Abstract
Aim
The objective of this study is to report, for the first time, quantitative data on CPR quality during the resuscitation of children under 8 years of age. We hypothesized that the CPR performed would often not achieve 2010 Pediatric Basic Life Support (BLS) Guidelines, but would improve with the addition of audiovisual feedback.
Methods
Prospective observational cohort evaluating CPR quality during chest compression (CC) events in children between 1 and 8 years of age. CPR recording defibrillators collected CPR data (rate (CC/min), depth (mm), CC fraction (CCF), leaning (% > 2.5 kg.)). Audiovisual feedback was according to 2010 Guidelines in a subset of patients. The primary outcome, “excellent CPR” was defined as a CC rate ≥ 100 and ≤ 120 CC/min, depth ≥ 50mm, CCF > 0.80, and < 20 % of CC with leaning.
Results
8 CC events resulted in 285 thirty-second epochs of CPR (15,960 CCs). Percentage of epochs achieving targets was 54% (153 / 285) for rate, 19% (54 / 285) for depth, 88% (250 / 285) for CCF, 79% (226 / 285) for leaning, and 8% (24 / 285) for excellent CPR. The median percentage of epochs per event achieving targets increased with audiovisual feedback for rate [88 (IQR: 79, 94) vs. 39 (IQR 18, 62) %; p=0.043] and excellent CPR [28 (IQR: 7.2, 52) vs. 0 (IQR: 0, 1) %; p=0.018].
Conclusions
In-hospital pediatric CPR often does not meet 2010 Pediatric BLS Guidelines, but compliance is better when audiovisual feedback is provided to rescuers.
Keywords: pediatric, cardiopulmonary resuscitation, quality appraisal
Introduction
In the United States, the number of children who receive in-hospital pediatric cardiopulmonary resuscitation (CPR) each year for cardiac arrest is in the thousands.1,2 Over the last decade, there have been substantial improvements in survival outcomes after pediatric arrest,3 but there are many children who will still suffer neurological sequelae post-event. As previous investigations have associated CPR quality with cardiac arrest outcome,4–9 interventions targeted to monitor and improve resuscitation quality are warranted.
Our group has previously established that CPR quality in older children and adolescents frequently does not achieve American Heart Association (AHA) Pediatric Basic Life Support (BLS)10 quality targets.11 However, these “children” are more similar in chest mechanics and compliance to adults than to younger children.12,13 Therefore, extrapolation of findings in these studies of CPR quality to younger children may not be appropriate. Unfortunately, the technology to quantitatively evaluate CPR quality in younger children is limited, highlighting a knowledge gap in the field of pediatric resuscitation science.
Therefore, the objective of this study was to evaluate quantitatively the quality of CPR performed during the resuscitation of young children between 1 and < 8 years of age as compared to the targets established by the 2010 Pediatric BLS Guidelines.10 We hypothesized that the CPR performed in these children would often not achieve Guideline targets, but would improve with the addition of audiovisual feedback.
Methods
Design
This investigation is a prospective in-hospital observational study of 30 months duration with the primary objective to evaluate quantitatively the quality of CPR performed during the resuscitation of young children between 1 and < 8 years of age. As a secondary objective, the effect of audiovisual feedback to improve CPR quality was evaluated. The study protocol including consent procedures was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Reporting of quantitative CPR data from the prospective quality improvement database was exempt from IRB review (see below). Data collection procedures were completed in compliance with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA) to ensure subject confidentiality.
Study Population
Pediatric intensive care unit (PICU) chest compression (CC) events in children between 1 and < 8 years of age where a CPR recording defibrillator was deployed during resuscitation were included in the analysis. All events – both pulseless arrest and bradycardia with poor perfusion – were considered. At our institution, CCs are provided primarily by registered nurses, resident and fellow physician trainees, and respiratory therapists, all who have been active participants in our daily CPR refresher training program14,15 and post-cardiac arrest debriefing quality improvement initiatives.16
CPR Recording Defibrillators
At our institution, the Heartstart MRx defibrillator with Q-CPR option, jointly designed by Philips Health Care (Andover, MA) and Laerdal Medical (Stavanger, Norway) is routinely deployed during clinical care to collect quantitative CPR data and to provide real-time feedback if the CPR is not meeting Pediatric BLS guidelines. This defibrillator is Food and Drug Administration cleared for use in children ≥ 8 years of age, but can be used “off-label” in younger children at the discretion of clinicians. In this study of younger children (< 8 years), an investigational device (IDE) defibrillator, similar in appearance and clinical function to the Heartstart MRx was deployed that had two modifications: 1) the compression pad placed on the victim’s chest to record CC data was sized to coincide with the smaller sternum of younger children17 and 2) audiovisual feedback was silenced (evidence-based quantitative feedback targets were a knowledge gap at the time of study initiation). Prospective screening for IDE device deployment was attempted twice daily (8AM and 4PM). At these times, subjects who were identified as high risk of cardiac arrest during a 10–15 minute, multi-disciplinary staff “safety-huddle” were approached for written consent. As screening / consent was not staffed 24 hours per day, there were subjects who had an event prior to screening. In such cases, bedside providers may have made the clinical decision to deploy the Heartstart MRx defibrillator with Q-CPR option “off-label” as they would in any arrest in an older child / adult. Quantitative CPR data collected in this manner was extracted and recorded in a de-identified quality improvement database, and represents CPR quality with assistance of audiovisual feedback (AVF). It is important to note that there were no subjects who declined prospective consent for IDE device deployment who had the Heartstart MRx with Q-CPR option deployed during their subsequent resuscitation.
Outcome Variables
Quantitative CPR was downloaded from the CPR recording defibrillators within 24 hours of each event. A Microsoft Windows based software program, Q-CPR Review (Version 2.1.0.0, Laerdal Medical, Stavanger, Norway), was used for initial examination and extraction of the quantitative CPR quality data. CPR quality parameters included CC rate (CC/min) and depth (mm), CC fraction (i.e., the percentage of time during pulseless arrest that compressions are provided) and percentage of CC with significant leaning (> 2.5 kg18). In accordance with previous publications on CPR quality, an average of each parameter was calculated using Q-CPR Review for each event and for each 30-second epoch of resuscitation. Compliance with 2010 Guidelines was defined as: rate ≥ 100 and ≤ 120 CC/min; depth ≥ 50mm; CC fraction > 0.80; leaning ≤ 20% of compressions. AVF provided by the Heartstart MRx with Q-CPR option drove CPR quality to a CC depth ≥ 50mm, CC rate ≥ 100 and ≤ 120 CC/min, < 2.5 kg of residual leaning force, and CC interruptions < 15s.
Statistical Analysis
Standard descriptive summaries, appropriate for the underlying distribution of the variable, were calculated. We defined a composite variable, “excellent CPR,” as a CC rate ≥ 100 and ≤ 120 CC/min, depth ≥ 50mm, CCF > 0.80, and < 20 % of CC with leaning. First, we calculated the percentage of epochs that met compliance targets overall and by group. Because our sample size was not adequate to account for the correlation of CPR epochs within events, epoch-level percentages are reported for descriptive purposes only. For the primary statistical analysis, we calculated a single summary per event as the percentage of epochs with excellent CPR (primary outcome) and compliant for each quality target individually (secondary outcomes). Percent of compliant epochs was compared across AVF vs. NoAVF groups using Wilcoxon rank-sum tests. Statistical analysis was completed using Stata (Version 12.0, StataCorp, College Station, TX).
Results
Between November 2011 and May 2013, 15 PICU cardiac arrests in children 1 to <8 years of age occurred at our institution, of which 8 (53%) had CPR recording defibrillators deployed during the resuscitation attempt: 4 events in the No Audiovisual Feedback (NoAVF) group (IDE device) and 4 events in the Audiovisual Feedback (AVF) group (“off-label” use of standard Heartstart MRx with Q-CPR option) (Figure 1). These events resulted in 285 thirty-second epochs of CPR (152 NAVF; 133 AVF). A total of 15,960 CCs were available for analysis. Table 1 contains subject and event demographics for the overall cohort and for the two feedback groups. Measured characteristics did not differ between the two feedback groups.
Figure 1.
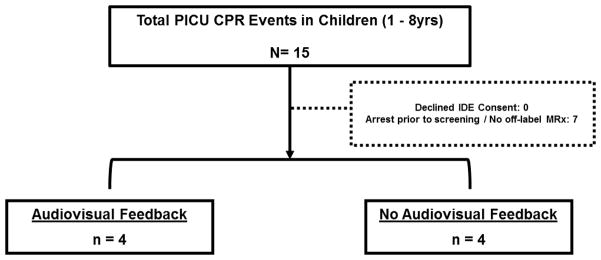
Study diagram. IDE refers to investigation device exemption.
Table 1.
Patient demographic and cardiac arrest event data.
| Overall Cohort | NoAVF | AVF | p* | |
|---|---|---|---|---|
|
| ||||
| Subject Demographic Data | n=8 | n=4 | n=4 | |
| Age: years mean ± SD | 4.9 ± 2 | 4.5 ± 3 | 5.4 ± 2 | 0.59 |
| Sex: male n (%) | 6 (75) | 4 (100) | 2 (50) | 0.43 |
|
| ||||
| Cardiac Arrest Event Data | ||||
| Time of Arrest n (%) | 0.49 | |||
| Day / Evening (7AM – 10:59PM)† | 4 (50) | 3 (75) | 1 (25) | |
| Night / Weekends* (11PM – 6:59AM) | 4 (50) | 1 (25) | 3 (75) | |
| Duration of CPR: minutes median (IQR) | 10.3 (7.4 – 29.9) | 19.4 (7.4, 29.9) | 9.4 (7.4, 24.8) | 0.99 |
| Initial Rhythm n (%) | 0.49 | |||
| Bradycardia | 5 (63) | 3 (75) | 2 (50) | |
| Asystole / PEA | 3 (38) | 1 (25) | 2 (50) | |
| Ventricular Fibrillation Pulseless Ventricular Tachycardia | 0 (0) | 0 (0) | 0 (0) | |
| Survival n (%) | ||||
| Return of Spontaneous Circulation | 4 (50) | 1 (25) | 3 (75) | 0.49 |
| Survival to Hospital Discharge | 2 (25) | 1 (25) | 1 (25) | 0.99 |
NoAVF indicates no audiovisual feedback; AVF, audiovisual feedback provided; ROSC, return of spontaneous circulation > 20 minutes; PEA, pulseless electrical activity.
p values for comparisons between NoAVF and AVF groups.
Weekend indicates time between Friday 11PM and Monday 6:59AM.
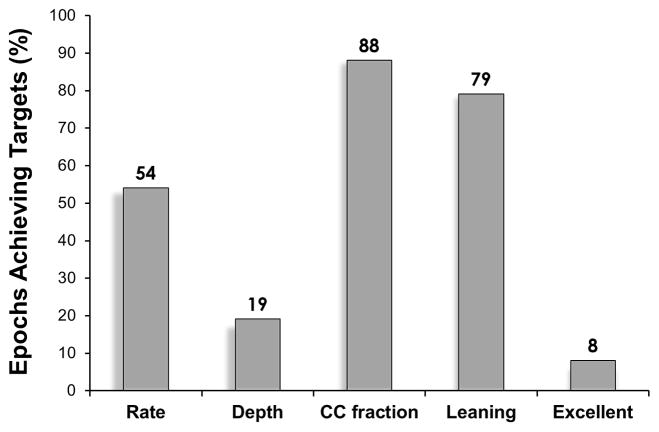
For the overall cohort at the event level, median rate was 115 (IQR 111, 121) CC/min, median CCF was 0.94 (IQR 0.91, 0.98), median percentage of CC with leaning was 7.0 (IQR 3.5, 10.9) %, and average CC depth was 45 ± 8 mm. Event level summary statistics for the two feedback groups were: AVF- median rate was 111 (IQR 108, 115) CC/min, median CCF was 0.94 (0.91, 0.97), median percentage of CC with leaning was 7.0 (IQR 3.1, 8.2) %, and average CC depth was 48 ± 4 mm; NoAVF- median rate was 121 (IQR 115, 137) CC/min, median CCF was 0.95 (0.80, 0.98), median percentage of CC with leaning was 9.7 (IQR 3.5, 35.5) %, and average CC depth was 42 ± 11 mm. Force measurements and mattress compensated CC depths (i.e., actual chest deflection during chest compression) were available for IDE device events only. Force measurements were: median peak force was 29.7 (IQR 25.9, 34.6) kg; median residual leaning force was 1.96 (IQR 1.2, 3.3) kg. The average mattress compensated CC depth19 was: 28 ± 6mm. Overall, percentage of epochs achieving compliance targets was 54% (153 / 285) for rate, 19% (54 / 285) for depth, 88% (250 / 285) for CCF, 79% (226 / 285) for leaning, and 8% (24 / 285) for excellent CPR (Figure 2).
Figure 2.
Percentage of CPR epochs achieving targets for depth ≥ 50mm, rate ≥ 100 and ≤ 120 CC/min, CC fraction > 0.80, and leaning < 20% of compressions. Excellent indicates CPR having all 4 CPR elements achieving targets.
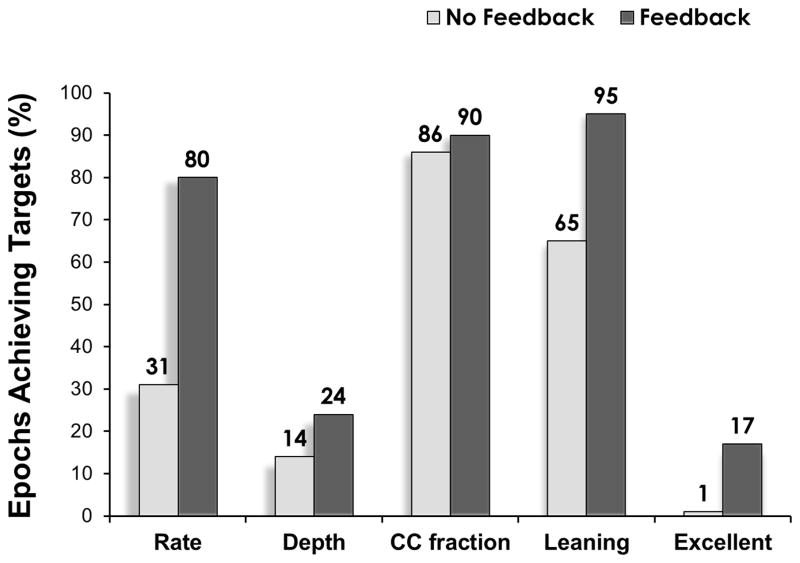
The percentage of epochs meeting compliance targets in the AVF group in comparison to NoAVF was for rate (80% vs. 31%), depth (24% vs. 14%), CCF (90% vs. 86%), leaning (95% vs. 65%), and excellent CPR (17% vs. 1%) (Figure 3). The median percentage of epochs per event achieving Guidelines increased with AVF compared to NoAVF for rate [88 (IQR: 79, 94) vs. 39 (IQR 18, 62) %; p=0.043] and excellent CPR [28 (IQR: 7.2, 52) vs. 0 (IQR: 0, 1) %; p=0.018]. Of note, the difference between AVF and NoAVF for rate was driven primarily by the avoidance of excessively fast CC rates (> 120 CC/min): 9.5 (IQR: 2.9, 19.3) vs. 55 (IQR: 32, 81) %; p=0.043).
Figure 3.
Percentage of CPR epochs achieving targets for depth ≥ 50mm, rate ≥ 100 and ≤ 120 CC/min, CC fraction > 0.80, and leaning < 20% of compressions. Excellent indicates CPR having all 4 CPR elements achieving targets.
Discussion
To our knowledge, this is the first study to report quantitatively the quality of cardiopulmonary resuscitation (CPR) performed during the resuscitation of young children (1 –< 8 years of age) during in-hospital resuscitations compared to the targets established by the 2010 American Heart Association (AHA) Pediatric BLS Guidelines.10 We found that often CPR quality does not meet Guideline targets, particularly for depth and rate in these children. Additionally, similar to previous adult studies,20–22 we demonstrated that real-time audiovisual feedback can improve resuscitation quality during pediatric resuscitation attempts.
Recent resuscitation science has focused on monitoring and improving resuscitation quality. This emphasis has been driven by several studies taken from animal and human adult literature that have associated improved survival with early vasopressor administration,23 prompt defibrillation,4,9,24 and high quality CPR with minimal interruptions.5–7,9 Unfortunately, our own study of older children and adolescents11 and several adult studies8,25–27 have demonstrated that achieving these targets during in-hospital resuscitation is difficult. In essence, there appears to be a gap between evidence-based, consensus-derived international treatment guidelines for CPR and the implementation of those guidelines at the bedside.
Improving CPR quality through feedback technologies has been investigated for decades,28–30 and appears to have benefit.31 There have been two interventional trials using historical controls20,21 and a cluster-randomized trial from the Resuscitation Outcomes Consortium22 that have demonstrated that feedback-enabled defibrillators can improve adult CPR quality. Similarly, Niles et. al. demonstrated that feedback devices can reduce leaning during the resuscitation of older children and adolescents.27 Although feedback devices consistently improve CPR metrics, their use has never been shown to improve actual patient outcomes. It is likely that although CPR is a vital component of any patient resuscitation, other important factors, not targeted with feedback devices (e.g., early recognition32,33, prompt defibrillation24) are playing a substantial role in ultimately determining long term patient outcome.
It is important to emphasize that this data represent some of the first substantial data collected from young children during resuscitation attempts. Since the 1994 publication by Berg et. al., which was limited to evaluating compression and ventilation rates29, there has been little published. Even previous reports by our own group have been quality parameters collected during the resuscitations of older children and adolescents (≥ 8 years of age),11,27,34,35 which are more similar to adults in chest compliance and Guideline recommendations.12,13 Therefore, while we are reporting a study of only 8 patients, given limitations in the available CPR monitoring technology, these represent the first precious data that may inform future pediatric Guideline development particularly in light of the findings of such poor depth compliance.
To that end, our group of investigators has demonstrated that the technology used in this study can overestimate actual thorax compression during CC by as much as 13 mm on soft beds due to mattress deflection.19 As such, compliance with 2010 Guidelines for CC depth in this investigation is actually worse than reported, despite an intensive, well-published, quality improvement initiative.14–16 There is no question that future studies should evaluate novel interventions, both educational and technological, to improve CPR quality, but these data should begin to raise doubt as to whether these depths can actually be attained clinically. While the Guidelines are developed using a rigorous evidence evaluation process,36 little data has been collected from young children in cardiac arrest. As an example, in the most recent Guideline revision, using CT37,38 and anthropometric data17 collected from healthy children, expert consensus decided that the real “risk” to the child is for providers to not push hard enough, so the recommended depth for children was increased, even though there was little evidence to suggest that we could achieve such depths. The current Guidelines, which recommend the same CC depth targets for 1 year olds and adults, may need to be re-evaluated, particularly when the average mattress compensated CC depth achieved in the IDE cohort was only 28mm.
This study has obvious limitations. First, this study was a small study, but as previously mentioned, collecting this data in young children is fraught with difficultly. Second, this study was completed in a clinical environment with a long history of CPR quality research, with an active interest and infrastructure to evaluate and improve resuscitation care. CPR quality performed more globally – in hospitals without feedback-enabled defibrillators, daily CPR refresher trainings14, and post-cardiac arrest debriefings16 – remains an unanswered question. This small report likely represents a best case scenario. Similarly, while we saw a benefit of audiovisual feedback, because of the non-randomized nature of the study, it may be confounded. In short, the decision to deploy a CPR monitoring defibrillator “off-label” may just represent a team highly committed to providing high quality CPR. And it may be this team that improved CPR quality, not the audiovisual feedback per se. Of note, no investigator of this study was involved in the off-label use of the MRx with Q-CPR option. Finally, due to the small number of arrests studied, associations between pediatric survival outcome and CPR quality could not be determined and is an important unanswered question.
Conclusions
In this small observational study, CPR quality often did not meet 2010 Guideline targets during pediatric resuscitation attempts, with depth and rate compliance being particularly problematic. Real-time audiovisual feedback resulted in modest improvements in resuscitation quality. Importantly, this study provides some of the first quantitative CPR quality data collected from young children; yet, many gaps still exist in the pediatric resuscitation knowledge base. In the future, larger studies must be designed with the objective to collect and associate pediatric survival outcomes with CPR quality, thereby transforming the landscape of pediatric resuscitation science and Guideline development from consensus-driven to evidence-based.
Acknowledgments
This study was supported by a Laerdal Medical Foundation Center of Excellence Grant and the Endowed Chair of Pediatric Critical Care Medicine at the Children’s Hospital of Philadelphia. We would like to thank Mette Stavland from Laerdal Medical for her support and guidance during this investigation. We would also like to thank all members of the Pediatric Intensive Care Unit multidisciplinary team for supporting resuscitation research at our institution.
Abbreviations
- AHA
American Heart Association
- CPR
cardiopulmonary resuscitation
- CC
chest compression
Footnotes
Conflicts of Interest: The authors acknowledge the following potential conflicts of interest. Vinay Nadkarni, Dana Niles, and Matt Maltese receive unrestricted research grant support from the Laerdal Foundation for Acute Care Medicine. Joar Eilevstjønn is an employee of Laerdal Medical. Robert Sutton is supported through a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K23HD062629).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parra DA, Totapally BR, Zahn E, et al. Outcome of cardiopulmonary resuscitation in a pediatric cardiac intensive care unit. Crit Care Med. 2000;28(9):3296–3300. doi: 10.1097/00003246-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25(12):1951–1955. doi: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: An analysis from Get with the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheskes S, Schmicker RH, Christenson J, et al. Perishock pause: An independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation. 2011;124:1, 58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–1247. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiell IG, Brown SP, Christenson J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med. 2012;40:4, 1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idris AH, Guffey D, Aufderheide TP, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125(24):3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: A prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 9.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Berg MD, Schexnayder SM, Chameides L, et al. Pediatric basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010;126(5):e1345–60. doi: 10.1542/peds.2010-2972C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton RM, Niles D, Nysaether J, et al. Quantitative analysis of CPR quality during inhospital resuscitation of older children and adolescents. Pediatrics. 2009;124(2):494–499. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 12.Dean JM, Koehler RC, Schleien CL, et al. Age-related effects of compression rate and duration in cardiopulmonary resuscitation. J Appl Physiol. 1990;68(2):554–560. doi: 10.1152/jappl.1990.68.2.554. [DOI] [PubMed] [Google Scholar]
- 13.Arbogast KB, Maltese MR, Nadkarni VM, Steen PA, Nysaether JB. Anterior-posterior thoracic force-deflection characteristics measured during cardiopulmonary resuscitation: Comparison to post-mortem human subject data. Stapp car crash journal. 2006;50:131–145. doi: 10.4271/2006-22-0006. [DOI] [PubMed] [Google Scholar]
- 14.Niles D, Donoghue A, Kalsi MS, et al. “Rolling refreshers”: A novel approach to maintain CPR psychomotor skill competence. Resuscitation. 2009;80(8):909–912. doi: 10.1016/j.resuscitation.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Sutton RM, Niles D, Meaney PA, et al. Low-dose, high-frequency CPR training improves skill retention of in-hospital pediatric providers. Pediatrics. 2011;128(1):e145–51. doi: 10.1542/peds.2010-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zebuhr C, Sutton RM, Morrison W, et al. Evaluation of quantitative debriefing after pediatric cardiac arrest. Resuscitation. 2012;83(9):1124–1128. doi: 10.1016/j.resuscitation.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton RM, Niles D, Nysaether J, et al. Pediatric CPR quality monitoring: Analysis of thoracic anthropometric data. Resuscitation. 2009;80(10):1137–1141. doi: 10.1016/j.resuscitation.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Zuercher M, Hilwig RW, Ranger-Moore J, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Crit Care Med. 2010;38(4):1141–1146. doi: 10.1097/CCM.0b013e3181ce1fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishisaki A, Nysaether J, Maltese M, et al. Effect of mattress deflection on CPR quality assessment for older children and adolescents. Resuscitation. 2009;80(5):540–545. doi: 10.1016/j.resuscitation.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: A prospective interventional study. Resuscitation. 2006;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Hostler D, Everson-Stewart S, Rea TD, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: Prospective, cluster-randomized trial. BMJ. 2011;342:512. doi: 10.1136/bmj.d512. http://dx.doi.org/10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuercher M, Kern KB, Indik JH, et al. Epinephrine improves 24-hour survival in a swine model of prolonged ventricular fibrillation demonstrating that early intraosseous is superior to delayed intravenous administration. Anesth Analg. 2011;112(4):884–890. doi: 10.1213/ANE.0b013e31820dc9ec. [DOI] [PubMed] [Google Scholar]
- 24.Chan PS, Krumholz HM, Nichol G, Nallamothu BK American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after inhospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 25.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 26.Fried DA, Leary M, Smith DA, et al. The prevalence of chest compression leaning during inhospital cardiopulmonary resuscitation. Resuscitation. 2011;82(8):1019–1024. doi: 10.1016/j.resuscitation.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niles D, Nysaether J, Nishisaki A, et al. Leaning is common during in-hospital pediatric CPR, and decreased with automated corrective feedback. Resuscitation. 2009;80(5):553–557. doi: 10.1016/j.resuscitation.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wik L, Myklebust H, Auestad BH, Steen PA. Retention of basic life support skills 6 months after training with an automated voice advisory manikin system without instructor involvement. Resuscitation. 2002;52(3):273–279. doi: 10.1016/s0300-9572(01)00476-2. [DOI] [PubMed] [Google Scholar]
- 29.Berg RA, Sanders AB, Milander M, Tellez D, Liu P, Beyda D. Efficacy of audio-prompted rate guidance in improving resuscitator performance of cardiopulmonary resuscitation on children. Acad Emerg Med. 1994;1(1):35–40. [PubMed] [Google Scholar]
- 30.Sutton RM, Donoghue A, Myklebust H, et al. The voice advisory manikin (VAM): An innovative approach to pediatric lay provider basic life support skill education. Resuscitation. 2007;75(1):161–168. doi: 10.1016/j.resuscitation.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Yeung J, Meeks R, Edelson D, Gao F, Soar J, Perkins GD. The use of CPR feedback/prompt devices during training and CPR performance: A systematic review. Resuscitation. 2009;80(7):743–751. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312. doi: 10.1097/PCC.0b013e318198b02c. [DOI] [PubMed] [Google Scholar]
- 33.Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: A systematic review and meta-analysis. Arch Intern Med. 2010;170(1):18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 34.McInnes AD, Sutton RM, Orioles A, et al. The first quantitative report of ventilation rate during in-hospital resuscitation of older children and adolescents. Resuscitation. 2011;82(8):1025–1029. doi: 10.1016/j.resuscitation.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton RM, Maltese MR, Niles D, et al. Quantitative analysis of chest compression interruptions during in-hospital resuscitation of older children and adolescents. Resuscitation. 2009;80(11):1259–1263. doi: 10.1016/j.resuscitation.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 36.International Liaison Committee on Resuscitation. The international liaison committee on resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: Pediatric basic and advanced life support. Pediatrics. 2006;117(5):e955–77. doi: 10.1542/peds.2006-0206. [DOI] [PubMed] [Google Scholar]
- 37.Kao PC, Chiang WC, Yang CW, et al. What is the correct depth of chest compression for infants and children? A radiological study. Pediatrics. 2009;124(1):49–55. doi: 10.1542/peds.2008-2536. [DOI] [PubMed] [Google Scholar]
- 38.Braga MS, Dominguez TE, Pollock AN, et al. Estimation of optimal CPR chest compression depth in children by using computer tomography. Pediatrics. 2009;124(1):e69–74. doi: 10.1542/peds.2009-0153. [DOI] [PubMed] [Google Scholar]