Abstract
Background
The mechanisms contributing to worsening of obstructive sleep apnea (OSA) during rapid eye movement (REM) sleep have been minimally studied. Reduced upper-airway muscle tone may be an important contributor. Because respiratory events and the associated blood gas changes can influence genioglossus (GG) activity, we compared GG activity between OSA patients and control subjects during REM sleep using continuous positive airway pressure (CPAP) to minimize the influences of upper-airway resistance (RUA) and blood gas disturbances on GG activity.
Methods
Twenty subjects (10 female subjects), 12 healthy individuals, and 8 OSA patients, were studied overnight. Sleep staging, epiglottic pressure, minute ventilation, and GG electromyogram (GGEMG) were recorded. GGEMG was compared between REM sleep with (phasic REM) and without (tonic REM) eye movements, non-REM (NREM) sleep, and wakefulness.
Results
Breathing frequency increased from stable NREM, to tonic REM to phasic REM sleep, whereas tidal volume and GGEMG decreased (ie, peak GGEMG: 3.0 ± 0.7 vs 1.7 ± 0.4 vs 1.2 ± 0.3% max, respectively; p < 0.001). Reductions in GGEMG during REM sleep were not different between OSA patients and control subjects or between genders.
Conclusions
When RUA and blood gas disturbances are minimized by CPAP, genioglossal activity is reduced in a stepwise manner from stable NREM, to tonic REM to phasic REM sleep to a similar extent in OSA and healthy individuals of both genders. Thus, an inherent abnormality in GG neural control in OSA patients during REM sleep is unlikely to explain the increased upper-airway collapse in this sleep stage. Rather, a generalized reduction in GG activity during REM likely renders individuals who are highly reliant on upper-airway dilator muscles vulnerable to pharyngeal collapse during REM sleep.
Keywords: dilator muscle, gender, lung, sleep-disordered breathing, upper airways
In the past few years, data from animal studies1 have importantly increased our understanding of the neurobiology of rapid eye movement (REM) sleep and have indicated that the characteristic features of REM sleep are controlled by independent neural processes. In patients with obstructive sleep apnea (OSA), respiratory events increase in frequency and duration, and are associated with more pronounced hypoxemia during REM compared to non-REM (NREM) sleep.2,3 Some patients have respiratory abnormalities exclusively during REM sleep, a phenomenon that appears to be more common in women than men.4,5 Because upper-airway dilator muscles are important in maintaining pharyngeal patency,6 reductions in the activity of these muscles may be an important contributor mediating REM-related apnea.
In adult healthy individuals, the expiratory (tonic) electromyographic activity of the largest upper-airway dilator muscle, the genioglossus (GG), was observed to be markedly reduced during REM sleep in a previous nonquantitative report.7 A subsequent study demonstrated reductions in peak and tonic GG electromyogram (GGEMG) compared to NREM sleep only during breaths in which active eye movements were present (phasic REM).8 Although these studies provide initial insight into the potential presence of REM-related reductions in GGEMG activity, only the latter provides quantitative data. Further, recordings were made only in a very small group of predominantly healthy male subjects (one female subject) and may have been underpowered to characterize GGEMG fully. Moreover, women appear to be particularly susceptible to REM-related apnea,5 perhaps indicating a fundamental gender difference in muscle control during REM sleep, thus emphasizing the importance of studying both sexes.
GGEMG recordings in OSA are complicated by respiratory events because fluctuations in blood gases, airway negative pressure, and variations in ventilatory drive can influence the measured values.9–12 Therefore, the application of continuous positive airway pressure (CPAP) is commonly used in respiratory physiology to assess mechanisms intrinsic to OSA while minimizing confounding influences of chemoreceptive and mechanoreceptive effects of repetitive apnea. GGEMG during REM sleep while on CPAP has only been investigated in two studies.13,14 Katz and White13 observed reductions in inspiratory (phasic) and tonic GGEMG activity during REM compared to stable NREM sleep in five children with OSA on CPAP with the greatest decrements being present during phasic REM periods. However, OSA pathogenesis differs between adults and children with children predominantly having sleep-disordered breathing during REM sleep. Schwartz and colleagues14 reported decreased tonic and phasic GGEMG activity in six adult OSA patients on CPAP during REM compared to NREM sleep. However, the effects of tonic vs phasic REM on GGEMG activity were not reported and gender differences were not investigated.
Thus, the studies of GGEMG during REM sleep on CPAP have included very few subjects, have lacked gender comparisons, and differences between healthy individuals and OSA patients have not been assessed. Therefore, the aim of the present study was to compare GGEMG activity during REM sleep between healthy individuals and patients with OSA while minimizing apnea effects by studying subjects on CPAP. This aim would allow us to test the hypothesis that REM sleep per se leads to greater genioglossal atonia in OSA patients compared to control subjects. A secondary aim was to examine whether GGEMG activity during REM sleep differs between genders, given the apparent female predisposition to REM-related apnea.
Materials and Methods
Subjects
Periods of wakefulness and stable NREM and REM sleep were identified (see data analysis section for further detail) in 20 subjects (10 women). Although some of our subjects participated in a prior experiment,15 none of the findings of the present study have been previously reported. Eight subjects (four women) had OSA and reported nightly use of CPAP for at least 3 months before participation in the study. The remaining subjects were healthy individuals (six women) without reported history of snoring or sleep-disordered breathing. However, polysomnography to rule out OSA definitively was not performed in the healthy subjects as part of this study. Female subjects were either postmenopausal (four subjects, two with OSA) or were studied in the follicular phase of the menstrual cycle. Other than OSA, all subjects were without a history of cardiopulmonary disease. The study was approved by the Human Research Committee of the Brigham and Women's Hospital, and participants gave informed written consent to participate.
Measurements and Equipment
Subjects were instrumented with EEG, left and right electrooculograms (EOGs), and submentalis electromyogram (EMG) for sleep staging and arousal scoring. The nostrils were decongested (0.05% oxymetazoline HCl), and the more patent nostril was anesthetized (4% lidocaine HCl) for insertion of a pressure-tipped catheter (model MCP-500; Millar; Houston, TX). The catheter was advanced 1 to 2 cm below the base of the tongue under direct visualization to measure epiglottic pressure (Pepi). Two fine-wire coated (Teflon; DuPont; Wilmington, DE) IM electrodes were inserted 3 to 4 mm on either side of the frenulum to a depth of approximately 1.5 cm after surface anesthesia (4% lidocaine HCl) to measure GGEMG activity. Subjects were fitted with a nasal mask (Gel Mask; Respironics; Murrysville, PA) with pneumotachograph (model 3700A; Hans Rudolf Inc; Kansas City, MO) and differential pressure transducer (Validyne Corp; Northridge, CA) for measurement of inspired flow and calculation of breath timing and minute ventilation (V̇E). End-tidal CO2 (Petco2) was also monitored from one nostril. The breathing circuit was connected to a CPAP device with a leak valve in series. Mask pressure was measured continuously via an additional pressure transducer attached to the mask. Arterial blood oxygen saturation via pulse oximetry and the ECG were measured throughout the study for safety purposes.
Data were acquired on a 1401 plus interface and Spike 2 software (Cambridge Electronic Design Ltd; Cambridge, UK). GGEMG was sampled at 1 kHz and filtered at 30 to 1,000 Hz. EEG and submentalis EMG were sampled at 250 Hz and the remaining channels at 125 Hz.
Protocol
Subjects arrived at the sleep laboratory 2 h before their usual bedtime after abstaining from alcohol and caffeine for at least 12 h. Once all the equipment had been fitted, subjects performed at least three swallows and three maximal tongue protrusions against the top teeth to determine the maximal activity of the GG muscle under verbal encouragement. Subjects were then asked to lie supine, to keep their eyes open, and to stay awake for 10 min (for baseline wakefulness data collection). CPAP was applied at the beginning of the baseline period at the prescribed level for OSA patients or 4 cm H2O for the healthy individuals. The lights were then switched off and subjects were given the opportunity to sleep. Once asleep, the CPAP was adjusted as necessary to eliminate inspiratory flow limitation based on the Pepi-flow relationship. Subjects slept in the supine position.
Data Analysis
Periods scored as REM sleep that were free from arousal (at least 3 min since an arousal/state change) or movement artifact were identified and analyzed on a breath-by-breath basis using custom-designed semiautomated software. Variables quantified included: breathing frequency (FB), Petco2, tidal volume (VT), V̇E, Pepi nadir during inspiration, and upper-airway resistance (RUA) [ie, Pepi – mask pressure] measured at a flow of 200 mL/s. Raw EMG recordings were rectified and moving time averaged (100 ms) for each subject. GG muscle activity was expressed as a percentage of the maximal activity level as determined during either swallow or protrusion maneuvers (the greater value was used for quantification). In addition, GGEMG was expressed as a percentage of the wakefulness level for each subject. For each breath, GGEMG activity was assessed as peak (maximal activity) and tonic (nadir muscle activity during expiration). Comparable numbers of breaths during wakefulness (absent from swallow or movement artifact and while on CPAP) and stable NREM sleep (at least 3 min since an arousal) were selected and analyzed in the same manner as the identified REM periods. In addition, only equivalent numbers of NREM breaths temporally closest to the identified REM sleep periods within the time window > 10 min from a REM transition (to avoid transition effects) and < 60 min from the REM transition were analyzed. During REM sleep each breath was categorized as either tonic REM (absence of any EOG deflections ≥ 50 μV within the breath cycle) or phasic REM (deflections in both EOG channels within the breath cycle with at least one ≥ 50 μV). This definition was similar to that described previously.8,16
Statistical Procedures
One-way repeated measures analysis of variance (ANOVA) was used to examine peak and tonic GGEMG activity across sleep states (wakefulness, NREM, tonic REM, and phasic REM) by means of a statistical software package (SPSS, version 12.1; SPSS Inc; Chicago, IL). Similarly, one-way repeated measures ANOVA was used to examine changes in ventilatory parameters across sleep states. Group (OSA vs healthy subjects) and gender comparisons for peak and tonic GGEMG across sleep states were explored using two-way repeated measures ANOVAs. Where significant ANOVA effects were observed, post hoc comparisons were performed using Dunn-Sidak adjusted Student paired t tests.17 Statistical significance was defined as p < 0.05. All data are reported as the mean ± SEM.
Results
Anthropometric Characteristics
The mean age and the body mass index of the 20 subjects were 42 ± 3 years and 29 ± 2 kg/m2, respectively. Male and female participants did not differ in age (40 ± 3 vs 44 ± 4 years, respectively; p = 0.452) or body mass index (29 ± 2 vs 29 ± 2 kg/m2, respectively; p = 0.879). OSA patients had severe disease with an apnea-hypopnea index of 51 ± 10 events per hour (range, 13 to 94 events per hour). OSA patients were older (48 ± 3 vs 37 ± 3 years, respectively; p = 0.047) and had higher body mass indexes than the non-OSA subjects (36 ± 2 vs 24 ± 1 kg/m2, respectively; p < 0.001).
CPAP, RUA, and Ventilatory Characteristics
CPAP, nadir Pepi, V̇E, and RUA were not different between sleep states. Petco2 increased from wakefulness to NREM sleep in the non-OSA group. The OSA patients were studied on higher CPAP and Pepi nadir was more negative during wakefulness compared to the non-OSA group. FB increased from wakefulness to stable NREM to tonic REM to phasic REM sleep, whereas VT progressively decreased (Table 1).
Table 1.
CPAP, RUA, and Ventilatory Characteristics Separated by Group*
| OSA (n = 8) |
Non-OSA (n = 12) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Wake | NREM | Tonic REM | Phasic REM | Wake | NREM | Tonic REM | Phasic REM |
| CPAP, cm H2O | 10.3 ± 1.0 | 11.6 ± 0.8 | 11.5 ± 0.9 | 11.5 ± 0.9 | 5.4 ± 0.4† | 6.0 ± 0.5† | 5.8 ± 0.4† | 5.8 ± 0.4† |
| Pepi nadir, cm H2O | –2.3 ± 0.3 | – 2.4 ± 0.7 | –1.9 ± 0.4 | –1.7 ± 0.3 | –1.3 ± 0.1† | –1.5 ± 0.2 | –1.7 ± 0.2 | –1.4 ± 0.2 |
| RUA, cm H2O/L/s | 0.9 ± 0.3 | 0.9 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.5 | 1.2 ± 0.2 | 1.0 ± 0.3 | 1.4 ± 0.3 | 1.1 ± 0.3 |
| PETCO2,mmHg | 40.7 ± 1.0 | 40.9 ± 1.2 | 40.9 ± 1.5 | 40.6± 1.4 | 42.1 ± 0.8‡ | 43.7 ± 0.7§ | 43.2 ± 0.9 | 42.0 ± 1.2 |
| FB, breaths/min | 16.2 ± 1.7∥¶ | 15.9 ± 1.0∥¶ | 17.6 ± 1.2‡§¶ | 19.5 ± 1.5‡§∥ | 13.4 ± 0.8¶ | 15.2 ± 0.6§¶ | 16.7 ± 0.8¶ | 18.5 ± 0.9‡§¶ |
| Vt, L | 0.54 ± 0.06‡§¶ | 0.45 ± 0.03§∥¶ | 0.46 ± 0.03§¶ | 0.40 ± 0.02‡§¶ | 0.51 ± 0.05¶ | 0.43 ± 0.02¶ | 0.40 ± 0.03¶ | 0.34 ± 0.02‡§¶ |
| Ve, L/min | 8.1 ± 0.7 | 7.2 ± 0.6 | 7.8 ± 0.6 | 7.5 ± 0.6 | 6.4 ± 0.3 | 6.3 ± 0.2 | 6.4 ± 0.3 | 6.0 ± 0.3 |
| Breaths analyzed, No. | 84 ± 23 | 188 ± 46 | 146 ± 37 | 51 ± 10 | 88 ± 31 | 146 ± 28 | 100 ± 23 | 45 ± 16 |
Values are given as the mean ± SEM.
Significant difference compared to equivalent sleep state in OSA. Other symbols refer to differences between sleep states within each group (OSA or non-OSA patients).
Significant difference compared to NREM.
Significant difference compared to wakefulness.
Significant difference compared to tonic REM.
Significant difference compared to phasic REM.
GGEMG Activity
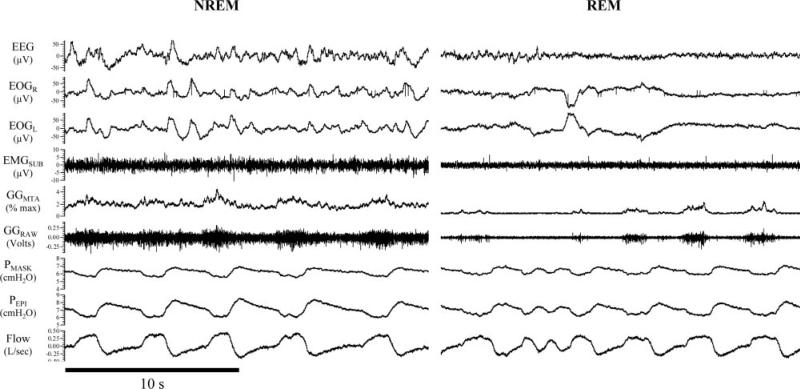
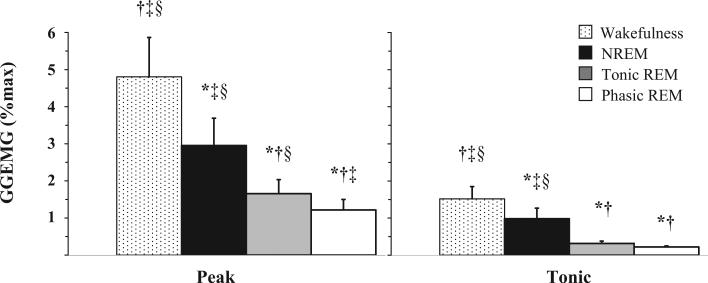
Figure 1 illustrates an example of the changes in GGEMG activity from stable NREM to REM sleep in one subject. There were no group differences (OSA vs non-OSA subjects) or gender differences in peak GGEMG activity (p = 0.395 and p = 0.902, respectively) or tonic GGEMG activity (p = 0.473 and p = 0.488, respectively), or in interaction effects for peak GGEMG activity (p = 0.631 and p = 0.473, respectively) or tonic GGEMG activity (p = 0.490 and p = 0.310, respectively) across sleep states when analyzed as a percentage of maximum activity (Table 2). Similar results were obtained when GGEMG was analyzed as a percentage of the wakefulness level with the exception that tonic GGEMG was reduced to a greater extent in men compared to women during stable NREM sleep (Table 2). Given the lack of differences observed between OSA and non-OSA subjects in GGEMG on CPAP in this study and the paucity of published GGEMG data during REM sleep, we elected to combine group data to explore state-related effects. Peak and tonic GGEMG activity decreased in a stepwise manner from wakefulness to stable NREM to tonic REM to phasic REM sleep (statistical significance indicated in Fig 2).
Figure 1.
An illustrative polysomnographic example demonstrating reduced GG muscle activity from NREM to REM sleep in a healthy 41-year-old woman. Note that GG activity is further reduced during REM sleep when ocular movements are present (phasic REM). EEG = EEG electrodes C3-A2; EOGR = right EOG; EOGL = left EOG; EMGSUB = submental EMG; GGMTA = 100-ms moving time averaged GGEMG; GGRAW = raw GGEMG; Flow = inspiratory (upward direction) and expiratory airflow.
Table 2.
Peak and Tonic GGEMG Activity Separated by Group and Gender Across Sleep States*
| REM Sleep |
||||
|---|---|---|---|---|
| Variables | Waking State | NREM Sleep | Tonic | Phasic |
| Peak GGEMG | ||||
| OSA patients (n = 8) | 4.5 ± 1.4 | 1.9 ± 0.6 (57 ± 12) | 1.0 ± 0.2 (31 ± 8) | 0.8 ± 0.2 (23 ± 5) |
| Non-OSA subjects (n = 12) | 5.0 ± 1.6 | 3.7 ± 1.1 (78 ± 8) | 2.1 ± 0.6 (56 ± 15) | 1.5 ± 0.4 (42 ± 11) |
| Men (n = 10) | 5.2 ± 1.4 | 2.5 ± 0.8 (55 ± 10) | 1.6 ± 0.6 (34 ± 7) | 1.1 ± 0.4 (26 ± 6) |
| Women (n = 10) | 4.4 ± 1.7 | 3.4 ± 1.2 (84 ± 8) | 1.7 ± 0.5 (57 ± 18) | 1.4 ± 0.5 (43 ± 13) |
| Tonic GGEMG | ||||
| OSA patients (n = 8) | 1.3 ± 0.3 | 0.6 ± 0.2 (61 ± 15) | 0.3 ± 0.1 (25 ± 5) | 0.2 ± 0.1 (23 ± 5) |
| Non-OSA subjects (n = 12) | 1.7 ± 0.5 | 1.2 ± 0.5 (78 ± 13) | 0.4 ± 0.1 (33 ± 7) | 0.2 ± 0.0 (24 ± 5) |
| Men (n = 10) | 1.5 ± 0.3 | 0.6 ± 0.2 (44 ± 8)† | 0.3 ± 0.1 (24 ± 4) | 0.2 ± 0.0 (22 ± 5) |
| Women (n = 10) | 1.6 ± 0.6 | 1.4 ± 0.5 (98 ± 13) | 0.4 ± 0.1 (35 ± 7) | 0.2 ± 0.0 (26 ± 6) |
Values are given as the mean ± SEM of the percentage of maximum activity (and percentage of wakefulness level).
Denotes a significant difference compared to equivalent sleep state in women. Otherwise, there were no group (OSA patients vs non-OSA subjects) or gender differences in peak or tonic GGEMG activity or interaction effects across sleep states (refer to text for further detail and p values).
Figure 2.
GG peak and tonic muscle activity on CPAP during wakefulness, NREM, and tonic REM and phasic REM sleep. Data are expressed as a percentage of maximum activity (%max). * = Significant difference compared to wakefulness; † = significant difference compared to NREM; ‡ = significant difference compared to tonic REM; § = significant difference compared to phasic REM (n = 20).
Discussion
The main finding of this study was that phasic and tonic GGEMG activity decreased considerably from wakefulness to stable NREM to REM sleep in both healthy subjects and patients with OSA on CPAP. The presence of eye movements during REM sleep was associated with further decrements in peak GGEMG activity. These data are in concordance with the few previous studies in this area, but they extend these prior observations by fully characterizing the extent of these reductions and determining that there were no major differences in the magnitude of reduction in GGEMG between OSA patients and healthy individuals or between genders.
GG Muscle Activity During NREM and REM Sleep
GG muscle activity is known to be reduced at sleep onset in healthy individuals and OSA patients,18,19 and in most healthy individuals, GG muscle activity recovers following the α-theta transition yielding a higher value during stable NREM sleep as compared to wakefulness.20,21 From the sparse data available in adult healthy individuals, uncertainty remains as to which components of GG muscle activity are reduced during REM sleep. One study7 indicated reductions in tonic activity, another in peak and tonic activity but only during phasic REM,8 and another study22 indicated the presence of irregular bursts of GG activity.
The findings of the current study demonstrating marked reductions in peak and tonic GGEMG during tonic REM, and further reductions during phasic REM, are in concordance with data collected in five children with OSA studied on CPAP.13 The extent of the GGEMG reductions demonstrated in the present study may not have been observed in the study by Wiegand and colleagues8 due to their relatively small sample size. However, it is also possible that differences between prior studies may be explained by our use of CPAP. Indeed, the reductions we observed in GG activity from wakefulness to stable NREM sleep are consistent with previous studies using CPAP,23,24 and they further suggest that the increase in GG in the natural situation (no CPAP) is due to increased negative airway pressure and/or CO2. Given that airway pressure and CO2 were not different between NREM and REM sleep, the further marked reductions in GGEMG during REM sleep likely reflect a state-dependent decrease in neural output to GG, chemoresponsiveness, and/or reduced responsiveness to the negative pharyngeal pressure generated during tidal breathing. Indeed, profound inhibition of the GG negative upper-airway pressure reflex has been reported25,26 with further decrements present during phasic eye movements.25
Potential Mechanisms Mediating Reduced GG Muscle Activity During REM
The presence of eye movements during REM sleep has long been associated with ventilatory suppression,27–29 suggesting that respiratory muscles are suppressed during eye movements. Indeed, there is an extensive animal literature investigating the underlying mechanisms responsible for reduced muscle tone during REM sleep.30–34 One hypothesis for the presence of eye movements leading to GGEMG inhibition during REM sleep posits a global neural inhibition associated with the presence of pontogeniculate-occipital waves.35 Indeed, P waves have recently been quantified in a human subject.36 However, contrary to previous beliefs, they only appear to be coincident with active eye movements less than half of the time.36 Nonetheless, our data do suggest further inhibition of VT and GG activity in association with eye movements even on CPAP when potentially confounding influences (negative pressure and blood gas changes resulting from airway collapse) are minimized.
Possible Relevance to OSA
The findings of the current study suggest that reduced neural output to the largest upper-airway dilator muscle during REM sleep likely contributes to increased apnea severity in this sleep state. The potential role of other mechanisms remains complex and at times somewhat counterintuitive. From the limited physiologic studies available in humans, REM sleep is associated with decreased chemosensitivity,37,38 profound impairment of the GG negative pressure reflex,25,26 and although complicated by the many confounding effects of apnea, reduced GGEMG in OSA patients during respiratory events.39 These factors should lead to a more collapsible upper airway and a worsening of blood gas disturbances during apnea in REM sleep. However, although the cross-sectional area of the upper airway decreases at the level of the oropharynx during phasic REM sleep, the collapsibility of the pharyngeal airway does not appear to differ from wakefulness to REM sleep.16,40 In addition, the passive critical closing pressure of the upper airway appears similar between REM and non-REM sleep in OSA patients despite reduced GGEMG activity during REM.14 Furthermore, the arousal response to respiratory loading appears to have a lower threshold during REM sleep, which would tend to reduce rather than prolong apnea duration.41–44 Thus, additional studies are required to understand the underlying physiologic mechanisms contributing to increased apnea severity during REM sleep. Nonetheless, these data suggest that reduced GGEMG during REM sleep, particularly in the presence of active ocular movements, may play an important role.
The lack of difference in the REM-related reduction in GGEMG activity between healthy individuals and OSA patients studied while on CPAP does not support the concept that OSA patients have a fundamental difference in GGEMG control during REM that puts them at greater risk of collapse than control subjects. Rather, a generalized reduction in GGEMG that occurs in all individuals during REM will render individuals that are highly reliant on upper-airway dilator muscles to maintain a patent airway (eg, an individual with an anatomically narrow upper airway) most vulnerable to airway collapse in this sleep state.
Although the prevalence of sleep-disordered breathing is greater in men than women,45 evidence suggests that sleep-disordered breathing may be more commonly isolated to REM sleep in female OSA patients compared to male patients.5 However, more recent evidence does not support this finding and suggests that REM-related OSA may be part of the spectrum of disease severity whereby its presence is most common in mild to moderate cases.46 The lack of a difference in GG activity during REM sleep between genders in the current study is consistent with this hypothesis. The potential for inherent differences between genders in other physiologic traits that may lead to gender-related differences in REM sleep OSA severity, such as GG reflex responsiveness to negative pressure, remains unknown. Alternatively, women may be protected from NREM but not REM-related apnea via mechanisms that are currently not well understood. The finding that women have less of a reduction in GGEMG from wakefulness to stable NREM sleep on CPAP (when expressed as a percentage of the wakefulness level) in the current study raises the possibility that upper-airway muscles may play a protective role in women in particular.
Methodological Considerations
Although we observed no differences in the GGEMG reductions during REM sleep between the OSA vs healthy subjects, this may not be the case in different subject populations. First, prior CPAP therapy, as used in this study, may lead to improved dilator muscle function (eg, via reversal of airway edema or improvements in upper-airway sensory function).47,48 Thus, responses may differ in untreated OSA patients. Second, the OSA patients in this study were not preselected on the basis of REM apnea predominance, and the pathophysiology may vary in this group. Third, OSA patients were older and heavier, and some evidence suggests that both these factors alone can importantly influence upper-airway dilator muscle function.49,50 Although the use of CPAP in both groups in the current study may have minimized some of these potential confounding effects, additional studies are required to explore these possibilities definitively.
The lack of gender and group (OSA vs healthy subjects) differences observed in this study may be due to insufficient statistical power. This possibility cannot be discounted, but should true differences be present, they would appear to be small and of minimal clinical importance. Indeed, our results suggest that to detect significant group differences with 90% power in peak GGEMG activity during REM sleep between genders would require on the order of 4,000 subjects in each group (based on a true difference of 0.12% maximum and an SD of 1.64, as measured in this study). In addition, we estimate that approximately 70 subjects in each group would be required to observe differences between OSA patients and healthy subjects should they exist (based on a true difference of 1.08% maximum and a SD of 1.91, as measured in this study). Obviously, such studies are not feasible.
In addition, one could argue that GG is only one of the many important upper-airway dilator muscles. Given our goal of understanding of the neural control of the upper-airway muscles, we believe that improvements in our understanding of GG control likely reflect other phasic muscles under hypoglossal control. However, clearly other upper-airway muscles also influence pharyngeal mechanics, and their responses during REM sleep may differ from GG. Hence, further research in this area is required.
Summary
This study has demonstrated that GGEMG activity decreases to a similar extent from wakefulness to stable NREM to tonic REM to phasic REM sleep in healthy men and women and patients with OSA on CPAP. Reduced GGEMG activity (and probably that of other upper-airway muscles) during REM sleep is likely an important factor contributing to sleep-disordered breathing in this sleep stage, particularly in those highly reliant on muscle activity to maintain a patent airway.
Acknowledgments
This study was supported by National Institutes of Health grants P50 HL60292, R01 HL085188-01, RO1-HL73146, AG024837-01, and RR01032; and by American Heart Association grants 0635318N and 0840159N. Dr. Eckert is supported by the Thoracic Society of Australia and New Zealand/Allen and Hanbury's Respiratory Research Fellowship.
Abbreviations
- ANOVA
analysis of variance
- CPAP
continuous positive airway pressure
- EMG
electromyogram
- EOG
electrooculogram
- FB
breathing frequency
- GG
genioglossus
- GGEMG
genioglossus electromyogram
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- Pepi
epiglottic pressure
- Petco2
end-tidal CO2
- REM
rapid eye movement
- RUA
upper-airway resistance
- V̇E
minute ventilation
- VT
tidal volume
Footnotes
Dr. Malhotra has received consulting and/or research income from Respironics, Restore/Medtronic, NMT Medical, Apnex Medical, Itamar Medical, Pfizer, Cephalon, and Inspiration Medical. Dr. White is Chief Medical Officer for Respironics Inc. Drs. Eckert, Lo, and Jordan have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
References
- 1.Lu J, Sherman D, Devor M, et al. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 2.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–436. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 3.Series F, Cormier Y, La Forge J. Influence of apnea type and sleep stage on nocturnal postapneic desaturation. Am Rev Respir Dis. 1990;141:1522–1526. doi: 10.1164/ajrccm/141.6.1522. [DOI] [PubMed] [Google Scholar]
- 4.Kass JE, Akers SM, Bartter TC, et al. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–169. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 6.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 7.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 8.Wiegand L, Zwillich CW, Wiegand D, et al. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 9.Lo YL, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–477. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis. 1981;124:215–217. doi: 10.1164/arrd.1981.124.3.215. [DOI] [PubMed] [Google Scholar]
- 11.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 12.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 13.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–560. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 15.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley JA, Zahn BR, Babcock MA, et al. The effect of rapid eye movement (REM) sleep on upper airway mechanics in normal human subjects. J Physiol. 1998;510:963–976. doi: 10.1111/j.1469-7793.1998.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludbrook J. On making multiple comparisons in clinical and experimental pharmacology and physiology. Clin Exp Pharmacol Physiol. 1991;18:379–392. doi: 10.1111/j.1440-1681.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 18.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 19.Worsnop C, Kay A, Pierce R, et al. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 20.Tangel DJ, Mezzanotte WS, Sandberg EJ, et al. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 21.Basner RC, Ringler J, Schwartzstein RM, et al. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 22.Chokroverty S. Phasic tongue movements in human rapid eye-movement sleep. Neurology. 1980;30:665–668. doi: 10.1212/wnl.30.6.665. [DOI] [PubMed] [Google Scholar]
- 23.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–558. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 24.Henke KG. Upper airway muscle activity and upper airway resistance in young adults during sleep. J Appl Physiol. 1998;84:486–491. doi: 10.1152/jappl.1998.84.2.486. [DOI] [PubMed] [Google Scholar]
- 25.Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckert DJ, McEvoy RD, George KE, et al. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–1205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould GA, Gugger M, Molloy J, et al. Breathing pattern and eye movement density during REM sleep in humans. Am Rev Respir Dis. 1988;138:874–877. doi: 10.1164/ajrccm/138.4.874. [DOI] [PubMed] [Google Scholar]
- 28.Aserinsky E. Periodic respiratory pattern occurring in conjunction with eye movements during sleep. Science. 1965;150:763–766. doi: 10.1126/science.150.3697.763-a. [DOI] [PubMed] [Google Scholar]
- 29.Millman RP, Knight H, Kline LR, et al. Changes in compartmental ventilation in association with eye movements during REM sleep. J Appl Physiol. 1988;65:1196–1202. doi: 10.1152/jappl.1988.65.3.1196. [DOI] [PubMed] [Google Scholar]
- 30.Orem J, Lydic R. Upper airway function during sleep and wakefulness: experimental studies on normal and anesthetized cats. Sleep. 1978;1:49–68. doi: 10.1093/sleep/1.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Megirian D, Hinrichsen CF, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- 32.Horner RL. Control of genioglossus muscle by sleep state-dependent neuromodulators. Adv Exp Med Biol. 2008;605:262–267. doi: 10.1007/978-0-387-73693-8_46. [DOI] [PubMed] [Google Scholar]
- 33.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradren ergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 35.Orem J. Neuronal mechanisms of respiration in REM sleep. Sleep. 1980;3:251–267. doi: 10.1093/sleep/3.3-4.251. [DOI] [PubMed] [Google Scholar]
- 36.Lim AS, Lozano AM, Moro E, et al. Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons. Sleep. 2007;30:823–827. doi: 10.1093/sleep/30.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas NJ, White DP, Weil JV, et al. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982;125:286–289. doi: 10.1164/arrd.1982.125.3.286. [DOI] [PubMed] [Google Scholar]
- 38.White DP, Douglas NJ, Pickett CK, et al. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis. 1982;126:530–533. doi: 10.1164/arrd.1982.126.3.530. [DOI] [PubMed] [Google Scholar]
- 39.Okabe S, Hida W, Kikuchi Y, et al. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- 40.Rowley JA, Sanders CS, Zahn BR, et al. Effect of REM sleep on retroglossal cross-sectional area and compliance in normal subjects. J Appl Physiol. 2001;91:239–248. doi: 10.1152/jappl.2001.91.1.239. [DOI] [PubMed] [Google Scholar]
- 41.Gugger M, Bogershausen S, Schaffler L. Arousal responses to added inspiratory resistance during REM and non-REM sleep in normal subjects. Thorax. 1993;48:125–129. doi: 10.1136/thx.48.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. J Appl Physiol. 1983;55:1113–1119. doi: 10.1152/jappl.1983.55.4.1113. [DOI] [PubMed] [Google Scholar]
- 43.Berry RB, Asyali MA, McNellis MI, et al. Within-night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apnea. J Appl Physiol. 1998;85:1434–1441. doi: 10.1152/jappl.1998.85.4.1434. [DOI] [PubMed] [Google Scholar]
- 44.Krieger J, Sforza E, Boudewijns A, et al. Respiratory effort during obstructive sleep apnea: role of age and sleep state. Chest. 1997;112:875–884. doi: 10.1378/chest.112.4.875. [DOI] [PubMed] [Google Scholar]
- 45.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 46.Haba-Rubio J, Janssens JP, Rochat T, et al. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–3357. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 47.Kimoff RJ, Sforza E, Champagne V, et al. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 48.Carrera M, Barbe F, Sauleda J, et al. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1960–1966. doi: 10.1164/ajrccm.159.6.9809052. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72, e79–14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrera M, Barbe F, Sauleda J, et al. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J. 2004;23:425–429. doi: 10.1183/09031936.04.00099404. [DOI] [PubMed] [Google Scholar]