Abstract
Ubiquitin-dependent degradation of hormone receptors is emerging as a key mechanism that regulates the magnitude and duration of hormonal effects on cells and tissues. The pituitary hormone prolactin (PRL) is involved in regulating cell differentiation, proliferation, and survival. PRL engages its receptor (PRLR) to initiate various signaling cascades, including the phosphorylation and activation of Stat5. We found that PRL promotes interaction between PRLR and the F-box protein β-TrCP2, which functions as a substrate recognition subunit of the SCFβ-TrCP E3 ubiquitin ligase. This interaction requires PRLR phosphorylation and the integrity of serine 349 within a conserved motif, which is similar to conserved motifs present in other substrates of SCFβ-TrCP. The PRLRS349A mutant is resistant to ubiquitination and is more stable than its wild-type counterpart. Phosphorylated PRLR undergoes ubiquitination by SCFβ-TrCP in vitro. Knockdown of β-TrCP expression inhibits the ubiquitination and degradation of PRLR and promotes PRL-dependent phosphorylation of Stat5 as well as Stat5-dependent transcription in cells. Furthermore, the activation of Stat5 and the stimulation of cell growth by PRL are augmented in cells expressing the PRLRS349A mutant. These data indicate that PRLR is a novel SCFβ-TrCP substrate and implicate β-TrCP as an important negative regulator of PRL signaling and cellular responses to this hormone.
Pituitary hormones play multiple roles in maintaining homeostasis, and aberrations in the regulation of the magnitude and duration of their effects on cells often lead to diseases. Prolactin (PRL) is a peptide hormone known to regulate diverse physiological functions via its effects on cellular processes such as proliferation, differentiation, and cell survival. Although more than 300 functions are attributed to PRL, its name comes from its pivotal role in mammary gland development and lactation (2, 15, 18). PRL is also known to be secreted by certain nonendocrine tissues (e.g., breast cancer cells) and, accordingly, to act as a cytokine (1, 8). PRL mediates its activities by engaging the PRL receptor (PRLR), whose dimerization leads to the activation of various signaling pathways, including Jak2-Stat5, phosphoinositol 3-kinase, and mitogen-activated protein kinase pathways. Multiple splicing forms of PRLRs are found in human cells, among which the long form appears to be the most functional in transducing the entire repertoire of PRL signals, whereas the intermediate and short forms elicit only partial activation or no activation of PRL-dependent signal transduction pathways (reviewed in references 2, 5, and 7).
Limitation of the extent and duration of hormonal signaling in cells is required for the physiological regulation of cellular responses. Negative regulation of PRL signaling appears to play an important role in cellular physiology, as abnormal activation of PRL signaling results in uncontrollable cell proliferation in vitro (35) and abnormal development of mammary glands and deficient lactation in vivo (19). Recently described mechanisms mediating negative regulation of the PRL-induced Jak-Stat pathway include the inactivation of Jak2 and Stat5 by specific phosphatases, suppressors of cytokine signaling, and protein inhibitors of activated STAT (reviewed in reference 6). In addition, modulation of this and other PRL-dependent signal transduction pathways is thought to rely on down-regulation of PRLR in response to the ligand (11, 13), which was shown to occur via PRLR endocytosis and lysosomal degradation (12, 14, 17). However, the determination of the role of PRLR down-regulation in restricting the extent of PRL signaling, as well as the delineation of the mechanisms that mediate PRLR down-regulation, remains a work in progress.
Ubiquitin-mediated proteolysis plays a universal role in the irreversible negative regulation of various signaling pathways (52), including those induced by pituitary hormones (46). Whereas the polyubiquitination of proteins leads to their degradation by 26S proteasome complexes, the oligoubiquitination of plasma membrane proteins targets them for endocytosis and degradation in lysosomes (25, 26). In both instances, the fate of protein substrates depends on the activity of specific E3 ubiquitin protein ligases that recognize these substrates and mediate their ubiquitination. The ubiquitin pathway is essential for the endocytosis and degradation of the receptor for growth hormone (GHR), although the ubiquitination of GHR per se seems not to be required (reviewed in references 46 and 47). The role of the ubiquitination of PRLR in its down-regulation and degradation has not previously been determined, and putative E3 ubiquitin protein ligases that catalyze such ubiquitination have not previously been identified.
By analogy with other receptors whose down-regulation and degradation is mediated by ubiquitination of their intracellular domains (25, 26), signals for the degradation of PRLR are expected to be present within its cytoplasmic tail. The sequences of the cytoplasmic tails of the long form of PRLR are poorly conserved among various species, but we noticed that the DSGRGS sequence, which is similar to a recognition motif for the SCFβ-TrCP E3 ligase, is present in all species whose PRLR sequences have been determined (Table 1). The β-TrCP1 (39) and HOS/β-TrCP2 (16, 48) members of the β-TrCP/Fbw1 subfamily of F-box proteins (4, 53) are known to recognize the phosphorylated DSG(X)2+nS motif within their substrates, which include the IκB (16, 23, 44, 48, 54, 55), β-catenin (16, 22, 29, 34, 54), ATF4 (33), Emi1 (21, 40), Cdc25A (3, 28), Dlg (38), and IFNAR1 (32) proteins. β-TrCP proteins recognize phosphorylated substrate and recruit the core Skp1-Cul1-Roc1/Rbx1 ubiquitin ligase complex to ubiquitinate the substrates (reviewed in reference 10). Unlike the predominantly nuclear β-TrCP1 protein, the β-TrCP2 member of the β-TrCP subfamily exhibits mostly cytoplasmic localization (9, 33).
TABLE 1.
Conserved HOS/β-TrCP2 recognition motifs in PRLRs from different species
| Species | Putative HOS/β-TrCP2 recognition motif | Position of recognition motif (amino acids) |
|---|---|---|
| Homo sapiens | DSGRGS | 348-353 |
| Cebus apella | DSGRGS | 348-353 |
| Callithrix jacchus | DSGRGS | 348-353 |
| Mus musculus | DSGHGS | 343-348 |
| Rattus norvegicus | DSGHGS | 343-348 |
| Oryctolagus cuniculus | DSGRGS | 348-353 |
| Ovis aries | DSGRGS | 348-353 |
| Bos taurus | DSGRGS | 348-353 |
| Cervus elaphus | DSGRGS | 348-353 |
| Columba livia | DSGRGS | 553-558 |
| Meleagris gallopavo | DSGRGS | 552-557 |
| Gallus gallus | DSGRGS | 552-557 |
| Xenopus laevis | DSGRGS | 347-352 |
| Carassius auratus | DSGRGS | 342-346 |
| Paralichtys olivaceus | DSGRGS | 349-354 |
| Cynops pyrrhogaster | DSGRGS | 347-352 |
| Oreochromis niloticus | DSGRGS | 348-353 |
| Oncorhynchus mykiss | DSGRGS | 346-351 |
We sought to investigate whether SCFβ-TrCP E3 ligase may play a role in regulating the stability of PRLR and PRL signaling. Here, we report that β-TrCP2 interacts with the long form of human PRLR in a manner dependent on ligand treatment and phosphorylation of PRLR. The integrity of the DSGRGS motif within PRLR is required for β-TrCP2 binding as well as for PRLR ubiquitination and PRLR degradation, which also depend on the expression of β-TrCP1 and β-TrCP2. Stabilization of PRLR via either the knock-down of β-TrCP or the expression of the PRLRS349A mutant, which is insensitive to β-TrCP-mediated regulation, up-regulates the extent of PRL signaling. We discuss the role of the SCFβ-TrCP E3 ligase in the negative regulation of cellular responses to PRL.
MATERIALS AND METHODS
Materials.
Recombinant human PRL was kindly provided by A. F. Parlow (National Hormone Pituitary Program). ATP, puromycin, methylamine HCl (all from Sigma), and protein phosphatase λ (New England Biolabs) were purchased. Recombinant E2 (UbcH5C) was a gift of Z.-Q. Pan. E1 and ubiquitin (Affiniti Inc.) were purchased.
DNA constructs.
Human pCDNA3-PRLR-Flag (35) was kindly provided by S. M. Anderson. The replacement of serine 349 with alanine was carried out with a QuikChange site-directed mutagenesis kit (Stratagene). Glutathione S-transferase (GST)-tagged bacterial expression constructs were prepared by subcloning sequences encoding C-terminal amino acids 259 to 622 (the cytoplasmic tail) of PRLR into the pGEX-2T vector (Amersham). Hemagglutinin (HA)-tagged ubiquitin (50) and Stat5-driven luciferase reporter (pLHRE-luc) (43) were generous gifts from D. Bohmann and P. A. Kelly. HA-tagged β-TrCP1 (kindly provided by R. Benarous) (33), β-TrCP2- and β-TrCP2ΔN-encoding plasmids (16), and the constructs for the expression of Flag-Cul1, Skp1, and Roc1 (49) were previously described. Plasmids for the expression of GFP (Clontech), Renilla luciferase, and β-galactosidase (Promega) were purchased. The plasmid for the expression of short hairpin RNA (shRNA) directed against both β-TrCP1 and β-TrCP2 (shBTR1,2) was kindly provided by J. W. Harper (28). Plasmids for the specific knock-down of β-TrCP2 (shBTR2) and the control shRNA construct (shCON) were constructed on a backbone of pSilencer 1.0-U6 vector (Ambion) by using the sequence of chemically synthesized duplex small interfering RNA described elsewhere (32). Sequences that formed the hairpin duplexes were 5′-GAGGCCATCAGAAGGAAACTTCAAGAGAGTTTCCTTCTGATGGCCTC-3′ for shBTR2 and 5′-GAGGCCATCAGTGGGAAACTTCAAGAGAGTTTCCCACTGATGGCCTC-3′ for shCON. The effects of shRNA constructs were verified by immunoblotting analysis.
Tissue culture and transfections.
293T human embryo kidney cells were kindly provided by Z. Ronai. Hamster CHO-K1 cells and mouse embryo fibroblasts from β-TrCP1 knockout mice (21) were generous gifts from R. J. Eisenberg and M. Pagano. Cells were grown in Dulbecco modified Eagle medium (DMEM) in the presence of 10% fetal bovine serum (FBS) and antibiotics at 37°C and at 5% CO2. Transfections were performed by the calcium phosphate procedure or lipofection (with Lipofectamine Plus or Lipofectamine 2000 [Invitrogen]) 24 to 48 h before harvesting. Stable mass cultures of CHO-K1 cells expressing PRLR proteins were obtained by cotransfecting PRLR constructs with pBABE-puro vector, followed by selection in medium containing puromycin (1 μg/ml).
Antibodies and immunotechniques.
Antibodies against HA (Roche), Flag (M2; Sigma), PRLR (Affinity BioReagents, Inc., or Neomarkers, Inc.), Skp2 (Zymed), GST (Santa Cruz), and phospho-Stat5 and Stat5 (Cell Signaling Technology) were purchased. HOS-N antibody, which specifically reacts with β-TrCP2, and HOS-C antibody, which recognizes both β-TrCP1 and β-TrCP2, were previously described (45). Secondary antibodies conjugated with horseradish peroxidase (Chemicon) were purchased. Immunoprecipitation and immunoblotting procedures were described elsewhere (16). Digital images were prepared with Adobe Photoshop 7.0 software.
In vitro binding assay.
Recombinant PRLR proteins expressed in 293T cells (grown in the presence of 10% FBS) were immunopurified with Flag antibody and protein A beads, stringently washed with stripping buffer containing 50 mM Tris HCl (pH 7.5), 1 M NaCl, 50 mM NaF, 10 nM okadaic acid, and 0.1% Nonidet P-40, and equilibrated with binding buffer (50 mM Tris HCl [pH 7.5], 100 mM NaCl, 50 mM NaF, 10 nM okadaic acid, 0.1% Nonidet P-40). For treatment with phosphatase λ, the beads were washed with the binding buffer without phosphatase inhibitors and incubated with the phosphatase λ for 1 h at 37°C, followed by washes in stripping buffer and reequilibration with binding buffer. Flag-PRLR proteins immobilized on the beads were incubated with in vitro-translated and 35S-labeled β-TrCP2 for 60 min at 4°C. The beads were extensively washed with binding buffer, and associated proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
In a separate experiment, 293T cells were starved overnight in medium containing 0.5% FBS and treated with PRL (200 ng/ml) for 0 to 120 min (as indicated in Fig. 2B) and lysates were prepared from harvested cells. GST-PRLR proteins were expressed and purified from bacterial cells by using glutathione-Sepharose. Purified bacterial proteins (1 μg) immobilized on glutathione beads were phosphorylated with 293T cell lysates (25 μg) of 293T cells in the presence or absence of ATP for 30 min at 30°C, followed by stringent washing with stripping buffer and reequilibration with binding buffer. GST-PRLR proteins immobilized on the beads were incubated with in vitro-translated and 35S-labeled β-TrCP2 for 60 min at 4°C. The beads were extensively washed with binding buffer, and associated proteins were analyzed by SDS-PAGE, autoradiography, and immunoblotting with GST antibody.
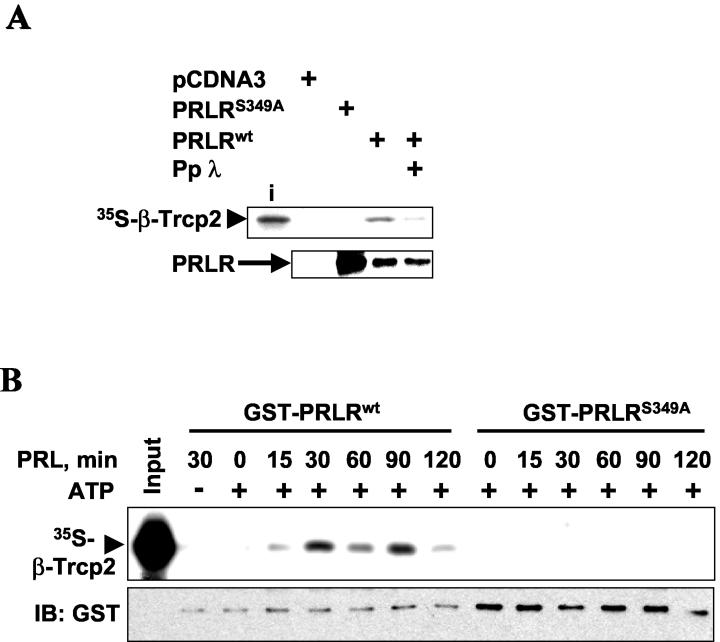
FIG. 2.
Binding of PRLR to β-TrCP2 in vitro depends on PRLR phosphorylation. (A) Binding of in vitro-translated and 35S-labeled β-TrCP2 to Flag-PRLR proteins expressed in 293T cells (grown in the presence of 10% FBS) and immunopurified with Flag antibody before or after treatment with protein phosphatase λ (Pp λ). Aliquots of reaction mixtures were analyzed by autoradiography (top) and immunoblotting with Flag antibody (bottom). The input of radiolabeled β-TrCP2 (i) is also shown. (B) Purified GST-PRLR proteins were incubated with lysates from 293T cells (serum starved and then treated with PRL for the indicated times) in the presence or absence of ATP as described in Materials and Methods. The binding of in vitro-translated and 35S-labeled β-TrCP2 to immobilized GST-PRLR proteins was analyzed by means of SDS-PAGE and autoradiography (top). The input of radioactive β-TrCP2 is also shown. Aliquots of these reaction mixtures were analyzed by immunoblotting (IB) with GST antibody to control the loading of GST-PRLR proteins (bottom).
Ubiquitination and degradation assays.
Cells were grown in the presence of 10% FBS, which contains substantial levels of fetal PRL (41). For in vivo ubiquitination assays, 293T cells were cotransfected with HA-tagged ubiquitin and Flag-tagged PRLR. Cells were harvested in a boiling solution of SDS (1% in Tris-buffered saline) and further disrupted by sonication. Lysates were diluted 10-fold with Triton X-100 solution (1% in Tris-buffered saline), incubated with protein A beads for 1 h, and centrifuged. Supernatants were analyzed by immunoprecipitation and immunoblotting with the indicated antibodies. For the in vitro ubiquitination assay, GST-PRLR proteins were expressed in bacteria, purified by using glutathione beads, and preincubated (1 μg) with 25-μg extracts from 293T cells treated with PRL (200 ng/ml for 20 min) in a kinase buffer containing 50 mM Tris HCl (pH 7.5), 5 mM MgCl2, 0.5 mM dithiothreitol, 5 mM NaF, 10 nM okadaic acid, 50 μM ATP, and 10 μM [32P-γ]ATP for 30 min at 30°C. Proteins were captured on SCFβ-TrCP isolated from transfected 293T cells with HA antibody (Roche) and protein A beads (Invitrogen) as previously described (49) and washed with buffer containing 300 mM NaCl, 0.5% Nonidet P-40, and phosphatase inhibitors. Ubiquitination reactions were carried out with a total volume of 20 μl containing 2 μg of ubiquitin, 20 pmol of E1, and 100 pmol of E2 as well as 2 mM ATP at 37°C for 60 min. Boiling in SDS-sample buffer terminated the reactions, and the products were analyzed by SDS-PAGE and autoradiography. Pulse-chase analysis was carried out with 293T cells as described elsewhere (16). Briefly, cells were grown in 100-mm-diameter dishes and transfected with the indicated plasmids. After starvation in DMEM lacking methionine and cysteine and metabolic labeling with a [35S]methionine-[35S]cysteine mixture (Perkin-Elmer), cells were harvested at each chase time point with complete DMEM supplemented with FBS (0.5%), PRL (20 ng/ml), and unlabeled methionine and cysteine (2 mM). Harvested cells were lysed, and PRLR proteins were immunoprecipitated with Flag or PRLR antibody, separated on SDS-PAGE gel, and analyzed by autoradiography.
Luciferase reporter assays.
293T or CHO cells were transfected with pLHRE-luc and Renilla luciferase vectors (Promega) as well as with small interfering RNA plasmids where indicated, starved in medium with 0.5% serum overnight, and treated with PRL (200 ng/ml) for 24 h before harvesting. Luciferase activities were determined with the aid of a Dual luciferase kit (Promega).
Cell proliferation.
CHO stable mass cultures were seeded into 96-well plates (5 × 103 of the trypan blue-negative live cells per well) in medium containing 0.5% FBS, and cell proliferation was assessed after 3 days of incubation with or without PRL (50 ng/ml) by using a colorimetric WST-1 cell proliferation kit (Roche) according to the manufacturer's recommendations. Acceleration of growth by PRL was calculated as [1 − (absorbance with PRL/absorbance without PRL)] × 100%.
RESULTS
PRLR is capable of interacting with β-TrCP2.
β-TrCPs bind to their substrates by recognizing a specific DSG(X)2+nS motif, within which the serine residues are phosphorylated (16, 48). We noticed that the long form of human PRLR contains a putative β-TrCP recognition motif (DSGRGS) within its cytoplasmic tail. This motif is present in the ΔS1 splicing form of human PRLR (31) but in neither the short nor the intermediate form of the receptor. Although the overall sequence of the intracellular domain of PRLR is poorly conserved among different species (6), the DSGRGS sequence is present in all species whose PRLR sequence has been identified to date (Table 1).
We tested whether β-TrCP2 interacts with PRLR by means of coimmunoprecipitation with the lysates of 293T cells grown under low-serum conditions (0.5% FBS). Analysis of these reactions revealed that β-TrCP2 was indeed found in immunoprecipitates with PRLR antibody but not with an irrelevant monoclonal (Flag) antibody (Fig. 1A, lanes 2 and 3). We could not detect an interaction between endogenous β-TrCP2 and PRLR in a reciprocal coimmunoprecipitation experiment (data not shown) either because of possible interfering effects of anti-β-TrCP antibody or because of low levels of PRLR in 293T cells and/or the transience of the interaction. Treatment of 293T cells with PRL noticeably increased the extent of the binding of β-TrCP2 to PRLR (Fig. 1A, lane 3 versus lane 6). These results suggest that β-TrCP2 interacts with PRLR and that treatment with the hormone ligand promotes this interaction.
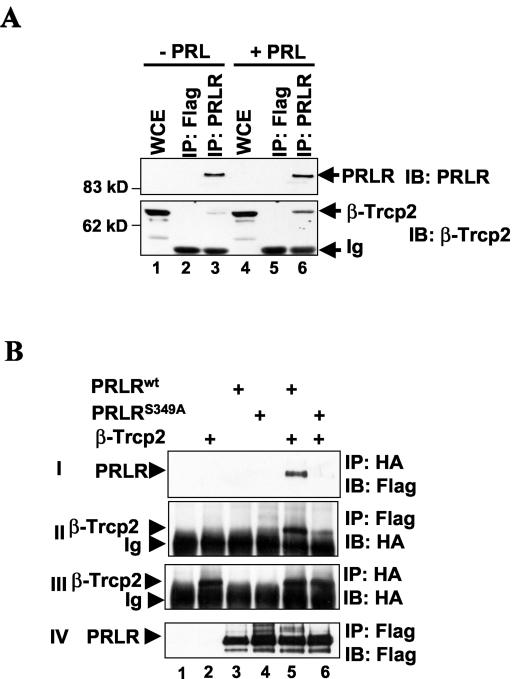
FIG. 1.
PRLR interacts with β-TrCP2 in vivo. (A) 293T cells were starved in medium containing 0.5% FBS overnight and treated with PRL (200 ng/ml) for 30 min as indicated. Cells were harvested and the levels of endogenous PRLR and β-TrCP2 in whole-cell extracts (WCE, 150 μg) or immunoprecipitates (IP) (from 5 mg) with PRLR antibody or irrelevant monoclonal antibody (Flag) were analyzed by immunoblotting (IB) with PRLR antibody (top) and β-TrCP2-specific HOS-N antibody (bottom). Ig, heavy chain of immunoglobulins. The positions of molecular mass markers are also shown. (B) 293T cells were cotransfected with plasmids for the expression of HA-tagged β-TrCP2 and Flag-tagged PRLR proteins as indicated and grown in the presence of 10% FBS. The interaction between the expressed proteins was assessed by immunoprecipitation followed by immunoblotting as indicated. Ig, heavy chain of immunoglobulins.
We next sought to determine whether the putative β-TrCP recognition motif within the cytoplasmic tail of PRLR is required for interaction between PRLR and β-TrCP2. We expressed HA-tagged β-TrCP2 and Flag-tagged PRLR in 293T cells grown in the presence of 10% FBS to supply PRL. We found that recombinant HA-β-TrCP2 and Flag-PRLRwt were coimmunoprecipitated with their respective antibodies in both direct and reciprocal immunoprecipitation experiments (Fig. 1B). Mutation of the potentially phosphorylated serine 349 within the β-TrCP recognition motif noticeably impaired the ability of the PRLRS349A mutant protein to bind to β-TrCP2 (Fig. 1B, panels I and II, lane 5 versus lane 6). Residual binding of β-TrCP2 to the PRLRS349A mutant (Fig. 1B, panel II, lane 6) can probably be attributed to the recruitment of β-TrCP2 by endogenous proteins that may interact with PRLRS349A (e.g., endogenous PRLR). These data confirm that β-TrCP2 is capable of interacting with PRLR and indicate that this interaction requires the integrity of the β-TrCP recognition motif, which may require phosphorylation of PRLR within this motif.
Phosphorylation of PRLR, which is promoted by the ligand, is required for the ability of PRLR to interact with β-TrCP2.
We further tested the role of PRLR phosphorylation in binding to β-TrCP2. Recombinant Flag-PRLRwt purified from 293T cells interacted with β-TrCP2 in vitro, but no interaction was observed with the Flag-PRLRS349A mutant (Fig. 2A). Phosphatase treatment of Flag-PRLRwt decreased its ability to bind β-TrCP2 in vitro (Fig. 2A), indicating that specific phosphorylation of PRLR was essential for the ability of PRLR to interact with β-TrCP2. This result also indicates that recombinant Flag-PRLR overexpressed in 293T cells which were grown in medium supplemented with 10% FBS was already phosphorylated and capable of binding to β-TrCP2 in the absence of added ligand. This finding does not rule out the possibility of a role for PRL in β-TrCP2-PRLR interaction, since PRL is contained in FBS (41) and the overexpression of receptors alone (e.g., tumor necrosis factor alpha receptor [24] or IFNAR1 [32]) is known to mediate signaling events.
To test the role of PRL in the PRLR phosphorylation that enables β-TrCP2-PRLR interaction, we prepared extracts from serum-starved cells at various time points after treatment with PRL. We further used these extracts as a source of kinase activity to phosphorylate immobilized bacterially produced GST-PRLR proteins, followed by repeated stringent washing and an in vitro β-TrCP2 binding assay. Binding of β-TrCP2 was detected with GST-PRLRwt protein phosphorylated with cell extracts from PRL-treated cells, with peak of activity being observed at 30 and 90 min after the addition of PRL (Fig. 2B, lanes 4 to 8). Importantly, the omission of ATP from the kinase reaction prevented interaction with β-TrCP2, indicating that GST-PRLR needs to be phosphorylated to exhibit affinity for β-TrCP2 (lane 2). Binding to β-TrCP2 was also not detected with the GST-PRLRS349A mutant (Fig. 2B, lanes 9 to 14). These data suggest that the phosphorylation of PRLR at Ser349 by a kinase, whose activity is increased by the hormone ligand treatment, is required for the interaction of PRLR with β-TrCP2.
Ubiquitination of PRLR depends on β-TrCP2.
The binding of β-TrCP2 to specifically phosphorylated substrates such as IκB, β-catenin, and IFNAR1 often results in the ubiquitination of such proteins. To determine a role for β-TrCP in the ubiquitination of PRLR, we assessed the effects of decreasing β-TrCP expression by the shRNA-mediated knock-down approach. Transfection of 293T cells with control shRNA (shCON) that differs from shBTR2 by a 2-bp substitution (see Materials and Methods) did not decrease β-TrCP levels (Fig. 3A). shRNA directed against β-TrCP2 (shBTR2) (see Materials and Methods) or that directed against both β-TrCP forms (shBTR1,2) (28) suppressed the expression of β-TrCP proteins in 293T cells as measured by immunoblotting with the antibody, which recognizes β-TrCP1 and β-TrCP2 (45). These shRNA constructs did not affect the steady-state levels of another F-box protein, Skp2 (Fig. 3A). In the absence of good-quality antibody specifically recognizing β-TrCP1, we used the expression of HA-tagged β-TrCP proteins to further characterize the effects of shRNA constructs. As seen in Fig. 3B, shBTR1,2 inhibited the expression of both β-TrCP1 and β-TrCP2 (lanes 7 and 4). These data are consistent with results recently reported by the group of J. W. Harper (28). shBTR2 decreased the levels of β-TrCP2 without substantially affecting the expression of β-TrCP1 (Fig. 3B, lanes 3 and 6).
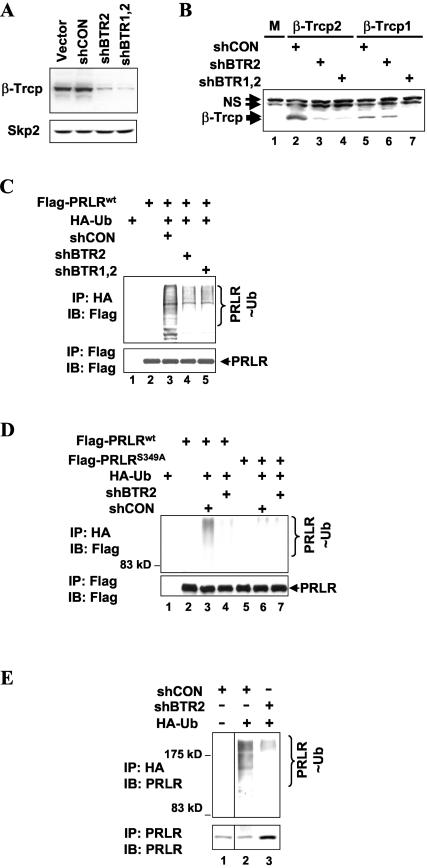
FIG. 3.
β-TrCP2 is required for ubiquitination of PRLR in vivo. (A) Characterization of shRNA directed against β-TrCP proteins. 293T cells were transfected with empty vector, irrelevant shRNA (shCON), or shRNA directed against β-TrCP2 (shBTR2) or against both β-TrCP1 and β-TrCP2 (shBTR1,2). Levels of β-TrCP proteins (top) were analyzed by immunoblotting with HOS-C antibody, which recognizes both β-TrCP1 and β-TrCP2 (45). Levels of Skp2 (bottom) were assessed by immunoblotting with Skp2 antibody. (B) 293T cells were cotransfected with HA-tagged β-TrCP1 or HA-β-TrCP2 and shRNA constructs as described for panel A. Levels of β-TrCP proteins were analyzed by immunoblotting with HA antibody. M, mock transfected cells; NS, nonspecific bands. (C) In vivo ubiquitination of Flag-PRLR in 293T cells cotransfected with HA-tagged ubiquitin and shRNA constructs as indicated and grown in the presence of 10% FBS. Immunoprecipitation (IP) reactions with HA (top) or Flag (bottom) antibodies were analyzed by means of immunoblotting (IB) with Flag antibody. Ubiquitinated Flag-PRLR species (PRLR∼Ub) are indicated. (D) In vivo ubiquitination of Flag-PRLR (the wild type or theS349A mutant) in 293T cells cotransfected with HA-tagged ubiquitin and shRNA constructs as indicated and grown in the presence of 10% FBS. Immunoprecipitation reactions with HA (top) or Flag (bottom) antibodies and analysis were carried out as described for panel C. (E) In vivo ubiquitination of endogenous PRLR in 293T cells transfected with HA-tagged ubiquitin and shRNA constructs as indicated and grown in the presence of 10% FBS. Endogenous PRLR immunoprecipitated with PRLR antibody (bottom) and ubiquitinated proteins immunoprecipitated with HA antibody (top) were analyzed by immunoblotting with PRLR antibody. Ubiquitinated Flag-PRLR species (PRLR∼Ub;) are indicated.
Transfection of 293T cells with recombinant Flag-tagged PRLR and HA-tagged ubiquitin with subsequent denaturing immunopurification of ubiquitinated proteins revealed that Flag-PRLR underwent efficient ubiquitination under these conditions (Fig. 3C, lane 3). Cotransfection with either shBTR2 or shBTR1,2 noticeably attenuated the ubiquitination of Flag-PRLR to similar extents (Fig. 3C, lanes 4 and 5). These data provide genetic evidence that the ubiquitination of endogenous PRLR in cells depends largely on β-TrCP.
To further confirm the role that β-TrCP might play in the ubiquitination of PRLR, we compared the extent of the ubiquitination of Flag-tagged PRLRwt with that of the PRLRS349A mutant, whose interaction with β-TrCP2 is impaired (Fig. 1B and 2A). The ubiquitination of this mutant was substantially decreased compared to the ubiquitination of wild-type PRLR (Fig. 3D, lane 6 versus lane 3), and the residual ubiquitination of the PRLRS349A mutant was not affected by shBTR2 (lane 6 versus lane 7). These results suggest that binding to β-TrCP is important for PRLR ubiquitination, although the possibility of a contribution of β-TrCP-independent mechanisms cannot be ruled out.
In addition to its effects on recombinant PRLR protein, the transfection of shBTR2 inhibited the extent of the ubiquitination of endogenous PRLR in 293T cells (Fig. 3E, lane 2 versus lane 3). Taken together, the data presented in Fig. 3 support the conclusion that β-TrCPs play a major role in the ubiquitination of PRLR in cells.
We next sought to investigate whether SCFβ-TrCP E3 ligase is capable of direct ubiquitination of PRLR. The extracts from 293T cells pretreated with PRL were used to phosphorylate GST-PRLR proteins in the presence of [32P-γ]ATP. These proteins were then captured on immobilized recombinant SCFβ-TrCP2 ubiquitin ligase. Whereas the GST-PRLRS349A mutant protein was phosphorylated to an extent similar to that of its wild-type counterpart (data not shown), no binding between this mutant and SCFβ-TrCP2 was detected (Fig. 4, lane 1). This result is in line with the data showing that the interaction of the PRLRS349A mutant with β-TrCP2 is impaired (Fig. 1B and 2). Similarly, omitting β-TrCP2 from immobilized complexes prevented the capture of phospho-GST-PRLR as well as the recruitment of Cul1 (Fig. 4, lane 4) and precluded testing of the ubiquitination of GST-PRLR under these experimental conditions.
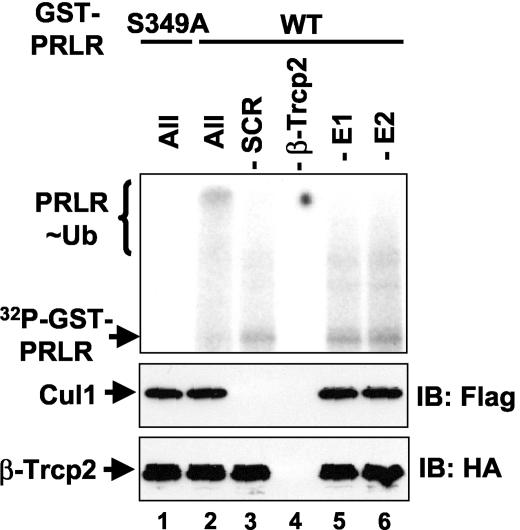
FIG. 4.
SCFβ-TrCP directly ubiquitinates phosphorylated PRLR in vitro. In vitro ubiquitination of GST-PRLR proteins (the wild type [WT] or the S349A mutant) phosphorylated in the presence of [32P-γ]ATP by extracts from 293T cells (pretreated with PRL) and prebound to SCFβ-TrCP2 complexes (lanes 1 to 2 and 5 to 6) or to β-TrCP2 (−SCR; lane 3) or Skp1-Cul1-Roc1 (−β-TrCP2; lane 4) alone immobilized on HA-agarose. After nonbound proteins were washed away, the ubiquitination reaction was carried out in the presence of ubiquitin, ATP, and E1 and E2 (as indicated) and analyzed by means of autoradiography (top). Ubiquitinated GST-PRLR species (PRLR∼Ub) are indicated. Aliquots of the reaction mixture were also analyzed with Flag antibody to detect Cul1 (middle) or with HA antibody to detect β-TrCP2 (bottom). IB, immunoblot.
Incubation of GST-PRLRwt protein captured on SCFβ-TrCP in the presence of ATP, ubiquitin, E1, and E2 allowed recapitulation of PRLR ubiquitination in vitro. This reaction yielded a high-molecular-weight smear characteristic of ubiquitinated proteins (Fig. 4, lane 2). The omission of either E1 or E2 reduced the intensity of this smear (lanes 5 and 6), indicating that it represented in vitro-ubiquitinated phospho-GST-PRLR. The extent of ubiquitination was also decreased when Skp1, Cullin1, and Roc1 were omitted from the immobilized material (lane 3, upper and middle panels). These observations provide biochemical evidence suggesting that phosphorylated PRLR is a bona fide substrate for SCFβ-TrCP E3 ubiquitin protein ligase.
β-TrCPs regulate proteolysis of PRLR.
Ubiquitination of plasma membrane receptors is known to result in their internalization and degradation via the lysosomal pathway. We measured the rate of PRLR degradation by pulse-chase analysis and found that treatment of cells with inhibitors of the lysosomal pathway (methylamine HCl, MA) led to stabilization of PRLR (Fig. 5A). This result implicates the lysosomal pathway in the proteolysis of PRLR.
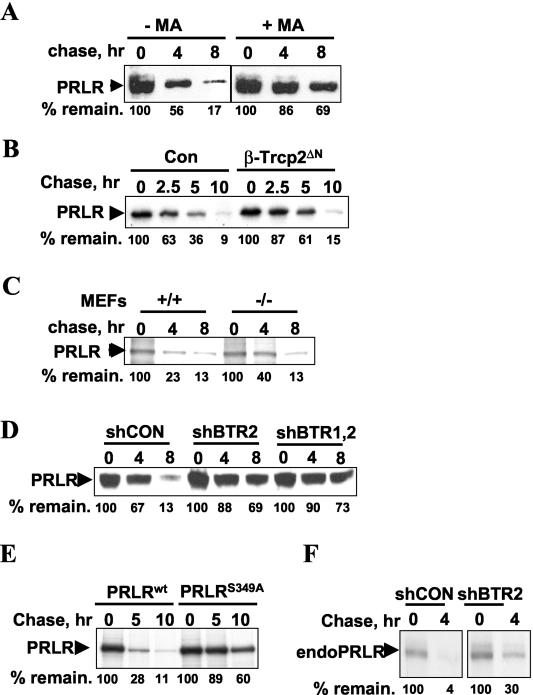
FIG. 5.
β-TrCP2 is involved in PRLR proteolysis. (A) Pulse-chase analysis of Flag-PRLR degradation in 293T cells grown in the presence of 10% FBS, metabolically labeled with [35S]methionine-[35S]cysteine, and treated or not treated with an inhibitor of lysosomal pathway methylamine HCl (MA, 40 mM) at the beginning of the chase as indicated. Cells were harvested at different chase time points with unlabeled methionine and cysteine. PRLR was immunoprecipitated with Flag antibody and analyzed by using autoradiography. Percentages of remaining PRLR (compared to the amount at time point 0, 100%) (% remain.) are shown at the bottom. (B) Pulse-chase analysis of human Flag-PRLRwt protein expressed in 293T cells with or without the dominant-negative β-TrCP2ΔN mutant and analyzed as described for panel A. Con, cells were cotransfected with an empty vector. (C) Pulse-chase analysis of Flag-PRLR degradation in mouse embryo fibroblasts (MEFs) derived either from wild-type (+/+) mice or from animals with genetic elimination of β-TrCP1 (−/−). Degradation of Flag-PRLR was analyzed as described for panel A. (D) 293T cells were transfected with Flag-PRLRwt in the presence of the indicated shRNA constructs. The degradation of Flag-PRLR was analyzed as described for panel A. (E) The indicated wild-type and S349A mutant Flag-PRLR proteins were expressed in 293T cells, and their degradation was analyzed as described for panel A. (F) 293T cells (grown in the presence of 10% FBS) were transfected with the indicated shRNA constructs, and the degradation of endogenous PRLR was analyzed by pulse-chase with PRLR antibody.
We next determined whether β-TrCP-mediated ubiquitination of PRLR is of importance in regulating PRLR protein stability. Flag-PRLRwt expressed in 293T cells exhibited a half-life of ∼3 h. Coexpression of the dominant-negative β-TrCP2ΔN mutant, which is known to compete with endogenous β-TrCP2 and β-TrCP1 for substrates and to inhibit the activities SCFβ-TrCP ubiquitin ligase (16, 48), noticeably delayed the degradation of Flag-PRLRwt (Fig. 5B). This result indicates that β-TrCPs are required for the efficient degradation of PRLR.
We next sought genetic evidence for the role of β-TrCP in regulating PRLR proteolysis. We observed that the rate of Flag-PRLRwt degradation was modestly decreased in mouse embryo fibroblasts derived from β-TrCP1 knockout mice (Fig. 5C). Since the stability of β-TrCP substrates in these cells is thought to be regulated by β-TrCP2 (21), we used the shRNA approach to confirm the role of β-TrCP in PRLR degradation. Flag-PRLRwt was noticeably stabilized by the coexpression of shRNA either directed against β-TrCP2 alone or directed against both β-TrCP1 and β-TrCP2 (Fig. 5D). These data suggest that SCFβ-TrCP E3 ligase plays a key role in regulating PRLR stability.
To further confirm the role of β-TrCP in the proteolysis of PRLR, we compared the rate of degradation of Flag-tagged PRLRwt with that of the PRLRS349A mutant, which poorly interacts with β-TrCP2 (Fig. 1A and 2B). This mutant exhibited substantially higher stability (half-life, >10 h) than the wild-type PRLR (half-life, ∼3 h) (Fig. 5E). In addition, proteolysis of endogenous PRLR was impaired in cells transfected with shBTR2 (Fig. 5F). These observations support other data presented here and are consistent with the idea that β-TrCP plays an important role in regulating the degradation of PRLR.
Role of β-TrCP in regulating cellular responses to PRL.
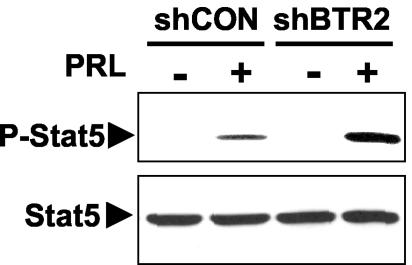
Considering the role of β-TrCP in regulating the stability of PRLR, as well as the fact that this receptor is required for most of the cellular effects elicited by PRL, we sought to determine whether β-TrCP is involved in the control of the extent of PRL signaling. For these experiments, we used the shBTR2 construct, which inhibited the ubiquitination and degradation of PRLR to an extent similar to that of the effects of shRNA against both β-TrCP forms (Fig. 3C and 5D). Transfection of 293T cells with shBTR2 noticeably augmented PRL-induced phosphorylation of Stat5 at Tyr694 (Fig. 6A). The measurement of the PRL-induced transcriptional activity of Stat5 as evaluated by the Stat5-driven luciferase reporter assay revealed activity levels (normalized per the activity of Renilla luciferase and calculated in arbitrary units) of 110 ± 13 units in cells transfected with shCON. The expression of the shBTR2 construct increased the activity of the Stat5-dependent reporter to 260 ± 58 units (P = 0.029, Student's t test). These findings indicate that β-TrCP contributes to negative regulation of the extent of PRL signaling.
FIG. 6.
β-TrCPs contribute to negative regulation of PRL signaling. 293T cells were transfected with the indicated shRNA constructs, serum starved, and treated with PRL (200 ng/ml for 20 min) as indicated. Specific tyrosine phosphorylation of endogenous Stat5 (P-Stat5, top) and overall levels of Stat5 (bottom) were analyzed by means of immunoblotting with the respective antibodies.
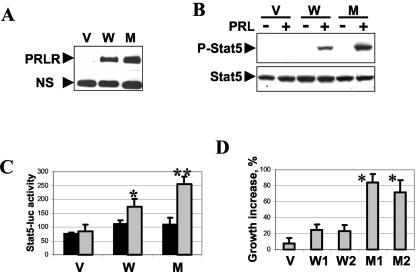
In further experiments aimed at determining the role of β-TrCP in cellular responses to PRL, we used hamster CHO cells, which are known to express low levels of endogenous PRLR (17). These cells were stably transfected either with empty pCDNA vector or with plasmids encoding Flag-tagged human PRLR (the wild type or the S349A mutant). Analysis of recombinant PRLR expression revealed that the PRLRS349A mutant, whose interaction with β-TrCP2 was impaired (Fig. 1 and 2) and which was more stable than PRLRwt (Fig. 5C), accumulated in CHO mass cultures to a greater extent than its wild-type counterpart (Fig. 7A). Both PRL-induced activating phosphorylation (Fig. 7B) and transcriptional activation (Fig. 7C) of Stat5 were augmented in cells expressing the PRLRS349A stable mutant. These data indicate that PRL signaling is increased under conditions in which PRLR is insensitive to ubiquitination by SCFβ-TrCP.
FIG. 7.
Expression of the stable PRLRS349A mutant elicits increased cellular responses to PRL in CHO hamster cells. (A) Levels of Flag-PRLR expression in mass cultures of CHO cells stably transfected with empty vector (V), Flag-PRLRwt (W), or the Flag-PRLRS349A mutant (M) were analyzed by immunoblotting with Flag antibody. (B) Specific tyrosine phosphorylation (P-Stat5, top) and total levels of Stat5 (bottom) in CHO cells (stably transfected as described for panel A) upon serum starvation and treatment with PRL were analyzed by immunoblotting with the respective antibodies. (C) Stably transfected CHO cells (as described for panel A) were cotransfected with Stat5-driven firefly luciferase reporter and Renilla luciferase vector, serum starved, and treated with PRL as indicated. Dual luciferase activity was measured after 24 h of incubation with (gray bars) or without (black bars) PRL. The graph depicts normalized levels of Stat5-driven luciferase activity (Stat5-luc activity) calculated from three independent experiments (each carried out in quadruplicate) in arbitrary units. *, P < 0.05 for the comparison with cells transfected with empty vector; **, P < 0.05 for the comparison with cells transfected with PRLRwt (Student's t test). (D) Effect of PRL on acceleration of growth of CHO cells stably transfected with empty vector, two independently selected mass cultures expressing wild-type PRLR (W1 and W2), or two independently selected mass cultures expressing the PRLRS349A mutant (M1 and M2). The graph depicts percentages of growth acceleration with PRL, calculated as described in Materials and Methods with data from three independent experiments (each carried out in quadruplicate). *, P < 0.01 for the comparison with either W1 or W2 (Student's t test).
We next assessed the effect of PRL on the proliferation of CHO cells stably expressing human PRLR proteins. The addition of PRL modestly increased the proliferation of two independently selected cultures of CHO cells transfected with PRLRwt (W1 and W2 in Fig. 7D) compared with that of cells transfected with an empty vector (V in Fig. 7D). A significantly higher rate of acceleration of growth with PRL was observed in CHO cell cultures transfected with the PRLRS349A mutant (M1 and M2 in Fig. 7D). These findings indicate that cellular proliferative responses to PRL signaling are increased when PRL signaling is resistant to negative regulation elicited through PRLR ubiquitination mediated by SCFβ-TrCP E3 ubiquitin ligases.
DISCUSSION
The data presented here support an important role of the SCFβ-TrCP E3 ubiquitin protein ligase in the negative regulation of PRL signaling. Our findings delineate the putative mechanisms underlying this regulation, including ligand-dependent binding of PRLR to SCFβ-TrCP E3 ubiquitin protein ligase (Fig. 1 and 2) and ubiquitination of PRLR by this ligase (Fig. 3 and 4) followed by degradation of PRLR (Fig. 5). These events manifest themselves in restricting the extent of cellular responses to PRL, including PRL-induced phosphorylation and transcriptional activation of Stat5 as well as acceleration of cell proliferation by PRL (Fig. 6 and 7). SCFβ-TrCP-dependent regulation of PRL is likely to contribute to negative regulation of the effects of this pituitary hormone or cytokine on cells in concert with the effects of specific phosphatases, suppressors of cytokine signaling, and protein inhibitors of activated STAT (reviewed in reference 6). To the best of our knowledge, SCFβ-TrCP is the first ubiquitin ligase identified to date which regulates the extent of cellular responses to a polypeptide pituitary hormone. However, the possibility of a potential role for other ubiquitin ligases in this process cannot be excluded.
Our findings characterize PRLR as a novel substrate for SCFβ-TrCP E3 protein ligase. Substrate recognition of this ligase is mediated by the closely related F-box proteins β-TrCP1 and β-TrCP2. These proteins are interchangeable in biochemical assays and experiments with β-TrCP overexpression (10). Knock-down experiments with shRNA showed that, in 293T cells, the inhibition of β-TrCP2 levels attenuates the ubiquitination and degradation of PRLR protein to an extent similar to that of the effects of shRNA directed against both β-TrCP1 and β-TrCP2 (Fig. 3 and 5). This result indicates that β-TrCP2 may represent a major regulator of PRLR ubiquitination and of PRLR stability in these cells (Fig. 3 and 5), although the possibility of a more prominent role for β-TrCP1 in other cell types and/or under various physiological or pathological conditions should not be ruled out. The predominantly cytoplasmic localization of β-TrCP2 compared to the nuclear localization of β-TrCP1 (9, 33) may provide a plausible explanation of why β-TrCP2 might be preferred by cytoplasmic substrates of SCFβ-TrCP such as IFNAR1 (32) and PRLR (this study). Modest stabilization of PRLR was observed in embryo fibroblasts from mice with genetic elimination of β-TrCP1 (Fig. 5C). Since knock-down experiments rarely (if ever) allow complete inhibition of protein expression, the future development of β-TrCP2 knockout animal models will enable the resolution of the issues of redundancy and relative substrate specificity for these two forms of β-TrCP.
Ubiquitination of PRLR by SCFβ-TrCP E3 ligase depends on interaction between PRLR and an F-box protein subunit of the ligase (i.e., β-TrCP). This interaction is promoted by PRL, suggesting that β-TrCP2-mediated ubiquitination may play a role in the down-regulation of PRLR in response to the ligand. As is the case for other SCFβ-TrCP substrates, PRLR ubiquitination and proteolysis appear to depend on specific phosphorylation of PRLR within the β-TrCP2 recognition motif. Our data indicate that PRL treatment induces activity of a yet-to-be-identified kinase(s) that phosphorylates PRLR with the DSGRGS motif and enables the recruitment of E3 ligase by PRLR as well as PRLR ubiquitination and degradation. Future studies will identify the nature of such a kinase and determine its role in regulating the extent of PRL signaling.
In human cells, PRL is known to interact with various splicing forms of PRLR (2, 6, 7). Given the presence of the DSGRGS sequence, it is expected that the SCFβ-TrCP-dependent pathway regulates proteolysis of the long form of PRLR as well as its ΔS1 variant (31). At the same time, the down-regulation of the intermediate and short forms of PRLR, which lack the β-TrCP2 recognition motif, may be regulated by other E3 ligases, or these forms may undergo endocytosis and degradation in a ubiquitin-independent manner. For example, multiple endocytosis motifs (including phenylalanine and dileucine motifs) were found to bring about internalization of short forms of PRLR as well as of truncation mutants of the long form (37, 51). Since intermediate or short forms of human PRLR are either partially or entirely deficient in mediating the signal transduction pathways induced by PRL (30, 42), it is expected that β-TrCP2-dependent control of stability of the long-form PRLR is important for regulating a complete repertoire of PRL signaling.
Future studies are required to determine how the ubiquitination of PRLR by SCFβ-TrCP E3 ligase leads to the proteolysis of PRLR. Whereas β-TrCP-dependent ubiquitination of IFNAR1 targets this protein for endocytosis and degradation by a lysosomal pathway (32), other known substrates of SCFβ-TrCP are degraded by 26S proteasome complexes (10). The treatment of cells with inhibitors of the lysosomal pathway substantially increased the half-life of Flag-PRLRwt in our assays (Fig. 5A). This result is in line with evidence that lysosomotropic agents prevent down-regulation of PRLR by its ligand (14). Thus, it is likely that β-TrCP-mediated ubiquitination of PRLR targets this protein for degradation via the lysosomal pathway.
Conjugation of ubiquitin to the plasma membrane-localized receptors often provides them with a potent internalization signal (reviewed in reference 27). Interestingly, it has been shown that bovine PRLR mutants, which lack a substantial portion of the cytoplasmic tail encompassing the β-TrCP recognition motif, undergo efficient endocytosis (37). In addition, the removal of a large portion of the cytoplasmic tail of GHR, which is closely related to PRLR and also contains a similar β-TrCP recognition motif (DSGRTS), does not impair the internalization of these GHR mutants (20). However, a putative role for β-TrCP-mediated ubiquitination in the internalization of endogenous PRLR (as well as that of GHR) cannot be entirely ruled out, since it remains possible that the endocytosis of native receptor protein is antagonized by a factor(s) whose effects rely on the integrity of sequences that are absent in such mutants. In addition, β-TrCP-dependent ubiquitination may play a role in sorting already-internalized PRLR and targeting it to lysosomes for degradation. A similar function in the regulation of growth factor receptor proteolysis is attributed to c-Cbl E3 ubiquitin ligase (36).
Putative roles of β-TrCP in PRLR endocytosis and postinternalization sorting remain to be determined. The development of antibodies specifically recognizing long and ΔS1 forms of PRLR will improve the feasibility of this analysis in future studies. However, it is important to note that, regardless of the mode of PRLR proteolysis, the β-TrCP-dependent pathway is stimulated by PRL (Fig. 1A and 2B). These data suggest an important physiological role of the β-TrCP-mediated mechanism in the down-regulation of PRLR levels and the restriction of the extent of PRL signaling.
Future studies will also determine whether β-TrCP-dependent regulation of PRLR stability and PRL signaling is important in restricting the effects of PRL on breast tissue. It is conceivable that these mechanisms may be utilized in limiting the extent of mammary gland development during pregnancy or in the gland's postlactation involution. In addition, considering that breast cancer cells secrete PRL and are subjected to its autocrine regulation (1, 8), putative aberrations in the β-TrCP-mediated pathway in these cells would be expected to lead to sustained stimulation of their growth.
Acknowledgments
We thank S. M. Anderson, R. Benarous, D. Bohmann, R. J. Eisenberg, J. W. Harper, P. A. Kelly, M. Pagano, Z.-Q. Pan, A. F. Parlow, and Z. Ronai for providing reagents. We are indebted to C. V. Clevenger for valuable criticism and comments and to M. Meyer for help with manuscript preparation.
This work was supported in part by The University of Pennsylvania Cancer Center Pilot Grant and NCI grant CA 92900 (to S.Y.F.) and American Cancer Society award RSG-02-140-01-CNE (to V.S.S.).
REFERENCES
- 1.Ben-Jonathan, N., K. Liby, M. McFarland, and M. Zinger. 2002. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol. Metab. 13:245-250. [DOI] [PubMed] [Google Scholar]
- 2.Bole-Feysot, C., V. Goffin, M. Edery, N. Binart, and P. A. Kelly. 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19:225-268. [DOI] [PubMed] [Google Scholar]
- 3.Busino, L., M. Donzelli, M. Chiesa, D. Guardavaccaro, D. Ganoth, N. V. Dorrello, A. Hershko, M. Pagano, and G. F. Draetta. 2003. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426:87-91. [DOI] [PubMed] [Google Scholar]
- 4.Cenciarelli, C., D. S. Chiaur, D. Guardavaccaro, W. Parks, M. Vidal, and M. Pagano. 1999. Identification of a family of human F-box proteins. Curr. Biol. 9:1177-1179. [DOI] [PubMed] [Google Scholar]
- 5.Clevenger, C. V., D. O. Freier, and J. B. Kline. 1998. Prolactin receptor signal transduction in cells of the immune system. J. Endocrinol. 157:187-197. [DOI] [PubMed] [Google Scholar]
- 6.Clevenger, C. V., P. A. Furth, S. E. Hankinson, and L. A. Schuler. 2003. The role of prolactin in mammary carcinoma. Endocr. Rev. 24:1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevenger, C. V., and J. B. Kline. 2001. Prolactin receptor signal transduction. Lupus 10:706-718. [DOI] [PubMed] [Google Scholar]
- 8.Clevenger, C. V., and T. L. Plank. 1997. Prolactin as an autocrine/paracrine factor in breast tissue. J. Mammary Gland Biol. Neoplasia 2:59-68. [DOI] [PubMed] [Google Scholar]
- 9.Davis, M., A. Hatzubai, J. S. Andersen, E. Ben-Shushan, G. Z. Fisher, A. Yaron, A. Bauskin, F. Mercurio, M. Mann, and Y. Ben-Neriah. 2002. Pseudosubstrate regulation of the SCFβ-TrCP ubiquitin ligase by hnRNP-U. Genes Dev. 16:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshaies, R. J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15:435-467. [DOI] [PubMed] [Google Scholar]
- 11.Djiane, J., H. Clauser, and P. A. Kelly. 1979. Rapid down-regulation of prolactin receptors in mammary gland and liver. Biochem. Biophys. Res. Commun. 90:1371-1378. [DOI] [PubMed] [Google Scholar]
- 12.Djiane, J., C. Delouis, and P. A. Kelly. 1982. Prolactin receptor turnover in explants of pseudopregnant rabbit mammary gland. Mol. Cell. Endocrinol. 25:163-170. [DOI] [PubMed] [Google Scholar]
- 13.Djiane, J., L. M. Houdebine, and P. A. Kelly. 1981. Down-regulation of prolactin receptors in rabbit mammary gland: differential subcellular localization. Proc. Soc. Exp. Biol. Med. 168:378-381. [DOI] [PubMed] [Google Scholar]
- 14.Djiane, J., P. A. Kelly, and L. M. Houdebine. 1980. Effects of lysosomotropic agents, cytochalasin B and colchicine on the “down-regulation” of prolactin receptors in mammary gland explants. Mol. Cell. Endocrinol. 18:87-98. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, M. E., B. Kanyicska, A. Lerant, and G. Nagy. 2000. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80:1523-1631. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs, S. Y., A. Chen, Y. Xiong, Z. Q. Pan, and Z. Ronai. 1999. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IκB and beta-catenin. Oncogene 18:2039-2046. [DOI] [PubMed] [Google Scholar]
- 17.Genty, N., J. Paly, M. Edery, P. A. Kelly, J. Djiane, and R. Salesse. 1994. Endocytosis and degradation of prolactin and its receptor in Chinese hamster ovary cells stably transfected with prolactin receptor cDNA. Mol. Cell. Endocrinol. 99:221-228. [DOI] [PubMed] [Google Scholar]
- 18.Goffin, V., N. Binart, P. Touraine, and P. A. Kelly. 2002. Prolactin: the new biology of an old hormone. Annu. Rev. Physiol. 64:47-67. [DOI] [PubMed] [Google Scholar]
- 19.Gourdou, I., J. Paly, C. Hue-Beauvais, L. Pessemesse, J. Clark, and J. Djiane. 2004. Expression by transgenesis of a constitutively active mutant form of the prolactin receptor induces premature abnormal development of the mouse mammary gland and lactation failure. Biol. Reprod. 70:718-728. [DOI] [PubMed] [Google Scholar]
- 20.Govers, R., P. van Kerkhof, A. L. Schwartz, and G. J. Strous. 1998. Di-leucine-mediated internalization of ligand by a truncated growth hormone receptor is independent of the ubiquitin conjugation system. J. Biol. Chem. 273:16426-16433. [DOI] [PubMed] [Google Scholar]
- 21.Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P. K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4:799-812. [DOI] [PubMed] [Google Scholar]
- 22.Hart, M., J. P. Concordet, I. Lassot, I. Albert, R. del los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama, S., M. Kitagawa, K. Nakayama, M. Shirane, M. Matsumoto, K. Hattori, H. Higashi, H. Nakano, K. Okumura, K. Onoe, and R. A. Good. 1999. Ubiquitin-dependent degradation of IκBα is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc. Natl. Acad. Sci. USA 96:3859-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller, R. A., K. Song, N. Fan, and D. J. Chang. 1992. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell 70:47-56. [DOI] [PubMed] [Google Scholar]
- 25.Hicke, L. 1999. Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 9:107-112. [DOI] [PubMed] [Google Scholar]
- 26.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 27.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 28.Jin, J., T. Shirogane, L. Xu, G. Nalepa, J. Qin, S. J. Elledge, and J. W. Harper. 2003. SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17:3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline, J. B., H. Roehrs, and C. V. Clevenger. 1999. Functional characterization of the intermediate isoform of the human prolactin receptor. J. Biol. Chem. 274:35461-35468. [DOI] [PubMed] [Google Scholar]
- 31.Kline, J. B., M. A. Rycyzyn, and C. V. Clevenger. 2002. Characterization of a novel and functional human prolactin receptor isoform (deltaS1PRLr) containing only one extracellular fibronectin-like domain. Mol. Endocrinol. 16:2310-2322. [DOI] [PubMed] [Google Scholar]
- 32.Kumar, K. G., W. Tang, A. K. Ravindranath, W. A. Clark, E. Croze, and S. Y. Fuchs. 2003. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 22:5480-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassot, I., E. Segeral, C. Berlioz-Torrent, H. Durand, L. Groussin, T. Hai, R. Benarous, and F. Margottin-Goguet. 2001. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCFβTrCP ubiquitin ligase. Mol. Cell. Biol. 21:2192-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latres, E., D. S. Chiaur, and M. Pagano. 1999. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18:849-854. [DOI] [PubMed] [Google Scholar]
- 35.Lee, R. C., J. A. Walters, M. E. Reyland, and S. M. Anderson. 1999. Constitutive activation of the prolactin receptor results in the induction of growth factor-independent proliferation and constitutive activation of signaling molecules. J. Biol. Chem. 274:10024-10034. [DOI] [PubMed] [Google Scholar]
- 36.Levkowitz, G., H. Waterman, E. Zamir, Z. Kam, S. Oved, W. Y. Langdon, L. Beguinot, B. Geiger, and Y. Yarden. 1998. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, J. C., P. Scott, G. J. Strous, and L. A. Schuler. 2002. Multiple internalization motifs differentially used by prolactin receptor isoforms mediate similar endocytic pathways. Mol. Endocrinol. 16:2515-2527. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani, F., and L. Banks. 2003. Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the β-TrCP ubiquitin ligase receptor. J. Biol. Chem. 278:42477-42486. [DOI] [PubMed] [Google Scholar]
- 39.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 40.Margottin-Goguet, F., J. Y. Hsu, A. Loktev, H. M. Hsieh, J. D. Reimann, and P. K. Jackson. 2003. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4:813-826. [DOI] [PubMed] [Google Scholar]
- 41.Root, A. W. 1976. Growth hormone and prolactin in the fetus. Prog. Clin. Biol. Res. 10:107-126. [PubMed] [Google Scholar]
- 42.Ross, R. J., N. Esposito, X. Y. Shen, S. Von Laue, S. L. Chew, P. R. Dobson, M. C. Postel-Vinay, and J. Finidori. 1997. A short isoform of the human growth hormone receptor functions as a dominant negative inhibitor of the full-length receptor and generates large amounts of binding protein. Mol. Endocrinol. 11:265-273. [DOI] [PubMed] [Google Scholar]
- 43.Sotiropoulos, A., S. Moutoussamy, F. Renaudie, M. Clauss, C. Kayser, F. Gouilleux, P. A. Kelly, and J. Finidori. 1996. Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor. Mol. Endocrinol. 10:998-1009. [DOI] [PubMed] [Google Scholar]
- 44.Spencer, E., J. Jiang, and Z. J. Chen. 1999. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 13:284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegelman, V. S., W. Tang, M. Katoh, T. J. Slaga, and S. Y. Fuchs. 2002. Inhibition of HOS expression and activities by Wnt pathway. Oncogene 21:856-860. [DOI] [PubMed] [Google Scholar]
- 46.Strous, G. J., and J. Gent. 2002. Dimerization, ubiquitylation and endocytosis go together in growth hormone receptor function. FEBS Lett. 529:102-109. [DOI] [PubMed] [Google Scholar]
- 47.Strous, G. J., and P. van Kerkhof. 2002. The ubiquitin-proteasome pathway and the regulation of growth hormone receptor availability. Mol. Cell. Endocrinol. 197:143-151. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, H., T. Chiba, M. Kobayashi, M. Takeuchi, T. Suzuki, A. Ichiyama, T. Ikenoue, M. Omata, K. Furuichi, and K. Tanaka. 1999. IκBα ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, βTrCP1 and βTrCP2. Biochem. Biophys. Res. Commun. 256:127-132. [DOI] [PubMed] [Google Scholar]
- 49.Tan, P., S. Y. Fuchs, A. Chen, K. Wu, C. Gomez, Z. Ronai, and Z. Q. Pan. 1999. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell 3:527-533. [DOI] [PubMed] [Google Scholar]
- 50.Treier, M., L. M. Staszewski, and D. Bohmann. 1994. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78:787-798. [DOI] [PubMed] [Google Scholar]
- 51.Vincent, V., V. Goffin, M. Rozakis-Adcock, J. P. Mornon, and P. A. Kelly. 1997. Identification of cytoplasmic motifs required for short prolactin receptor internalization. J. Biol. Chem. 272:7062-7068. [DOI] [PubMed] [Google Scholar]
- 52.Weissman, A. M. 2001. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2:169-178. [DOI] [PubMed] [Google Scholar]
- 53.Winston, J. T., D. M. Koepp, C. Zhu, S. J. Elledge, and J. W. Harper. 1999. A family of mammalian F-box proteins. Curr. Biol. 9:1180-1182. [DOI] [PubMed] [Google Scholar]
- 54.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaron, A., A. Hatzubai, M. Davis, I. Lavon, S. Amit, A. M. Manning, J. S. Andersen, M. Mann, F. Mercurio, and Y. Ben-Neriah. 1998. Identification of the receptor component of the IκBα-ubiquitin ligase. Nature 396:590-594. [DOI] [PubMed] [Google Scholar]