Abstract
Background
Studies to date showing an association between cannabis use and schizophrenia-spectrum disorders are of relatively small sample sizes with limitations in generalizability. The present study addresses this gap by examining the relationship between cannabis use and psychotic-like symptoms in a large representative community sample.
Method
Data were derived from the 2004 – 2005 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC, Wave 2), a large, nationally representative sample of 34 653 adults from the United States population. We evaluated the association between lifetime cannabis use, psychosis, and schizotypal personality features.
Results
The prevalence of psychosis and schizotypal personality disorder increased significantly with greater cannabis use in a dose-dependent manner. The association between cannabis use and psychosis was 1.27 (95% CI 1.03–1.57) for lifetime cannabis use, 1.79 (95% CI 1.35–2.38) for lifetime cannabis abuse, and 3.69 (95% CI 2.49–5.47) for lifetime cannabis dependence. There was a similar dose-response relationship between the extent of cannabis use and schizotypal personality disorder (OR = 2.02 for lifetime cannabis use, 95% CI 1.69–2.42; OR = 2.83 for lifetime cannabis abuse, 95% CI 2.33–2.43; OR = 7.32 for lifetime cannabis dependence, 95% CI 5.51–9.72). Likelihood of individual schizotypal features increased significantly with increased extent of cannabis use in a dose-dependent manner.
Conclusion
This is the first population-based study to examine the association between lifetime cannabis use, psychosis, and schizotypal personality traits. These results add to evidence that cannabis use may be a risk factor for psychosis liability.
Keywords: cannabis, epidemiology, psychosis, schizotypal, NESARC
1. INTRODUCTION
Cannabis is the most widely used illicit substance in the United States (Substance Abuse and Mental Health Services Administration, 2010) and the most commonly used illicit drug among patients with schizophrenia. Accumulating evidence from longitudinal epidemiologic studies also suggests that cannabis use may increase the risk of schizophrenia (Andreasson et al., 1987; Arseneault et al., 2002; Degenhardt et al., 2001; Fergusson et al., 2003; Henquet et al., 2005; Tien and Anthony, 1990; van Os et al., 2002; Zammit et al., 2002), serving as a component cause of the disorder, meaning one of a constellation of complex factors that may hasten the development of psychotic symptoms while neither necessary nor sufficient to do so alone (Compton et al., 2009).
One approach to explore the relationship between cannabis use and psychotic symptoms is to examine cannabis use as a correlate of schizotypal personality disorder (SPD), characterized by a set of dimensional traits that are thought to contribute to risk for psychosis. SPD traits have been reported to be more prevalent in relatives of patients with schizophrenia (Appels et al., 2004; Kendler et al., 1995) and may share some of the same genetic underpinnings as schizophrenia (Fanous et al., 2007). Individuals with SPD also exhibit social deficits similar to, but less prominent than, those found in schizophrenia (Dickey et al., 2005).
An emerging body of research suggests an association between schizotypy and cannabis use. Several small studies of university students have found associations between cannabis use and positive schizotypal features (Bailey and Swallow, 2004; Barkus and Lewis, 2008; Dumas et al., 2002; Esterberg et al., 2009; Mass et al., 2001; Najolia et al., 2012; Nunn et al., 2001; Schiffman et al., 2005; Skosnik et al., 2001; Williams et al., 1996). One study of 40 college undergraduates found a significant positive correlation between schizotypy scores and cannabis use (Skosnik et al., 2001). Another study (Bailey and Swallow, 2004) found an association between cannabis use and the presence of positive, negative, and disorganized schizotypal traits among 60 undergraduates. However, other studies have shown that cannabis users have lower negative schizotypal traits than non-users (Nunn et al., 2001; Schiffman et al., 2005).
Understanding the relationship between cannabis use and SPD is important for enhancing our understanding of cannabis use as a risk factor for schizophrenia-spectrum disorders and has implications for prevention and treatment, especially in light of evidence showing that interventions targeting personality factors can significantly reduce substance use (Conrod et al., 2008). While studies to date provide a foundation for establishing an association between cannabis use and schizotypy, previous studies have shown both positive and negative associations between cannabis use and domains of schizotypy, as described above. Discrepancy in previous research challenges our understanding of the relationship between cannabis use and schizotypy. One possible explanation for the discrepancy in previous studies is that all of them are limited by relatively sample sizes and limited statistical power, as well as limitations in generalizability. None has examined whether the extent of cannabis use is associated with likelihood of having SPD features. We aim to clarify the discrepancy in previous research on cannabis use and schizotypy using an epidemiologic approach that extends current evidence based on small survey samples. The present study examines – in a large, representative U.S. sample – the relationship between cannabis use and both psychosis and SPD, and whether there is a dose-response relationship between the extent of cannabis use and psychosis and specific schizotypal personality traits.
2. METHODS
2.1 Sample and procedures
Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) was used as the source of data. The NESARC is a large, nationally representative survey of people living in the 50 states of the U.S. and the District of Columbia, including citizens and noncitizens, aged 18 years and older (Grant, 2003; Grant et al., 2005; Grant et al., 2004). Wave 1 of the NESARC was conducted in 2001 and 2002 with households and non-institutional group quarters, with a total of 43 093 respondents and response rate of 81.2%. The Wave 1 NESARC assessed Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) alcohol and specific drug use disorders, mood and anxiety disorders, and some personality disorders (avoidant, dependent, obsessive-compulsive, paranoid, schizoid, histrionic, and antisocial). In Wave 2, conducted in 2004 – 2005, attempts were made to interview all 43 093 respondents from Wave 1. After excluding individuals ineligible for Wave 2 (e.g., deceased), the Wave 2 response rate was 86.7%, reflecting 34,653 completed Wave 2 interviews. The Wave 2 interview assessed DSM-IV alcohol and specific drug use disorders and mood/anxiety disorders assessed in Wave 1, in addition to schizotypal, borderline, and narcissistic personality disorders, posttraumatic stress disorder and attention-deficit/hyperactivity disorder. The current study is an analysis of Wave 2 NESARC data (SPD was not assessed in Wave 1).
The weighted data were adjusted to represent the U.S. civilian population based on the 2000 Census. All of the potential respondents were informed about the nature of the survey, the statistical uses of data, the voluntary nature of their participation, and federal laws regarding the confidentiality of the identifiable survey information. Additional details about the NESARC methodology can be found elsewhere (Grant, 2003).
2.2 Diagnostic assessment
The Alcohol Use Disorder and Associated Disabilities Interview Schedule, DSM-IV Version (AUDADIS-IV) (Grant et al., 2003), a structured diagnostic interview designed for administration by professional interviewers, was used to assess lifetime and past-year DSM-IV disorders. In addition to questions to diagnose mood, anxiety, psychotic, and personality disorders, the AUDADIS-IV included questions on an extensive list of symptoms that separately operationalized DSM-IV criteria for substance use disorders, including drug-specific abuse and dependence for cannabis and nine other classes of drugs. Information on the reliability and validity of the AUDADIS-IV has been previously published (Grant et al., 2003; Ruan et al., 2008). Due to concerns about the feasibility of assessing psychotic diagnoses in general population surveys (Kendler et al., 1996; Kessler et al., 2005) as well as the length of the interview, a diagnosis of lifetime schizophrenia or a psychotic illness or episode (SPIE) was given to any person who answered yes to the question, “Did a doctor or other health professional ever diagnose you with schizophrenia or a psychotic illness or episode?” This method, which was validated in a prior study (Supina and Patten, 2006), provides prevalence rates of schizophrenia similar to those estimated in the North American population (Jablensky, 1997).
The diagnosis of personality disorders requires evaluation of long-term patterns of functioning (American Psychiatric Association, 1994). Diagnoses of SPD in the AUDADISIV were made accordingly. All NESARC respondents were asked a series of SPD symptom questions about how they felt or acted most of the time throughout their lives, regardless of the situation or whom they were with at the time. They were instructed not to include symptoms occurring only when they were depressed, manic, anxious, drinking heavily, using medicines or drugs, experiencing withdrawal symptoms (defined earlier in the interview), or physically ill.
Multiple symptom items were used to operationalize the criteria for DSM-IV personality disorders, including SPD. Personality disorder symptom items (Grant et al., 2003) were similar to those appearing in the Structured Clinical Interview for DSM-IV Axis II Disorders (First et al., 1997), the International Personality Disorder Examination (Loranger, 1999), and the Diagnostic Interview for DSM-IV Personality Disorders (Zanari et al., 1996).
The reliability of AUDADIS-IV personality disorder diagnoses and symptoms scales was assessed in large test-retest studies conducted as part of the Wave 1 and Wave 2 NESARC surveys. The test-retest reliability of the SPD section of the measure has been reported as .67 in smaller samples, with average test and retest interval of 5.9 weeks (Ruan et al., 2008). The internal consistency of the SPD section of the NESARC data is .83, which supports the reliability of the measure in the NESARC (Ahmed et al, 2013). The test-retest reliabilities ranged from fair to good (κ = 0.40 to 0.71) for other personality disorders and from 0.79 to 0.83 for schizophrenia or psychotic episode (Grant et al., 2003), with little variability in test and retest intervals between sites, ranging from 3 to 20 weeks with average of approximately 10.2 weeks.
2.3 Statistical Analyses
Among Wave 2 respondents, we compared demographic characteristics (sex, age, and race) of lifetime cannabis users (defined as at least one episode of cannabis use during a person’s lifetime) and non-users. We also determined the percentage of participants with lifetime cannabis use, SPIE, and SPD in all Wave 2 respondents. Logistic regression analyses were used to obtain odds ratios (ORs) measuring the association between lifetime cannabis use, abuse, and dependence (using no lifetime cannabis use as the reference group) with psychosis and SPD. Logistic regression analyses also yielded ORs measuring the association between lifetime cannabis use, abuse, and dependence and likelihood of individual symptoms of SPD to examine whether the strength of association varied by symptom.
Standard errors and 95% confidence intervals for all analyses were estimated using SUDAAN (Research Triangle Park, 2008) to account for design effects of the NESARC. In examining associations between lifetime cannabis use and SPIE and SPD, adjustments were made for sociodemographic characteristics (sex, age, and race).
To be comprehensive in our analyses, we conducted the same analyses with individuals with lifetime cannabis use and individuals with past-year cannabis use. The pattern of results was the same for both sets of analyses, although due to the smaller sample size, some of the analyses using past-year users had lower power. Therefore, we present the results of the lifetime analyses. The results of the past-year analyses are available upon request.
3. RESULTS
Table 1 shows the descriptive sample characteristics of lifetime cannabis users and nonusers. Compared to non-users (n = 27 025), lifetime cannabis users (n = 7 438) were significantly more likely to be male. There were also significant differences with regard to age and race. Lifetime cannabis users were more likely to be younger (30–44 years old), and they were more likely to be white compared to cannabis non-users.
Table 1.
Descriptive Sample Characteristics of Cannabis Users and Nonusers
| Lifetime Cannabis Users (N=7,438) |
Nonusers (N=27,025) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | % | 95% CI | % | 95% CI | OR | 95% CI | |||
| Sex | |||||||||
| Male | 58.51 | 57.12 | 59.89 | 44.83 | 44.06 | 45.61 | 1.74 | 1.63 | 1.85 |
| Female | 41.49 | 40.11 | 42.88 | 55.17 | 54.39 | 55.94 | 1.00 | 1.00 | 1.00 |
| Age | |||||||||
| 18–29 years old | 23.47 | 22.13 | 24.87 | 14.29 | 13.62 | 14.98 | 1.00 | 1.00 | 1.00 |
| 30–44 years old | 39.33 | 37.94 | 40.74 | 26.90 | 26.03 | 27.79 | 0.89 | 0.80 | 0.99 |
| 45+ years old | 37.20 | 35.79 | 38.63 | 58.81 | 57.78 | 59.84 | 0.39 | 0.35 | 0.42 |
| Race | |||||||||
| White | 76.51 | 74.11 | 78.76 | 69.29 | 65.80 | 72.58 | 1.00 | 1.00 | 1.00 |
| Non-White | 23.49 | 21.24 | 25.89 | 30.71 | 27.42 | 34.20 | 0.69 | 0.63 | 0.77 |
Table 2 shows the percentage of lifetime cannabis users in the entire sample (n = 34,653) categorized as: no use, 77.53%; use, 12.82%; abuse, 7.91%; or dependence, 1.74%). SPIE was reported in 3.12% of the sample, and SPD was diagnosed in 3.93%.
Table 2.
Descriptive Sample Characteristics of Cannabis Users and Nonusers
| All Subjects (N=34,653) | |||
|---|---|---|---|
| Characteristics | % | 95% CI | |
| Cannabis use (lifetime) | |||
| No use | 77.53 | 76.46 | 78.58 |
| Use | 12.82 | 12.15 | 13.52 |
| Abuse | 7.91 | 7.42 | 8.42 |
| Dependence | 1.74 | 1.53 | 1.97 |
| Cannabis use (past-year) | |||
| No use | 95.43 | 95.05 | 95.78 |
| Use | 2.97 | 2.71 | 3.25 |
| Abuse | 1.23 | 1.07 | 1.40 |
| Dependence | 0.37 | 0.29 | 0.49 |
| Schizophrenia or a psychotic illness or episode | 3.12 | 2.80 | 3.47 |
| Schizotypal personality disorder | 3.93 | 3.63 | 4.26 |
There was a dose-response relationship between the level of cannabis use and SPIE (Table 3). Compared to individuals without lifetime cannabis use, the OR for SPIE was 1.10 for lifetime cannabis use, 1.45 for lifetime cannabis abuse, and 2.72 for lifetime cannabis dependence. The association between cannabis use and SPIE remained significant after adjusting for sociodemographic characteristics (OR = 1.27 for lifetime cannabis use, 1.79 for lifetime cannabis abuse, and 3.69 for lifetime cannabis dependence).
Table 3.
Associations between Cannabis Use, Psychosis, and SPD
| All Subjects | ||||||
|---|---|---|---|---|---|---|
| Comparison | OR | 95% CI | AO R* |
95% CI | ||
| Cannabis use (lifetime) vs. likelihood of psychosis | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 1.10 | 0.89 | 1.35 | 1.27 | 1.03 | 1.57 |
| Abuse | 1.45 | 1.09 | 1.92 | 1.79 | 1.35 | 2.38 |
| Dependence | 2.72 | 1.83 | 4.05 | 3.69 | 2.49 | 5.47 |
| All Subjects | ||||||
| Comparison | OR | 95% CI |
AO R* |
95% CI | ||
| Cannabis use (lifetime) vs. likelihood of schizotypal personality disorder | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.03 | 1.70 | 2.42 | 2.02 | 1.69 | 2.42 |
| Abuse | 2.88 | 2.38 | 3.48 | 2.83 | 2.33 | 3.43 |
| Dependence | 7.97 | 6.00 | 10.60 | 7.32 | 5.51 | 9.72 |
adjusted for sociodemographic characteristics
There was also a dose-response relationship between the level of cannabis use and SPD (Table 3) that was stronger than that between level of cannabis use and SPIE. After adjusting for sociodemographic characteristics, the OR for SPD was 2.02 for lifetime cannabis use, 2.83 for lifetime cannabis abuse, and 7.32 for lifetime cannabis dependence.
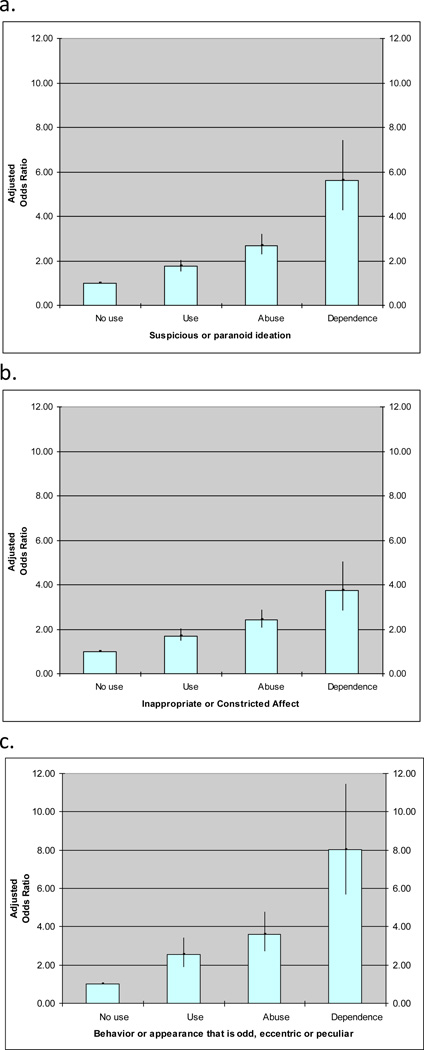
In the analysis of individual SPD symptoms (Table 4), cannabis use predicted a significantly greater prevalence of almost all domains of schizotypy, increasing with severity of use, even after controlling for sociodemographic characteristics. In Figure 1, the symptoms more strongly associated with severity of cannabis use (categorized as use, abuse, or dependence) were: suspicious or paranoid ideation (AOR = 1.75, 2.69, and 5.62, respectively); inappropriate or constricted affect (AOR = 1.70, 2.42, 3.76, respectively); and behavior or appearance that is odd, eccentric, or peculiar (AOR = 2.54, 3.59, and 8.04, respectively). Cannabis use also predicted a significantly greater likelihood of other SPD features (ideas of reference, odd beliefs or magical thinking, unusual perceptual disturbances, lack of close friends or confidants other than first-degree relatives, and excessive social anxiety). A dose-response relationship was apparent for each of the nine SPD features.
Table 4.
Associations between Cannabis Use and Features of Schizotypal Personality Disorder (SPD)
| All Subjects | ||||||
|---|---|---|---|---|---|---|
| Comparison | OR | 95% CI | AO R* |
95% CI | ||
| Cannabis use (lifetime) vs. Symptoms of SPD | ||||||
| Ideas of reference | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 0.93 | 0.63 | 1.38 | 0.95 | 0.63 | 1.43 |
| Abuse | 2.13 | 1.44 | 3.15 | 2.26 | 1.51 | 3.38 |
| Dependence | 5.20 | 3.12 | 8.66 | 5.15 | 3.04 | 8.75 |
| Odd beliefs or magical thinking | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.08 | 1.63 | 2.65 | 2.21 | 1.73 | 2.82 |
| Abuse | 2.38 | 1.83 | 3.10 | 2.55 | 1.94 | 3.36 |
| Dependence | 6.36 | 4.42 | 9.15 | 6.66 | 4.60 | 9.65 |
| Unusual perceptual experiences | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.07 | 1.47 | 2.92 | 2.16 | 1.53 | 3.06 |
| Abuse | 2.21 | 1.43 | 3.40 | 2.39 | 1.52 | 3.77 |
| Dependence | 6.55 | 3.96 | 10.85 | 6.47 | 3.86 | 10.85 |
| Odd thinking and speech | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.58 | 1.88 | 3.52 | 2.62 | 1.91 | 3.60 |
| Abuse | 3.69 | 2.63 | 5.18 | 3.79 | 2.66 | 5.39 |
| Dependence | 8.26 | 5.47 | 12.49 | 8.47 | 5.53 | 12.97 |
| Suspicious or paranoid ideation | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 1.81 | 1.56 | 2.10 | 1.75 | 1.50 | 2.03 |
| Abuse | 2.72 | 2.31 | 3.20 | 2.69 | 2.27 | 3.18 |
| Dependence | 6.12 | 4.64 | 8.08 | 5.62 | 4.27 | 7.40 |
| Inappropriate or constricted affect | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 1.80 | 1.53 | 2.12 | 1.70 | 1.45 | 2.01 |
| Abuse | 2.71 | 2.29 | 3.20 | 2.42 | 2.04 | 2.87 |
| Dependence | 4.35 | 3.25 | 5.81 | 3.76 | 2.81 | 5.02 |
| Behavior or appearance that is odd, eccentric or peculiar | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.48 | 1.86 | 3.31 | 2.54 | 1.89 | 3.40 |
| Abuse | 3.51 | 2.68 | 4.60 | 3.59 | 2.71 | 4.76 |
| Dependence | 7.94 | 5.61 | 11.25 | 8.04 | 5.66 | 11.42 |
| Lack of close friends or confidants other than first degree relatives | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.48 | 1.90 | 3.24 | 2.42 | 1.83 | 3.20 |
| Abuse | 2.73 | 1.93 | 3.85 | 2.76 | 1.93 | 3.96 |
| Dependence | 5.40 | 3.09 | 9.42 | 5.33 | 3.06 | 9.31 |
| Excessive social anxiety | ||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Use | 2.03 | 1.49 | 2.78 | 2.04 | 1.48 | 2.82 |
| Abuse | 2.49 | 1.81 | 3.42 | 2.62 | 1.90 | 3.60 |
| Dependence | 9.18 | 5.91 | 14.26 | 9.32 | 5.99 | 14.49 |
adjusted for sociodemographic characteristics
Figure 1.
Relationship between cannabis use, abuse, and dependence and three of nine features of schizotypal personality disorder in NESARC Wave 2 (n = 34,653). (a) Suspicious or paranoid ideation; (b) Inappropriate or constricted affect; (c) Behavior or appearance that is odd, eccentric, or peculiar.
4. DISCUSSION
This is the first population-based study to examine the association between lifetime cannabis use, self-reported history of psychotic illness or episode (SPIE), schizotypal personality disorder (SPD), and schizotypal features, further implicating cannabis use as a possible risk factor for psychosis liability. The results indicate that the risk of both psychosis and SPD increases with greater use of cannabis, in a dose-dependent manner. Compared to non-users, greater cannabis use showed significantly elevated risk of having been diagnosed with SPIE and elevated risk of all SPD symptoms, even after adjusting for sociodemographic characteristics. Although the cross-sectional design of the study limits inferences about causality, together with previous studies (Andreasson et al., 1987; Arseneault et al., 2002; Degenhardt et al., 2001; Fergusson et al., 2003; Henquet et al., 2005; Tien and Anthony, 1990; van Os et al., 2002; Zammit et al., 2002) our findings suggest that cannabis use may contribute to the etiopathogenesis of psychotic features.
The association between cannabis use and SPIE and SPD found in this study could be explained by three mechanisms: (1) Direct pharmacological effects of cannabis lead to psychosis or schizotypal traits; (2) Psychosis or schizotypal traits lead to cannabis use as a means for individuals to cope with these symptoms; or (3) Another associative factor influences both tendency toward psychosis or schizotypal traits and cannabis use. Concerning the first potential mechanism, it has been well established that cannabis use can increase positive symptoms in individuals with psychosis (D'Souza et al., 2005; Henquet et al., 2005; Negrete et al., 1986), a relationship that could be mediated by dopaminergic hyperactivity. Cannabinoids increase the activity of dopaminergic neurons in the ventral tegmental area within the mesolimbic pathway (Ameri, 1999). While there is limited support for a separate clinical diagnosis of “cannabis psychosis” (Thornicroft, 1990), the ability of cannabis to increase activity of the mesolimbic dopaminergic system could explain the short-term persistence of psychotic-like effects after cannabis intoxication (D'Souza et al., 2009).
An alterative explanation to the pharmacological effects of cannabis is that individuals with SPIE or SPD may use cannabis in an attempt to alleviate their symptoms. Case reports have suggested that cannabidiol, a component of cannabis, might exert antipsychotic effects in individuals with psychosis (Zuardi et al., 2006; Zuardi et al., 1995), and experimental studies suggest that cannabidiol may reduce psychosis-like effects of Δ9-tetrahydrocannabinol and synthetic analogs (Bhattacharyya et al., 2010; Leweke et al., 2000). Evoking the “self-medication” hypothesis in schizophrenia (Peralta and Cuesta, 1992), individuals with SPIE or SPD could therefore attempt to reduce psychotic-like symptoms (or other illness features) by using cannabis (Khantzian, 1997; Schneier and Siris, 1987).
A third potential explanation for the observed association between psychosis, schizotypal traits, and cannabis use could be a co-occurrence without any causal link but with a common etiopathological factor. Based on the cannabinoid hypothesis of schizophrenia (Leweke et al., 1999; Schneider et al., 1998), perturbations in the endogenous cannabinoid system could lead to both a vulnerability for schizophrenia-spectrum symptoms and to a vulnerability for cannabis use. Each of these hypotheses appears viable, and they are not mutually exclusive.
In addition to showing an association between cannabis use and higher scores of measures of schizotypy (Bailey and Swallow, 2004; Barkus and Lewis, 2008; Dumas et al., 2002; Esterberg et al., 2009; Najolia et al., 2012; Nunn et al., 2001; Schiffman et al., 2005; Skosnik et al., 2001; Williams et al., 1996), the current study suggests a broader association between cannabis use and SPD across all domains of schizotypy. The nine features of SPD appear to belong to three broad psychopathological domains (Raine, 2006): positive/cognitive-perceptual (paranoid ideation, magical thinking, unusual perceptual disturbances, ideas of reference), negative/interpersonal (constricted affect, lack of close friends, excessive social anxiety), and disorganized features (odd/eccentric behavior, odd speech). Among non-clinical young adult populations, cannabis use has previously been associated with higher rates of positive and disorganized, but not negative/interpersonal, schizotypy traits (Bailey and Swallow, 2004; Cohen et al., 2011; Earleywine, 2006; Mass et al., 2001; Schiffman et al., 2005; Williams et al., 1996). The current study shows that cannabis use is associated with schizotypy across all three SPD symptoms clusters, including negative/interpersonal traits (constricted affect, lack of close friends, excessive social anxiety). In fact, excessive social anxiety was a SPD symptom found in this study to be strongly associated with the extent of cannabis use (cannabis use AOR = 2.04, 95% CI = 1.48–2.82; abuse AOR = 2.62, 95% CI = 1.90–3.60; and dependence AOR = 9.32, 95% CI = 5.99–14.49). This finding is clinically significant in light of studies showing that there is a positive relationship between social anxiety and cannabis use disorder (Agosti et al., 2002; Buckner et al., 2012; Buckner and Schmidt, 2008) and cannabis-related problems (Buckner et al., 2011; Buckner et al., 2006a; Buckner and Schmidt, 2008, 2009; Buckner et al., 2006b; Marmorstein et al., 2010) in the general population.
As noted, the current study cannot determine if schizotypal traits predispose subjects to use cannabis or if cannabis use increases schizotypal traits. One previous study used a longitudinal design to explore the temporal relationship between cannabis use and the development of SPD symptoms (Anglin et al., 2012). Prospective data from 804 participants in that study showed that the initiation of cannabis use prior to age 14 strongly predicted SPD symptoms in adulthood, independent of early adolescent SPD symptoms, major depression, anxiety disorder, other drug use, and cigarette use. The sample size in that study did not provide adequate statistical power to make firm conclusions regarding specific SPD features. However, those data, together with the findings of the present cross-sectional analysis, suggest the need to investigate in a developmental context the mechanisms that underlie the association of cannabis use and SPD symptoms.
Methodological limitations inherent to the current study design should be considered. First, the diagnosis of SPIE was based on individuals’ self-report by asking them if a physician or other health professional had ever diagnosed them with schizophrenia or psychotic disorder. It should be acknowledged that self-report of these diagnoses is questionably reliable. Yet self-report method described above has been used in previous studies, and results are consistent with accepted prevalence rates of psychotic disorders in the North American population (McMillan et al., 2009; Supina and Patten, 2006). These reports have shown similar rates of self-reported schizophrenia in population-based samples as those found in several studies relying on clinical diagnoses, with similar age and sex distribution.
A second methodological is that it is possible that some aspects of schizotypy may not be fully recognized by those who have such traits. Fourth, the cross-sectional nature of the study limits inferences about causality. Finally, although the NESARC sampling frame included group quarters, some special populations, such as individuals under 18 years of age and those incarcerated, homeless, or hospitalized during the interview periods, were not included in the sample.
In summary, this is the first population-based epidemiologic study to examine the association of cannabis use, psychosis, and schizotypy, showing that prevalence of both psychosis and SPD increases with greater severity of cannabis use in a dose-dependent manner. Compared to non-users, cannabis users had a significantly elevated prevalence of SPD symptoms across all domains (positive/cognitive-perceptual, negative/interpersonal, and disorganized), adding to evidence that cannabis use may contribute to the etiopathogenesis of schizophrenia-related symptoms. There is a need to examine the biological underpinnings of the relationship between cannabis use and the development of schizophrenia-spectrum symptoms, which may ultimately inform the development of more effective interventions for individuals with cannabis use and schizophrenia-spectrum disorders.
Acknowledgment
Role of Funding Sources
This work was supported by the National Institute on Drug Abuse (DA019606, DA023200 and DA023973 to C.B. and DA007294 to F.R.L.); National Institute of Mental Health (MH082773 and MH076051 to C.B. and MH081011 to M.T.C.); New York State Psychiatric Institute (C.B, F.R.L, and G.P.D.).
Original data set for the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) is available from the National Institute on Alcohol Abuse and Alcoholism (http://www.niaaa.nih.gov).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
GD and CB were extensively involved in the conception and planning of the focus and content of this manuscript. GD undertook the literature search and completed the first draft of the paper. SW conducted the statistical analysis for this study. GD worked closely with CB in subsequent revisions of the manuscript. MC and FL provided feedback and provided additional content to the body of the report. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Contributor Information
Glen P. Davis, Email: davisgl@nyspi.columbia.edu.
Michael T. Compton, Email: mcompton@mfa.gwu.edu.
References
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28(4):643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Ahmed AO, Green A, Goodrum NM, Doane NJ, Birgenheir D, Buckley PF. Does a latent class underlie schizotypal personality disorder? Implications for schizophrenia. J Abnorm Psychol. 2013;122(2):475–491. doi: 10.1037/a0032713. [DOI] [PubMed] [Google Scholar]
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58(4):315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 1994. [Google Scholar]
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Anglin DM, Corcoran CM, Brown AS, Chen H, Lighty Q, Brook JS, Cohen PR. Early cannabis use and schizotypal personality disorder symptoms from adolescence to middle adulthood. Schizophr Res. 2012;137(1–3):45–49. doi: 10.1016/j.schres.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appels MC, Sitskoorn MM, Vollema MG, Kahn RS. Elevated levels of schizotypal features in parents of patients with a family history of schizophrenia spectrum disorders. Schizophr Bull. 2004;30(4):781–790. doi: 10.1093/oxfordjournals.schbul.a007131. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Bmj. 2002;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EL, Swallow BL. The relationship between cannabis use and schizotypal symptoms. Eur Psychiatry. 2004;19(2):113–114. doi: 10.1016/j.eurpsy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Barkus E, Lewis S. Schizotypy and psychosis-like experiences from recreational cannabis in a non-clinical sample. Psychol Med. 2008;38(9):1267–1276. doi: 10.1017/S0033291707002619. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, CM OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Schmidt NB. Social anxiety and marijuana-related problems: the role of social avoidance. Addict Behav. 2011;36(1–2):129–132. doi: 10.1016/j.addbeh.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Schneier FR, Liu SM, Wang S, Blanco C. The relationship between cannabis use disorders and social anxiety disorder in the National Epidemiological Study of Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2012;124(1–2):128–134. doi: 10.1016/j.drugalcdep.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Mallott MA, Schmidt NB, Taylor J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. J Anxiety Disord. 2006a;20(8):1087–1102. doi: 10.1016/j.janxdis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB. Marijuana effect expectancies: relations to social anxiety and marijuana use problems. Addict Behav. 2008;33(11):1477–1483. doi: 10.1016/j.addbeh.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB. Social anxiety disorder and marijuana use problems: the mediating role of marijuana effect expectancies. Depress Anxiety. 2009;26(9):864–870. doi: 10.1002/da.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Bobadilla L, Taylor J. Social anxiety and problematic cannabis use: evaluating the moderating role of stress reactivity and perceived coping. Behav Res Ther. 2006b;44(7):1007–1015. doi: 10.1016/j.brat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Buckner JD, Najolia GM, Stewart DW. Cannabis and psychometrically-defined schizotypy: use, problems and treatment considerations. J Psychiatr Res. 2011;45(4):548–554. doi: 10.1016/j.jpsychires.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, Stewart T, Walker EF. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry. 2009;166(11):1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos N, Mackie C. Personality-targeted interventions delay the growth of adolescent drinking and binge drinking. J Child Psychol Psychiatry. 2008;49(2):181–190. doi: 10.1111/j.1469-7610.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259(7):413–431. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Niznikiewicz MA, Voglmaier MM, Seidman LJ, Kim S, Shenton ME. Clinical, cognitive, and social characteristics of a sample of neuroleptic-naive persons with schizotypal personality disorder. Schizophr Res. 2005;78(2–3):297–308. doi: 10.1016/j.schres.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P, Saoud M, Bouafia S, Gutknecht C, Ecochard R, Dalery J, Rochet T, d'Amato T. Cannabis use correlates with schizotypal personality traits in healthy students. Psychiatry Res. 2002;109(1):27–35. doi: 10.1016/s0165-1781(01)00358-4. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Schizotypy, marijuana, and differential item functioning. Hum Psychopharmacol. 2006;21(7):455–461. doi: 10.1002/hup.802. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Goulding SM, McClure-Tone EB, Compton MT. Schizotypy and nicotine, alcohol, and cannabis use in a non-psychiatric sample. Addict Behav. 2009;34(4):374–379. doi: 10.1016/j.addbeh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Gardner CO, Webb BT, Straub RE, O'Neill FA, Walsh D, Riley BP, Kendler KS. Significant correlation in linkage signals from genome-wide scans of schizophrenia and schizotypy. Mol Psychiatry. 2007;12(10):958–965. doi: 10.1038/sj.mp.4001996. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15–21. doi: 10.1017/s0033291702006402. [DOI] [PubMed] [Google Scholar]
- First MB, G M, Spitzer RL. User’s Guide for the Structured Clinical Interview for DSM-IV Personality Disorders. 1997 [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71(1):7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, Dawson DA, Smith S, Saha TD, Huang B. The epidemiology of social anxiety disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(11):1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2004;65(7):948–958. doi: 10.4088/jcp.v65n0711. [DOI] [PubMed] [Google Scholar]
- Grant BF, Moore TC, Shepard J, Kaplan K. Source and Accuracy Satement: WAve 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Bethesda MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van Os J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj. 2005;330(7481):11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A. The 100-year epidemiology of schizophrenia. Schizophr Res. 1997;28(2–3):111–125. doi: 10.1016/s0920-9964(97)85354-6. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53(11):1022–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52(4):296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Demler O, Falloon IR, Gagnon E, Guyer M, Howes MJ, Kendler KS, Shi L, Walters E, Wu EQ. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R) Biol Psychiatry. 2005;58(8):668–676. doi: 10.1016/j.biopsych.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10(8):1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM. Different effects of nabilone and cannabidiol on binocular depth inversion in Man. Pharmacol Biochem Behav. 2000;66(1):175–181. doi: 10.1016/s0091-3057(00)00201-x. [DOI] [PubMed] [Google Scholar]
- Loranger AW. International Personality Disorder Examination: DSM-IV and ICD-10 Interviews. Odessa, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- Marmorstein NR, White HR, Loeber R, Stouthamer-Loeber M. Anxiety as a predictor of age at first use of substances and progression to substance use problems among boys. J Abnorm Child Psychol. 2010;38(2):211–224. doi: 10.1007/s10802-009-9360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass R, Bardong C, Kindl K, Dahme B. Relationship between cannabis use, schizotypal traits, and cognitive function in healthy subjects. Psychopathology. 2001;34(4):209–214. doi: 10.1159/000049309. [DOI] [PubMed] [Google Scholar]
- McMillan GP, Timken DS, Lapidus J, et al. Underdiagnosis of comorbid mental illness in repeat DUI offenders mandated to treatment. J Subst Abuse Treat. 2008;34:320–325. doi: 10.1016/j.jsat.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najolia GM, Buckner JD, Cohen AS. Cannabis use and schizotypy: The role of social anxiety and other negative affective states. Psychiatry Res. 2012 doi: 10.1016/j.psychres.2012.07.042. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychol Med. 1986;16(3):515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- Nunn JA, Rizza F, Peters ER. The incidence of schizotypy among cannabis and alcohol users. J Nerv Ment Dis. 2001;189(11):741–748. doi: 10.1097/00005053-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. Acta Psychiatr Scand. 1992;85(2):127–130. doi: 10.1111/j.1600-0447.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, Dawson DA, Huang B, Stinson FS, Grant BF. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92(1–3):27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J, Nakamura B, Earleywine M, LaBrie J. Symptoms of schizotypy precede cannabis use. Psychiatry Res. 2005;134(1):37–42. doi: 10.1016/j.psychres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Schneider U, Leweke FM, Mueller-Vahl KR, Emrich HM. Cannabinoid/anandamide system and schizophrenia: is there evidence for association? Pharmacopsychiatry. 1998;31(Suppl 2):110–113. doi: 10.1055/s-2007-979355. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Siris SG. A review of psychoactive substance use and abuse in schizophrenia. Patterns of drug choice. J Nerv Ment Dis. 1987;175(11):641–652. doi: 10.1097/00005053-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res. 2001;48(1):83–92. doi: 10.1016/s0920-9964(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health, Volume I: Summary of National Findings. 2010. [Google Scholar]
- Supina AL, Patten SB. Self-reported diagnoses of schizophrenia and psychotic disorders may be valuable for monitoring and surveillance. Can J Psychiatry. 2006;51(4):256–259. doi: 10.1177/070674370605100407. [DOI] [PubMed] [Google Scholar]
- Thornicroft G. Cannabis and psychosis. Is there epidemiological evidence for an association? Br J Psychiatry. 1990;157:25–33. doi: 10.1192/bjp.157.1.25. [DOI] [PubMed] [Google Scholar]
- Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis. 1990;178(8):473–480. [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Williams JH, Wellman NA, Rawlins JN. Cannabis use correlates with schizotypy in healthy people. Addiction. 1996;91(6):869–877. [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. Bmj. 2002;325(7374):1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanari MC, F F, Sickel AE. The Diagnostic Interview for DSM-IV Personality Disorders. Belmont, MA: McLean Hospital, Laboratory for the Study of Adult Development; 1996. [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39(4):421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485–486. [PubMed] [Google Scholar]