Abstract
Variation in the early postnatal social environment can have lasting effects on hypothalamic-pituitary-adrenal (HPA) axis stress responses. Both rats and macaque monkeys subjected to low quality or abusive maternal care during the early postnatal period have more pronounced HPA responses to environmental stressors throughout development and into adulthood compared to animals reared in higher quality early maternal environments. However, little is known about the relative contributions to HPA stress response styles in developing offspring in species in which offspring care is routinely provided by group members other than the mother, such as in cooperatively breeding mammals. Marmoset monkeys exhibit cooperative offspring rearing, with fathers and older siblings providing care in addition to that provided by the mother. We evaluated the effects of early maternal, paternal, and older sibling care on HPA responses to social separation across development in captive white-faced marmoset offspring (Callithrix geoffroyi). We monitored offspring care by mothers, fathers, and older siblings in marmosets for the first 60 days of life. Later in development, each marmoset experienced three standardized social separation/novelty exposure stressors at 6, 12, and 18 months of age. During separation, we collected urine samples and analyzed them via enzyme immunoassay for cortisol levels. Infants that received higher rates of rejections from the entire family group showed higher cortisol responses to social separation. This relationship was found when mothers, fathers, and older siblings, were analyzed separately as well. No differences in cortisol responses were found between offspring that received high and low rates of carrying or high and low rates of licking and grooming by any group member. In the cooperatively breeding marmoset, early social cues from multiple classes of caregivers may influence HPA stress responses throughout the lifespan.
Variation in early postnatal environments can permanently influence neuroendocrine responses to environmental stressors. In particular, differences in the nature of the early social environment can cause lasting changes in hypothalamic-pituitary-adrenal (HPA) axis stress responses. In rats, repeated maternal separation for extended periods of time and/or low rates of maternal licking-grooming and arch-backed nursing during the early postnatal period results in increased production of corticotropin-releasing hormone (CRH; released in the paraventricular nucleus of the hypothalamus), adrenocorticotropic hormone (ACTH; secreted by the pituitary), and glucocorticoids (corticosterone; secreted by the adrenal glands) to stressful stimuli in adulthood (Bhatnagar and Meaney, 1995; Levine et al., 1957; Liu et al., 2000, 1997; Meaney et al., 1996; Plotsky and Meaney, 1993; Viau et al., 1993). Short periods of maternal separation, however, can elicit higher rates of licking and grooming from rat mothers, which appears to blunt rises in these HPA products (Plotsky and Meaney, 1993). In humans, low quality early social environments are also associated with blunted early morning rises in cortisol and increased HPA responses to stress (Flinn et al., 2011; Gunnar and Quevedo, 2007; Tarullo and Gunnar, 2006), which is associated with several mood disorders (Arborelius et al., 1999; Lupien et al., 2009; Nestler et al., 2002) and other deleterious health outcomes such as decreased immune function, heart disease, gastrointestinal illnesses, and exacerbation of autoimmune disorders, to name a few (DeVries et al., 2007; McEwen, 1998; Sapolsky et al., 2000). The health and fitness of an individual, therefore, is significantly impacted by the quality of the early postnatal social environment.
Investigations in nonhuman primates have, for the most part, supported the results from rodent experiments and correlational human studies. Rhesus macaques (Macaca mulatta) have been the primary nonhuman primate model used in exploring the influence of the early environment on the development of HPA stress response styles (reviewed in Parker and Maestripieri, 2011; Stevens et al., 2009). Compared to normally mother-reared monkeys, rhesus macaques reared with a peer group (a less-ideal, low-quality environment) show no differences in baseline plasma cortisol levels, but an increased cortisol response to social separation at 6 and 18 months of age compared to their mother-reared peers (Higley et al., 1992). Moreover, peer-reared females exhibited a higher ACTH response to alcohol infusion compared to their peer-reared male counterparts, but no differences in ACTH responses were found between peer-reared and mother-reared offspring overall (Barr et al., 2004a). In contrast to rodent studies, which found that early adverse environments result in increased HPA stress responses, one study demonstrated that peer-reared rhesus monkeys had lower baseline plasma cortisol along with lower cortisol levels after a period of chronic separation stress compared to mother-reared infants (Barr, Newman, Shannon, et al. 2004). Another investigation found that peer-reared macaques had lower plasma ACTH and cortisol responses to housing transition stress than mother-reared monkeys (Clarke, 1993). This discrepancy between the rat and macaque findings may be due in part to more severe forms of parental neglect or maltreatment (complete parental absence in the case of the monkeys versus short-term, repeated maternal separation in rats) causing chronic hypersecretion of stress-related hormones, which in turn may cause desensitization of the HPA stress response through alterations in the sensitivity of negative feedback in the HPA axis (reviewed in Sanchez, 2006). It also remains possible that different developmental timeframes are a key factor in HPA development differences seen across these groups. Rats have relatively underdeveloped nervous systems at birth compared to primates (Clancy et al., 2007), and they exhibit a hypoactive period of HPA activity for two weeks following birth (Sapolsky and Meaney, 1986); therefore, environmental influences during the postnatal period may not have analogous effects across these groups.
Little attention has been paid to the possibility that differences in classes of offspring caregivers, particularly during critical periods of development, may also contribute to differences in HPA response styles across species. In both rats and macaque monkeys, mothers are primary caregivers during periods of highest offspring dependency (Smuts and Gubernick, 1992). While interactions between infant macaques and adult males, older siblings, and other adult females have been observed (Thierry et al., 2004), this type of so-called alloparental care is much more extensive in New World monkeys, such as marmosets (genus Callithrix). Marmosets and other calltrichine primates exhibit a cooperatively breeding social structure in which all group members—mothers, fathers, and older siblings—assume a substantial role in infant care (Solomon and French, 1996). The marmoset, therefore, provides a valuable model in which to test the relative contributions of infant care provided by various group members on developmental outcomes such as stress responsivity. The marmoset HPA stress response is also sensitive to acute social cues from various group members. Social relationships within this family-like arrangement can either buffer HPA stress responses (Rukstalis and French, 2005) or intensify HPA activation during intragroup conflict (Smith and French, 1997). Marmosets exposed to stressors show increases in anxiety-like behavior, altered HPA axis function, and impaired social interactions (French et al., 2007; Johnson et al., 1996b; Smith et al., 2011). Therefore, the marmoset is an excellent animal model in testing questions related to the social environment and HPA axis function and development.
We previously demonstrated that developing marmosets have demonstrable individual differences in the excretion of stress hormones in response to a standardized stressor, and that these individual differences were highly consistent within individuals across major phases of lifespan development (independent from caregivers) for prepubertal (6 months of age), peripubertal (12 months) and postpubertal young adults (18 months; French et al., 2012). In investigating the sources of individual variation in HPA stress responses, we found that individual marmosets within a twin litter do not show concordant HPA responses to social separation with their co-twins (French et al., 2012). We therefore postulated that shared intrauterine environments and shared genes (average of 50% among fraternal twins) cannot completely account for individual differences in stress response styles in this species. Similar to studies in rodents, humans, and other nonhuman primates, empirical evidence suggests that marmoset HPA stress response styles are sensitive to variation in early environments. For example, infant marmosets (Callithrix jacchus) that were repeatedly separated from their caregivers (30 to 120 minutes) between 2 and 28 days of life showed lower baseline levels of urinary cortisol as juveniles than control animals (Dettling et al., 2002), but no differences were found in cortisol responses during social separation challenges. In addition, normative variation in parental quality, as opposed to experimental parental deprivation, is also related to differences in HPA stress response styles in marmosets. In response to an exogenous CRH challenge, plasma cortisol responses were lower in marmoset infants that experienced some form of parental abuse either from mothers or fathers during the early postnatal period compared to infants from non-abusive families (Johnson et al., 1996a). In the closely related Goeldi’s monkey (Callimico goeldii), higher levels of parental aggression (threat vocalizations, biting, or removing an infant that is being carried) between 3–12 weeks of life were related to higher urinary cortisol responses to social separation in offspring at 32–35 weeks of age (Dettling et al., 1998). After repeated separation challenges, Goeldi's monkey offspring that received higher rates of parental aggression early in life showed less behavioral and HPA adaption to the stressful event than offspring receiving lower rates of parental aggression. While these results suggest that the early social environment plays a key role in HPA axis function and development, it is still unclear as to which social cues contribute significantly to the development of HPA stress response styles in cooperatively breeding species. That is, are the alterations in HPA stress responses in cooperatively breeding species a result of the quality of maternal interactions, paternal interactions, or total quality and quantity of offspring care provided by all caregivers during the critical period of infancy? Marmoset fathers and older siblings often play a substantial role in infant rearing (Nunes et al., 2001, 2000; Yamamoto, 1993; Yamamoto et al., 2008). We therefore postulated that the differences in the care provided by fathers and other group members could potentially constitute a significant source of variation in later HPA stress response styles in this cooperatively breeding primate.
The purpose of the current study was to investigate the nature of differences in early offspring care in marmosets and to assess the relationship between these differences and HPA responses to social separation stress across development in marmoset monkeys (C. geoffroyi). We used a social separation stress paradigm at different developmental time points to assess HPA responsivity in marmoset offspring and examined if differences in paternal and/or alloparental care, in addition to maternal care, were associated with differences in HPA stress responses. Specifically, we investigated the relationships between HPA stress responses and infant carrying behavior, licking and grooming, and infant rejections across these different group members. To the extent that HPA stress response styles are sensitive to variation in early infant care in marmosets, we predicted that offspring that received lower rates of grooming and anogenital licking and were subjected to more infant rejections across care providers would have higher urinary cortisol responses across development to social separation compared to infants who received higher rates of licking and grooming and lower rates of rejections.
Methods
Subjects and housing
A total of 37 (21 male; 16 female) white-faced marmosets (C. geoffroyi) were used in the study. Marmosets typically give birth to fraternal twins, and of the subjects used, 29 were from twin pairs (one male from a twin pair died before testing) and 8 were singletons. Animals were socially housed in family groups of 3 to 9 at the University of Nebraska at Omaha Callitrichid Research Center. Twenty-three of the 37 offspring in this study had between 1 and 6 older siblings present at the time of their birth, while the remaining 14 did not have older siblings present at the time of their birth. Older siblings ranged in ages from 6 months to 2 years. Animals were houses in wire-mesh enclosures no smaller than 1m × 2m × 2m with no less than 1m3 per animal. Cages were furnished with branches, a nest box, and various enrichment devices. Animals had access to water ad libitum and were fed Zupreem® Marmoset diet and Mazuri® fiber blocks each day, supplemented with varying combinations of meal worms, crickets, various fruits, yogurt and eggs. Adequate steps were taken to ensure minimal pain and discomfort, and all procedures complied with and were approved by the University of Nebraska Medical Center/University of Nebraska at Omaha Institutional Animal Care and Use Committee (IACUC: 07-033-05). Further details of husbandry and housing conditions can be found in Schaffner et al. (1995).
Behavioral observations
Behavioral observations (20 min each) were conducted two to five times per week for all offspring during the first two months (1 to 60 days) of age. Several observers collected behavioral data. Prior to data collection, observers were trained and inter-rater reliability was assessed. Observers were allowed to collect behavioral data once they achieved 90% or higher reliability with the other observers. During behavioral observations, a trained observer sat approximately 1 m from the home cage with a laptop computer and recorded behaviors with either Observer 5.0 or Observer XT 8.0 (Noldus Technologies, Houston, TX). All occurrences of infant care behaviors and the identity of the group member performing the target behavior were recorded during observations. The time spent carrying as a proportion of the total observation time for each infant was recorded for all members of the group. Each infant was scored individually; thus in the case of twins, if a caregiver was carrying both infants, each infant received a separate score for the duration of time spent carrying by that caregiver. We also collected the frequencies of infant rejections, infant grooming, and infant anogenital licking for each caregiver present for each observation. An infant rejection was scored when a caregiver, carrying an infant, actively displaced the infant by pushing, pulling or otherwise removing the infant from its body. An infant grooming bout was scored when a caregiver manipulated the coat of an infant with its teeth or hands. An anogenital licking bout was scored when a caregiver licked the anogenital region of an infant. A new infant grooming bout or anogenital licking bout was scored only if the behavior had not been performed during the previous five second interval. Each infant was identified with a unique dye mark to track parental care for each member of the set of twin offspring.
Social separation challenges
Animals underwent social separation stress challenges at six, 12, and 18 months of age, which represent juvenile, sub-adult, and young adult developmental stages, respectively (Yamamoto, 1993). Not all 37 animals completed the separation challenges; we were able to obtain data for 37, 35, and 28 monkeys at 6-, 12-, and 18-months of age, respectively. During the challenge, the animal was removed from its natal group and housed alone in a smaller cage for eight h. One day prior to removal from the home cage and immediately prior to the removal, a first-void urine sample was collected from each animal under stress-free conditions in their respective home cage between 0600 and 0800 hours. Animals were habituated to investigators entering their cages and were trained to urinate into hand-held pans for a food reward. Urine from the pans was then transferred to a clean microcentrifuge vial, centrifuged for 2 min at 2500 rpm to remove any sediment, and the supernatant portion of the sample was then transferred to a clean vial and frozen at −20° C until it was assayed for hormone concentrations. At 0900 hrs, the animal was removed from the home cage and placed in a small wire mesh separation cage measuring 0.5 m × 0.5 m × 0.5 m. Separation cages were then moved to a separate, novel, and quiet room away from the home cage. These cages provided separated marmosets with access to food and water, and a water bottle filled with diluted apple juice to facilitate urination. Animals remained in the separation cage until 1700 h (total of 8 h of separation). Animals were returned to their natal family group and familiar cage at 1700 h. This type of social separation has been shown to induce a measurable and significant increase in urinary glucocorticoid excretion relative to normal circadian variation (Johnson et al., 1996b; Rukstalis and French, 2005; Smith and French, 1997).
To collect urine samples during the time the marmoset was separated from its natal group, separation cages were placed on top of clean plastic sheets. Every hour from 1000 to 1700 h a trained technician would enter the room and collect urine from the plastic with a pipette, providing a total of eight urine collection time points during separation. Plastic sheets were replaced after each urine collection time point. The day following social separation, another first-void urine sample (between 0600 and 0800 h) was collected in the home cage as described above.
Cortisol assay
To measure urinary glucocorticoid excretion, we used a cortisol enzyme immunoassay that has previously been developed and validated for marmoset urine (Smith and French, 1997). Briefly, 96-well microtiter plates were coated with rabbit anticortisol antibody and 50 µl of standard and urine sample (diluted 1:6,400 in distilled, deionized water) was added. Standards were serially diluted in distilled and deionized water, and ranged from 1000–7.8 pg/well. Labeled cortisol (horseradish peroxidase, HRP) was added to each well, and the plates were incubated for 2 h. We separated free from bound hormone in each well by washing the plate four times with saline-buffered detergent solution (Tween 20; Sigma Chemicals, St. Louis, MO) and then added 100 µl of substrate (ABTS and H2O2) and allowed the plate to develop until the B0 wells reached an optical density of approximately 1.0. The sample concentration was determined by interpolating optical densities onto a four-parameter sigmoidal fit function. Interassay coefficients of variation (CV), determined from high and low concentration pool samples ran on each plate, were 12.28% and 14.90%, respectively. Intraassay CVs for the high and low pools 8.94% and 9.78%, respectively. To minimize procedural variability, all samples for a single individual were measured in the same block of assays whenever possible. To control for variable fluid intake and output, creatinine (Cr) concentrations were determined with a modified Jaffe reaction colorimetric assay, and the concentration of cortisol in the urine sample (in µg/ml) was divided by the creatinine concentration (in mg/ml) to yield values of cortisol in µg/mg Cr.
Data analyses
We calculated three measures of infant care quality. First, we calculated the proportion of time spent carrying each infant for each group member for all observations. Second, infant grooming and anogenital licking for each observation were summed, yielding a total licking and grooming (LG) score for each infant during each observation. Last, we summed infant rejections (IR) by all group members for each observation. We calculated carrying, LG, and IR scores for mothers, fathers, older siblings, and summed scores across all caregivers present. Proportion of time spent carrying, LG, and IR scores were then averaged across all observations conducted during the first 60 days of life for each infant. We used median splits to classify infants based on these scores into two categories for each variable; i.e., high- and low-carrying, high- and low-LG, high- and low-IR. In order to test whether or not carrying, LG, and IR were independent of one another, we conducted Pearson’s chi-square analyses using the frequencies of carrying, LG, and IR group members within infant care categories (e.g., high- and low-maternal IR and high- and low-maternal LG).
A total of six measures of urinary cortisol levels were calculated from samples taken before, during, and after social separation. A baseline cortisol level was calculated by averaging the first-void urine sample taken one day prior to and on the day of separation immediately prior to removal of the subjects from their home cages. Cortisol levels for samples collected during the 8-hr separation period were averaged into 2-hr time blocks, yielding a total of four data points across the duration of the separation challenge. The sixth and final time point for urinary cortisol measurement was calculated from the first-void morning sample collected the day following the separation challenge. These six data points provided HPA stress reactivity profiles for each separation challenge for each infant at 6, 12, and 18 months of age, and provided measurements to compare urinary cortisol at baseline, maximum responses during separation stress, and post-stress recovery levels.
We then evaluated urinary cortisol responses to social separation stress with a series of 2 (sex) × 2 (infant care category: high/low carry, high/low IR, high/low LG) × 6 (time) mixed-model, repeated measures analyses of covariance (ANCOVA) tests using the general linear model procedure in SPSS 19. Family size (not counting infants) was used as a covariate in analyses. Family size is highly correlated with maternal parity in our sample (r=0.92), so it was omitted as a covariate in order to reduce multicollinearity in analyses. We conducted post hoc independent sample t-tests to compare cortisol levels between groups at each time point in the presence of a significant group x time interaction; i.e., at baseline, during separation stress, and at post-stress recovery. Planned independent sample t-tests were conducted on all baseline and recovery cortisol levels. All analyses were two-tailed and the criterion for statistical significance was set at p≤0.05.
Results
Infant care behaviors
Table 1 shows means, standard deviations, and ranges of infant care behaviors performed by mothers, fathers, older siblings, and total caregiving during the first 60 days of offspring life. The data reveal that while overall infants are being carried by caregivers a substantial portion of the time (approximately 75% of the time), there is substantial variation among infants in the identity of the primary caregiver. Further, there are also considerable differences among infants in the rates of both positive caregiver interactions (LG) and negative interactions with caregivers (IR).
Table 1.
Infant care behaviors
| N | Mean | SD | Range | |
|---|---|---|---|---|
| Time Carried by Mother | 37 | 30.3% | 20.7% | 3–98 % |
| Frequency Maternal LG | 37 | 0.174 | 0.152 | 0.00–0.62 |
| Frequency Maternal IR | 37 | 0.078 | 0.109 | 0.00–0.53 |
| Time Carried by Father | 37 | 34.2% | 24.6% | 0–80% |
| Frequency Paternal LG | 37 | 0.248 | 0.231 | 0.00–1.06 |
| Frequency Paternal IR | 37 | 0.065 | 0.102 | 0.00–0.44 |
| Time Carried by Siblings | 231 | 16.4% | 16.1% | 0–48% |
| Frequency Sibling LG | 23 | 0.340 | 0.258 | 0.00–0.88 |
| Frequency Sbiling IR | 23 | 0.134 | 0.179 | 0.00–0.62 |
| Time Carried Total | 37 | 74.1% | 13.9% | 53–98% |
| Frequency Total LG | 37 | 0.627 | 0.344 | 0.07–1.79 |
| Frequency Total IR | 37 | 0.224 | 0.238 | 0.00–0.75 |
Note: Values shown are averages per 20 min observation from 1–60 days postnatal.
Averages for siblings include only those family groups that contained at least one older sibling that could potentially provide care to dependent offspring.
Independence of Infant Care Behaviors
The amount of time spent carrying was independent of the number of IRs infants received. Pearson’s chi-square analyses indicated that the numbers of animals in the high and low carrying groups were equally distributed in the high and low IR groups for mothers [χ2 (1) = .026, p=873], fathers [χ2(1) =.232, p=630], siblings [χ 2(1) = 2.112, p=.146], and overall as families [χ 2(1) =.669, p=.413]. The amount of time spent carrying was independent of the rates of infant LG for most measures. Analyses indicated that the numbers of animals in the high and low carrying groups were equally distributed in the high and low LG groups mothers [χ2 (1) = 1.337, p=248], fathers [χ2(1) =.248, p=.618], and siblings [χ 2(1) = .034, p=855]. However, offspring in the high total carrying group were significantly more likely to be in the low total LG group and vice versa [χ2(1) = 7.797, p=.005].The number of IRs infants received was independent of the amount of LG received. Pearson’s chi-square analyses indicated that the numbers of animals in the high and low IR groups were equally distributed in the high and low LG groups for mothers [χ2 (1) = .669, p=.413], fathers [χ2 (1) = .703, p=.402], siblings [χ2 (1) = .088, p=767], and overall as families [χ2 (1) = 2.179, p=.140].
Early Care and Stress-induced Cortisol Changes
Urinary cortisol levels across the separation challenge differed between offspring in the overall high- and low-IR groups as indicated by significant overall IR category (i.e., IR by all caregivers) by time interactions at 6 months [F(5, 160)=3.38, p=0.006], and 18 months [F(5, 115)=3.203, p=0.010]. Overall, urinary cortisol levels for the 12 month separation challenge were higher in offspring in the high-IR group than the low-IR group [main effect: F(1, 30)=6.31, p=0.02], but there was no significant group by time interaction at 12 months [F(5, 150)=1.751, p=0.126]. Figures 1a–c show that urinary cortisol increased during the separation challenge in both IR groups and that offspring that experienced high rates of IR showed greater cortisol increases during separation, compared to offspring that experienced low rates of IR early in life. No differences in baseline cortisol were found between overall high- and low-IR groups [6 months: t(35)=0.82, p=0.42; 12 months: t(33)=0.79, p=0.44; 18 months: t(26)=0.70, p=0.49]. Recovery cortisol levels at 12 months tended to be higher in offspring in the overall high-IR group [t(33)=2.00, p=0.054], though not statistically significant, while recovery levels for the 6 month [t(35)=1.01, p=0.32] and 18 month separation challenges did not differ [t(26)=1.60, p=0.12]. Furthermore, no significant differences were found in cortisol levels during any time point of social separation between infants from high- and low-LG families (Figures 1d–f) or from high- and low-carrying families (Figures 1g–i).
Figure 1.
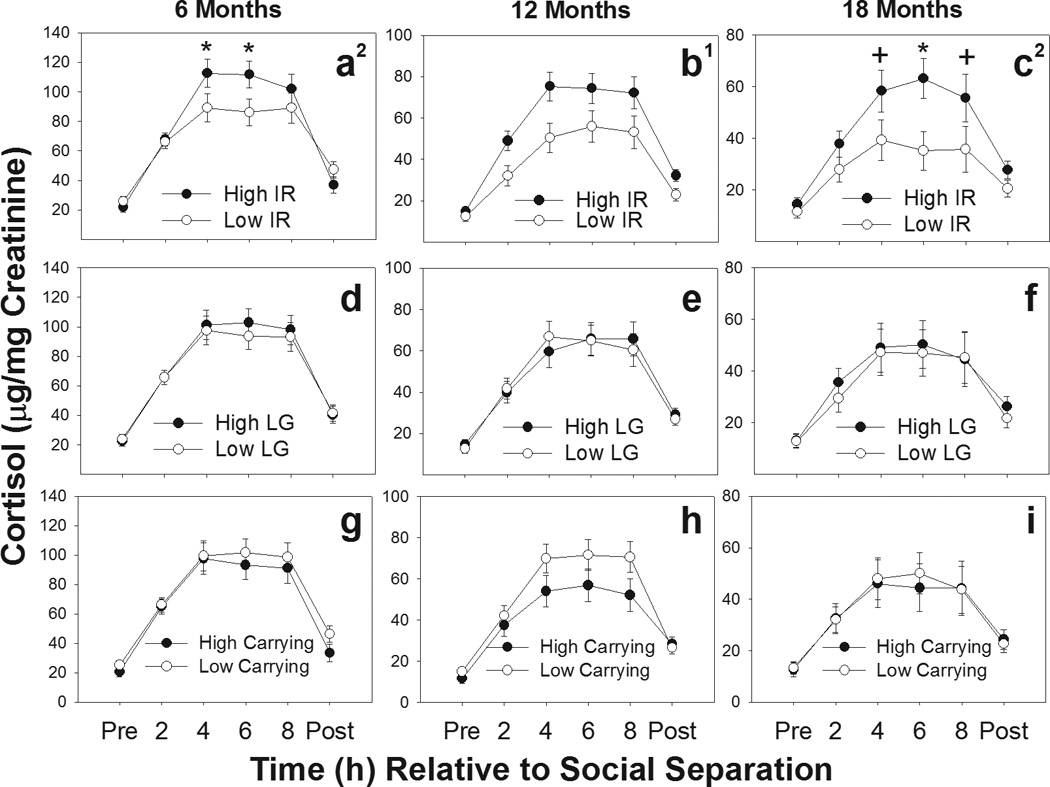
Urinary cortisol levels during social separation challenges at 6, 12, and 18 months of age for offspring receiving high (●) and low (○) rates of carrying (a-c), licking and grooming (LG; d-f), and infant rejections (IR; g-i) from the entire family group. Offspring from high-IR families had higher urinary cortisol levels across the duration of social separation compared to offspring from low-IR families. 1Significant main effect of group; 2Significant group by time interaction. *p<.05; + p<.10.
We then analyzed glucocorticoid excretion during the stressor as a function of care provided by the various group members, and the analyses reveal that differential IR by all potential caregivers was associated with differential HPA function. Cortisol levels as a consequence of differential treatment of offspring by mothers was noted, as indicated by significant maternal IR category by time interactions at 6 months [F(5, 160)=3.30, p=0.007] and 18 months [F(5, 115)=3.68, p=0.004]. Total urinary cortisol levels for the 12 month separation challenge tended to be higher in offspring in the high maternal IR group than the low maternal IR group [main effect trend: F(1, 30)=3.16, p=0.086], but there was no significant group x time interaction at 12 months [F(5, 150)=1.870, p=0.103]. Figures 2a–c reveal that urinary cortisol increased during the separation challenge in both groups and that offspring who experienced high rates of maternal IR show greater cortisol increases during separation, compared to offspring that experienced low rates of IR early in life. No differences in baseline cortisol were found between maternal high- and low-IR groups at any time point tested [6 months: t(35)=1.21, p=0.23; 12 months: t(33)=0.65, p=0.52; 18 months: t(26)=0.56, p=0.57]. Recovery cortisol levels at 18 months of age were higher in offspring from high-IR mothers [t(26)=2.19, p=.038], but not at any other time point [6 months: t(35)=0.99, p= 0.33; 12 months: t(33)=1.48, p=0.15]. No significant differences were found in separation stress cortisol levels between infants from high- and low-LG mothers or high- and low-carrying mothers for any time point of separation (data not shown; see online supplemental material).
Figure 2.
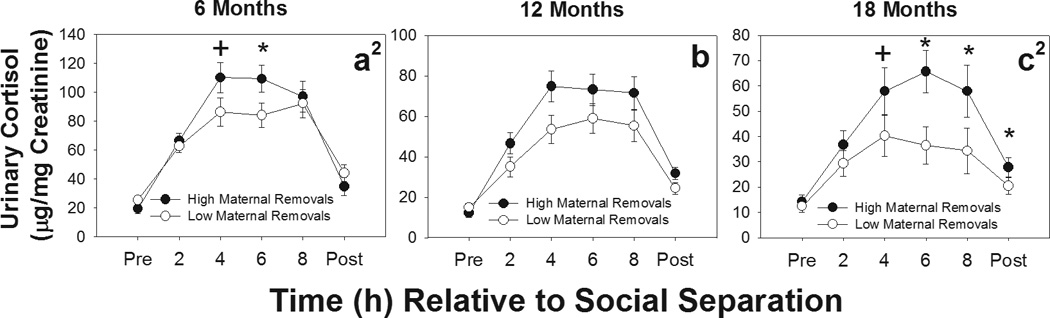
Urinary cortisol levels during social separation challenges at 6, 12, and 18 months of age for offspring receiving high (●) and low (○) rates of infant rejections (IR) from their mothers. Offspring from high-IR mothers had higher urinary cortisol levels across the duration o social separation compared to offspring from low-IR mothers. 1Significant main effect of group; 2Significant group by time interaction. *p<.05; + p<.10.
Cortisol levels across the separation challenge differed as a function of differential early treatment by fathers as indicated by significant paternal IR category by time interactions at 12 months [F(5, 150)=3.14, p=0.01], and 18 months [F(5, 115)= 2.68, p=0.025], but not at 6 months [F(5, 160)=0.49, p=0.79]. Figures 3a–c show that urinary cortisol increased during the separation challenge in both groups and that, compared to offspring that experienced low rates of IR early in life, offspring that experienced high rates of maternal IR show greater cortisol increases during separation. There were no significant differences in baseline cortisol between paternal high- and low-IR groups at any time point tested [6 months: t(35)=0.62, p=0.54; 12 months: t(33)=0.64, p=0.53; 18 months: t(26)=0.13, p=0.90]. Similarly, there were no significant differences in recovery cortisol levels between paternal high- and low-IR groups at any time point tested [6 months: t(35)=0.95, p=0.35; 12 months: t(33)=0.54, p=0.59; 18 months: t(26)=0.02, p=0.98]. Urinary cortisol levels during separation did not differ between offspring that experienced high- or low-LG or high- or low-carrying rates by fathers at 6, 12, or 18 months (data not shown; see online supplemental material).
Figure 3.
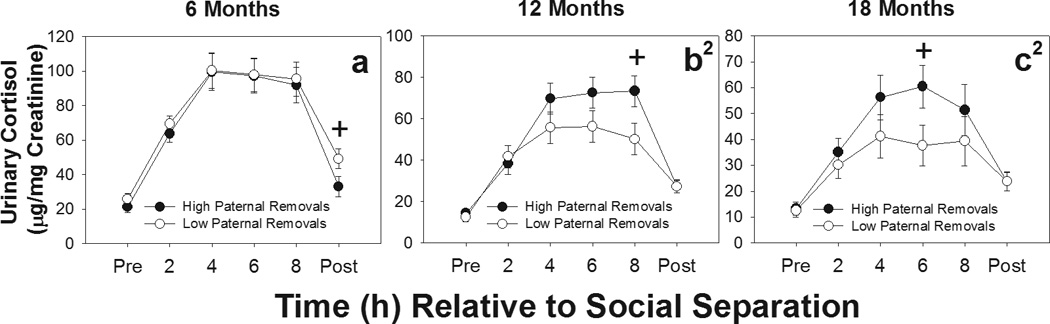
Urinary cortisol levels during social separation challenges at 6, 12, and 18 months of age for offspring receiving high (●) and low (○) rates of infant rejections (IR) from fathers. Offspring from high-IR fathers had higher urinary cortisol levels across the duration of social separation compared to offspring from low-IR families at 12 and 18 months, but not 6 months. 1Significant main effect of group; 2Significant group by time interaction. *p<.05; + p<.10.
We then evaluated the effects of care by older siblings on stress responses. Offpsirng with older siblings present in the natal group did not differ in the amount of overall IR received (t(35)=0.48, p=.64) or time spent being carried by any group member (t(35)=−1.04, 0.30). However, offspring with older siblings present did receive more overall LG from caregivers (t(35)=2.24, p=0.03). In evaluating the effects of early sibling care on stress responses, we limited our analyses to only those offspring that had older siblings in the natal group during infancy. We found that cortisol levels across the separation challenge differed between offspring in the sibling high- and low-IR groups as indicated by significant sibling IR category by time interactions only at 18 months [F(5, 75)=2.62, p=0.031], but not at 6 months [F(5, 90)=.45, p=0.881] or 12 months [F(5, 100)=1.24, p=0.30]. Figures 4a–c shows that at 18 months offspring in the high sibling IR group had higher cortisol levels during separation than offspring in the low sibling IR group. No differences were found between sibling IR groups in either baseline cortisol [6 months: t(21)=0.42, p=0.68; 12 months: t(23)=0.03, p=0.98; 18 months: t(19)=0.75, p=0.33] or recovery cortisol levels [6 months: t(21)=0.77, p=0.45; 12 months: t(23)=0.80, p=0.43; 18 months: t(19)=0.72, p=0.48] during any of the separation challenges. Urinary cortisol levels during separation did not differ between offspring that experienced high- or low-LG by siblings or high- and low-carrying rates by siblings at 6, 12, or 18 months (data not shown; see online supplemental material).
Figure 4.
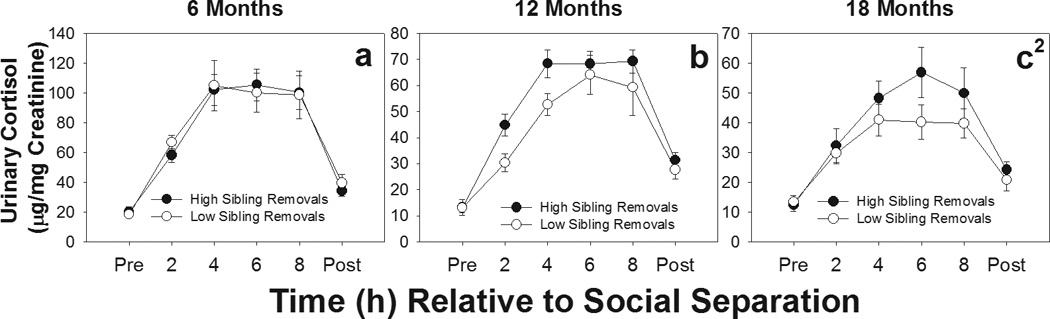
Urinary cortisol levels during social separation challenges at 6, 12, and 18 months of age for offspring receiving high (●) and low (○) rates of infant rejections (IR) from siblings. Offspring from high-IR siblings had higher urinary cortisol levels across the duration of social separation compared to offspring from low-IR families at 18 months, but not at 6 or 12 months; 1Significant main effect of group; 2Significant group by time interaction. *p<.05; + p<.10.
Sex Differences
Urinary cortisol levels during social separation at 6, 12, and 18 months did not differ between males and females as a function of any early care measure analyzed, nor were there any sex differences in baseline or recovery cortisol levels (data not shown).
Discussion
A degree of plasticity exists in which variation in the early social environment can program HPA responses to stressful stimuli throughout an individual’s lifetime (Caldji et al., 2001; Liu et al., 1997; Meaney et al., 1996). Rodent and nonhuman primate studies have shown that differences in early maternal care can alter stress response styles throughout the lifespan in offspring. In the current study, we tested the hypothesis that infant care provided by other group members, in addition to mothers, is also associated with later-life stress response styles in the cooperatively breeding marmoset monkey. Although there is substantial variation among families in how much fathers carry infants early in life (Nunes et al., 2001, 2000), we found no relationship between social stress responses and the proportion of time that infants were carried by fathers or any other group member. Likewise, we found no relationship between differences in early licking and grooming received by infants from any group member and later stress response styles. However, marmoset offspring that received higher rates of rejection while being carried from all family members during the first 60 days of life had more pronounced urinary cortisol responses to social separation that extended at least through three major developmental stages: prepubertal young (6 months), peripubertal (12 months), and young adult (18 months). Further analyses suggested that infant rejections performed by mothers, fathers, and older siblings all contributed to this difference. Offspring born to high-IR mothers showed significantly higher urinary cortisol responses to social separation, compared to offspring that experienced low rates of maternal IR as infants. A similar pattern was seen with paternal care: offspring born to high-IR fathers showed significantly higher urinary cortisol responses to social separation at 12 and 18 months, but not 6 months of age. Furthermore, offspring that received higher rates of IR from older siblings showed more pronounced cortisol responses during social separation at 18 months of age, but not at 6 or 12 months. Taken together, these data suggest that the development of the HPA stress response is sensitive to early-life social cues from multiple group members in the cooperatively breeding marmoset.
Liu et al. (1997) argue that it is adaptive for offspring to program fundamental biological responses to environmental stimuli based on early social cues from primary caregivers. This, in turn, allows the animal to respond to various threats unique to that environment. For instance, in an unstable environment with scarce resources or other external stressors, a rat mother is less likely to spend time licking and grooming her pups and is more likely to be away from the nest for long periods of time (foraging for scarce food, for instance). Thus, for rat pups, low rates of licking and grooming and extended periods of maternal separation would be two cues indicating that the environment in which they live is, to some extent, unstable. Therefore, adopting a more acute sensitivity to environmental stressors, in this case, may better prepare the offspring for an unpredictable environment by promoting vigilance and thus increasing the likelihood of survival (reviewed in Matthews, 2002). In rats, infants would receive the vast majority of such social cues from their mothers, since they are the primary caregivers in these species. In macaque monkeys, while infants do interact with group members other than the mothers in late infancy, early infancy is characterized by markedly high rates of mother-infant interactions. In cooperatively breeding animals, offspring receive a large amount of socio-environmental information from multiple group members that are substantially involved in infant rearing. Our results suggest that the developing HPA axis of cooperatively breeding marmosets is sensitive to the quality of care, particularly in the form of infant rejection, from mothers, fathers, and to some extent, older siblings. Given that marmoset fathers and older siblings play such a critical role in infant rearing, it is perhaps not surprising to find associations that are similar to the well-known relationships between maternal care and HPA development. We previously characterized a substantial amount of variability in the both the quality and quantity of infant care provided by fathers (Nunes et al., 2001), which appears to be dependent on several factors. Marmoset fathers display maximal levels of paternal care 3–4 weeks after parturition (Cavanaugh and French, 2013; Nunes et al., 2000), coinciding with a decline in maternal care and changes in steroid hormones (Nunes et al., 2001). Thus, marmoset offspring are exposed to a considerable amount of variation in infant care, and our results suggest that HPA axis development can be influenced by the care provided across all group members.
It is unclear precisely which environmental factors might contribute to variations in early parental care by the natal group, which in turn could influence stress reactivity styles of offspring in marmosets. While separation from the mothers could have nutritional consequences that would alter HPA activity, it is unlikely that infants in our sample experienced sufficiently long maternal separations to induce such changes. Our data support this, albeit indirectly, since there was no relationship between the amount of time spent carrying by mothers (or any other caregiver) and later HPA reactivity. Infants can only nurse when they are being carried by mothers, and if there were nutritional consequences that lead to changes in HPA stress functioning, we would expect to find a relationship between maternal carrying and stress cortisol levels, which was not the case. An alternative hypothesis might instead account for various group stability factors. Given that intra-group aggression in callitrichines can be a source of conflict resulting in the ostracism of group members (e.g., cotton-top tamarin: Snowdon and Pickhard, 1999), it is also likely that group stability plays a major role in how callitrichines interact with offspring. Infants born to unstable social groups would likely experience high rates of rejections from stressed caregivers who may also be more prone to aggressive acts. This, is turn, might alter HPA development, which would be manifested during stressful events as a more pronounced HPA stress response. However, rates of intragroup conflict are very low in our marmoset colony and the current study did not measure any variables related to group stability, so this hypothesis remains admittedly speculative.
Nonetheless, our findings are consistent with other studies that have found that early exposure to lower quality infant care is associated with more pronounced HPA responses to stress later in life in primates. When separated from social groups, peer-reared rhesus macaques had more pronounced plasma cortisol responses, but similar baseline levels, compared to mother-reared animals (Higley et al., 1992). Contrast this with the findings of Dettling et al., (2002), in which, compared to normally-reared controls, early deprived marmosets showed lower basal plasma cortisol levels, but similar cortisol responses to social stress. This difference may reflect the effects of total social deprivation (in the case of early deprivation: Dettling et al., 2002) versus parental deprivation (in the case of peer-reared macaque monkeys: Higley et al., 1992; or marmoset offspring born to high IR mothers and fathers: current study). This is further supported by the finding that peer-reared macaques had decreased basal cortisol after chronic social separation (Barr et al., 2004b). Decreased baseline cortisol suggests HPA over-regulation through negative feedback or decreased adrenal sensitivity to ACTH. In our sample, there were few and inconsistent differences in baseline cortisol levels between offspring experiencing either high or low IR, indicating that normal resting state HPA regulation was primarily conserved. This would suggest, then, that within a normal range in quality of infant care, resting-state HPA axis functioning is not disrupted. HPA stress responses, on the other hand, appear to be sensitive to relatively small variations within a normative range in infant care quality. Most primate studies to date have found differences in HPA stress responses in offspring subjected to substantial early interventions (e.g., peer group rearing in macaques: Higley et al., 1992) or very poor quality infant care (e.g., infant abuse; marmosets: Johnson et al., 1996a; macaques: reviewed in Parker and Maestripieri, 2011). It is interesting to note that in our sample, offspring appeared to be sensitive to changes in their early environment even within the boundaries of normative variation in infant care.
There were no instances in which sex of the offspring moderated the effects of early parental experience on HPA axis development. This is somewhat surprising because there is some degree of sexual dimorphism in the adult stress response and cortisol awakening response of marmosets (Smith and French, 1997). While there are some accounts of differences in how male and female infants are treated (e.g., carrying rates: Yamamoto et al., 2008), other studies have found that callitrichine primates do not vary the amount of care provided based on the sex of the offspring (e.g., Cleveland and Snowdon, 1984; Tardif et al., 1992). In addition, and most similar to our findings, Dettling et al. (2002) also found no sex differences in HPA stress responses among young (18 weeks) marmosets, whether early-deprived or normally-reared. Little is know, however, about other infant characteristics such as size or nutritional status that might also affect how parents interact with infants.
Interestingly, we found that infant rejections, but not licking and grooming, have an effect on HPA responsivity. This is consistent with other primate studies that have found little to no relationship between sociopositive infant care behaviors (e.g., carrying, grooming, anogenital licking, etc.) and later HPA stress response styles (macaque monkeys: review in Parker and Maestripieri, 2011; Stevens et al., 2009; marmoset: Johnson et al., 1996a; Goeldi’s monkey: Dettling et al., 1998). Instead, these studies reveal that variation in the experience of dependent offspring with poor quality infant care is associated with more pronounced HPA responses to stressful events. This may reflect the quality of each category of behavior as a measure of infant care. Licking and grooming, for example, are distinct, brief events of increased attention toward the infant. Infant rejections, by contrast, constitute a physical removal of warmth and security, sometimes including a brief struggle between caregiver and infant. Furthermore, after a rejection, the infant must move about on its own, find another suitable caregiver, or wait to be retrieved. Thus, infant rejections would more likely result in greater changes in the infant’s arousal and greater HPA activation. We argue, therefore, that the resulting activation of the HPA axis during infant rejection is more likely to lead to postnatal programming of the HPA axis, while the relative lack of change in HPA activity resulting from normal variation in LG would have lesser or even no effects on later-life HPA stress responses. (See supplemental online material for a video example of an adolescent silvery marmoset (Callithrix argentata) performing an infant rejection; courtesy THIRTEEN/WNET, 2002). It is also possible that, in our sample, the directionality of the relationship between infant care and HPA reactivity is reversed; i.e., more reactive infants might have elicited more removals from caregivers. Infants were not randomly assigned to groups and the amount of carrying, licking and grooming, and infant rejections was not manipulated. Interpretation of these results should therefore be done with these caveats in mind.
Future investigations might attempt to delineate the mechanisms by which various caregivers in cooperatively breeding animals can alter offspring’s HPA stress responses. It is likely that, similar to relationships found between maternal care and HPA stress responses, early infant care provided by different group members interacts with several key genetic polymorphisms to produce phenotypic variation in HPA stress response styles. Candidate markers would include variation in genes coding for glucocorticoid and mineralocorticoid receptors (DeRijk et al., 2010) and GABAergic (Uhart et al., 2004) or monoaminergic (Barr et al., 2004b; McCormack et al., 2009) neurotransmitter system components. Evidence to date also supports the hypothesis that early experiences operate on many neural target sites through epigenetic factors, providing a mechanism by which genes and environments interact to produce variation in individual HPA stress phenotypes through a variety of mechanisms, including changes in glucocorticoid (GC) and mineralocorticoid (MC) receptors in the hippocampus (Meaney and Szyf, 2005; Weaver et al., 2004). There is compelling evidence that experimentally-induced early life adversity in marmosets leads to important changes in brain function relevant for stress reactivity. Most relevant to the present paper, early experimental deprivation in infant marmosets also leads in adolescence to reduced GC and MR mRNA expression in the hippocampus (particularly CA1−2), but not in the prefrontal cortex or the hypothalamus (Arabadzisz et al., 2010). Given the central role of the neural systems for regulating stress sensitivity, and their sensitivity to early social life, these changes are hence prime candidates for mechanisms by which normative variation in the quality of early offspring care received by marmoset infants in our study led to substantial and sustained changes in HPA function lasting even to adulthood.
In summary, the early postnatal period represents a critical period of development for key components of neuroendocrine systems and other physiological-behavioral pathways. We found evidence suggesting that, during early life, multiple caregivers can influence the HPA responses to social stress in offspring reared by cooperatively breeding family groups. It is likely that neuroendocrine pathways and other developmental trajectories of cooperatively breeding mammals are influenced by social cues from the entire family group. Such a mechanism would allow for more finely tuned adaptations to unique environments based on early-life exposure to complex socio-environmental information.
Supplementary Material
Acknowledgements
We thank Heather Jensen, Danny Revers and Liz Gunkelman for their excellence in animal care and husbandry. We would also like to thank the UNO Neuroscience and Behavior Journal Club and the journal’s reviewers for providing helpful comments on previous versions of this manuscript. The research program from which these data are derived was initiated by Jeffrey E. Fite, and was supported by funds from the National Institutes of Health (HD 42882) and the National Science Foundation (IBN 00-91030) awarded to JAF. All procedures were approved by the University of Nebraska at Omaha/Medical center Institutional Animal Care and Use Committee and adhered to all local, state, and national laws regulating research on nonhuman primates.
Funding body agreements and policies
The research program from which these data are derived was initiated by Jeffrey E. Fite and Jeffrey A. French, and was supported by funds from the National Institutes of Health (HD 42882) and the National Science Foundation (IBN 00-91030) awarded to Jeffrey A. French.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
In submitting this manuscript, all authors declare no conflict of interest, monetary or otherwise
Contributors
Andrew K. Birnie assisted in data collection, conducted the statistical analyses, and wrote the majority of the manuscript; Jack Taylor and Jon-Ryan Cavanaugh assisted in data collection and wrote portions of the manuscript; Jeffrey French designed and oversaw implementation of the study and wrote portions of the manuscript
References
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, Pryce CR. Primate Early Life Stress Leads to Long-Term Mild Hippocampal Decreases in Corticosteroid Receptor Expression. Biological Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early Experience and Sex Interact to Influence Limbic-Hypothalamic-Pituitary-Adrenal-Axis Function After Acute Alcohol Administration in Rhesus Macaques (Macaca mulatta) Alcoholism: Clinical and Experimental Research. 2004a;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. Journal of neuroendocrinology. 1995;7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Liu D, Sharma S, Diorio J, Francis D, Meaney MJ, Plotsky PM. Development of individual differences in behavioral and endocrine responses to stress: role of the postnatal environment. Comprehensive Physiology. 2001 [Google Scholar]
- Cavanaugh J, French JA. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Hormones and behavior. 2013;64:551–558. doi: 10.1016/j.yhbeh.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. NeuroToxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Developmental Psychobiology. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus o. oedipus) Animal Behaviour. 1984;32:432–444. [Google Scholar]
- DeRijk RH, De Kloet ER, Zitman FG, Van Leeuwen N. Mineralocorticoid receptor gene variants as determinants of HPA axis regulation and behavior. Pediatric Adrenal Diseases. 2010;20:137–148. doi: 10.1159/000321235. [DOI] [PubMed] [Google Scholar]
- Dettling A, Pryce CR, Martin RD, Döbeli M. Physiological responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi’s monkeys (Callimico goeldii) Developmental psychobiology. 1998;33:21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology Biochemistry and behavior. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Craft TKS, Glasper ER, Neigh GN, Alexander JK. Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Nepomnaschy PA, Muehlenbein MP, Ponzi D. Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neuroscience & Biobehavioral Reviews. 2011;35:1611–1629. doi: 10.1016/j.neubiorev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- French JA, Fite JE, Jensen H, Oparowski K, Rukstalis MR, Fix H, Jones B, Maxwell H, Pacer M, Power ML. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) American journal of primatology. 2007;69:877–889. doi: 10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Smith AS, Gleason AM, Birnie AK, Mustoe A, Korgan A. Stress reactivity in young marmosets (Callithrix geoffroyi): Ontogeny, stability, and lack of concordance among co-twins. Hormones and behavior. 2012;61:196–203. doi: 10.1016/j.yhbeh.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Progress in Brain Research. 2007;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatric research. 1996a;39:999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus) Biological Psychiatry. 1996b;40:317–337. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- Levine S, Alpert M, Lewis G. Infantile experience and the maturation of the pituitary adrenal axis. Science. 1957;126 doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. Journal of Neuroendocrinology. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of traumatic stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo–pituitary–adrenal axis. Trends in Endocrinology & Metabolism. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early Environmental Regulation of Forebrain Glucocorticoid Receptor Gene Expression: Implications for Adrenocortical Responses to Stress. Developmental neuroscience. 1996;18:61–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in clinical neuroscience. 2005;7:103. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Animal Behaviour. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among Paternal Behavior, Steroid Hormones, and Parental Experience in Male Marmosets (Callithrix kuhlii) Hormones and behavior. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience & Biobehavioral Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Hormones and behavior. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiology & behavior. 2011;104:955–961. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiology & behavior. 1997;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Gubernick DJ. Father-child Relations: Cultural and Biosocial Contexts. New York: Walter de Gruyter; 1992. Male-infant relationships in nonhuman primates: Paternal investment or mating effort; pp. 1–30. [Google Scholar]
- Snowdon CT, Pickhard JJ. Family feuds: severe aggression among cooperatively breeding cotton-top tamarins. International journal of primatology. 1999;20:651–663. [Google Scholar]
- Solomon NG, French JA. Cooperative breeding in mammals. Cambridge University Press; 1996. [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:114–127. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Infant-care Behavior of Non-reproductive Helpers in a Communal-care Primate, the Cotton-top Tamarin (Saguinus oedipus) Ethology. 1992;92:155–167. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Thierry B, Singh M, Kaumanns W. Macaque societies: a model for the study of social organization. Cambridge University Press; 2004. [Google Scholar]
- Uhart M, McCaul ME, Oswald LM, Choi L, Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Molecular psychiatry. 2004;9:998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. The Journal of neuroscience. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. Marmosets and Tamarins: Systematics, Behaviour and Ecology. Oxford: Oxford University Press; 1993. From dependence to sexual maturity: The behavioural ontogeny of Callitrichidae; pp. 235–250. [Google Scholar]
- Yamamoto ME, Albuquerque FS, Lopes NA, Ferreira ES. Differential infant carrying in captive and wild common marmosets (Callithrix jacchus) Acta ethologica. 2008;11:95–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


