Abstract
High treatment-related mortality and high graft failure rate are serious concerns in HLA-mismatched umbilical cord blood (UCB) transplantation with myeloablative conditioning. We conducted a prospective trial of dual UCB transplantation using modified myeloablation consisting of total body irradiation (TBI; 1350 cGy) and fludarabine (160 mg/m2). Twenty-seven patients (median age, 33 years; range, 20–58 years) with hematologic malignancies were enrolled. The median combined cryopreserved total nucleated cell (TNC) dose was 4.3 × 107/kg (range, 3.2–7.7 × 107/kg). The cumulative incidences of neutrophil (≥500/μl) and platelet (≥50,000/μl) engraftment were 80% (95% confidence interval (CI), 58–91%) and 68% (95% CI, 46–83%), respectively. Among engrafted patients, a single cord blood unit was predominant by 100 days post transplantation. A higher cryopreserved and infused TNC dose and infused CD3+ cell dose were significant factors associated with the predominant UCB unit (P = 0.032, 0.020, and 0.042, respectively). Treatment-related mortality and relapse rates at 2 years were 28% (95% CI, 12–47%) and 20% (95% CI, 7–37%), respectively. Cumulative incidences of grades II–IV and grades III–IV acute GVHD were 37% (95% CI, 20–55%) and 11% (95% CI, 3–26%), respectively, and that of chronic GVHD was 31% (95% CI, 15–49%). With a median follow-up of 23 months, overall and disease-free survival rates at 2 years were 58% (95% CI, 34–75%) and 52% (95% CI, 29–70%), respectively. This study supports the use of TBI 1350cGy/fludarabine as an alternative to conventional myeloablative conditioning for dual UCB transplantation.
Keywords: adult, dual umbilical cord blood transplantation, myeloablative, fludarabine, total body irradiation
Introduction
Unrelated umbilical cord blood (UCB) has emerged as a viable alternative source for hematopoietic stem cells for pediatric and adult patients in need of allogeneic stem cell transplantation [1-12]. However, the high incidence of treatment-related mortality and graft failure have historically been significant obstacles to wider application of myeloablative UCB transplantation for adult patients [6-10]. In an early multi-center retrospective study comparing outcomes of myeloablative UCB transplantation to unrelated donor transplantation, the neutrophil engraftment rate was lower (<70%) and treatment-related mortality was higher (63%, 95 of 150 patients) in the UCB transplantation cohort [7].
Multiple strategies have been employed to reduce treatment-related mortality associated with allogeneic stem cell transplantation. Although non-myeloablative conditioning regimens have been used in UCB transplantation as well as in related or unrelated donor transplantation to reduce treatment-related mortality [13-17], patients with high-risk hematologic malignancies would be expected to have an increased risk of disease relapse following this approach [18, 19]. Therefore, there is critical need for additional myeloablative conditioning regimens that can exert potent antitumor activity and sufficient immunosuppression to facilitate mismatched unrelated cord blood engraftment, but are less toxic than conventional myeloablative conditioning regimens such as total body irradiation (TBI), fludarabine and cyclophosphamide [20].
Here, we report the results from a consecutive cohort of patients who received dual UCB transplantation after conditioning with myeloablative TBI (1350 cGy) and fludarabine (160 mg/m2).
Methods
Inclusion criteria
The inclusion criteria were as follows: (1) patients aged between 14 years and 65 years; (2) patients with high-risk lymphoblastic leukemia (ALL) (Philadelphia chromosome positive) or acute myelogenous leukemia (AML) (complex cytogenetic abnormality or antecedent hematologic malignancy) in first complete remission (CR1), or second or higher complete remission (≥CR2); (3) patients with chronic myelogenous leukemia (CML) in chronic phase or accelerated phase; (4) patients with myelodysplastic syndrome (MDS) with an International Prognostic Scoring System risk category of INT-1 or greater; (5) patients with non-Hodgkin’s lymphoma (NHL) or Hodgkin’s lymphoma with high-risk disease in CR1, ≥CR2, or first partial remission, or those with chemotherapy-resistant relapse; (6) patients with an Eastern Cooperative Oncology Group performance status ≤ 2; (7) patients with adequate organ function (creatinine, <2.0 mg/dl; aspartate aminotransaminases and alanine aminotransferase, <4 x normal value; bilirubin, <2.0 mg/dl; forced vital capacity and forced expiratory volume in 1 second values, >50% of the value predicted for age; carbon monoxide diffusing capacity, >50% of the predicted value; ejection fraction, >45%); (8) patients without a 6/6 or a 5/6 HLA-A, -B, or -DR antigen-matched related donor; and (9) patients without an 8/8 HLA-A, -B, -C, and -DRB1 allele-matched unrelated donor by high resolution typing. CR of leukemia was defined as the absence of any residual disease confirmed by morphology and flow cytometry, while CR of lymphoma was defined according to the response criteria proposed by Cheson et al [21]. The study was approved by the institutional review boards of the Duke University School of Medicine and the University of British Columbia. Written informed consent was obtained from all patients prior to transplantation.
Treatment plan
The conditioning regimen consisted of TBI 150 cGy twice a day, total 9 fractions (days -9 to -5), and fludarabine (Flu)(Berlex Laboratories, Wayne, NJ, USA) 40 mg/m2/day i.v. × 4 days (days -5 to -2). The dose to the lungs was attenuated (range 8-10Gy based on degree of pulmonary dysfunction) in all patients using the arms and brass compensators. Patients received 2 partially matched UCB grafts that were at least 4 of 6 HLA-matched with the recipient and 3 of 6 HLA-matched between grafts (low resolution A, B, high resolution DRB1). Each red cell depleted graft contained a minimum cryopreserved total nucleated cell (TNC) dose of 1.5 × 107/kg and each red cell containing unit contained a minimum cryopreserved TNC dose of 2.0 × 107/kg. UCB units were thawed and washed before transplantation using the methods described by Rubinstein et al. [22], and two cord blood units were infused sequentially with an arbitrary order and no mandatory time interval. The colony-forming unit (CFU) content (CFU-GM, CFU-GEMM, and BFU-E), number of CD3+ and CD34+ cells, and TNC count were determined for each washed cord blood unit. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus dosed to achieve serum levels of 10–20 ng/mL for at least 6 months and mycophenolate mofetil (MMF) 1000 mg twice daily until at least 60 days following transplantation. Granulocyte colony-stimulating factor was administered until the neutrophil count exceeded 1000/mm3. Voriconazole, acyclovir, and ciprofloxacin were given as anti-infective prophylaxis for at least 3 months. Total parenteral nutrition (TPN) was started when caloric intake of patients was negligible. For the 24 patients treated at Duke University Medical Center, donor chimerism was sequentially determined using quantitative PCR amplification of informative microsatellites on DNA isolated from CD3+ and CD15+ peripheral blood or bone marrow cells.
Statistical analysis
The primary endpoint for this trial was donor stem cell engraftment. The secondary endpoints were (1) platelet engraftment; incidence of (2) acute and chronic GVHD; (3) treatment-related mortality; (4) relapse; and (5) disease-free and overall survival. TPN usage was also evaluated as a post hoc analysis. Day of donor stem cell engraftment was defined as the first of 3 consecutive days achieving a neutrophil count of ≥500/μl on or before transplant day 50. Day of platelet engraftment was defined as the first of 3 consecutive days achieving a platelet count of ≥20,000/μl and ≥50,000/μl without platelet transfusion support on or before transplant day 100. Patients with early death or relapse without engraftment before day 21 were excluded from the engraftment analysis. Day to TPN usage was measured from the first day of conditioning to the first day of TPN requirement before transplant day 30. Acute or chronic GVHD was characterized using standard criteria [23, 24]. Disease-free survival was measured from the day of transplant until the documented recurrence of disease or death from any cause. To eliminate the effects of competing risk, cumulative incidence was assessed using methods described elsewhere [25]. In the analysis of the cumulative incidence of neutrophil and platelet engraftment, a competing event was defined as relapse or death without an event of interest, whereas in the analysis of relapse, acute and chronic GVHD, and TPN usage, a competing event was defined as death without an event of interest. Relapse was defined as a competing risk in the analysis of treatment-related mortality. Disease-free and overall survival was estimated using the Kaplan-Meier method. We defined a predominant UCB unit as sustained ≥70% myeloid and lymphoid chimerism after transplantation. The Wilcoxon signed rank test was used to evaluate the effect of cell dose and HLA compatibility on engraftment of a predominant single cord unit. All tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata version 11 (Stata Corp., College Station, TX, USA).
Results
Patients and graft characteristics
A total of 27 patients with a median age of 33 years (range 20–58 years) and body weight of 74.7 kg (range, 37–112 kg) were treated on study between April 2006 and January 2010. Pre-transplant disease characteristics are shown in Table 1. The UCB graft characteristics are shown in Table 2. The combined cryopreserved and infused TNC counts of the 2 UCB units were 4.3 × 107/kg (range: 3.2–7.7 × 107/kg) and 3.8 × 107/kg (2.6–6.0 × 107/kg), respectively. Seventy-four percent of patients received one 4/6 HLA-A, -B, and -DRB1 matched unit and one 4/6 or 5/6 matched unit.
Table 1.
Patient characteristics for the 27 recipients of dual umbilical cord blood transplantation
| Characteristics | n = 27 |
|---|---|
| Age (years), median (range) | 33 (20 - 58) |
| Sex | |
| Male | 11 (41%) |
| Female | 16 (59%) |
| Weight (kg), median (range) | 74.7 (37 - 112) |
| Acute myelogenous leukemia | 15 (56%) |
| CR1 | 1 |
| CR2 | 14 |
| Myelodysplastic syndrome | 3 (11%) |
| RA | 1 |
| RAEB-1 | 1 |
| RAEB-2 | 1 |
| Chronic myelogenous leukemia | |
| CP | 1 (4%) |
| Acute lymphoblastic leukemia | 5 (19%) |
| CR1 | 2 |
| CR2 | 3 |
| Non Hodgkin’s lymphoma | 3 (11%) |
| CR1 | 1 |
| CR2 | 1 |
| Relapse | 1 |
| CMV serostatus | |
| Positive | 17 (63%) |
| Negative | 8 (30%) |
| Indeterminate | 2 (7%) |
CR, complete remission; CP, chronic phase; RA, refractory anemia; RAEB, refractory anemia with excessive blasts.
Data are counts of individuals unless specified otherwise.
Table 2.
Graft characteristics for the 27 recipients of dual umbilical cord blood transplantation
| Characteristics | |
|---|---|
| *Cryopreserved TNC, ×107/kg, median (range) (n=27) | 4.3 (3.2-7.7) |
| *Infused TNC, ×107/kg, median (range) (n=26) | 3.8 (2.6-6.0) |
| *Infused CD34+, ×105/kg, median (range) (n=26) | 1.2 (0.3-4.9) |
| *Infused CD3+, ×106/kg, median (range) (n=24) | 6.9 (3.6-11.6) |
| *Infused CFU, ×104/kg, median (range) (n=27) | 14.3 (0.0-68.0) |
| HLA matching** | |
| 6/6 + 6/6, n (%) | 1 (4) |
| 5/6 + 6/6, n (%) | 4 (15) |
| 5/6 + 5/6, n (%) | 2 (7) |
| 4/6 + 5/6, n (%) | 10 (37) |
| 4/6 + 4/6, n (%) | 10 (37) |
TNC indicates total nucleated cell; CFU, colony-forming unit
Values reflect combined umbilical cord blood graft
HLA matching was expressed as the degree of matching between recipient and each umbilical cord blood unit for HLA-A, HLA-B (low resolution typing) and HLA-DRB1 (high resolution typing)
Engraftment
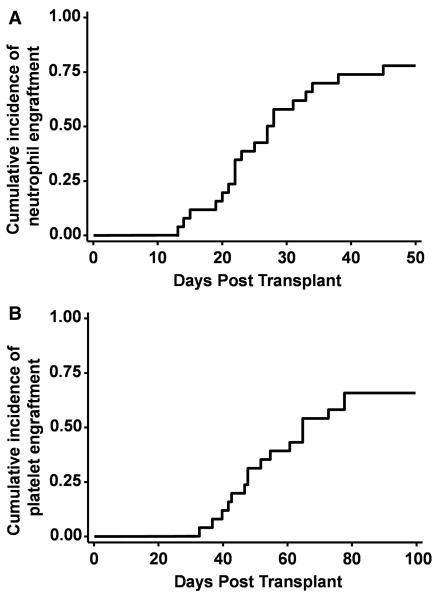
Neutrophil engraftment was achieved in 20 patients at a median of 24 days following transplantation (range, 13–45 days). Two patients were excluded from the engraftment analysis; one patient with AML in CR2 relapsed at day 14 after transplantation and died at day 38 due to disease progression. The other patient died day 20 after transplantation due to multi-organ failure arising from cardiovascular collapse. The cumulative incidence of neutrophil engraftment at day 50 was 80% (95% CI, 58–91%) for the 25 evaluable patients (Figure 1). Three patients experienced primary graft failure, and hematopoiesis was restored in two patients with either autologous (n = 1) or haploidentical allogeneic (n = 1) hematopoietic stem cells. Platelet engraftment of ≥20,000/μl was achieved in 19 patients in a median of 37 days (range, 20–66 days), and ≥50,000/μl in 17 patients in a median of 52 days (range, 33–78 days). The cumulative incidences of platelet engraftment of ≥20,000/μl and ≥50,000/μl by day 100 were 76% (95% CI, 54–88%) and 68% (95% CI, 46–83%), respectively (Figure 1).
Figure 1.
Cumulative incidence of (A) neutrophil and (B) platelet engraftment of 50,000/μl
A comparison of the cryopreserved and infused TNC dose, infused CD34+ and CD3+ cell count, and CFU content in the dominant and non-dominant units is shown in Figure 2. A higher cryopreserved and infused TNC and infused CD3+ cell dose was associated with establishment of the predominant UCB unit (cryopreserved TNC, P = 0.032; infused TNC, P = 0.020; CD3+, P = 0.042) (Figure 2). There was no association between the degree of HLA compatibility and establishment of a predominant UCB unit (P = 0.206).
Figure 2.
Dot plots showing the impact of total nucleated cell dose, CD34+ and CD3+ cell dose and CFU content on establishment of the predominant unit and non-dominant units for each patient. (A) Cryopreserved total nucleated cell dose (n=20), median predominant unit 2.5 × 107/kg (range, 1.7–3.6 × 107/kg), median non-dominant unit, 1.8 × 107/kg (range, 1.2–4.2 × 107/kg). (B) Infused total nucleated cell count (n=19), median dominant unit 2.1 × 107/kg (range, 1.2–3.6 × 107/kg), median non-dominant unit, 1.5 × 107/kg (range, 1.0–3.1 × 107/kg). (C) Infused CD34+ cell dose (n=20), median dominant unit 0.7 × 105/kg (range, 0.2–1.6 × 105/kg), median non-dominant unit 0.5 × 105/kg (range, 0.1–2.5 × 105/kg). (D) CD3+ cell count (n=17), median dominant unit 3.5 × 106/kg (range, 2.2–7.9 × 106/kg), median non-dominant unit 3.4 × 106/kg (range, 1.3–5.1 × 106/kg). (E) Colony forming unit content (n=20), median dominant unit 6.5 × 104/kg (range, 0.3–28.7 × 104/kg), median non-dominant unit 4.5 × 104/kg (range, 0.0–42.6 × 104/kg).
GVHD
Acute GVHD Grades II and III occurred in 7 and 3 patients, respectively. No patient developed grade IV acute GVHD. The cumulative incidences of grades II–IV and grades III–IV acute GVHD were 37% (95%CI, 20–55%) and 11% (95% CI, 3–26%), respectively (Figure 3). Chronic GVHD occurred in 8 patients, with a cumulative incidence of 31% (95% CI, 15–49%). There was no death attributable to acute or chronic GVHD. All but one episode of acute GVHD occurred prior to day 60, when mycophenolate mofetil was scheduled to be discontinued.
Figure 3.
Cumulative incidence of (A) acute and (B) chronic GVHD
Total parenteral nutrition
We evaluated the cumulative incidence of post-transplant TPN usage as a surrogate marker of regimen-related toxicity. The cumulative incidence of TPN usage was 56% (95% CI, 35–72%) at day 30.
Survival, treatment-related mortality, and relapse
With median follow-up of 23 months (range, 3.6–50.4 months) among survivors, the 2-year overall and disease-free survival rates were 58% (95% CI, 34–75%) and 52% (95% CI, 29–70%), respectively (Figure 4). Six patients died within 100 days following transplantation due to fungal infection (n = 1), diffuse alveolar hemorrhage (n = 2), disease progression (n = 1), multi-organ failure (n = 1), and graft failure (n = 1), while 4 patients died after 100 days post-transplant due to disease progression (n = 2), viral infection (n = 1), and unknown reason (n = 1). At time of last follow-up, 3 patients were alive with disease. The cumulative incidence of treatment-related mortality was 19% (95% CI, 7–35%) at 180 days and 28% (95% CI, 12–47%) at 2 years (Figure 4). The cumulative incidence of relapse was 20% (95% CI, 7–37%) at 2 years (Figure 4). Among 7 patients aged ≥50 years old, 6 patients are alive with a median follow-up of 24 months (range, 3.6–35.0 months) and 1 patient died due to disease progression at day 402. One of eight patients with lymphoid malignancies relapsed, while five of nineteen patients with myeloid malignancies relapsed.
Figure 4.
(A) Overall and (B) disease-free survival, (C) treatment-related mortality, and (D) relapse rates
Myeloid and lymphoid chimerism
The predominant unit provided a median of 90% donor myeloid and lymphoid chimerism at 1 month following transplantation. Myeloid chimerism of the non-dominant unit was still detectable at 12 months following transplantation in 2 patients (Figure 5). This has remained stable with nearly 2 years of follow-up in both patients.
Figure 5.
Sequential change of myeloid chimerism for 2 patients (panel A & B) who had long-lasting dual cord blood chimerism
Discussion
To reduce regimen-related toxicity but maintain antitumor activity in UCB transplantation, we combined a myeloablative dose of TBI with fludarabine instead of the more commonly used TBI/fludarabine/cyclophosphamide combination. With this myeloablative regimen, the cumulative incidence of neutrophil engraftment was 80% with a median of 24 days, which is comparable to that reported in previous multi-center retrospective studies analyzing the outcomes of myeloablative UCB transplantation [7-10]. This engraftment rate is inferior to what has been reported in adult dual cord blood transplantation studies from Japan [26, 27]. The reason for this difference is unclear but may be attributable to the genetic homogeneity of the Japanese population [28]. The Minnesota group reported the largest series of patients who received dual UCB transplantation after myeloablative conditioning consisting of TBI/fludarabine/cyclophosphamide [29]. For 93 patients who received dual UCB transplantation with this approach, the cumulative incidences of neutrophil and platelet engraftment (≥50,000/μl) were 86% and 63%, respectively [29], which is slightly higher than the neutrophil engraftment in our approach (80%) but slightly lower with respect to platelet engraftment (68%). While improving tolerability of the preparative regimen, it is possible that elimination of cyclophosphamide negatively impacted the engraftment rate. Larger studies are needed to determine if the observed differences in engraftment are significant.
It is difficult to compare regimen toxicity and tolerability due to the lack of objective methods for measurement. We hypothesized that the elimination of high dose cyclophosphamide from this myeloablative preparative regimen would improve tolerability in terms of severity of mucositis, and a reduction in treatment-related mortality. Using our conditioning regimen, we obtained a treatment-related mortality rate of 19% at 6 months and 28% at 2 years, which is lower than in the earlier reports of myeloablative UCB transplantation (40–50% at 2 years) [8, 10] and comparable to other reports of UCB transplantation after reduced-intensity conditioning [13-16]. Brunstein et al. [14] reported a treatment-related mortality rate of 19% at 6 months and 26% at 3 years in dual UCB transplantation after reduced-intensity conditioning using fludarabine (200 mg/m2), cyclophosphamide (50 mg/kg), and low-dose TBI (200 cGy). Although a recent report of dual UCB transplantation after myeloablative conditioning with TBI/fludarabine/cyclophosphamide showed a low treatment-related morality rate of 29% at 1 year [29], the median age of this cohort (median, 24 years; range 9–57 years) was lower than that of our cohort (median, 33 years; range 20–58 years), thus making it difficult to compare the results. We observed no treatment-related death in the 7 patients over age 50 years. Larger studies will be needed to confirm what appears to be a favorable toxicity profile for a select group of older patients. One of the weaknesses of the study is that we did not objectively measure the severity of mucositis. As a surrogate for mucositis measurement, we evaluated the incidence of TPN usage after transplantation, and found a cumulative incidence of TPN usage of just 56%. This suggests that treatment-related toxicity, including mucosal damage, may be milder than what is expected following conventional myeloablative regimens, where TPN usage typically approaches 100%. However, the criteria for initiating TPN varies across transplant centers, and the rate of TPN usage and degree of mucosal damage might be affected not only by the conditioning regimen but also by the use of MMF instead of methotrexate for GVHD prophylaxis. Therefore, definitive conclusion cannot be drawn from this finding.
Use of a myeloablative dose of TBI (1350 cGy) with fludarabine retains myeloablative properties based on the fact that no patient experienced autologous myeloid hematopoietic reconstitution, and assures maintenance of potent antitumor activity. The relapse rate in our study (20% at 2 years) is comparable to, or better than previous reports of UCB transplantation after conventional or reduced-intensity conditioning [7, 8, 10, 14], although the heterogeneity of patient diagnoses limits the broad applicability of this finding. Brunstein et al. reported a relapse rate of 31% at 3 years after UCB transplantation using reduced-intensity conditioning for patients with a similarly diverse spectrum hematologic malignancies [14]. Due to the comparable treatment-related mortality and low relapse rate, we obtained respectable 2-year overall survival (58%) and relapse-free survival (52%) rates for patients with hematologic diseases at a high risk of relapse.
After dual UCB transplantation, mixed UCB chimerism has been observed for a few weeks or months, but converts to complete single UCB chimerism by one year in almost all patients [16, 20]. We report here the phenomenon of stable dual cord blood chimerism observed in two of our study patients. Definitive graft characteristics that predict for the predominant unit are unknown. A higher CD3+ dose has been associated with the predominant unit in two prior studies [20, 30]. The current study confirms this observation. Prior reports have also implicated the order of cord blood graft infusion, CD34+ cell viability, and the presence of an immunologic graft versus graft effect mediated by effector CD8+ T-cells, as the driving force behind the emergence of a predominant graft [13, 30, 31]. We could not verify the importance of CD34+ cell viability in this study because we performed a post-thaw wash of the UCB units resulting in high (≥90%) viability of all cell types (data not shown). In contrast to other reports of both myeloablative and non-myeloablative dual cord blood transplantation [14, 20], we found that the graft with the higher TNC content was more likely become the predominant unit. However, there was a weak correlation between CD3 content and cryopreserved (R=0.52, P<0.01) or infused (R=0.48, P<0.01) TNC dose. Given this weak but significant correlation, we cannot determine which of these factors is the most important determinant for engraftment.
In order to maintain consistency with the statistical methods employed by comparable UCB transplant studies [14, 20, 29, 32], we excluded two patients with very early toxic death and relapse from the cumulative incidence calculations. While this deviates from the classical methods for calculation of cumulative incidence [33], we believe this to be a rational approach since these patients do not reflect the effect of this regimen on engraftment. This methodological difference should be considered when comparing the engraftment rates between studies. In the early multi-center retrospective study of myeloablative UCB transplantation, the neutrophil engraftment was 70–75% in the analysis for all patients including early toxic death cases [7, 8]. If the two patients with early toxic death were included in our analysis, neutrophil engraftment rate would be 74% (95% CI, 53–87%).
In conclusion, we find that the modified myeloablative regimen of TBI (1350cGy)/fludarabine provides stem cell engraftment rates comparable to more conventional myeloablative regimens. In contrast to other reports, we found that graft size predicts for the predominant UCB unit. This study supports the use of TBI 1350cGy/fludarabine as an alternative to conventional myeloablative conditioning for dual UCB transplantation and provides justification for larger studies.
ACKNOWLEDGMENTS
The study investigators wish to thank the nurse practitioners, physician’s assistants, ward and clinic nurses, and staff of the Duke Adult Stem Cell Transplant Program for their outstanding care of the patients described in this report. J.K. is a Research Fellow of the Japan Society for the Promotion of Science.
N.C. and M.H. have relevant financial relationships as follows; Genzyme, Research Funding (N.C), and Genzyme, Honoraria and Research Funding (M.H.).
Footnotes
Financial disclosure: None
Other authors have no relevant financial relationship to disclose.
REFERENCES
- 1.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 3.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 5.Chao NJ, Koh LP, Long GD, et al. Adult recipients of umbilical cord blood transplants after nonmyeloablative preparative regimens. Biol Blood Marrow Transplant. 2004;10:569–575. doi: 10.1016/j.bbmt.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Long GD, Laughlin M, Madan B, et al. Unrelated umbilical cord blood transplantation in adult patients. Biol Blood Marrow Transplant. 2003;9:772–780. doi: 10.1016/j.bbmt.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 8.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 9.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Arcese W, Rocha V, Labopin M, et al. Unrelated cord blood transplants in adults with hematologic malignancies. Haematologica. 2006;91:223–230. [PubMed] [Google Scholar]
- 11.Takahashi S, Iseki T, Ooi J, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104:3813–3820. doi: 10.1182/blood-2004-03-1001. [DOI] [PubMed] [Google Scholar]
- 12.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 13.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyakoshi S, Yuji K, Kami M, et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res. 2004;10:3586–3592. doi: 10.1158/1078-0432.CCR-03-0754. [DOI] [PubMed] [Google Scholar]
- 16.Cutler C, Ballen K. Reduced-intensity conditioning and umbilical cord blood transplantation in adults. Bone Marrow Transplant. 2009;44:667–671. doi: 10.1038/bmt.2009.283. [DOI] [PubMed] [Google Scholar]
- 17.Miyakoshi S, Kami M, Tanimoto T, et al. Tacrolimus as prophylaxis for acute graft-versus-host disease in reduced intensity cord blood transplantation for adult patients with advanced hematologic diseases. Transplantation. 2007;84:316–322. doi: 10.1097/01.tp.0000269796.23593.16. [DOI] [PubMed] [Google Scholar]
- 18.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 19.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 20.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Ooi J, Takahashi S, Tomonari A, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2008;14:1341–1347. doi: 10.1016/j.bbmt.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Ooi J, Takahashi S, Tomonari A, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with ALL. Bone Marrow Transplant. 2009;43:455–459. doi: 10.1038/bmt.2008.347. [DOI] [PubMed] [Google Scholar]
- 28.Oh H, Loberiza FR, Jr., Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–1416. doi: 10.1182/blood-2004-06-2385. [DOI] [PubMed] [Google Scholar]
- 29.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16:500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–765. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]