had less than 7 CD34+ cells/μl after 5 daily doses of G-CSF and thus had apheresis deferred until the following day. Following the first dose of plerixafor, these patients had a median of 18 CD34+ cells/μl (range 1–55 CD34+ cells/μl), representing a median 4 fold (range 1–8 fold) increase from the prior day. The median cumulative CD34+ cell dose recovered from all study patients was 2.3 × 106 CD34+ cells/kg (range 0–6 × 106 CD34+ cells/kg ). Four of 20 patients (20%) failed preemptive dosing of plerixafor and thus had inadequate collection of peripheral blood CD34+ cells.
Of the 4 patients who failed to respond to preemptive dosing of plerixafor, all subsequently went on to provide a transplantable CD34+ cell dose with additional mobilization sessions. Two collected with chemotherapy mobilization and two with a second G-CSF/plerixafor combination.
There were 5 patients with multiple myeloma who were enrolled in the study and had prior therapy with lenalidomide (median 5 cycles, range 4–15 cycles). Three of the 5 patients were successfully rescued with plerixafor dosing on protocol.
Toxicity and Engraftment
There were no toxicities from plerixafor therapy that exceeded WHO grade I. Hyperleukocytosis (WBC >60 × 109) was not observed in any of the subjects. Fourteen of 15 patients from whom ≥ 2 ×106 CD34+ cells/kg were obtained subsequently underwent high dose chemotherapy and autologous stem cell rescue. One patient with multiple myeloma opted for a delay in high dose therapy. All transplanted patients who received autologous cells collected with the combination of G-CSF and plerixafor experienced prompt hematopoietic recovery. The median time to neutrophil engraftment (>500/μl) and platelet engraftment (>50,000/μl) was 11 days and 19 days, respectively following transplantation. There were no cases of primary or secondary graft failure.
Discussion
Twenty patients with a pre-defined suboptimal mobilization response to 5 daily doses of G-CSF 10μg/kg were enrolled on this study. With the use of preemptive plerixafor dosing, a transplantable stem cell dose was obtained from 15 of 20 patients (75%), preventing the need for a second mobilization session. Consistent with observations from larger phase III studies, plerixafor administration proved to be safe and effective with no significant toxicities associated with its administration. All patients undergoing stem cell transplantation using cells collected with the aid of plerixafor experienced prompt and robust engraftment.
In an era of continued escalation of the cost of healthcare, it is critical to study new methods of blending both optimal and cost-effective treatment approaches. There are multiple opportunities for healthcare costs to escalate when candidates for autologous SCT are poor mobilizers. Some patients will require additional days of apheresis that, even then, may not provide an adequate CD34+ cell dose. Other patients will require a second stem cell mobilization and apheresis session using G-CSF alone or in combination with chemotherapy or possibly a bone marrow harvest procedure performed in the operating room. Patients transplanted with suboptimal CD34+ cell dose also experience prolonged time to engraftment with increased needs for transfusion support, antibiotics and hospitalization. Finally, there is the emotional toll placed on the patient when optimal therapy (high dose therapy with autologous stem cell rescue) cannot be provided due to the inability to collect adequate numbers of hematopoietic progenitors. Plerixafor, used in a judicious and cost-effective manner may reduce the costs associated with a poor mobilization response.
Using the average wholesale price as reported in the Redbook 2009 edition, Shaughnessy and colleagues estimated the average sale price of a vial (a single dose for a patient ≤70kg) of plerixafor to be $6250.00.12 Despite the high cost, plerixafor has the potential to provide considerable cost savings by reducing both the number of apheresis sessions and the number of failed mobilization attempts. Additional cost savings could be achieved if plerixafor usage is limited to patients who have an inadequate mobilization response to G-CSF alone. 13 The current standard of care is to administer plerixafor on day 4 of G-CSF. However, the peak mobilization response to G-CSF occurs on day 5 of subcutaneous administration at a dose of 10μg/kg. 9, 10 Therefore, administration of plerixafor on day 4 of G-CSF comes before peak mobilization to G-CSF can be determined. The rational for the preemptive plerixafor usage described in this study is that it allows for peak mobilization to G-CSF to be assessed in all patients prior to plerixafor usage.
The impact of plerixafor on stem cell mobilization was examined in the context of a phase 3 randomized trial comparing the combination of G-CSF and day 4 dosing of plerixafor with G-CSF alone in autologous SCT candidates with non-Hodgkin lymphoma1. For patients randomized to the G-CSF/plerixafor mobilization, 87% provided ≥ 2 × 106 CD34+ cells/kg compared to 47% of those patients who received G-CSF alone. Patients from either arm who failed to mobilize (<0.8 × 106 CD34+ cells/kg in 2 collections or <2 × 106 CD34+ cells in 4 collections) were offered a second rescue mobilization at a later date using plerixafor given on day 4 of G-CSF in an open label fashion. As reported by Micallef and colleagues, successful collection (>2 × 106 CD34+ cells/kg) was achieved in 63% of patients who failed mobilization in the G-CSF alone arm 11. In the present study, 75% of the poor mobilizers were successfully collected using a preemptive plerixafor rescue approach that does not require a second session of mobilization and apheresis.
Important differences in the two studies should be noted. For our study, patients with non-Hodgkin lymphoma, Hodgkin lymphoma and multiple myeloma were eligible for participation, while the Micallef study only included patients with non-Hodgkin lymphoma. Our study mandated determination of a “poor mobilizer” based on the circulating CD34+ level after 5 daily doses of G-CSF and 0 or 1 apheresis sessions. In contrast, the Micallef study allowed for 2–4 sessions of apheresis before declaring the patient a poor mobilizer. Thus, the latter study identified a more accurately defined population of poor mobilizers. But even with the acknowledgment that with further apheresis sessions, some of the study patients may have provided an adequate CD34+ cell dose without the addition of plerixafor treatment, there remain many advantages to an up-front preemptive plerixafor rescue approach. First is that it reduces the likelihood of a costly and more time consuming second session of mobilization. Second, those patients who responded adequately to G-CSF alone do not incur the additional and unnecessary cost of plerixafor administration.
In the time since plerixafor has become available for clinical use, many groups have described novel approaches to utilization of this agent following cytokine only, or chemotherapy plus cytokine mobilization strategies.13–17 Two groups have proposed a preemptive strategy for plerixafor administration based on circulating levels of CD34+ cells on the fourth day of G-CSF administration.13, 17 The advantage of this approach is that the peak mobilizing effect of both plerixafor and cytokine is synchronized. The disadvantage is that it may be premature to designate a patient as a “poor mobilizer” and in need of preemptive plerixafor after only 4 doses of cytokine.
We conclude that a mobilization rescue strategy consisting of preemptive plerixafor given on day 5 of G-CSF administration in patients who demonstrate an inadequate mobilization response to G-CSF alone is safe and effective in the majority of patients. This preemptive strategy allows for the peak mobilization response to G-CSF to be assessed and plerixafor administered to the patients who have declared themselves as poor mobilizers. We propose that the preemptive plerixafor dosing strategy should be part of further pharmacoeconomic studies comparing this approach to up-front use of plerixafor for autologous stem cell transplantation candidates.
Acknowledgments
The authors would like to thank the apheresis staff of the Duke Adult Stem Cell Transplant program for their outstanding work and contribution to this study. Research funding for this study was supported by Genzyme Corporation
Footnotes
Conflict of Interest
N.C. and M.H. have relevant financial relationships as follows; Genzyme, Research Funding (N.C), and Genzyme, Honoraria and Research Funding (M.H.). Other authors have no relevant financial relationship to disclose.
References
- 1.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 2.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 3.Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44(5):815–20. doi: 10.1080/1042819031000067585. [DOI] [PubMed] [Google Scholar]
- 4.Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. Prediction of mobilisation failure in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33(9):907–12. doi: 10.1038/sj.bmt.1704466. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar S, Weshi AE, Rahal M, Khafaga Y, Tbakhi A, Humaidan H, et al. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma. 2008;49(4):769–78. doi: 10.1080/10428190701843213. [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527(1–3):255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 8.Rosenkilde MM, Gerlach LO, Hatse S, Skerlj RT, Schols D, Bridger GJ, et al. Molecular mechanism of action of monocyclam versus bicyclam non-peptide antagonists in the CXCR4 chemokine receptor. J Biol Chem. 2007;282(37):27354–65. doi: 10.1074/jbc.M704739200. [DOI] [PubMed] [Google Scholar]
- 9.Stroncek DF, Clay ME, Herr G, Smith J, Jaszcz WB, Ilstrup S, et al. The kinetics of G-CSF mobilization of CD34+ cells in healthy people. Transfus Med. 1997;7(1):19–24. doi: 10.1046/j.1365-3148.1997.d01-75.x. [DOI] [PubMed] [Google Scholar]
- 10.Grigg AP, Roberts AW, Raunow H, Houghton S, Layton JE, Boyd AW, et al. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86(12):4437–45. [PubMed] [Google Scholar]
- 11.Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15(12):1578–86. doi: 10.1016/j.bbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, Macpherson J, Winkler K, et al. Cost and Clinical Analysis of Autologous Hematopoietic Stem Cell Mobilization with G-CSF and Plerixafor compared to G-CSF and Cyclophosphamide. Biol Blood Marrow Transplant. 2011;17(5):729–36. doi: 10.1016/j.bbmt.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011;46(1):64–9. doi: 10.1038/bmt.2010.78. [DOI] [PubMed] [Google Scholar]
- 14.Basak GW, Mikala G, Koristek Z, Jaksic O, Basic-Kinda S, Cegledi A, et al. Plerixafor to rescue failing chemotherapy-based stem cell mobilization: it’s not too late. Leukemia & lymphoma. 2011 doi: 10.3109/10428194.2011.578312. e-pub ahead of print 15 June 2011. [DOI] [PubMed] [Google Scholar]
- 15.Duong HK, Bolwell BJ, Rybicki L, Koo A, Hsi ED, Figueroa P, et al. Predicting hematopoietic stem cell mobilization failure in patients with multiple myeloma: a simple method using day 1 CD34+ cell yield. J Clin Apher. 2011;26(3):111–5. doi: 10.1002/jca.20278. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of “just-in-time” salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03136.x. e-pub ahead of print 16 April 2011. [DOI] [PubMed] [Google Scholar]
- 17.Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03206.x. e-pub ahead of print 11 June 2011. [DOI] [PubMed] [Google Scholar]