Abstract
Calcium-binding protein 4 (CaBP4) regulates voltage-gated Ca2+ channels in retinal rod cells and specific mutations within CaBP4 are associated with congenital stationary night blindness type 2. We report complete NMR chemical shift assignments of the Ca2+-saturated form of CaBP4 with Ca2+ bound at EF1, EF3 and EF4 (BMRB no. 18877).
Keywords: calcium, CaBP4, retina, EF-hand, CaV1.4
Biological Context
Neuronal calcium-binding proteins (CaBP1–8, (Haynes et al., 2012)) belong to a subclass of the calmodulin (CaM) superfamily (Ikura, 1996) and regulate particular Ca2+ channel targets in the brain and retina (Haeseleer et al., 2000). Multiple isoforms of CaBPs are localized in different neuronal cell types and perform specialized roles in signal transduction (Haynes et al., 2012; Haynes et al., 2004). The CaBP1 isoform regulates Ca2+-dependent activity of inositol 1,4,5-trisphosphate receptors (InsP3Rs) in the brain (Li et al., 2013; Yang et al., 2002), whereas CaBP4 regulates voltage-gated Ca2+ channels (CaV1.4) in retinal photoreceptor cells (Haeseleer et al., 2004). Mutations in both CaV1.4 (Mansergh et al., 2005) and CaBP4 (Aldahmesh et al., 2010) have been identified in patients suffering from congenital stationary night blindness type 2 (CSNB2), indicating that defects in Ca2+-dependent regulation of CaV1.4 by CaBP4 are closely associated with CSNB2. Indeed, deletion of CaBP4 in the mouse leads to a CSNB2-like phenotype (Haeseleer et al., 2004). Thus, understanding the structural interaction of CaBP4 with CaV1.4 may provide insights for developing therapeutic agents for treating CSNB2.
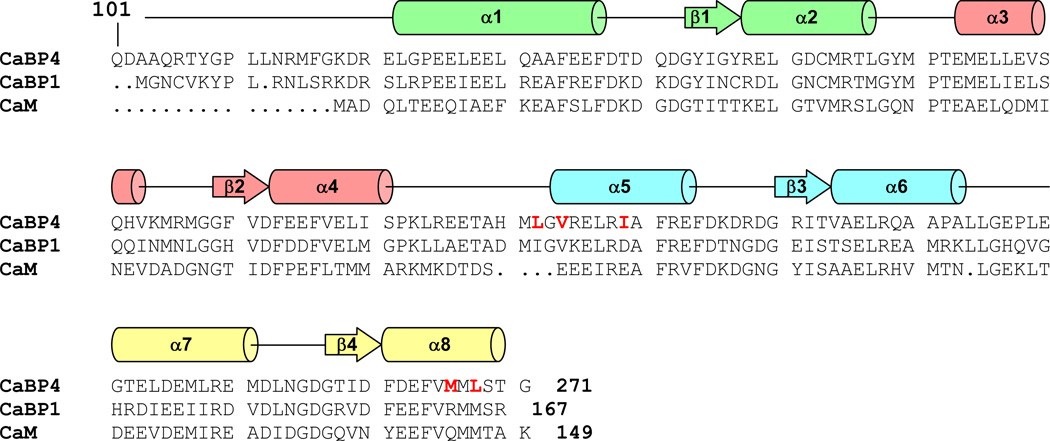
CaBP4 is structurally similar to CaBP1 and CaM (Fig. 1). Like CaBP1, CaBP4 contains two globular domains (N- and C-lobe) that each contains a pair of EF-hand motifs connected by a central linker (Haeseleer et al., 2000). The second EF-hand in CaBP4 lacks conserved residues in the binding loop and is predicted to not bind Ca2+ (Haeseleer et al., 2000). CaBP4 interacts structurally with the C-terminal region of CaV1.4 (Shaltiel et al., 2012) and this Ca2+-dependent interaction has been implicated in the modulation of voltage-dependent CaV1.4 activation (Haeseleer et al., 2004). We report here the NMR assignments of CaBP4 with Ca2+-bound at EF1, EF3 and EF4, as a first step toward elucidating its atomic-level structure and Ca2+-dependent regulatory mechanism of CaV1.4
Figure 1.
Alignment of the primary sequence of mouse CaBP4, CaBP1, and calmodulin. Secondary structural elements indicated schematically were derived from analysis of NMR chemical shift index (CSI) (Wishart et al., 1992). The four EF-hands (EF1, EF2, EF3 and EF4) are highlighted green, salmon, cyan, and yellow, respectively. CaBP4 residues highlighted in red exhibit markedly different backbone chemical shift values compared to those of CaBP1 (Li et al., 2009).
Methods and Experiments
Expression and Purification of Mouse CaBP4
The full-length mouse CaBP4 (residues 1–271) has limited solubility and was not amenable to high resolution structural analysis by NMR. The first 99 residues from the N-terminus of CaBP4 was shown previously to be unstructured, because this region was extensively cleaved in limited proteolysis studies (Shaltiel et al., 2012). Removal of the first 99 residues of CaBP4 caused marked improvement in protein solubility and did not affect target or Ca2+ binding. Therefore, all NMR experiments in this study were performed on an N-terminal deletion construct of mouse CaBP4 (consisting of residues 100–271), which binds functionally to Ca2+ and exhibits Ca2+-dependent binding to CaV1.4. The N-terminal deletion construct of CaBP4 (residues 100–271) with an N-terminal 6His-tag was cloned between the Ndel and BamHI restriction site of vector pET28a, using PCR primer forward 5’GGAATTCCATATGCAGCAGGATGCTGCCCAAAGGAC3’ and reverse 5’CGCGGATCCCTAGCCCGTAGATAGCATCATTAC3’. A plasmid pET28a vector harboring CaBP4 residues 100–271 was transformed into Rosetta 2 (DE3) cells (Novagen). The bacterial cells were pre-cultured in 30 mL LB media including antibiotics (34µg/ml chloramphenicol and 100 µg/ml kanamycin) at 37°C until the optical density at 600nm (A600) reached 1.0. The cells were then transferred into M9 or D2O based M9 minimal medium including antibiotics, 15NH4Cl and D-[13C] glucose and grown at 37°C. The uniformly 15N−, 13C/15N- or 2H/13C/15N-labeled protein expression was induced by the addition of IPTG (at a final concentration of 0.7mM) to cells when A600 was equal to 0.7. The bacterial cells were grown overnight after induction and harvested by centrifugation. The cell pellet was suspended and sonicated in lysis buffer containing 20 mM Tris (pH = 7.5), 5 mM imidazole, 0.5 M NaCl and 0.1 mM PMSF. The supernatant after ultracentrifugation was loaded onto Ni Sepharose column (His Trap FF, GE Healthcare) pre-equilibrated with buffer containing 20mM Tris (pH = 7.5), 5 mM imidazole and 0.5 M NaCl. The CaBP4 protein eluted from the Ni Sepharose column using an elution buffer containing 20 mM Tris (pH = 7.5), 500 mM imidazole, 0.5 M NaCl. Selected fractions containing CaBP4 were purified using a size exclusion column (Superdex 75prep, Amersham) pre-equilibrated with buffer containing 20 mM Tris (pH = 7.5), 1 mM EDTA, 1 mM DTT and 150 mM NaCl. The 6His-tag on CaBP4 was cleaved by thrombin (Novagen) treatment for 1h at room temperature. After cleavage of the 6His-tag, the final CaBP4 sample was purified using size-exclusion chromatography. The final identity and purity (> 95%) of protein was verified by SDS-PAGE.
NMR spectroscopy
All NMR experiments were performed using a Bruker Advance 800 MHz or 600 MHz spectrometer equipped with a triple resonance cryo-probe at 37°C. Samples for NMR analysis were prepared by dissolving 15N, 2H/15N/13C, and 13C/15N labeled CaBP4 (residues 100–271) (0.5 mM) in 0.3 ml of a 90/10% H2O/D2O solution with 10 mM [2H11]Tris (pH = 7.4), 20 mM KCl, 1 mM 2H-labeled dithiothreitol and 5 mM CaCl2. Backbone chemical shift assignments and side chain assignments were accomplished with 15N-1H HSQC, HNCO, HN(CA)CO, TROSY_CACBCONH, and TROSY_HNCACB, HCCH-TOCSY, HBHACONH, 15N-HSQC-TOCSY experiments as described by (Ikura et al., 1990). Stereospecific assignments of valine and leucine methyl groups were obtained by 13C-edited CT-HSQC experiments performed on protein samples with directed 13C labeling. NMR data were processed using NMRPipe software package and analyzed using SPARKY.
Assignments and Data Deposition
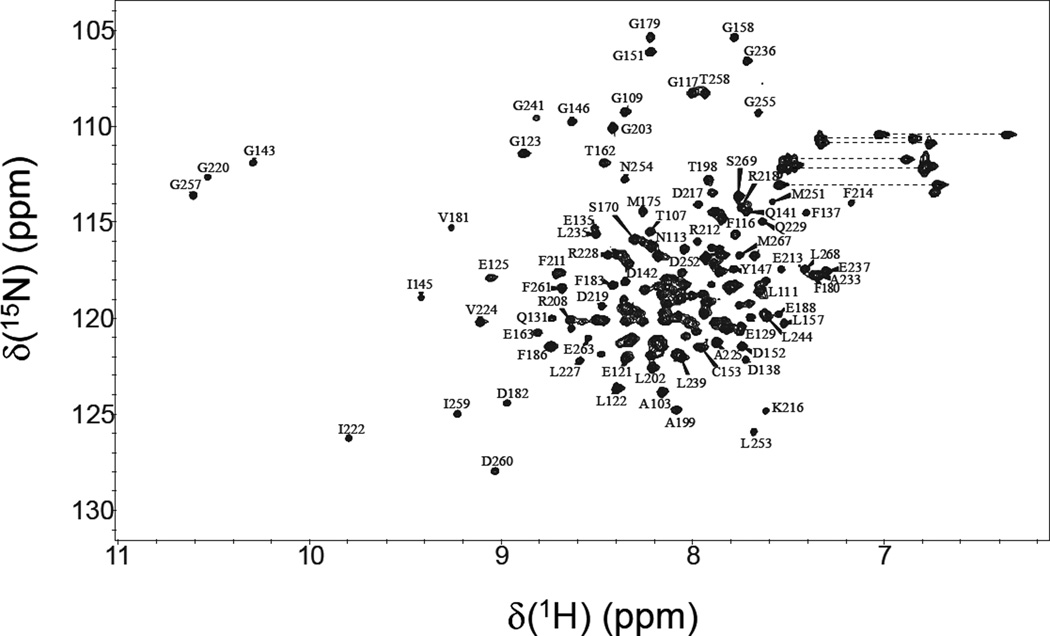
Figure 2 presents 15N-1H HSQC spectrum of Ca2+-saturated CaBP4 to illustrate representative backbone resonance assignments. NMR assignments were based on 3D heteronuclear NMR experiments performed on 13C/15N-labeled CaBP4 (residues 100–271). The first 20 residues from the amino-terminus (residues 100–120), residues 177–178 (EF2 loop), and residues 192–195 (domain linker) all exhibited weak NMR signals with random coil chemical shifts, indicative of structural disorder in these regions. The remaining residues in the core region (residues 121–271) exhibited highly dispersed NMR signals with uniform intensities, indicative of a well-defined three-dimensional protein structure. More than 95% of the backbone resonances (1HN, 15N, 13Cα, 13Cβ, and 13CO) and ~80% of aliphatic side chain resonances were assigned for residues in structured regions, including stereospecific assignment of valine and leucine methyl resonances. Three downfield shifted amide proton resonances at ~10.5 ppm are assigned to Gly143, Gly220 and Gly257, which demonstrate that Ca2+ is bound functionally at EF1, EF3 and EF4, in contrast to CaBP1 that binds tightly to Ca2+ only at EF3 and EF4 (Li et al., 2009). CaBP4 contains glutamate at the 12-position of EF1 (E149) in contrast to CaBP1 that contains aspartate (D46), which might explain why EF1 in CaBP4 binds tightly to Ca2+ whereas EF1 in CaBP1 binds to Mg2+ (Li et al., 2009). The chemical shift assignments (1H, 15N, 13C) of Ca2+ saturated CaBP4 have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 18877.
Figure 2.
Two-dimensional 15N-1H HSQC spectrum of Ca2+-saturated CaBP4 recorded at 800-MHz 1H frequency. The protein sample (0.5 mM) was uniformly labeled with nitrogen-15 and was dissolved in 0.3 ml of a 90% H2O/10% [2H] H2O solution containing 10 mM [2H11] Tris (pH 7.4), 1 mM [2H10] dithiothreitol, and 5 mM CaCl2. Under these conditions, CaBP4 contains Ca2+ bound at EF1, EF3 and EF4 as detected by the three downfield NMR resonances assigned to G143, G220 and G257.
The chemical shift index (CSI) of each amino acid residue reveals a protein secondary structure in CaBP4 (Fig. 1) similar to that observed previously in CaBP1 (Li et al., 2009). CaBP4 contains 8 α-helices and two antiparallel β-sheets (α1: 122–137; β1: 143–146; α2: 147–157; α3: 163–173; β2: 179–182; α4: 183–191; α5: 203–213; β3: 220–223; α6: 224–233; α7: 241–251; β4: 257–260; α8: 261–270). A few NMR assignments reported here for Ca2+ saturated CaBP4 are somewhat different from the corresponding chemical shifts reported previously for CaBP1 (Li et al., 2009). The most noteworthy chemical shift differences are observed for residues in CaBP1 that are known to interact with InsP3Rs (highlighted red in Fig. 1). We suggest that these binding site residues in CaBP1 may occupy a different structural environment in CaBP4 and might explain why CaBP4 is not able to bind to InsP3Rs.
Acknowledgements
We thank Jerry Dallas for technical support and help with NMR experiments. Work supported by NIH grant (EY012347) to J.B.A.
References
- Aldahmesh MA, Al-Owain M, Alqahtani F, Hazzaa S, Alkuraya FS. A null mutation in CABP4 causes Leber's congenital amaurosis-like phenotype. Mol. Vis. 2010;16:207–212. [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Reike F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat. Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CL, Erdjument H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, McCue HV, Burgoyne RD. Evolution and functional diversity of the Calcium Binding Proteins (CaBPs) Front. Mol. Neurosci. 2012;5:e9. doi: 10.3389/fnmol.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J. Biol. Chem. 2004;279:547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
- Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem. Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- Ikura M, Kay LE, Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonanc three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990;29:4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Li C, Chan J, Haeseleer F, Mikoshiba K, Palczewski K, Ikura M, Ames JB. Structural insights into Ca2+-dependent regulation of inositol 1,4,5-trisphosphate receptors by CaBP1. J. Biol. Chem. 2009;284:2472–2481. doi: 10.1074/jbc.M806513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Enomoto M, Rossi AM, Seo MD, Rahman T, Stathopulos PB, Taylor CW, Ikura M, Ames JB. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc. Natl. Acad. Sci. U S A. 2013;110:8507–8512. doi: 10.1073/pnas.1220847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Shaltiel L, Paparizos C, Fenske S, Hassan S, Gruner C, Rötzer K, Biel M, Wahl-Schott CA. Complex regulation of voltage-dependent activation and inactivation properties of retinal voltage-gated Cav1.4 L-type Ca2+ channels by Ca2+-binding protein 4 (CaBP4) J. Biol. Chem. 2012;287:36312–36321. doi: 10.1074/jbc.M112.392811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD, Richards FM. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992;31:1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak DO, Vardi N, Palczewski K, Haeseleer F, Foskett JK. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc. Natl. Acad. Sci. U S A. 2002;99:7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]