Abstract
Thin (actin) filament accessory proteins are thought to be the regulatory force for muscle contraction in cardiac muscle; however, compelling new evidence suggests that thick (myosin) filament regulatory proteins are emerging as having independent and important roles in regulating cardiac muscle contraction. Key to these new findings is a growing body of evidence that point to an influential and more recently, direct role for ventricular myosin light chain-2 (MLC2v) phosphorylation in regulating cardiac muscle contraction, function and disease. This includes the discovery and characterization of a cardiac-specific myosin light chain kinase capable of phosphorylating MLC2v as well as a myosin phosphatase that dephosphorylates MLC2v in the heart, which provides added mechanistic insights on MLC2v regulation within cardiac muscle. Here we review evidence for an emerging and critical role for MLC2v phosphorylation in regulating cardiac myosin cycling kinetics, function and disease, based on recent studies performed in genetic mouse models and humans. We further provide new perspectives on future avenues for targeting these pathways as therapies in alleviating cardiac disease.
A. Cardiac Thick Filament: Major Players and Emerging Role for MLC2v Phosphorylation
A.I. Structural Components of the Cardiac Motor
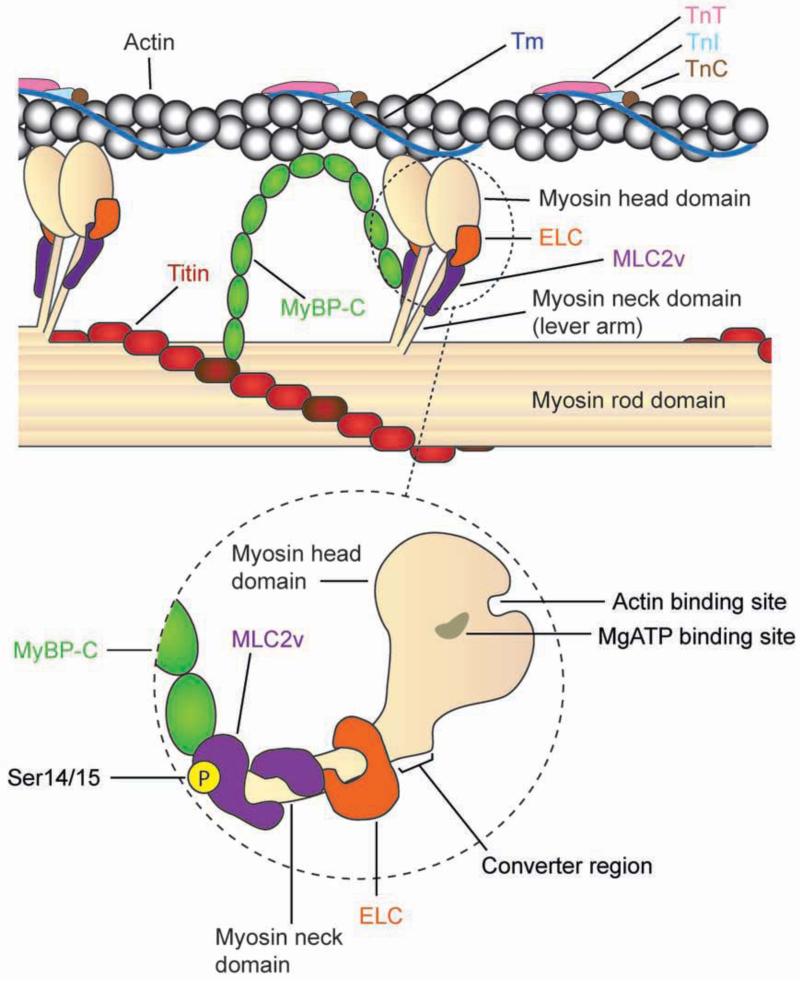
The major components within the cardiac thick filament that control muscle contraction, include cardiac myosin and its regulatory proteins, (i) essential light chain (ELC), (ii) myosin regulatory light chain-2 (ventricular myosin light chain-2 (MLC2v) in cardiac muscle) and (iii) myosin-binding protein C (MyBP-C). Substructure analyses of myosin revealed that it is a hexameric protein consisting of a pair of myosin heavy chains and two pairs of light chains (two ELC and two MLC2) (Warrick and Spudich, 1987). The structural domains within myosin help to define its role in muscle contraction. These include the (i) myosin head domain, which forms two globular heads at the N-terminus and reveals binding sites for actin, ATP and divalent cations, (ii) myosin neck domain, which reveal binding sites where the light chains, ELC and MLC2, fold and bind around myosin to regulate myosin motility through formation of the convertor domain and (iii) myosin rod (tail) domain, which forms a dimerized coiled-coil α-helix at the C-terminus, while revealing binding sites for MyBP-C and titin within the sarcomere as well as serving as the backbone to position myosin to interact with actin (England and Loughana, 2013; Rayment et al., 1993; Warrick and Spudich, 1987). A recent study has highlighted a novel interaction between the cardiac-specific domain (C0 domain) of MyBP-C and MLC2 (Ratti et al., 2011). These results add to the complexity of myosin regulation but also shed new light on how several cardiomyopathy linked mutations in MyBP-C could also impact muscle contraction via its effects on MLC2v and its phosphorylation (Ratti et al., 2011). These studies add to a growing body of evidence that point to an increasingly critical role for MLC2v (Ser14/Ser15) phosphorylation in regulating cardiac muscle contraction, function and disease (Tables 1 and 2), which is the main focus of this review. A schemata highlighting the locations of major actin accessory and myosin regulatory proteins, including MLC2v and its major phosphorylation sites, within the context of thin and thick filaments is shown in Figure 1. Our review provides an up-to-date summary of the molecular, biochemical and functional studies performed in mouse models and humans that have solidified an essential role for MLC2v phosphorylation in cardiac muscle function and disease. We further abbreviate myosin regulatory light chain-2 to MLC2v as a means to denote the major isoform found in ventricular muscle, which is the focus of this review.
Table 1. Summary of Human Studies Implicating a Role for MLC2v Phosphorylation in Cardiac Physiology and Disease.
| Cardiac Physiology and Disease |
Influence on MLC2v Phosphorylation | Reference |
|---|---|---|
| Heart Failure | Dephosphorylation of MLC2v in failing hearts versus controls |
(Morano, 1992) |
| Hypertrophic Cardiomyopathy (midventricular variant) |
Identification of MLC2v mutations (Ala13Thr, Glu22Lys) in close proximity to Ser15 phosphorylation site in human patients |
(Poetter et al., 1996) |
| Hypertrophic Cardiomyopathy (midventricular variant) |
Biochemical characterization of purified MLC2v mutations (Ala13Thr, Glu22Lys) were shown to cause a loss in MLC2v phosphorylation versus controls (non-mutated MLC2v) |
(Szczesna et al., 2001) |
| Heart Failure | Decreased MLC2v phosphorylation in failing versus non-failing hearts |
(van der Velden et al., 2001; van der Velden et al. , 2003a; van der Velden et al., 2003b) |
| Left Ventricular Torsion | Identification of spatial gradients of MLC2v phosphorylation and MLCK across human heart |
(Davis et al., 2001) |
| Hypertrophic Cardiomyopathy (midventricular variant) |
Identification of MLCK (Ala87Val; Ala95Glu) mutations in human patients that impact MLC2v phosphorylation |
(Davis et al., 2001) |
| Hypertrophic Cardiomyopathy |
Decrease in MLC2v phosphorylation in hypertrophic versus donor hearts. Level of decrease similar to failing hearts |
(Jacques et al., 2008) |
| Dilated, non-ischemic Cardiomyopathy |
Decrease in MLC2v (Ser15) phosphorylation in failing versus non-failing hearts |
(Scruggs et al., 2010) |
| Heart Failure | Increased MLC2v phosphorylation in compensatory adaptive phase of failing versus non-failing hearts |
(Toepfer et al., 2013) |
Table 2. Summary of Genetic Mouse Models Implicating a Direct Role for MLC2v Phosphorylation in Cardiac Function and Disease.
| Genetic Mouse Model | Influence on MLC2v Phosphorylation |
Phenotype | Reference |
|---|---|---|---|
| Myosin Light Chain-2v Phosphorylation | |||
| Cardiac-specific transgenic mouse model overexpressing MLC2v Ser14Ala/Ser15Ala/Ser19Ala |
50-100% replacement of endogenous with mutant protein depending on line, causing reduction in MLC2v phosphorylation |
Mice display atrial defects, mild ventricular defects (blunted contractile parameters following dobutamine stress) as well as tricuspid valve insufficiency coincident with RV dilation with age. |
(Sanbe et al., 1999) |
| 100% replacement of endogenous with mutant protein was shown to lead to 34% reduction in MLC2v phosphorylation in one line |
Mice display systolic dysfunction, blunted contractile parameters following dobutamine stress, altered skinned fiber muscle mechanics and decreased baseline TnI, MyBP-C and MLC2v phosphorylation (the latter of which is increased by dobutamine) |
(Scruggs et al., 2009) | |
| MLC2v Ser14Ala/Ser15Ala knock-in mouse model |
Complete loss of MLC2v (Ser14/Ser15) phosphorylation |
Mice exhibit dilated cardiomyopathy and sudden death preceded by LV torsional defects and papillary muscle twitch relaxation defects (independent of changes in calcium transients) leading to subendocardial mechanical strain defects. Subset exhibit subendocardial fibrosis and calcification associated with β-MHC expression. Mice have worsened and differential (eccentric as opposed to concentric) response to pressure overload- induced hypertrophy. Computational and mouse models highlight myosin independent mechanisms for driving cardiac muscle function. |
(Sheikh et al., 2012) |
| Myosin Light Chain Kinase | |||
| Cardiac myosin light chain kinase hypomorphic (neo/neo) mouse model |
> 95% reduction in MLC2v phosphorylation |
Mice display RV and LV enlargement, hypertrophy, fibrosis and reduced cardiac function. Isoproterenol increased MLC2v- phosphorylation and had no further effects in mice. |
(Ding et al., 2010) |
| Cardiac myosin light chain kinase conventional knockout mouse model |
Complete loss of MLC2v phosphorylation |
Mice display features of dilated cardiomyopathy as well as LV torsion defects and cardiomyocyte contractile defects, independent of changes in calcium transients. No fibrosis observed. Mice display heart failure and death in response to pressure overload and swimming-induced hypertrophy. |
(Warren et al., 2012) |
| Cardiac-specific transgenic mouse model overexpressing skeletal myosin light chain kinase |
Increase in both MLC2v and non- muscle MLC2 phosphorylation |
Mice do not exhibit basal cardiac defects. Mice display attenuated response to stress- induced cardiac hypertrophy (treadmill exercise, isoproterenol). No effects on calcium-calmodulin-mediated hypertrophic signaling pathways. |
(Huang et al., 2008) |
| Cardiac-specific transgenic mouse model overexpressing cardiac myosin light chain kinase |
8-16% increase in MLC2v phosphorylation |
Mice have attenuated response to pressure- overload induced hypertrophy. |
(Warren et al., 2012) |
| Myosin Phosphatase | |||
| Cardiac-specific transgenic mouse model overexpressing myosin phosphatase 2 |
15% reduction in MLC2v phosphorylation |
Mice display cardiomyopathy (LV chamber dilation, impaired cardiac function, increased BNP & β-MHC expression). Cardiac muscle defects (calcium desensitization to contraction and cardiomyocyte ultrastructural defects linked to degeneration and disarray) also observed in mice. |
(Mizutani et al., 2010) |
Figure 1.
Schemata of actin-myosin interactions in cardiac muscle, which emphasize the locations of major myosin regulatory proteins. The dashed circle is a magnified representation of the myosin head and neck domains, which highlight (i) the interactions of myosin binding protein C (MyBP-C) with ventricular myosin light chain-2 (MLC2v) as well as locations of MLC2v and essential light chain (ELC) within the myosin neck domain to form the myosin convertor region, important for myosin motility, (ii) the actin and MgATP binding sites within the myosin head domain are the sites that interact with actin and (iii) MLC2v phosphorylation (Ser14/15) site which regulates myosin head diffusion from the backbone and myosin lever arm stiffness to promote actin-myosin interactions. MyBP-C interactions within both actin and the myosin rod domain are also depicted, including its interaction with titin. Tm; Tropomyosin, TnT; Troponin T, TnI; Troponin I, TnC; Troponin C.
A.II. Myosin Light Chain-2v Regulation via Ser14/Ser15 Phosphorylation
Myosin regulatory light chain-2 (MLC2v) is an ~19 kDa protein (160 amino acids in length), initially discovered in 1969 (Weeds, 1969) as a member of the EF-hand Ca2+ binding protein superfamily since it contains a divalent cation binding site (Grabarek, 2006). Phosphorylation of MLC2 at Ser19 is the primary mechanism activating the actin-activated myosin ATPase to regulate smooth muscle contraction (Ikebe et al., 1986). Although it was reported that phosphorylation may be a major mechanism underlying its regulation in cardiac muscle (Frearson and Perry, 1975), it was only recently that the role and in particular, the phospho-specific sites critical for MLC2v regulation in cardiac muscle were revealed in vivo. Elegant studies by Scruggs et al. utilized an in-solution proteomics approach to show that MLC2v is doubly (Ser14/Ser15) phosphorylated in mouse hearts, whereas it is singly (Ser15) phosphorylated in human hearts (Scruggs et al., 2010). Expanding upon these studies, we showed through the use of novel genetic knock-in mutant mice that endogenous regulation of MLC2v phosphorylation in the mouse heart is dependent upon both Ser14/Ser15 sites since a single mutation of MLC2v Ser15 to Ala15 in mice lead to a compensatory increase in MLC2v Ser14 phosphorylation (Sheikh et al., 2012).
A.III. MLC2v Phosphorylation “On” and “Off” Switches in the Heart
The major kinase phosphorylating MLC2v was recently identified in mice and humans as the cardiac myosin light chain kinase (MLCK), which is encoded by Mylk3 (Chan et al., 2008; Seguchi et al., 2007). Initial purification of MLCK from the heart revealed its dependence on Ca2+ and calmodulin (Walsh et al., 1979). Intriguingly, although cardiac MLCK contains a Ca2+ and calmodulin-binding domain, it can phosphorylate MLC2v in the absence of both (Chan et al., 2008). This is in contrast to smooth and skeletal MLCKs, which are also found in the heart but show dependence on Ca2+ and calmodulin activity (Davis et al., 2001; Kamm and Stull, 2011). Interestingly, conventional loss of skeletal MLCK in mice was shown to have no effect on MLC2v phosphorylation and heart weight to body weight ratios (Zhi et al., 2005). In addition, conventional ablation of the long isoform of smooth muscle MLCK in mice resulted in no major effects on cardiac function (Ohlmann et al., 2005). These data suggest that skeletal and smooth muscle MLCKs do not play a role in regulating MLC2v phosphorylation and cardiac function. Furthermore, these studies reinforce the importance of cardiac MLCK as the unequivocal kinase for MLC2v in the heart, since two independent studies using cardiac MLCK hypomorphic and null mice demonstrated loss of MLC2v phosphorylation in the mouse heart (Ding et al., 2010; Warren et al., 2012). Additionally, cardiac MLCK knockout mice also display striking cardiac abnormalities, which phenocopy our non-phosphorylatable MLC2v (Ser14Ala/Ser15Ala) knock-in mutant mouse model (Sheikh et al., 2012; Warren et al., 2012). Alternate kinases have been found that add to the complexity of MLC2v regulation. Zipper-interacting protein kinase (ZIPK), which is a Ca2+ independent serine/threonine protein kinase was shown to phosphorylate the MLC2v Ser15 site in cardiomyocytes in vitro and heart in vivo (Chang et al., 2010). However, the biological relevance and specificity of ZIPK to target MLC2v in the heart, in light of the role of the cardiac MLCK, remain to be explored. Regardless, these new findings point to cardiac MLCK as the important regulator of the phosphorylation “on switch” for MLC2v activity. Phosphatase control of MLC2v phosphorylation in the heart has also been described. The most compelling evidence comes from studies in cardiac muscle cells which show that MLC2v can be dephosphorylated by the phosphatase, type 1 phosphatase subunit-catalytic δ isoform (PP1C-δ), which serves as the holoenzyme for myosin phosphatase (Okamoto et al., 2006). Consistent with these findings, independent studies showed that cardiac-specific overexpression of a target subunit for myosin phosphatase 2 (MYPT2: major isoform in the heart) that complexes with PP1C-δ, in mice resulted in increased cardiac PP1C-δ activity as well as decreased MLC2v phosphorylation associated with cardiac abnormalities (Mizutani et al., 2010), providing strong evidence for a mechanistic “off switch” for MLC2v phosphorylation in the heart.
B. MLC2v Phosphorylation: At the Heart of Crossbridge Cycling Kinetics and Cardiac Myofilament Function
Compelling new evidence using integrated computational and genetic mouse models provided important mechanistic insights into a direct, independent and indispensable role for MLC2v phosphorylation in regulating calcium-dependent cardiac muscle contraction and function (Sheikh et al., 2012). Most importantly we were able to show the fluidity of these mechanisms to drive biological findings in cardiac muscle at increasing levels of complexity starting from the cross-bridge, to the myofilament, and then whole heart using these integrated approaches (Sheikh et al., 2012). At the cross-bridge level, we showed that MLC2v phosphorylation regulates cross-bridge cycling kinetics by increasing lever arm stiffness at the myosin neck to prolong cross bridge duty cycle (Sheikh et al., 2012). These effects occur in concert with phosphorylation promoting the electrostatic diffusion effects at the myosin head, which drive the accumulated myosins to be cooperatively recruited to neighboring actin binding sites to sustain thin filament activation as a means to fine-tune myofilament Ca2+ sensitivity to force (Sheikh et al., 2012). These studies provided further biological proof to mechanisms involving a role for MLC2 phosphorylation in increasing myosin head diffusion and myosin lever arm stiffness (Colson et al., 2010; Greenberg et al., 2009; Khromov et al., 1998; Levine et al., 1996; Metzger et al. 1989, Sweeney et al., 1994). Recent atomic and crystal structure studies performed on human cardiac muscle myosin filament and MLC, respectively, lent further ultrastructural support to these mechanisms (AL Khayat et al., 2013; Brown et al., 2011).
Proposals for a role for MLC2 phosphorylation in striated muscle (myofilament) function stemmed from studies performed by Stull and colleagues using MLCK showing that the potentiation of isometric twitch force correlated with the extent of MLC phosphorylation in skeletal muscle (Sweeney et al., 1993). Elegant studies by Moss and colleagues deconstructed the effects of MLCK (and thus MLC2v phosphorylation) on skinned adult cardiac myofilament function to include effects on (i) increasing maximum contraction force, (ii) increasing Ca2+sensitivity of contraction force, and (iii) no changes in the kinetics of force redevelopment following stretch (Olsson et al., 2004; Stelzer et al., 2006). We show that best fits for all the effects of MLC2v phosphorylation on cardiac myofilaments as described above could only be obtained through computational models, when both an increase in myosin head diffusion and lever arm stiffness were included as direct mechanisms for MLC2v phosphorylation (Sheikh et al., 2012). Interestingly, Solaro and colleagues also reported a role for MLC2v phosphorylation in regulating myosin cycling kinetics, by showing decreased crossbridge detachment rates (tension-cost) in cardiac fibers of a cardiac-specific transgenic mouse model overexpressing a non-phosphorylatable MLC2v, with 34% reduction in MLC2v phosphorylation (Scruggs et al., 2009). This study suggests that MLC2v phosphorylation speeds up myosin cycling kinetics, which is in contrast to our study via our non-phosphorylatable MLC2v knock-in mutant mice (Sheikh et al., 2012), which suggests that MLC2v phosphorylation slows down myosin cycling kinetics and prolongs duty cycle.. Also, no effects on Ca2+ sensitivity to force could be observed in the transgenic model, which was thought to be due to a compensatory increase in cardiac troponin I and MyBP-C phosphorylation observed in mouse hearts (Scruggs et al., 2009). This compensatory response may possibly also account for differing effects on myosin cycling kinetics between models (Scruggs et al., 2012; Sheikh et al., 2012). Independent studies using this same non-phosphorylatable MLC2v transgenic model reported no effects on frequency force- and pressure-frequency relations in cardiac muscle, while reporting a decrease in cardiac TnI phosphorylation and an increase in cardiac SERCA2 expression in adult mice (Dias et al., 2006). New studies have also revealed new roles for MLC2v phosphorylation in cardiac muscle shortening speed and velocity (Toepfer et al., 2013). Interestingly, we show that loss of MLC2v phosphorylation mediated mechanisms, which were inherent to both the computational model and our non-phosphorylatable MLC2v (Ser14Ala/Ser15Ala) knock-in mutant mouse model at the level of cardiac twitch dynamics, resulted in twitch relaxation defects (magnitude and timing), which were observed in the absence of ultrastructural and functional defects in the mouse heart (Sheikh et al., 2012). Most importantly, we show these relaxation kinetic defects were observed in the absence of changes in Ca2+ transients, providing striking evidence of an adaptable as opposed to previously believed static relationship between Ca2+ transients and muscle twitch tension (Sheikh et al., 2012). Specifically, decreased phosphorylation causes myosin to spend less time in strongly bound state, thereby decreasing ability of myosin to sustain thin filament activation during declining calcium concentrations, leading to accelerated twitch relaxation (Sheikh et al., 2012). Lack of effects on Ca2+ transients were similarly observed in a non-phosphorylatable MLC2v transgenic mouse model and cardiac MLCK null mice (Dias et al., 2006; Warren et al., 2012). Altogether these studies provided the first indication that myosin (MLC2v) phosphorylation can regulate myosin motility and ensuing effects which impact cooperativity to recruit myosins to neighboring actins to sustain thin filament activation and tune Ca2+ sensitive of force independent of effects of calcium (Sheikh et al., 2012). These results provide a new view of how myosin regulatory proteins (MLC2v) can independently regulate Ca2+ sensitivity of the filament to regulate cardiac muscle contraction (Sheikh et al., 2012).
C. Reconstructing a Role For MLC2v Phosphorylation in Adult Cardiac Function and Disease
MLC2v plays an essential role in early embryonic cardiac development and function (Chen et al., 1998); however, it was not until recent studies that its regulatory effects following phosphorylation were shown to play an essential role in adult heart physiology and function. Specifically, a growing number of genetic mouse models alongside human studies have paved the way towards identifying an essential role for MLC2v phosphorylation in adult cardiac torsion, function and disease in vivo (Tables 1 and 2). Integral to these findings is the discovery that MLC2v phosphorylation along with its kinase, cardiac MLCK, are expressed as gradients across the heart from endocardium (low phosphorylation) to epicardium (high phosphorylation) (Davis et al., 2001; Hidalgo et al., 2006; Sheikh et al., 2012; Warren et al., 2012). Interestingly, expression of the MLC2v phosphorylation gradient is more clearly apparent in the heart when physiological diastolic pressures are maintained (Hidalgo et al., 2006; Sheikh et al., 2012), which may also explain why these patterns may be masked or not as sharply observed in some cases (Huang et al., 2008; Warren et al., 2012). Although the relevance of these gradients was unclear, Epstein and colleagues hypothesized that they may impact cardiac torsion due to the relative spatial orientation of endocardial versus epicardial myofibers (Davis et al., 2001). We and others showed by performing tagged MRI on non-phosphorylatable MLC2v knock-in mutant mice and a cardiac MLCK null mouse model, that MLC2v phosphorylation is critical in regulating left ventricular torsion (Sheikh et al., 2012; Warren et al., 2012). Loss of MLC2v phosphorylation gradients and associated mechanisms in a computational model was sufficient to not only phenocopy the torsional defects observed in non-phosphorylatable MLC2v knock-in mutant mice prior to disease onset, but also provided novel insights on the impact of this loss on the mechanical vulnerability of the subendocardium to increased workload, which helped explain the subsequent subendocardial fibrosis and calcification observed in a subset of mutant mice (Sheikh et al., 2012). These results demonstrated how underlying variations in myosin cycling kinetics and contractile properties imposed by MLC2v phosphorylation-mediated mechanisms contribute to the differential regulation of epicardial versus endocardial myofiber tension development and recovery to control cardiac torsion and myofiber strain mechanics (Sheikh et al., 2012). We further show that defects in torsion and myofiber strain mechanics in non-phosphorylatable MLC2v knock-in mutant mice, are early disease predictors of ensuing dilated cardiomyopathy, heart failure and premature death observed in mutant mice (Sheikh et al., 2012). Interestingly, independent studies in a non-phosphorylatable MLC2v transgenic mouse model that resulted in a reduction in MLC2v phosphorylation, revealed atrial defects coupled with mild ventricular defects that could be exacerbated with age and β-adrenergic stress (Sanbe et al., 1999; Scruggs et al., 2009). Differences in cardiac phenotypes from non-phosphorylatable MLC2v models likely reflected differences in strategies to generate genetic mouse models as well as level of MLC2v phosphorylation knockdown reported (Sanbe et al., 1999; Scruggs et al., 2009; Sheikh et al., 2012). However, in agreement with findings from non-phosphorylatable MLC2v knock-in mutant mice (Sheikh et al., 2012), studies utilizing cardiac MLCK hypomorphic and null mice, resulting in reduction and loss of MLC2v phosphorylation, respectively, also revealed cardiac abnormalities reminiscent of dilated cardiomyopathy in mice (Ding et al., 2010; Warren et al., 2012). Fibrosis and upregulation of fetal gene markers were also observed in hearts from cardiac MLCK hypomorphic mice but could not be detected in cardiac MLCK null hearts (Ding et al., 2012; Warren et al., 2012), suggesting that the presence of the neomycin cassette may play a role in the severity of the cardiac phenotype observed (Ding et al., 2012; Warren et al., 2012). However, we also show that a majority of non-phosphorylatable MLC2v knock-in mutant mice did not display fibrosis or changes in fetal gene markers despite severe cardiac dysfunction, consistent with observations in cardiac MLCK null mice (Sheikh et al., 2012; Warren et al., 2012). These results further suggest that MLC2v phosphorylation can target pathways underlying cardiac muscle function distinct from compensatory mechanisms underlying disease (fibrosis and fetal gene markers). Further support came from examination of hearts from cardiac-specific myosin phosphatase 2 overexpressing transgenic mice, which led to a reduction in MLC2v phosphorylation and demonstrated cardiac features reminiscent of dilated cardiomyopathy (Mizutani et al., 2010). These results altogether support a critical role for MLC2v phosphorylation in cardiac torsion and function, but more importantly, in the setting of dilated cardiomyopathy and heart failure. These studies provide direct validation to human studies, which implicated direct effects of loss of MLC2v phosphorylation in the pathogenesis of human dilated cardiomyopathy and heart failure (Table 1).
Studies have also implicated a role for MLC2v phosphorylation in familial hypertrophic cardiomyopathy (FHC) as mutations near the Ser15 phosphorylation site of MLC2v as well as in MLCK have been identified to be associated with a rare variant of human hypertrophic cardiomyopathy and MLC2v phosphorylation was shown to be dysregulated in compensatory phases of hypertrophic cardiomyopathy (Table 1). To date, results have been limited to transgenic mouse models and have not been able to conclusively attribute a direct role for these disease-associated mutations on regulatory effects involving MLC2v phosphorylation. For instance, cardiac-specific overexpression of the human MLC2v mutation A13T, which is close to the Ser15 phosphorylation site, in mice leads to cardiac hypertrophy, myocyte disarray and fibrosis, but was believed to be due to a “poison-peptide” dominant-negative response since low (10%) incorporation of the mutant protein was reported in transgenic mouse hearts (Kazmierczak et al., 2012). In this case, no significant change in MLC2v phosphorylation was reported in transgenic hearts from controls (Kazmierczak et al., 2012). Although indirect, transgenic mice overexpressing the human MLC2v R58Q mutation, which impacts Ca2+ binding affinity and is associated with FHC, was also shown to lead to a reduction in MLC2v phosphorylation in hearts (Abraham et al., 2009). Mice exhibited early signs of FHC, which included diastolic dysfunction that progressed with age (Abraham et al., 2009). Subsequent studies suggested that the R58Q mutation could affect properties of the myosin neck domain leading to altered load-dependent kinetics of myosin (Greenberg et al., 2010). Since MLC2v phosphorylation-mediated mechanisms impact myosin neck domain by increasing lever arm stiffness (Greenberg et al., 2009; Sheikh et al., 2012), it remains to be determined whether the R58Q mutation may impact properties of the myosin neck domain via loss of MLC2v phosphorylation. Interestingly, cardiac-specific expression of the human MLC2v D166V mutation in mice, which also impacts Ca2+ binding affinity and is associated with FHC, was shown to also result in loss of MLC2v phosphorylation in mouse hearts (Muthu et al., 2012). Cardiac papillary muscles from mice displayed contractile defects, which included an increase in Ca2+ sensitivity of contraction along with reduced myofibrillar ATPase and force (Muthu et al., 2012). Interestingly, in this case, expression of MLCK in an ex-vivo setting could reverse the detrimental contractile defects in cardiac muscle from mice, with the exception of force, suggesting that restoration of MLC2v phosphorylation is still an important target to consider in alleviating cardiac disease phenotypes associated with FHC (Muthu et al., 2012). Future studies aimed at generating new mouse models that can completely replace endogenous MLC2v or MLCK protein with disease mutants using knock-in strategies will be helpful to more clearly delineate the role and therapeutic outcomes of targeting MLC2v phosphorylation in these settings.
D. MLC2v Phosphorylation as a Potential Therapeutic Target for Alleviating Cardiac Disease
A role for MLC2v phosphorylation in the adaptive response to stress-induced cardiac hypertrophy and disease was also identified when both young non-phosphorylatable MLC2v knock-in mutant mice, utilized at a stage when no structural defects or disease was observed, as well as cardiac MLCK null mice displayed an exacerbated response to cardiac dysfunction following pressure overload (Sheikh et al., 2012; Warren et al., 2012). Intriguingly, we showed that young mutant hearts displayed a differential growth response following short term (seven days) pressure overload, which involved eccentric (increased cell length) as opposed to typical concentric (increased cell width) hypertrophy observed in wild type mice, suggesting that MLC2v phosphorylation is involved in the intrinsic compensatory hypertrophic growth (thickening) response to stress (Sheikh et al., 2012). Future studies aimed at understanding how MLC2v phosphorylation regulates cardiomyocyte geometry in response to hypertrophic stress may also help unlock the preferential intracellular pathways regulating eccentric versus concentric hypertrophic responses following mechanical stress, which have remained elusive til now. Studies performed in cardiac MLCK null mice demonstrated long term effects of loss of MLC2v phosphorylation in the setting of pathological (pressure overload) and physiological (swimming) hypertrophy, which included an exacerbated response to heart failure leading to premature death in mice (Warren et al., 2012). Although the underlying mechanisms likely impinge on the effects of MLC2v phosphorylation on myosin cycling kinetics (Sheikh et al., 2012), Kasahara and colleagues further revealed new insights for a potential role for ubiquitin-mediated protein degradation pathways in the endogenous regulation of levels of cardiac MLCK in the setting of cardiac stress (Warren et al., 2012). These results ultimately highlight a cardioprotective role for MLC2v phosphorylation in both the adaptive and decompensated phases of heart failure following hypertrophic stress. Consistent with these findings, a transgenic mouse model overexpressing cardiac MLCK in the heart displayed an increase in MLC2v phosphorylation, while revealing an attenuated response to stress-induced hypertrophy mediated by pressure overload (Warren et al., 2012). Interestingly, overexpression of skeletal MLCK in the heart also attenuated catecholamine and exercise (treadmill)-induced hypertrophy in mice (Huang et al., 2008). These results provide striking evidence that increased MLC2v phosphorylation can be used as a therapeutic strategy to alleviate stress-induced cardiac disease. Future studies aimed at generating targeted knock-in mice, which constitutively express MLC2v phosphorylation in the heart, will be helpful to more specifically attribute the rescued effects to MLC2v phosphorylation, while providing a novel model to understand the direct underlying mechanisms important for this rescue.
D. Conclusions and Future Directions
New findings from genetic mouse models strongly suggest critical roles for MLC2v phosphorylation in cardiac myosin cycling kinetics, contraction, torsion, function and disease (Table 2). These studies have provided important relevance to human studies that highlighted a role for MLC2v phosphorylation in the pathogenesis of human cardiac disease (Table 1). Key to these findings is the identification of cardiac MLCK and myosin phosphatase, which provided additional proof towards an essential role for MLC2v phosphorylation in cardiac function and disease. Identification of spatial gradients of MLC2v phosphorylation in the heart also helped uncover defects in cardiac torsion and subendocardial mechanics as important early predictors of dilated cardiomyopathy as a consequence of loss of MLC2v phosphorylation mechanisms, which were recently identified through integrated computational and genetic mouse models. Characterization of transgenic models overexpressing MLCK also revealed a potential role for MLC2v phosphorylation as a therapeutic target to alleviate stress-induced cardiac disease. However, several key questions remain to be addressed to further our understanding of a role for MLC2v phosphorylation in cardiac muscle biology and disease. For example, at the mechanistic level, how do MLC2v phosphorylation-mediated mechanisms impinge on actin accessory proteins at the cross-bridge? At the biological level, what is the molecular basis for the spatial gradients of MLC2v phosphorylation in the heart? In the setting of cardiac disease, how does MLC2v phosphorylation and mechanics control effects of hypertrophic stimuli and disease mutations on cardiomyocyte geometry? As part of clinical implications, can MLC2v phosphorylation be directly exploited as a therapeutic target to alleviate cardiac disease and be used as an early diagnostic marker of cardiac disease? Future studies aimed at using multi-scale approaches involving newly generated knock-in mouse models alongside more sophisticated computational models, will help to propel the field forward by testing hypotheses and providing important mechanistic insights to understanding how complex cardiac disease etiologies can be directly regulated or intervened by MLC2v phosphorylation.
Acknowledgments
We thank Dr. Valeria Mezzano (UCSD, La Jolla, CA) for technical assistance with the figure. R.C.L. is a recipient of an American Heart Association Postdoctoral fellowship. F.S. and J.C are supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham TP, Jones M, Kazmierczak K, Liang HY, Pinheiro AC, Wagg CS, et al. Diastolic dysfunction in familial hypertrophic cardiomypathy transgenic model mice. Cardiovasc Res. 2009;82:84–92. doi: 10.1093/cvr/cvp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA. 2013;110:318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Kumar VS, O’Neall-Hennessey E, Reshetnikova L, Robinson H, Nguyen-McCarty M, et al. Visualizing key hinges and a potential major source of compliance in the lever arm of myosin. Proc Natl Acad Sci USA. 2011;108:114–19. doi: 10.1073/pnas.1016288107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Takeda M, Briggs LE, Graham ML, Lu JT, Horikoshi N, et al. Identification of cardiac-specific myosin light chain kinase. Circ Res. 2008;102:571–80. doi: 10.1161/CIRCRESAHA.107.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Chen G, Gerard RD, Kamm KE, Stull JT. Cardiac myosin is a substrate for Zipper-interacting protein kinase (ZIPK) J Biol Chem. 2010;285:5122–26. doi: 10.1074/jbc.C109.076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kubalak SW, Minamisawa S, Price RL, Becker KD, Hickey R, et al. Selective requirement of myosin light chain 2v in embryonic heart function. J Biol Chem. 1998;273:1252–56. doi: 10.1074/jbc.273.2.1252. [DOI] [PubMed] [Google Scholar]
- Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, et al. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588:981–93. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–41. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Peña JR, et al. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–39. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ding P, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull J. Cardiac myosin light chain kinase in necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem. 2010;285:40819–29. doi: 10.1074/jbc.M110.160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J, Loughna S. Heavy and light roles: myosin in the morphogenesis of the heart. Cell Mol Life Sci. 2013;70:1221–39. doi: 10.1007/s00018-012-1131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson N, Perry SV. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975;151:99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarek Z. Structural Basis for Diversity of the EF-hand Calcium-binding Proteins. J Mol Biol. 2006;359:509–525. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- Greenberg MJ, Kazmierczak K, Szczesna-Cordary D, Moore JR. Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc Natl Acad Sci USA. 2010;107:17403–08. doi: 10.1073/pnas.1009619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR. The molecular effects of skeletal myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R265–R274. doi: 10.1152/ajpregu.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Wu Y, Peng J, Siems WF, Campbell KB, Granzier H. Effect of diastolic pressure on MLC2v phosphorylation in the rat left ventricle. Arch Biochem Biophys. 2006;456:216–23. doi: 10.1016/j.abb.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Huang J, Shelton JM, Richardson JA, Kamm KE, Stull JT. Myosin regulatory light chain phosphorylation attenuates cardiac hypertrophy. J Biol Chem. 2008;283:19748–56. doi: 10.1074/jbc.M802605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M, Hartshorne DJ, Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem. 1986;261:36–39. [PubMed] [Google Scholar]
- Ishikawa Y, Kurotani R. Cardiac myosin light chain kinase: a new player in the regulation of myosin light-chain in the heart. Circ Res. 2008;102:516–18. doi: 10.1161/CIRCRESAHA.108.173005. [DOI] [PubMed] [Google Scholar]
- Jacques AM, Briceno N, Messer AE, Gallon CE, Jalilzadeh S, Garcia E, et al. The molecular phenotype of human cardiac myosin associated with hypertrophic obstructive cardiomyopathy. Cardiovasc Res. 2008;79:481–91. doi: 10.1093/cvr/cvn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Signaling to Myosin Regulatory Light Chain in Sarcomeres. J Biol Chem. 2011;286:9941–47. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromov AS, Somlyo AV, Somlyo AP. Thiophosphorylation of myosin light chain increases rigor stiffness of rabbit smooth muscle. J Physiol. 1998;512:345–50. doi: 10.1111/j.1469-7793.1998.345be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Muthu P, Huang W, Jones M, Wang Y, Szczesna-Cordary D. Myosin regulatory light chain mutation found in hypertrophic cardiomyopathy patients increases isometric force production in transgenic mice. Biochem J. 2012;442:95–103. doi: 10.1042/BJ20111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J. 1996;71:898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989;93:855–83. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano I. Effects of different expression and posttranslational modifications of myosin light chains on contractility of skinned human cardiac fibers. Basic Res Cardiol. 1992;87:129–41. doi: 10.1007/978-3-642-72474-9_11. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Okamoto R, Moriki N, Konishi K, Taniguchi M, Fujita S, et al. Overexpression of myosin phosphatase reduces Ca(2+) sensitivity of contraction and impairs cardiac function. Circ J. 2010;74:120–28. doi: 10.1253/circj.cj-09-0462. [DOI] [PubMed] [Google Scholar]
- Muthu P, Kazmierczak K, Jones M, Szczesna-Cordary D. The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts. J Cell Mol Med. 2012;16:911–19. doi: 10.1111/j.1582-4934.2011.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Patel J, Fitzsimons D, Walker J, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287:H2712–18. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Kato T, Mizoguchi A, Takahashi N, Nakakuki T, Mizutani H, et al. Characterization and function of MYPT2, a target subunit of myosin phosphatase in heart. Cell Signal. 2006;18:1408–1416. doi: 10.1016/j.cellsig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Poetter K, Jiang H, Hassandeh S, Master SR, Chang A, Dalakas MC, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem. 2011;286:12650–58. doi: 10.1074/jbc.M110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Fewell JG, Gulick J, Osinska H, Lorenz J, Hall DG, et al. Abnormal cardiac structure and function in mice expressing non-phosphorylatable cardiac regulatory myosin light chain 2. J Biol Chem. 1999;274:21085–94. doi: 10.1074/jbc.274.30.21085. [DOI] [PubMed] [Google Scholar]
- Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem. 2009;284:5097–106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, et al. A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Mol Cell Proteomics. 2010;9:1804–18. doi: 10.1074/mcp.M110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguchi O, Takashima S, Yamazaki S, Asakura M, Asano Y, Shintani Y, et al. A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. J Clin Invest. 2007;117:2812–24. doi: 10.1172/JCI30804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F, Ouyang K, Campbell SG, Lyon RC, Chuang J, Fitzsimons D, et al. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest. 2012;122:1209–21. doi: 10.1172/JCI61134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol. 2006;128:261–72. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–95. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA. 1994;91:1490–94. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna D, Ghosh D, Li Q, Gomes AV, Guzman G, Arana C, et al. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J Biol Chem. 2001;276:7086–92. doi: 10.1074/jbc.M009823200. [DOI] [PubMed] [Google Scholar]
- Toepfer C, Caorsi V, Kampourakis T, Sikkel MB, West TG, Leung MC, et al. Myosin regulatory light chain (RLC) phosphorylation change as a modulator of cardiac muscle contraction in disease. J Biol Chem. 2013 doi: 10.1074/jbc.M113.455444. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden J, Klein LJ, Zaremba R, Boontje NM, Huybregts MA, Stooker W, et al. Effects of calcium, inorganic phosphate, and pH on isometric force in single skinned cardiomyocytes from donor and failing human hearts. Circulation. 2001;104:1140–46. doi: 10.1161/hc3501.095485. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, et al. Increased Ca2+ sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003a;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, et al. The effect of myosin light chain 2 dephosphorylation on Ca2+ −sensitivity of force is enhanced in failing hearts. Cardiovasc Res. 2003b;57:505–14. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- Walsh MP, Vallet B, Autric F, Demaille JG. Purification and characterization of bovine cardiac calmodulin-dependent myosin light chain kinase. J Biol Chem. 1979;254:12136–12144. [PubMed] [Google Scholar]
- Warren SA, Briggs LE, Zeng H, Chuang J, Chang EI, Terada R, et al. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation. 2012;126:2575–88. doi: 10.1161/CIRCULATIONAHA.112.116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick HM, Spudich JA. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Weeds AG. Light chains of myosin. Nature. 1969;223:1362–1364. doi: 10.1038/2231362a0. [DOI] [PubMed] [Google Scholar]
- Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, et al. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci USA. 2005;102:17519–24. doi: 10.1073/pnas.0506846102. [DOI] [PMC free article] [PubMed] [Google Scholar]