Abstract
A critical requirement for research using model organisms is an appropriate, well-defined and consistent diet. There is currently no complete chemically defined (holidic) diet available for Drosophila melanogaster. We describe a holidic medium that is equal in performance to an oligidic diet optimized for adult fecundity and lifespan. It is also sufficient to support development over multiple generations, but at a reduced rate. During seven years of experiments, the holidic diet yielded more consistent experimental outcomes than oligidic food for adult fitness traits. Furthermore, nutrients and drugs are more accessible to flies in holidic medium and, similar to dietary restriction on oligidic food, amino acid dilution increases fly lifespan. We also report amino acid specific effects on food choice behavior and that folic acid from the microbiota is sufficient for development. These insights could not be gained using oligidic or meridic diets.
Introduction
In nature, fruitflies Drosophila melanogaster feed on fermenting fruit 1. They gain the majority of their nutrition from ingested microbes and experience relatively high concentrations of ethanol and organic acids 2. Thus, a simple diet of sucrose and lyophilized yeast, with a weak organic acid as preservative, is sufficient to support rapid growth, egg-laying and long life of Drosophila in the laboratory 3,4. The popularity of D. melanogaster in research has led to a variety of food recipes, which contain some combination of molasses, treacle, beet syrup, rolled oats, banana, potato starch, cornmeal, corn syrup, malt, soy, one of several mono- or di-saccharides and one of a variety of processed dead or fresh live yeasts (supplement to 5). At worst, these are reported simply as ‘standard’ media, but more often a recipe is given, but without the method of preparation or the nature and source of its components. Such nutritional variations could explain many inconsistent experimental outcomes between laboratories.
Work to specify the nutritional requirements of Drosophila can be traced to 1946 6, with incremental progress to 1956 with an exhaustive investigation of the optima for each of 13 different nutritional components required by Drosophila for development 7 and subsequently for adult egg-laying 4,8. More recently, semi-defined (meridic) media have been employed for longevity studies 9–12. However, the non-purified ingredients in these media can show substantial batch variation. We presently lack a defined synthetic medium for Drosophila made entirely from purified ingredients (holidic) that is adequate to support development, adult egg-laying and lifespan.
Here we describe such a medium and demonstrate that it is sufficient for development, albeit at a reduced rate. When adult flies are maintained on the diet, they are phenotypically similar to those kept on a natural yeast based (oligidic) diet. Importantly, we show that holidic medium can produce more stable experimental outcomes than oligidic media, thus improving the potential for comparability of studies between laboratories as well as providing a nutritionally explicit context for experiments where outcomes are particularly sensitive to the nutritional status of the flies. Furthermore, our holidic medium offered the opportunity to investigate the effects of subtle nutrient manipulations that could not be achieved otherwise. In particular, we found that egg-associated microbes inherited from the mother could overcome folic acid deficiency during development. Holidic medium also greatly improved the efficiency of oral drug delivery to the flies. Given the broad utility of this medium and its demonstrated advantages over oligidic diets, we propose that it serves as the starting point for many studies seeking a precisely controlled nutritional context.
Results
A holidic medium for adult Drosophila
During the last seven years of experimental work on Drosophila lifespan in our laboratory using the same experimental conditions, we have observed that lifespan has varied over a more than 25-day range (Fig. 1a). To minimize the nutritional variability arising from changes to natural ingredients, such as yeast, we developed a holidic medium suitable for measuring adult egg-laying, lifespan and behavior.
Figure 1.
A holidic diet for adult Drosophila. (a) Drosophila median lifespans. Under the same experimental conditions using an oligidic diet, fly lifespan varied over a 25-day range during the period September 2005 – July 2012; n = 65. (b) Adult lifespan of flies on holidic and oligidic diets. This trial, flies on the holidic diet were longer lived than on 1SY (P < 0.001, n = 100, Log-rank test). (c) Fitness traits of flies on holidic and oligidic diets. While average median lifespan on holidic and oligidic diets were no different (P = 0.52, n = 7, Wilcoxon rank-sum test), inter-trial variance tended to be lower, but was not significant (P = 0.059, n = 7, F-test). There was significantly less variance in egg-laying on the holidic diet (P = 0.03, n = 17, F-test), but at a ~25% lower level (P = 0.008, n = 17, Wilcoxon rank-sum test). Data shown collected May 2006 – November 2011. Lines connect cohorts in the same experiment. (d) Lifespan of insulin-mutant (wDah; dilp2-31,5) and control (wDah) flies on holidic and oligidic diets. Insulin mutants were longer lived than controls (P < 0.0001, n = 100, Cox proportional hazards) on both food types (effect of diet: P = 0.53, n = 100, Cox proportional hazards). There was a significant genotype x diet interaction (P = 0.004, n = 100, Cox proportional hazards) due to the enhanced lifespan of control flies on holidic diet.
The key features of our holidic medium that differentiate it from previously reported diets 4,7,8 are: first, the medium had an acetate buffer base and was adjusted to pH 4 - 4.5, rather than neutral, to match the pH of natural food sources 13 and of our laboratory sugar, yeast medium (SY) 14, and; second, it contains only purified ingredients (Table 1) to substitute for the non-defined components casein, lecithin and RNA extracts. Initially, we adopted proportions of nutrients identified as optimal in 7 and 15 (referred to as HUNTaa). In later experiments we found that the diet was improved by modifying the amino acid mixture to make it more similar to that found in yeast (referred to as Yaa).
Table 1.
Composition of holidic medium
| Ingredient | Stock | per liter |
Notes | Manufacturer, example order number |
|
|---|---|---|---|---|---|
| Gelling agent | Agar | 20 g | Difco, 214530 | ||
|
|
|||||
| Base | Buffer | 10x: | 100 ml | ||
| 30 ml/l glacial acetic acid | Fisher, A/0400/PB15 | ||||
| 30 g/l KH2PO4 | Sigma, P9791 | ||||
| 10 g/l NaHCO3a | Sigma, S8875 | ||||
|
|
|||||
| Sugar | Sucrose | 17.12 g | Sigma, S1888 | ||
|
|
|||||
| Amino acids | L-isoleucine | 1.82 g | Amounts for HUNTaa |
Sigma, I2752 | |
| L-leucine | 1.21 g | Sigma, L8912 | |||
| L-tyrosine | 0.42 g | Sigma, T3754 | |||
|
|
|||||
| Metal ions | CaCl2.6H2O | 1000x: 250 g/l | 1 ml | Sigma, C7902 | |
| CuSO4.5H2O | 1000x: 2.5g/l | 1 ml | Sigma, C7631 | ||
| FeSO4.7H2O | 1000x: 25 g/l | 1 ml | Sigma, F7002 | ||
| MgSO4 (anhydrous) | 1000x: 250 g/l | 1 ml | Sigma, M7506 | ||
| MnCl2.4H2O | 1000x: 1 g/l | 1 ml | Sigma, M3634 | ||
| ZnSO4.7H2O | 1000x: 25 g/l | 1 ml | Sigma, Z0251 | ||
|
|
|||||
| Cholesterol | Cholesterol | 20 mg/ml in EtOH | 5 or 15mlb |
Sigma, C8667 | |
|
|
|||||
| Water (milliQ) | 1 liter minus combined volume of additions to be added after autoclaving |
||||
|
| |||||
| Autoclave 15min at 120°C along with glassware (vials) for experiment and tubing for pump | |||||
| All additions below performed using sterile technique. | |||||
|
| |||||
| Amino acids | Essential amino acid stock solution |
8 g/l L-arginine | 60.51 mlc |
Amounts for HUNTaa |
Sigma, A5131 |
| 10 g/l L-histidine | Sigma, H8000 | ||||
| 19 g/l L-lysine(HCl) | Sigma, L5626 | ||||
| 8 g/l L-methionine | Sigma, M9625 | ||||
| 13 g/l L-phenylalanine | Sigma, P2126 | ||||
| 20 g/l L-threonine | Sigma, T8625 | ||||
| 5 g/l L-tryptophan | Sigma, T0254 | ||||
| 28 g/l L-valine | Sigma, V0500 | ||||
| Non-essential amino acid stock solution |
35 g/l L-alanine | 60.51 ml |
Amounts for HUNTaa |
Sigma, A7627 | |
| 17 g/l L-asparagine | Sigma, A0884 | ||||
| 17 g/l L-aspartic acid | Sigma, A6683 | ||||
| 1 g/l L-cysteine HCl | Sigma, C1276 | ||||
| 25 g/l L-glutamine | Sigma, G3126 | ||||
| 32 g/l glycine | Sigma, G7126 | ||||
| 15 g/l L-proline | Sigma, P0380 | ||||
| 19 g/l L-serine | Sigma, S4500 | ||||
| Sodium glutamate stock solution |
100 g/l sodium glutamate | 15.13 ml |
Amount for HUNTaa |
Sigma, G5889 | |
|
|
|||||
| Vitamins | Vitamin solution | 125 x: | 14 mld | ||
| 0.1 g/l thiamine (aneurin) | Sigma, T4625 | ||||
| 0.05 g/l riboflavin | Sigma, R4500 | ||||
| 0.6 g/l nicotinic acid | Sigma, N4126 | ||||
| 0.775 g/l Ca pantothenate | Sigma, P2250 | ||||
| 0.125 g/l pyridoxine (HCl) | Sigma, P9755 | ||||
| 0.01 g/l biotin | Sigma, B4501 | ||||
| Sodium folatee | 1000x: 0.5 g/l | 1ml | Sigma, F7876 | ||
|
|
|||||
| Other nutrients |
125x: | 8 ml | |||
| 6.25 g/l choline | Sigma, C1879 | ||||
| chloride | Sigma, I7508 | ||||
| 0.63 g/l myo-inositol | Sigma, I4125 | ||||
| 8.13 g/l inosine | Sigma, U3750 | ||||
| 7.5 g/l uridine | |||||
|
|
|||||
| Preservatives | Propionic acid | 6 ml | Sigma, P5561 | ||
| Nipagin | 100 g/l methyl 4- hydroxybenzoate in 95% EtOH |
15 ml | Clariant Nipagin M | ||
During preparation of the buffer stock, add NaHCO3 slowly as escaping CO2 will cause solution to foam vigorously. Despite this loss, its addition is necessary as omitting NaHCO3 causes flies to be short-lived.
5 ml used in all experiments except Fig. 1D, Fig. 3a&b, Fig. 4, Fig. 5, Supplementary Fig. 1, Supplementary Fig. 5.
The volumes shown for glutamate, essential and non-essential amino-acid solutions delivers 200mM biologically available nitrogen
50% more vitamin solution (to 21 ml/l improved the proportion of flies surviving from egg to adult, see Fig. 5b)
Folic acid brought into solution by drop-wise addition of 2 N NaOH solution
We found that holidic medium was adequate to support lifespan to a similar extent as flies on our longevity-promoting oligidic diet (1SY; Fig. 1b). Median lifespan between trials tended to be more stable on holidic medium than SY food, but this did not reach statistical significance (P=0.059, n=11). Egg-laying showed significantly less variation between replicate experiments (P=0.03, n=17) but at ~25% lower level than 1SY (Fig. 1c). Adjusting the proportions of amino acids to Yaa, both lifespan and egg-laying matched those on 1SY (Supplementary Fig. 1). We also assayed the lifespan of long-lived flies lacking three of the seven insulin-like peptides (dilp2, 3 and 5) 16 on both SY and holidic diets. Mutant flies were ~30% longer lived than controls on both media (Fig. 1d). Together, these data indicate that holidic medium is an adequate source of nutrients for adult lifespan and egg-laying and yields more consistent outcomes for these traits over time.
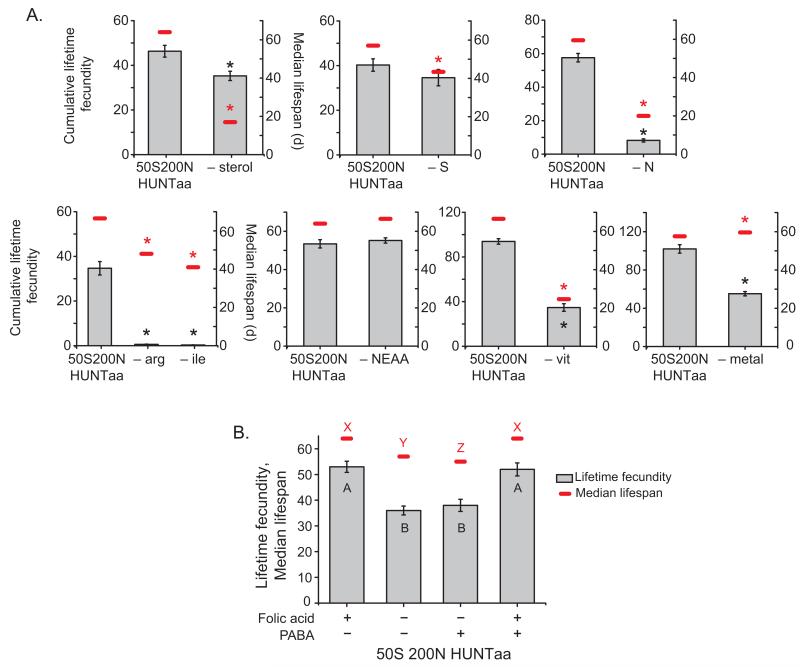
Compared with the medium containing all nutrients, omitting cholesterol, vitamins, amino acids or only the single essential amino acid arginine or isoleucine shortened lifespan by 30-70% and egg-laying by at least 30%. Omission of sugar did not affect egg-laying, but did reduce lifespan (Fig. 2a), similar to its effect in yeast-based foods 3, while a medium lacking all non-essential amino acids, except for glutamate which was provided to replace the nitrogen lost by omitting non-essential amino acids, had no detrimental effect on lifespan or egg-laying (Fig. 2a). Finally, we found that a medium containing only the ions and salts considered essential in 7 (K, P, Mg and Na) resulted in flies that were no shorter-lived than on a complete medium, but with a 50% reduction in lifetime egg-laying (Fig. 2a). Addition of Ca, Cu, Fe, Mn and Zn was required to establish normal egg-laying after the first week of adult life (Supplementary Fig. 2). Thus, each of the major nutrient groups in the holidic diet, except for the non-essential amino acids, was required for full egg-laying and lifespan.
Figure 2.
Nutrient-specific effects on Drosophila fitness traits. (a) From the holidic diet, omitting cholesterol (– sterol), sugar (– S), amino acids (– N), arginine alone (– arg), isoleucine alone (– ile) or vitamins (– vit) shortened lifespan (P < 0.001, n = 100, for all comparisons, Log-rank test). Cumulative egg-laying was reduced by omission of either cholesterol, amino acids, arginine alone, isoleucine alone or vitamins (P < 0.016, n = 10 for all comparisons, Wilcoxon rank-sum test). Omission of the non-essential amino acids (– NEAA) had no effect on longevity (P = 0.28, n = 100, Log-rank test) or egg-laying (P = 0.71, n = 10, Wilcoxon rank-sum test) and removal of Ca, Cu, Fe, Mn and Zn (– metal) resulted in reduced egg-laying (P = 0.009, n = 5, Wilcoxon rank-sum test), and a small, but significant increase in lifespan (P = 0.002, n = 100, Log-rank test). Asterisks indicate significance (P < 0.05) versus complete holidic diet control. Bars represent mean ± s.e.m. (b) Lifespan and egg laying for flies on media without folic acid. Omission of dietary folic acid significantly shortened lifespan (P < 0.016, n = 100, in both comparisons, Log-rank test) and reduced egg laying (P < 0.002, n = 10, in both comparisons, Wilcoxon rank-sum test). This was not rescued by addition of para-amino benzoic acid that microbes but not flies can convert to folic acid. Unique letters indicate significant differences. Bars represent mean ± s.e.m.
To ensure that the flies were acquiring their nutrition directly from the holidic medium rather than from microbes growing on it, we tested the effect of replacing folic acid with equimolar para-aminobenzoic acid (PABA), which microbes, but not flies, can readily convert to folate. Irrespective of the presence of PABA, absence of folic acid reduced both lifespan and lifetime egg-laying of the flies (Fig. 2b). These data indicate that the holidic medium itself is directly supplying sufficient nutrients to support egg-laying and lifespan of adult flies.
Dietary amino acids determine adult egg-laying and lifespan
Fly lifespan and fecundity are both altered by changes in the balance of sugar and yeast in SY food, where yeast is the flies’ only source of protein 17–19. The holidic medium allowed us to dissect this further by testing the effect of a concentration range of both sugar and amino acids (Supplementary Fig. 3). Increasing the sugar: amino acid ratio caused both lifespan and egg-laying to decline, while decreasing the sugar: amino acid ratio caused egg-laying to rise and lifespan to decline (Supplementary Fig. 3). We standardized sucrose at 50 mM (50S), because this led to significantly longer lifespan than did 0 mM sucrose, while any further increase reduced egg-laying. Adding amino acids at 200 mM biologically available nitrogen (200N HUNTaa; see Online Methods) produced both long lifespan and relatively high egg-laying. Further amino acid addition caused egg-laying to increase and lifespan to decrease. This inverse relationship between lifespan and egg-laying with dietary restriction, is also observed by changing either yeast 17 (Supplementary Fig. 3) or amino acid levels 14 in SY food.
Holidic medium to study behavior
We assessed the activity and sleep levels of flies kept on holidic medium. During a 72 hour period there was no difference in the activity patterns of flies between the two diets, whether assessed in real time (Fig. 3a andSupplementary Fig. 4) or as the cumulative time spent active or sleeping (Fig. 3b and Supplementary Fig. 4). These data establish a baseline to utilize the medium for examining the effects of specific nutrients on behavior.
Figure 3.
Holidic diet as a tool to study behavior (a, b) Activity and sleep profiles of flies on holidic and oligidic diets (a) (summed into 30 minute bins) for female and male (Supplementary Fig. 4) flies recorded for 72 h following a 24 h acclimation period. Black and white boxes represent light and dark periods. (b) Average sleep amount and total waking activity were summed during the 72 h recording. They did not significantly differ between diets (P > 0.35, n = 16, for both comparisons, Wilcoxon rank-sum test). Plotted data represent mean ± s.e.m. (c) Diet preference test. After maintenance on holidic diets lacking either sucrose or amino acids for a period of 3 or 6 days, flies were then assayed for their choice of sugar alone or yeast alone. Flies deprived of amino acids showed a significantly greater yeast preference (Yeast Preference Index; YPI) than those deprived of sugar (P < 0.0001, n = 13 for 3-day deprived and n = 14 for 6-day deprived flies, Wilcoxon rank-sum test).
After a period of yeast deprivation, flies will choose yeast over sugar 20, which is interpreted as compensation for protein deprivation. However, yeast provides many nutrients for flies as well as possessing hedonic properties that confound the interpretation of how and why flies choose it as a food source. To test if yeast preference is induced by a lack of amino acids, we maintained flies on holidic media lacking either sugar or amino acids and then gave them a choice of either sugar alone or yeast alone. After 3 or 6 days of nutrient-specific deprivation on holidic diets, the flies that were deprived of amino acids preferentially ingested yeast instead of sugar and those deprived of sugar, preferred sucrose over yeast (Fig. 3c). These data indicate that flies sense internal amino acid deficiency, leading to an enhanced preference for proteinaceous food. Moreover, these data highlight the suitability of the holidic diet to aid in dissecting the contribution of specific nutrients to shaping feeding behavior.
Drug bioavailability is enhanced in the holidic medium
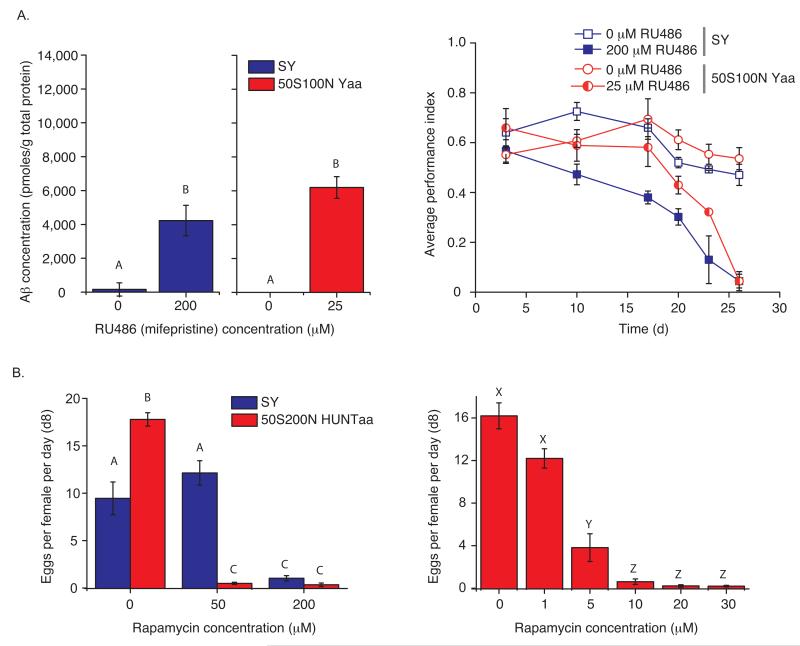
The Drosophila GeneSwitch 21 system can be used to direct the expression of cloned genes in response to a drug (RU486) administered in the food. We have used this system to generate an adult-onset fly model of Alzheimer’s disease 22. Using the holidic diet, we found Aβ42 expression was induced at lower doses of RU486 compared with drug additions to SY food and that for our standard dose of 200 μM RU486, ~3-fold more Aβ42 was found in flies on the holidic diet than on SY food (Supplementary Fig. 5). Furthermore, Aβ42 induction caused similarly detrimental effects on climbing ability between the two media (Supplementary Fig. 5). A more refined dose-response analysis showed that 25 μM RU486 in the holidic medium reproduced both Aβ42 expression levels and a similar climbing defect seen in flies on 200 μM RU486 in SY food (Fig. 4a).
Figure 4.
Drug bioavailability is increased on holidic medium. (a) RU486-induction of transgenic Aβ42 expression. Only 25 μM RU486 was required on holidic medium to match Aβ42 peptide expression found for flies feeding on oligidic diet with 200 μM RU486 (P = 0.05, n = 3, Student’s t-test). Climbing ability was similarly affected over time for flies on both media when Aβ42 was induced to the same level (diet by age interaction, P = 0.47, n = 3, 2-way ANOVA). There was a significant effect of diet (P = 0.0006, n = 3, 2-way ANOVA) due to the flies on holidic diet starting with a higher performance index, and age (P < 0.0001, n = 3, 2-way ANOVA). Supplementary fig. 5 shows a wider range of RU486 concentrations. Bars represent mean ± s.e.m. Unique letters indicate significant differences. (b) Effect of rapamycin on egg laying. Only 10 μM rapamycin was required to completely block egg-laying on holidic food (P < 0.0001, n = 5, Wilcoxon rank-sum test), whereas a dose of 200 μM was required in SY food (P = 0.001, n = 5, Wilcoxon rank-sum test). Unique letters indicate significant differences. Bars represent mean ± s.e.m.
Rapamycin inhibits Target of Rapamycin (TOR) kinase activity and suppresses egg-laying in Drosophila 23. The minimum dose required to completely suppress female egg-laying at one week was 10 μM in holidic diet, while on SY food it was somewhere between 50 – 200 μM (Fig. 4b). In fact, a dose as small as 5 μM rapamycin on the holidic diet was sufficient to substantially reduce egg-laying. Together, these data show that drug bioavailability was increased in holidic food.
Development on the holidic diet
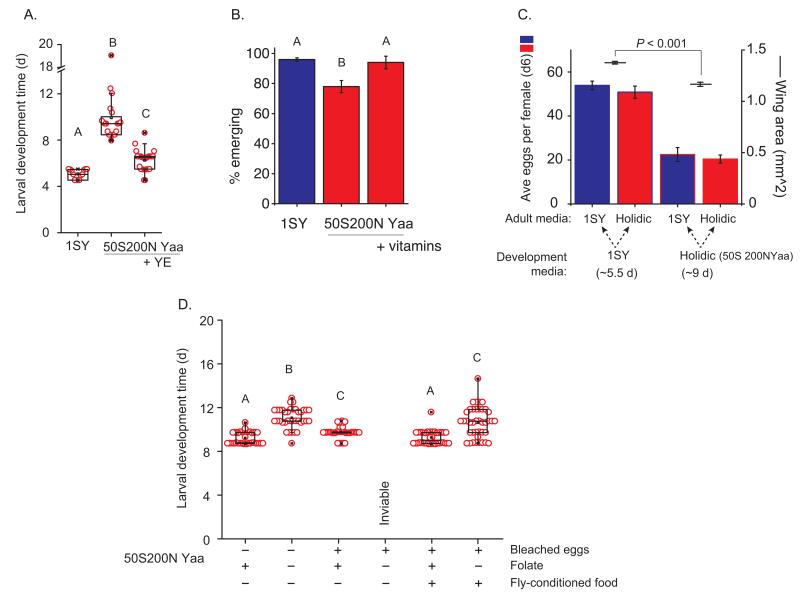
Average time for development from egg to the appearance of the first pupa was 9.9±0.3 days on holidic diet, considerably longer than on SY medium (Fig. 5a). Development time was not shortened by addition of extra amino acids, carbohydrates (as sucrose, glycogen or trehalose), carnitine, folic acid, nucleosides, choline or metal ions to the medium. Interestingly, addition of extra vitamins in the context of higher amino acid levels did improve the proportion of flies surviving from egg to adult (Fig. 5b), but this did not improve development time. Adding water-soluble yeast extract to the defined medium, which did not change the texture, reduced development time to 6.3±0.3 days (Fig. 5a), only one day slower than on SY food. The active ingredient(s) for this improvement is not known, but we conclude it acted to limit growth rather than being an essential element that was missing because, at the time of writing, we have been able to rear Drosophila on the medium for eleven successive generations with a stable developmental duration. We also found that the limiting nutrient could not be extracted with chloroform, is heat stable (95 °C for 135 minutes) and is smaller than 10 KDa indicating the critical element is not lipid, large protein or a heat labile vitamin.
Figure 5.
Development on holidic media. (a) Development was delayed on holidic medium (P < 0.0001, n = 13 oligidic diet (SY) and n = 20 holidic diet, Wilcoxon rank-sum test). The delay was significantly reduced by addition of water-soluble yeast extract (YE) (P < 0.0001, n = 29, Wilcoxon rank-sum test). (b) Adding 50% more vitamins to holidic food improved eclosion success (P < 0.05, n = 3, Student’s t-test) to match oligidic food. (c) Rearing conditions affect adult fitness. Wing area was smaller for flies reared on holidic medium (P < 0.001, n = 22 SY, n = 16 holidic diet, Wilcoxon rank-sum test). Holidic and oligidic diets supported egg laying equally although the larger flies reared on oligidic food laid more eggs than those from holidic food (effect of rearing diet P < 0.0001, n = 8; effect of adult diet, P = 0.31, n = 8; interaction P = 0.85, n = 8, Linear Model). (d) Folic acid requirement for development. Untreated or bleach treated eggs were transferred to sterile holidic media either with or without folic acid. Removing folic acid delayed development of untreated eggs (P < 0.0001, n = 40, Wilcoxon rank-sum test) and abolished development of bleach-treated eggs. Bleach treatment slowed development in the presence of folate (P = 0.015, n = 40, Wilcoxon rank-sum test), which was rescued by pre-exposing developmental medium to male flies. Within panels, unique letters indicate significant differences. Data expressed as means ± s.e.m.
After rearing flies on holidic diet, we measured their egg-laying and wing size, which is a proxy for body size. Flies that developed in SY food were ~18% bigger and had a ~2.5-fold higher egg laying capacity than those reared in holidic medium (Fig. 5c). However, once the flies reached adulthood, the holidic medium was an equally good source of nutrients for egg-laying as SY food.
Microbes can adhere to Drosophila eggs and promote larval growth under nutrient limiting conditions 24. These microbes can be removed by bleach-treating eggs to produce germ free animals 7. We reared larvae from both untreated and bleach-treated eggs on sterile holidic medium, with or without folic acid. Absence of folic acid from the medium caused a developmental delay of approximately two days for normal, untreated eggs, indicating that dietary folates are not required for larval development (Fig. 5d). Bleach-treatment delayed development on folic-acid containing food by ~0.5 day, and led to a complete failure to produce any pupae on medium without folic acid (Fig. 5d). This was not simply a detrimental effect of bleach treatment, because adding bleached eggs to defined medium that had previously been exposed to male flies and that had therefore presumably acquired their associated microbes, rescued development time back to the level of untreated eggs (Fig. 5d). Thus, Drosophila larvae require exogenous folic acid for development and this demand can be met either from dietary sources or from egg-associated microbes.
Discussion
We report a medium for Drosophila for which the chemical composition is entirely defined. Much of the original work to determine the nutritional requirements of Drosophila melanogaster employed meridic media 7,8. We found that the non-defined components of these meridic diets were likely to have contained nutritious impurities, the most obvious of which were trace metal ions that we found to be essential to sustain egg-laying beyond the first week of adult life. Our medium is the only holidic diet reported to support adult lifespan and egg-laying to the same extent as oligidic food that we have optimized for these traits 3. While a meridic medium that supported egg-laying to nearly the same extent as yeast-based food has been reported 8 there is no record of the effect on lifespan. Lifespan has been measured on several other meridic media 9,25–28, and one 9 supported lifespan to the same extent as yeast-based food. Unfortunately, egg-laying data are not available for this diet and there is unresolved diffreences between the authors and manufacturers about the exact concentration of some components (Harlan Teklad TD.04310)10.
Flies reared on our holidic medium were delayed, smaller and had lower egg laying capacity than those maintained on SY food. Reduced fecundity is a known correlate of smaller body size in many animals, including Drosophila 29, but the reasons for this effect are unknown. Thus current knowledge does little to indicate what might be limiting in our holidic diet. Given the problem of optimization in such complex nutritional space, we are posting the protocol at Nature Protocol Exchange for community feedback for improvement http://dx.doi.org/10.1038/protex.2013.082.
Two drugs that are widely used in Drosophila research had eight – ten-fold higher bioavailability in our holidic diet than in SY food. Given that these drugs, RU486 and rapamycin, cost ~eight-times and ~four-times more, respectively, than gold at its record high in September 2011, the cost advantage of enhanced bioavailability is obvious. Moreover, lower doses of drugs with poor palatability reduces the need to pre-starve flies before treatment and for drugs whose water solubility is low, enhanced bioavailability avoids the addition of toxic doses of vehicles, such as DMSO 30. Given the improved consistency in nutritional value supported by the holidic diet over time, our diet offers a more stable platform on which to undertake drug studies.
General patterns of fly activity and sleep are known to be nutritionally sensitive 31. When comparing flies maintained on holidic medium and SY food, we found no differences in activity patterns indicating that, from a behavioral point of view, the flies were experiencing nutritionally similar conditions on both diets. Activity patterns may change in response to diet quality to enhance foraging strategies. Classical models of foraging focus on maximizing net caloric gain, however organisms also possess the ability to adjust foraging behavior in response to physiological protein imbalances 32–34. We found that flies specifically deprived of amino acids choose to ingest yeast over a sugar medium, indicating they seek out yeast to find amino acids. This is perhaps not surprising given the cost to egg-laying of amino acid omission from the diet. It will be interesting to dissect the exact amino acid requirements for this response, as well as the molecular and neuronal mechanisms required for this behavior. Interestingly, when flies were maintained on holidic diet without sugar they chose to eat the sugar medium over the yeast medium, even though we observed no cost to lifetime egg-laying, and only a small reduction in lifespan, of being sugar deprived. This tendency to ingest sweet diets without apparent need for enhanced fitness, perhaps reflects the relatively lower cost of over-ingesting sugar, which can be stored as fat, compared with the cost of over-ingesting nitrogen, which must be excreted.
Many organisms are known to benefit from metabolites produced by associated gut microbes. Here, we show that Drosophila has an obligate requirement for exogenous folic acid during development and that this can be supplied either directly from the diet, or from inherited microbes that normally reside on the surface of eggs. This establishes the holidic medium as an ideal setting in which to characterize the exact nature of the relationship between flies and their inherited microbiota.
Online Methods
Media and flies
In all experiments except those for RU486 calibration, we used our laboratory stock of outbred wild type Drosophila melanogaster, Dahomey, which has been cured of Wolbachia by tetracycline treatment 1. Flies were maintained in large population cages with overlapping generations at 25 °C with a 12 h: 12 h light: dark cycle. UAS-ArcAβ42 and elavGS transgenic flies were backcrossed into w1118, as reported in 2 and wDah; dilp2-31,5 deletion mutants and Wolbachia-positive white Dahomey (wDah) controls are those reported in 3. All genetic constructs were back-crossed into the genetic background of their control for at least six generations before experiments were performed.
For all experiments using adult flies, other than food choice, flies were reared on sugar, yeast food (1SYBrewer’s; 1SY) as described in 4 for lifespan experiments. Egg collections were used to synchronize fly age as described in 5. Flies for the food choice assay were reared in a medium containing, per liter, 80 g cane molasses, 22 g beetroot syrup, 8 g agar, 80 g corn flour, 10 g soya flour, 18 g yeast extract, 8 ml propionic acid, 12 ml nipagin (15% in ethanol).
Holidic media
An open access editable version of this protocol is available through Nature Protocol Exchange at: http://dx.doi.org/10.1038/protex.2013.082. A description of the components of the holidic medium as well as supplier order numbers for all components can be found in Table 1. Preparation of the medium is performed in two stages. In the first, sucrose, agar, amino acids with low solubility (L-isoleucine, L-leucine and L-tyrosine) as well as stock solutions of buffer, metal ions and cholesterol are combined in a 1 liter autoclavable bottle with a magnetic stirrer and milliQ water up to 1 liter, minus the volume of solutions to be added after autoclaving. Following autoclaving at 120 °C for 15 min, the solution is allowed to cool at room temperature with stirring to ~65 °C. Stock solutions for the amino acids (Table 2), vitamins, nucleosides, choline, inositol and preservatives are then added as are the drugs mifepristone (Sigma) or rapamycin (LC Laboratories), where appropriate. With constant stirring, sterile tubing is used to dispense the solution into sterile vials. These are covered, allowed to cool for 90 min at room temperature and then stored at 4 °C until use. Feedback from other users of the medium have highlighted that this method of preparation may result in media that does not set. This varies with the autoclave used and can be resolved by adding the sterilized buffer base after autoclaving, indicating that it is caused by acid hydrolysis of the agar during autoclaving.
Table 2.
Amino acid stock solutions for the holidic medium
| Amino acid | biologically available Na |
HUNTaa (g/200 mlb)c |
Yaa (g/200ml) |
|---|---|---|---|
| Essential amino acid stock solution | |||
| F (L-phenylalanine) | 1 | 2.6 | 3.03 |
| H (L-histidine | 2 | 2 | 2.24 |
| K (L-lysine) | 1 | 3.8 | 5.74 |
| M (L-methionine) | 1 | 1.6 | 1.12 |
| R (L-arginine) | 2 | 1.6 | 4.7 |
| T (L-threonine) | 1 | 4 | 4.28 |
| V (L-valine) | 1 | 5.6 | 4.42 |
| W (L-tryptophan) | 1 | 1 | 1.45 |
| Non-essential amino acid stock solution | |||
| A (L-alanine) | 1 | 7 | 5.25 |
| C (L-cysteine) | 1 | 0.1 | |
| D (L-aspartate) | 1 | 3.4 | 2.78 |
| G (glycine) | 1 | 6.4 | 3.58 |
| N (L-asparagine) | 2 | 3.4 | 2.78 |
| P (L-proline) | 1 | 3 | 1.86 |
| Q (L-glutamine) | 2 | 5 | 6.02 |
| S (L-serine) | 1 | 3.8 | 2.51 |
| Other amino acid stock solutions | ml/ld | ml/l | |
|
| |||
| C (L-cysteine) (50mg/ml)e | 1 | 5.28 | |
| E (L-glutamate, Na salt) (100mg/ml)f | 1 | 15.13 | 18.21 |
| Added as solid before autoclavingg | g/l | g/l | |
|
| |||
| I (L-isoleucine) | 1 | 1.82 | 1.16 |
| L (L-leucine) | 1 | 1.21 | 1.64 |
| Y (L-tyrosine) | 1 | 0.42 | 0.84 |
Moles of nitrogen available if 1 mole of amino acid completely catabolized
all stock solution prepared in milliQ water and pH adjusted using NaOH
to deliver 200mM biologically available nitrogen, 60.51 ml of both the essential and non-essential amino acid solutions must be added to the medium as well as the indicated amounts of E, C, I, L, Y where they need to be added separately.
these volumes refer to ml of stock solution to be added per liter medium
For Yaa stock, C drops out during storage. A separate stock of C should be made and added to food directly when adding other amino acid solutions.
E solution is added separately for flexibility as is used to make up loss of bulk nitrogen when food is grossly imbalanced.
Due to relatively poor solubility, these three amino acids are added directly to the food before autoclaving.
Stock solutions are prepared in milliQ water, except for the cholesterol stock, which is prepared in absolute ethanol. The cholesterol stock, buffer stock, amino acid solutions and stock containing nucleosides, choline and inositol are stored at 4 °C, while the FeSO4, vitamin and folic acid stocks are stored at –20 °C. Before freezing of these latter stocks, we would typically make 1 liter and make aliquots of smaller volumes so that once thawed, they could be used quickly and without re-freezing. Before storing, amino acid stocks are pH adjusted to 4.5 using HCl. All aqueous solutions were filter sterilized by passing through a 0.22 μm syringe-fitted filter. Note also, that cholesterol precipitates out of solution during storage at 4°C, but it easily re-dissolves with stirring at room temperature. The amino acid ratio shown in Table 1 refers to HUNTaa. The proportions and amounts of the amino acid stock solutions as well as their value in terms of biologically available nitrogen can be found in Table 2.
Measuring development, egg-laying and lifespan
For development assays, young age-matched flies were allowed to lay eggs on grape juice plates overnight. 24 h later, 1st instar larvae were picked onto test media, which were kept at 25 °C. The appearance of pupae was scored twice a day.
To generate age-synchronized adult flies larvae developed on SY food at standard density, transferred to fresh SY food upon emerging as adults and allowed 48 h to mate. Under light CO2 anesthesia, females were separated from males and allocated to treatment vials at a density of ten flies per vial. Flies were transferred to fresh vials three times per week at which point deaths and censors were scored. Egg-laying was scored after flies occupied vials for ~18 h and the value expressed as the number of eggs per vial per female.
To generate germ-free larvae, eggs were bleach treated according to 6 in which eggs were transferred into a sterile mesh strainer and bleached in 2.7% sodium hypochlorite for two minutes, washed twice in 70% ethanol and rinsed twice in sterile diluted Phosphate Buffered Saline (PBS; 1:10) for one minute. Eggs were collected and transferred into a sterile 1.5 ml micro tube using an autoclaved brush whence 4 μl was transferred onto the appropriate food using a pipette. In order to rescue microbe loss, food vials were exposed to five male flies for 24 h. The male flies were removed before the eggs were placed on the food.
Multiple-generation fly rearing was performed using three replicate vials of 1SY in parallel with three replicate vials of 50S200N Yaa holidic medium. At each generation, flies were allowed to emerge on the food they had developed and transferred to fresh food of the same type to mate. After 3–5 days, 5 female and 5 male siblings were allocated to fresh vials for overnight egg laying, whereupon the adults were discarded and eggs allowed to develop. Time to adult emergence was recorded.
Assays for drug delivery, climbing and behaviors
For rapamycin and RU486 administration, flies were reared and sorted into single-sex groups as for lifespan experiments. Flies were introduced to drug-containing food two days post-eclosion. Negative geotaxis assays and ELISA for Aβ42 levels were performed at day 14, according to the methods described in 2.
The food choice assay was performed as described in 7. Groups of 1 – 5 day old flies (16 females and 5 males) were reared and maintained for 72 h after eclosion in the oligidic medium described above. Flies were transferred to holidic medium from which either sucrose or amino acids were omitted and maintained for 72 h at which point food preference was tested. Flies were given the choice between red sucrose (20 mM sucrose; 7.5 mg/ml agarose; 5 mg/ml Erytrosin B (Sigma-Aldrich); 10% PBS) and blue yeast (10% yeast (SAF instant yeast); 7.5 mg/ml agarose; 0.25 mg/ml Indigo carmine (Sigma-Aldrich); 10% PBS) medium. After visual inspection of the abdomen, each female fly was scored as having eaten red sucrose (red abdomen), blue yeast (blue abdomen), or both (red and blue or purple abdomen) media. Dye swap experiments were performed and amino acid deprived flies statistically significantly chose to eat yeast over sucrose in this condition when compared with flies kept on the medium without sugar (data not shown). The yeast preference index (YPI) for the whole female population in the assay was calculated as follows: (nblue yeast – nred sucrose)/(nred sucrose + nblue yeast + nboth). Fly rearing, maintenance, and behavioral testing were performed at 25 °C in a humidified and temperature controlled chamber.
For activity and sleep monitoring, young (2–4 day old) mated male and female Canton-S flies (n = 16 per group) were transferred to individual 5 mm polycarbonate vials containing either 1SY medium or 50S 200N HUNTaa holidic medium. Vials were transferred to a Trikinetics activity monitoring apparatus (Trikinetics) in an environmentally controlled chamber at 25 °C with a 12 h: 12 h light: dark cycle. Activity counts were summed every minute and sleep was defined as 5 or more minutes of continuous inactivity 8 The average sleep and waking activity (activity counts / minutes awake) were calculated for each fly.
Statistical analyses
All statistical analyses were performed using JMP (V9).
Reproducibility
Sample sizes are at least, and in many cases beyond, what is required to give confidence in the results. These are consistent with established norms for research on Drosophila and ageing. For lifespan and egg laying assays, individuals that developed in the same vessels were assigned evenly and systematically between experimental treatments. None of the assays required scoring blindly.
Supplementary Material
Acknowledgements
We would like to acknowledge the following funding: MDWP: the Royal Society (UF100158), the Biotechnology and Biological Sciences Research Council, UK (BB/I011544/1); RLG: Foundation for Science and Technology (postdoctoral fellowship SFRH/BPD/78947/2011) and EMBO (long-term postdoctoral fellowship ALTF 1602-2011); FK: Alzheimer’s Research, UK; SDP: This work utilized the Drosophila Aging Core of the Nathan Shock Center of Excellence in the Biology of Aging funded by the USA National Institute of Aging (P30-AG-013283); CR: Champalimaud Foundation, the BIAL foundation and the Foundation for Science and Technology (grant PTDC/BIABCM/118684/2010); LP: the Wellcome Trust UK (098565/Z/12/Z), Max Planck Society and the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement no. 268739.
Footnotes
Competing financial interests statement The authors declare no competing financial interests.
References
- 1.Shorrocks B. An ecological classification of European Drosophila species. Oecologia. 1977;345:335–345. doi: 10.1007/BF00345533. [DOI] [PubMed] [Google Scholar]
- 2.McKenzie J, McKechnie S. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia. 1979;40:299–309. doi: 10.1007/BF00345326. [DOI] [PubMed] [Google Scholar]
- 3.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62:1071–81. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sang JH. In: Genet. Biol. Drosoph. Ashburner M, Wright TRF, editors. Academic Press; 1978. pp. 159–192. [Google Scholar]
- 5.Piper MDW, Partridge L. Dietary restriction in drosophila: delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz J, St. Lawrence P, Newmeyer D. A chemically defined medium for the growth of Drosophila melanogaster. Anat. Rec. 1946;96:540. [PubMed] [Google Scholar]
- 7.Sang JH. The quantitative nutritional requirements of Drosophila melanogaster. J.Exp.Biol. 1956;33:45–72. [Google Scholar]
- 8.Sang J, King R. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J. Exp. Biol. 1961;38:793–809. [Google Scholar]
- 9.Troen A, et al. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Omaha) 2007;29:29–39. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troen AM, et al. Erratum to: Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Omaha) 2010;32:123–123. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shchedrina V. a, et al. Analyses of fruit flies that do not express selenoproteins or express a mouse selenoprotein, methionine sulfoxide reductase B1, reveal a role of selenoproteins in stress resistance. J. Biol. Chem. 2011;286:29449–29461. doi: 10.1074/jbc.M111.257600. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W-C, Micchelli C. a. Development and Characterization of a Chemically Defined Food for Drosophila. PLoS One. 2013;8:e67308. doi: 10.1371/journal.pone.0067308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakir M, Peridy O, Capy P, Pla E, David JR. Adaptation to alcoholic fermentation in Drosophila: a parallel selection imposed by environmental ethanol and acetic acid. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3621–5. doi: 10.1073/pnas.90.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–4. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt V. A qualitatively minimal amino acid diet for D. melanogaster. Drosoph. Inf. Serv. 1970;45:179. [Google Scholar]
- 16.Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2498–503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010;20:1000–5. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc.Natl.Acad.Sci.U.S.A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofola O, et al. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer’s disease. PLoS.Genet. 2010;6:e1001087. doi: 10.1371/journal.pgen.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem. Soc. Trans. 2011;39:460–5. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 24.Storelli G, et al. Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Hollingsworth MJ, Burcombe JV. The nutritional requirements for longevity in Drosophila. J.Insect Physiol. 1970;16:1017–1025. doi: 10.1016/0022-1910(70)90195-2. [DOI] [PubMed] [Google Scholar]
- 26.Van Herrewege J. Nutritional requirements of adult Drosophila melanogaster: the influence of the casein concentration on the duration of life. Exp.Gerontol. 1974;9:191–198. doi: 10.1016/0531-5565(74)90036-9. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Romero FJ, et al. Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality. J. Biol. Chem. 2001;276:29798–804. doi: 10.1074/jbc.M100422200. [DOI] [PubMed] [Google Scholar]
- 28.Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech.Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Lefranc A, Bundgaard R. The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas. 2000;132:243–247. doi: 10.1111/j.1601-5223.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- 30.Pandey U, Nichols C. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linford NJ, Chan TP, Pletcher SD. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 2012;8:e1002668. doi: 10.1371/journal.pgen.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front. Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson SJ, Raubenheimer D. The Nature of Nutrition. Princeton University Press; 2012. [Google Scholar]
- 34.Stephens DW, Brown JS, Ydenberg RC. Foraging. The University of Chicago Press; 2007. [Google Scholar]
Methods-only references
- 35.Toivonen JM, et al. No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS.Genet. 2007;3:e95. doi: 10.1371/journal.pgen.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofola O, et al. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer’s disease. PLoS.Genet. 2010;6:e1001087. doi: 10.1371/journal.pgen.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62:1071–81. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in bottle cultures. Dros.Inf.Serv. 2001;84:168–169. [Google Scholar]
- 40.Shin SC, et al. Drosophila Microbiome Modulates Host Developmental and Metabolic Homeostasis via Insulin Signaling. Science (80-. ) 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010;20:1000–5. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 42.Huber R, et al. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–39. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.