Abstract
Transcription of the U6 snRNA gene (SNR6) in Saccharomyces cerevisiae by RNA polymerase III (pol III) requires TFIIIC and its box A and B binding sites. In contrast, TFIIIC has little or no effect on SNR6 transcription with purified components in vitro due to direct recognition of the SNR6 TATA box by TFIIIB. When SNR6 was assembled into chromatin in vitro by use of the Drosophila melanogaster S-190 extract, transcription of these templates with highly purified yeast pol III, TFIIIC, and TFIIIB displayed a near-absolute requirement for TFIIIC but yielded a 5- to 15-fold-higher level of transcription relative to naked DNA (>100-fold activation over repressed chromatin). Analysis of chromatin structure demonstrated that TFIIIC binding leads to remodeling of U6 gene chromatin, resulting in positioning of a nucleosome between boxes A and B. The resulting folding of the intervening DNA into the nucleosome could bring the suboptimally spaced SNR6 box A and B elements into greater proximity and thus facilitate activation of transcription. In the absence of ATP, however, the binding of TFIIIC to box B in chromatin was not accompanied by remodeling and the transcription activation was ∼35% of that seen in its presence, implying that both TFIIIC binding and ATP-dependent chromatin remodeling were required for the full activation of the gene. Our results suggest that TFIIIC, which is a basal transcription factor of pol III, also plays a direct role in remodeling chromatin on the SNR6 gene.
Packing of DNA as chromatin generates gene-specific architectures that are instrumental in poising genes for or against immediate or eventual expression (23, 32, 43, 45, 61, 70). These gene-specific chromatin structures are generated in vivo by defined positioning of histone octamers (72). Chromatin is generally repressive for transcription (50, 71), but this repression can be overcome with help from chromatin structure modulators (41). The first step in returning an inactive gene to activity frequently involves the binding of an activator to its cognate binding site over the surface of a nucleosome; binding sites that are not exposed on the nucleosome surface can be unmasked by ubiquitous ATP-dependent chromatin remodeling factors working in conjunction with histone acetylation (1, 5, 11, 24, 28, 39, 63-65). Chromatin remodeling and histone modification have been largely analyzed in the context of RNA polymerase II (pol II) genes, but chromatin structure has also been shown to play an important role in pol III gene transcription (44, 67, 70).
pol III genes are characteristically short, with intragenic promoter elements (boxes A, B, and C) to which the core transcription factors TFIIIC and TFIIIA bind (15, 54, 68). TFIIIC is very large, and the nine Zn fingers of TFIIIA also cover an extended DNA site. This ability of pol III to nearly cover entire transcription units with its “own” transcription factors in competition with occupancy by nucleosomes suggests a relationship of pol III genes in vivo to their chromatin background that is quite different from that of RNA pol II genes. However, 5S rRNA genes also harbor nucleosome-localizing sequences, and nucleosome binding to these sites is in direct competition with TFIIIA binding (and TFIIIA-dependent TFIIIC binding). Nucleosomes and regularly spaced nucleosome arrays on 5S rRNA genes have also been extensively analyzed as obstacles to RNA chain elongation by diverse RNA polymerases, including pol III (19, 60, 66, 68, 70). The principal findings of these analyses show RNA polymerases transcribing through single nucleosomes and short arrays of nucleosomes but not histone H1-mediated higher-order structures or very long nucleosome arrays. The ability of nucleosomes to block assembly of transcriptional initiation complexes is enhanced by histone H1-mediated chromatin condensation and diminished by histone acetylation.
The structure of the yeast Saccharomyces cerevisiae U6 snRNA gene (SNR6) makes it particularly significant for examining the role of chromatin in transcription of pol III genes (31). This single-copy gene has a strong TATA box (6) to which the yeast transcription initiation factor TFIIIB (composed of subunits TATA binding protein [TBP], Bdp1, and Brf1) can bind autonomously (in the absence of competition by chromatin). This makes it possible to transcribe the U6 gene, as naked DNA, independently of TFIIIC (7, 40). Box A is in its canonical location, ∼20 bp downstream of the start site of SNR6 transcription, but box B is extragenic, located ∼200 bp downstream of box A (∼120 bp downstream of the transcriptional terminator [Fig. 1A]). Such large box A-box B separations are not compatible with stable, simultaneous box A and box B occupancy by TFIIIC: the higher-affinity box B is occupied by TFIIIC, but secondary sites with a favorable spacing tend to substitute for the U6 gene's proper box A (12). However, TFIIIC has been shown to be nearly essential for transcription in vitro in crude extracts (35), and both boxes A and B are essential for transcription in vivo (7, 10). Chromatin assembly in vitro (in Xenopus laevis egg extract) was reported to repress U6 gene transcription, and TFIIIC relieved this repression (9, 10). The chromatin structure of the U6 gene in vivo also correlates with its functional state: a 2-bp deletion in box B generated a rearrangement in chromatin organization (36), and a deletion reducing the distance between boxes A and B generated micrococcal nuclease (MNase)-hypersensitive sites in the TATA box region (17).
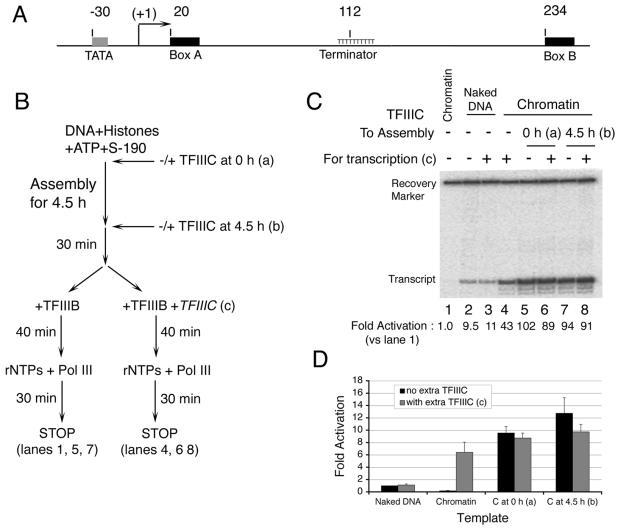
FIG. 1.
TFIIIC activates transcription of SNR6 chromatin to a high level. (A) The yeast U6 snRNA gene. Locations of important elements are indicated relative to the transcriptional start site (bent arrow) as +1. (B) The scheme of the experiment. TFIIIC was added at the beginning (a) or near the end (b) of chromatin assembly, and supplementary addition (TFIIIC) was made at assembly of promoter complexes (c) as specified in panels C and D. rNTPs, ribonucleoside triphosphates. (C) Transcription of naked DNA (lanes 2 and 3) or chromatin (lanes 1 and 4 to 8) was carried out without or with TFIIIC as outlined in panel B and indicated above each lane. The 98-nt primer extension product of the accurately initiated U6 transcript and the recovery marker are identified at the left side. Fold increase of transcript yield in each lane after normalization with respect to repressed chromatin (lane 1) is given at the bottom of the panel. (D) Quantitative comparisons: fold increase of transcript yield relative to lane 2 (naked DNA without TFIIIC), normalized for sample recovery. Standard deviations of three independent experiments are specified.
These relationships between chromatin structure and transcription of yeast SNR6—the unusual distance between its boxes A and B and its contrasting TFIIIC requirements for in vitro and in vivo transcription—suggest that, in order to allow TFIIIC binding in vivo, chromatin-mediated foreshortening of DNA may be required to bring the two TFIIIC binding sites together (17). We have studied this TFIIIC-mediated mechanism of derepression of transcription and have examined the chromatin structure of a transcriptionally active U6 gene. We have observed striking effects of TFIIIC on U6 gene transcription: a manyfold activation of chromatin transcription accompanied chromatin remodeling, with evidence for positioning of a nucleosome within the U6 gene, between boxes A and B.
MATERIALS AND METHODS
DNA.
Plasmid pCS6 contains bp −123 to +312 (relative to the transcriptional start site, +1) of the wild-type S. cerevisiae U6 snRNA (SNR6) gene (7). A schematic map of SNR6 in pCS6 is shown in Fig. 1A.
Chromatin assembly.
Chromatin having regularly spaced nucleosomes and lacking histone H1 was assembled with plasmid pCS6, Drosophila melanogaster core histones, and Drosophila embryo S-190 extract (8, 46). In a typical assembly, 1 μg of plasmid DNA was incubated at 27°C for 5 h with 0.9 to 1.5 μg of core histones and 1.2 mg of protein of the S-190 fraction (which also contains some histones) in 200 μl of buffer providing 10 mM HEPES-KOH (pH 7.5), 7 mM MgCl2, 30 mM NaCl, 3 mM ATP, 8.5 mM β-glycerophosphate, 30 mM creatine phosphate, and 1 μg of creatine kinase/ml. Where appropriate, TFIIIC was added (in excess to saturate its box B binding site) either 30 min before addition of histones and S-190, together with histones and S-190, or at 4.5 h into the 5-h assembly process. Prior to further analysis, the periodicity of nucleosomal arrays was tested by a nucleosome-dependent-supercoiling assay as well as formation of MNase-resistant nucleosomal DNA ladders (8). Aliquots of the same assembly mix were used in parallel for transcription, for protection from DNase I to detect binding of TFIIIC, and for mapping of the nucleosome position by protection against MNase.
In vitro transcription.
Yeast TFIIIB recombinant subunits TBP, Brf1, and Bdp1; TFIIIC (purified by DNA affinity chromatography); and pol III (Mono-Q fraction) were prepared and quantified as described previously (reference 26 and references therein). Quantities of TFIIIB subunits and TFIIIC are specified as femtomoles of protein active for TFIIIB- and TFIIIC-DNA complex formation, respectively (25). pol III is specified as femtomoles of enzyme active for specific transcription of the SUP4 tRNATyr gene (27). Transcription was carried out according to the scheme shown in Fig. 1B in 50 μl of reaction mixture containing 50 fmol of DNA (naked or chromatin), 100 fmol of TBP, 64 fmol of Brf1, and 150 fmol of Bdp1 with or without 125 fmol of TFIIIC. After TFIIIB-TFIIIC-DNA complex formation for 40 min at 23°C, 5 fmol of pol III and ribonucleoside triphosphates (to 500 μM each) were added for 30 min of RNA synthesis. Transcription buffer and chromatin assembly buffer contributed a reaction medium containing 10 mM Tris-Cl (pH 8.0), 5 mM HEPES-KOH (pH 7.5), 7 mM MgCl2, 45 mM NaCl, 3 mM dithiothreitol, and 100 μg of bovine serum albumin/ml. U6 transcript formation was visualized by primer extension of a complementary 5′-end-32P-labeled primer (the 5′ end of the primer is complementary to nucleotide [nt] 98 of the U6 transcript) by avian myeloblastosis virus reverse transcriptase (16). Primer extension products were resolved on 10% polyacrylamide-8 M urea gels and quantified by autoradiography and scanning or phosphorimage analysis. Peak areas were determined from full lane profiles following background subtraction based on the lowest density points above and below the band. This procedure overstates peak areas of bands that are just above background and provides a minimum estimate of activation relative to chromatin-repressed transcription.
Chromatin structure analysis.
Chromatin structure was analyzed at closer range and finer resolution by footprinting and at longer range by indirect end labeling (IEL). For IEL (73), assembled chromatin and naked DNA were subjected to partial MNase digestion and DNA (125 ng per sample) was deproteinized followed by secondary digestion with restriction endonucleases AlwNI (915 bp upstream of box A) and XmnI (347 bp downstream of box B). Southern blots of the resulting DNA fragments were probed with a labeled primer that hybridizes to the SNR6-proximal side of the AlwNI site for mapping MNase cuts relative to the AlwNI site. For footprinting, 125 ng of DNA was digested with 7.4 × 10−3 to 6.7 × 10−2 U (chromatin) or 1.5 × 10−4 to 9.3 × 10−4 U (naked DNA) of MNase for 5 min at room temperature. DNA (125 ng) was digested for 1 min at room temperature with 0.625 to 1.25 μg of DNase I for chromatin and 0.42 to 2.5 ng for naked DNA. All samples were deproteinized before and after primer extension. The partial digestion products were analyzed by primer extension with Vent Exo− DNA polymerase and 5′-end-32P-labeled primers. The extension products were resolved by electrophoresis on 6% polyacrylamide-8 M urea gels. Profiles of all structure analysis gels were generated using the Image Gauge program.
ATP depletion.
ATP was depleted after 4.5 h of assembly by addition of apyrase to 0.02 U of assembled chromatin/μl for 15 min at 27°C or by desalting 100 μl of the chromatin assembly mixture on a 1-ml Sephadex G-25 column (48) equilibrated in 10 mM HEPES-KOH (pH 7.5)-40 mM NaCl-7 mM MgCl2-10% (vol/vol) glycerol-1 mM dithiothreitol-10 mM β-glycerophosphate. Chromatin-containing fractions were pooled, and DNA recovery (80%) was estimated by agarose gel electrophoresis. The G-25 column resulted in a 2.5-fold dilution of chromatin DNA. ATP-depleted chromatin was subjected to structure analyses as specified above and to transcription as specified by the scheme in Fig. 6A with DNA amounts equivalent to those of normal chromatin.
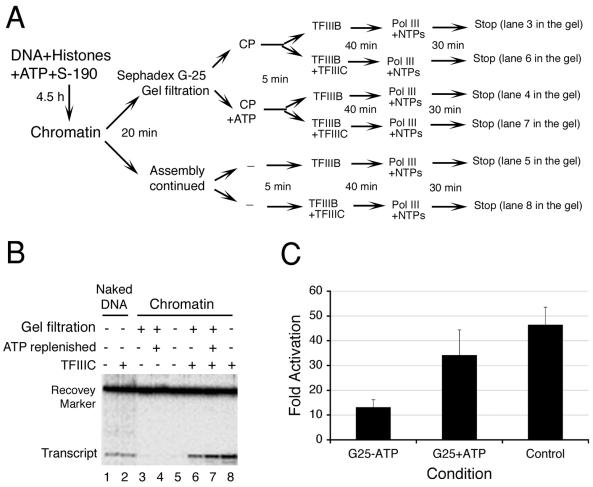
FIG. 6.
ATP requirement for TFIIIC-dependent transcription. (A) Experimental scheme of ATP depletion for transcription. pCS6 chromatin templates (lanes 3 to 8) were subjected to gel filtration to remove ATP. For lanes 4 and 7, chromatin was replenished with ATP and creatine phosphate (CP) prior to the addition of TFIIIB, with or without TFIIIC, for transcription. NTPs, nucleoside triphosphates. (B) ATP dependence of SNR6 chromatin transcription. Transcription according to the scheme in panel A is shown in lanes 3 to 8. Lanes 1 and 2 show naked DNA transcription with or without TFIIIC, as indicated. Positions of the transcript seen as a 98-nt primer extension product and the recovery marker on the gel are indicated. (C) Transcript yields were normalized for sample recovery. The fold increase represents the ratio of transcripts generated with and without TFIIIC under otherwise identical conditions specified below the graph. Error bars indicate standard deviations of four independent experiments.
RESULTS
TFIIIC and chromatin activate U6 transcription.
As shown previously (22, 35, 40), TFIIIC is not required for U6 gene transcription with highly purified components nor does TFIIIC stimulate transcription (Fig. 1C, compare lanes 2 and 3). Reconstitution of DNA into chromatin during a 5-h assembly process prior to the addition of TFIIIB (Fig. 1B) nearly abolished pol III transcription (Fig. 1C, lane 1), but transcription was restored when TFIIIC was added along with TFIIIB (lane 4). This result is similar to previously reported observations (9) showing that chromatin greatly represses TFIIIB-directed U6 transcription but that TFIIIC can partially reverse the repression. A notable difference in Fig. 1C is that transcription of chromatin in the presence of TFIIIC exceeded that of naked DNA (4.5-fold; compare lanes 3 and 4). Although the Drosophila S-190 extract may contain some TFIIIB, TFIIIC, and pol III, transcription of the U6 gene in the extract was completely dependent on exogenous yeast TFIIIB, TFIIIC, and pol III (data not shown).
The restoration of transcription of the U6 gene in chromatin by TFIIIC addition suggests a TFIIIC-dependent alteration of chromatin structure that allows accessibility for both TFIIIC and TFIIIB. Alternatively, a TFIIIB-dependent rearrangement of chromatin structure may occur that is insufficient for pol III binding and transcription but is sufficient for TFIIIC binding which, in turn, allows pol III access. Chromatin is dynamic in the presence of the S-190 extract's remodeling enzymes. If TFIIIC alone can induce a rearrangement of nucleosomes around the U6 gene, then addition of TFIIIC prior to TFIIIB, or at the outset of nucleosome deposition, may further enhance U6 transcription in chromatin. This is what was observed. Adding TFIIIC 30 min prior to TFIIIB (at the end of 4.5 h of assembly) generated an additional approximately twofold increase in transcription over that obtained when TFIIIB and TFIIIC were added together (compare lanes 4 and 7). Addition of TFIIIC at the outset of chromatin assembly further increased transcription only slightly, if at all (compare lanes 5 and 7). The modest additional increase of transcription when TFIIIC was added at the outset may reflect the limited residence time for TFIIIC on box B (half-life of ∼20 min [27]), but doubling the TFIIIC concentration during the final 40 min of TFIIIC-TFIIIB-DNA complex formation also had no additional stimulatory effect (compare lanes 8 and 6 with 5 and 7), implying either that saturation of box B in chromatin was achieved or that TFIIIB and/or pol III became limiting for transcription. Taken together, these results suggest that TFIIIC and a chromatin structure of the template combine to activate U6 transcription approximately 5- to 15-fold over that obtained with naked DNA (data from additional experiments are averaged in Fig. 1D). The activation of transcription mediated by TFIIIC relative to the chromatin-repressed TFIIIB-only reaction (lane 1, Fig. 1C) was found to be at least 50- to 100-fold (given that the residual transcription in lane 1, used as the denominator, is only barely distinguishable from the gel background; see Materials and Methods).
TFIIIC binds to chromatin, altering its structure.
The above transcription experiments suggested that TFIIIC is capable of binding to its high-affinity box B binding site in the context of dynamic chromatin and, in turn, altering chromatin structure so as to promote initiation complex assembly. This was examined directly in the experiments that follow (Fig. 2 to 4).
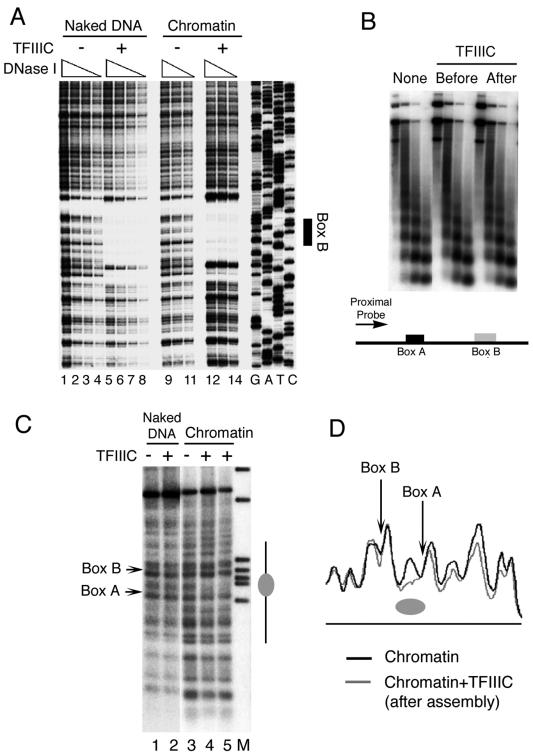
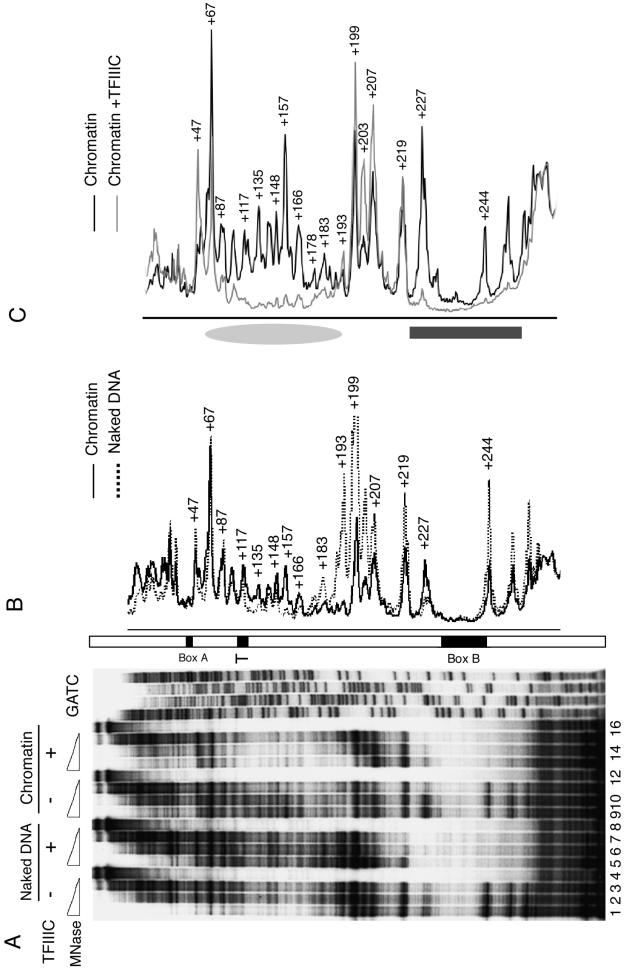
FIG. 2.
Chromatin structure and TFIIIC binding. (A) DNase I footprinting of pCS6 as naked DNA (lanes 1 to 8) or assembled into chromatin (lanes 9 to 14) was performed in the absence (lanes 1 to 4 and 9 to 11) or presence (lanes 5 to 8 and 12 to 14) of TFIIIC. Box B is identified on the right. The 5′ end of the primer extension probe was 135 bp away from the 3′ end of box B, complementary to the U6 gene top strand. (B) TFIIIC does not disrupt the periodicity of nucleosomal arrays. MNase-digested chromatin fragments, from assemblies in the absence or presence of TFIIIC added at different times, were purified, electrophoretically resolved, blotted, and hybridized to a probe complementary to DNA ∼50 bp upstream of the start site (proximal probe), as shown schematically at the bottom of the panel. (C) IEL analysis of chromatin structure on the U6 snRNA gene. Naked DNA (lanes 1 and 2) and chromatin (lanes 3 to 5) were digested with MNase and probed with a primer that hybridizes 894 bp upstream of the SNR6 TATA box. Lane M represents markers, lane 3 is the control chromatin sample, and lanes 4 and 5 had TFIIIC added prior to and after 4.5 h of chromatin assembly, respectively. Positions of boxes A and B are marked on the left. The gray ellipse on the right marks the nucleosome-size protection between boxes A and B. (D) Aligned profiles of lanes 5 and 3 of panel C. The gray ellipse marks the nucleosomal protection between boxes A and B.
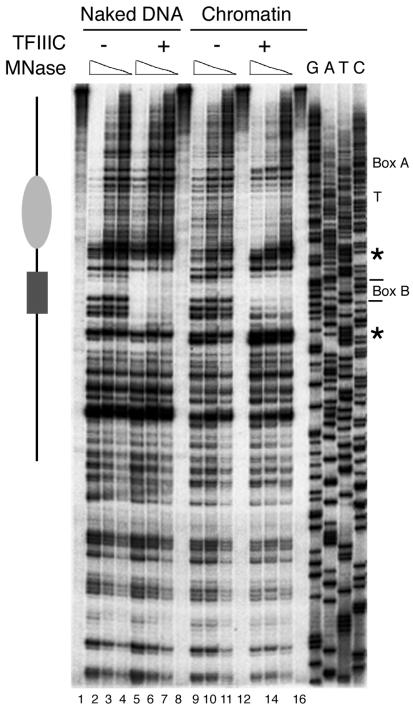
FIG. 4.
Structure of SNR6 gene chromatin downstream of box B. The partial MNase digestion pattern for naked DNA (lanes 1 to 8) or chromatin (lanes 9 to 16) with (lanes 5 to 8 and 13 to 16) or without (lanes 1 to 4 and 9 to 12) TFIIIC is shown. Positions of box A, the terminator (T), and box B are marked at the right side. The gray ellipse to the left spans the positioned nucleosome, and the black box spans protection due to TFIIIC. Asterisks denote hypersensitive sites flanking the TFIIIC-protected box B in chromatin, but not in naked DNA. The 3′ end of the primer extension probe was located 174 bp from the 3′ end of box B, complementary to the top strand, and was also used for the sequencing ladder.
First we examined the ability of TFIIIC to bind to its box B binding site in the context of chromatin by DNase I footprinting (Fig. 2A). TFIIIC added to the U6 plasmid DNA template assembled into chromatin was indeed capable of binding and protecting the box B region in chromatin with substantial occupancy (lanes 12 to 14 versus 9 to 11), comparable to that on naked DNA (lanes 12 to 14 versus 5 to 8). The MNase cleavage pattern of assembled chromatin shown in Fig. 2B demonstrates that the binding of TFIIIC to box B, either before or after chromatin assembly, did not disrupt the periodicity of nucleosomes on the plasmid DNA close to the gene (proximal probe) as well as distal to the U6 gene (data not shown). This indicates that TFIIIC bound to box B did not disturb nucleosome periodicity at a gross structural level. However, this did not rule out the possibility of removal-readjustment of a nucleosome around or on the TFIIIC binding sites. The IEL approach (46) was taken to examine more localized rearrangements of nucleosomes, if any.
Mapping of nucleosomes by nuclease digestion analysis.
IEL of DNA displays patterns of protection from double-strand MNase cleavage that can be interpreted to show changes in the positions of several nucleosomes at the same time. Figure 2C shows results of one such analysis for the chromatin assembled over plasmid pCS6. No change in the MNase digestion pattern of naked DNA was observed upon binding of TFIIIC to the SNR6 box B element (lanes 1 and 2) due to the low resolution of this footprinting format. In the absence of TFIIIC, the digestion pattern of chromatin in the vicinity of boxes A and B was similar to that of naked DNA (compare lanes 1 and 3), indicating no preferred positioning of nucleosomes. In the presence of TFIIIC, added before (lane 4) or near the end (lane 5) of chromatin assembly, a protected segment between box A and box B was seen (marked as a gray ellipse on the right). The IEL blots were calibrated using molecular weight markers to estimate the MNase cut positions on the gene. The protection generated by TFIIIC was found to be ∼180 bp long, indicating that it could be due to a positioned nucleosome. Aligning the profile of lane 5 with that of lane 3 confirmed the presence of a TFIIIC-dependent, positioned nucleosome between box A and box B (Fig. 2D, gray ellipse). Weaker protection was also seen upstream of box A (Fig. 2D), but further analysis could not confirm the positioning of nucleosomes in this region. Thus, the IEL approach showed that, irrespective of the time of addition of TFIIIC, the local chromatin structure of the gene changes to place at least one positioned nucleosome in the SNR6 gene.
High-resolution analysis of chromatin remodeling by TFIIIC.
MNase protection was also used to map the TFIIIC-dependent nucleosome positioning at higher resolution. Four sets of MNase titrations were used to compare the partial digestion products of naked DNA and chromatin in the absence or presence of TFIIIC (Fig. 3A). The footprint of TFIIIC at box B in both naked DNA (compare lanes 5 to 8 with 1 to 4) and in chromatin (compare lanes 9 to 12 with 13 to 16) was readily apparent in pCS6 DNA digested with MNase. Comparison of the MNase partial digestion profiles of chromatin and of naked DNA (Fig. 3B) displays both chromatin-mediated protection upstream of box B (bp +178 to +219) and enhanced susceptibility to MNase cleavage (bp +157 and +227). Comparison of the partial MNase digestion products of chromatin in the absence and in the presence of TFIIIC showed an ∼140-bp (approximately nucleosome core DNA size) protected segment between ∼bp +50 and +190 (Fig. 3A, compare lanes 9 to 11 with lanes 13 to 15; profile in Fig. 3C, marked by a gray ellipse; the dark gray box spans protection over box B). The different size estimates for the TFIIIC-mediated nucleosomal protection between IEL (Fig. 2C) and high-resolution footprinting (Fig. 3; 180 and 140 bp, respectively) reflect, at least in part, the requirement for double-strand cleavage in the former case and single-strand cleavage in the latter. The localization of the positioned nucleosome by higher-resolution footprinting places the transcriptional terminator of the U6 gene (bp 109 to 118) near the nucleosomal dyad at bp +120. This positioning of a nucleosome on the intragenic region of pCS6, from ∼bp +50 to +190, also overlaps the reported in vivo protection (bp +94 to +198) on the gene (17).
FIG. 3.
Analysis of chromatin remodeling by MNase digestion. (A) Higher-resolution footprinting. Chromatin without or with an 11-fold molar excess of TFIIIC added at the start of assembly (lanes 9 to 16) was subjected to three levels of MNase digestion and compared with naked DNA without (lanes 1 to 3) or with (lanes 5 to 7) TFIIIC. Lanes 4, 8, 12, and 16 show primer extension on undigested DNA. The 5′ end of the primer extension probe was 31 bp away from the 3′ end of box B, complementary to the U6 gene top strand. The GATC ladder was generated with the same primer. Positions of box B, the transcription terminator (T), and box A are marked on an open bar. (B and C) A nucleosome-size footprint on chromatin in the presence of TFIIIC. Aligned digestion profiles are taken from panel A. (B) Naked DNA (lane 2) and chromatin (lane 10) without TFIIIC. (C) Chromatin without TFIIIC (lane 9) and with TFIIIC (lane 14). Numbers denote positions of MNase cuts with reference to the transcriptional initiation site; +244 denotes the single cut in box B. Protection in the region of box B is marked with a dark gray box, while the gray ellipse marks the position of nucleosomal protection in the presence of TFIIIC.
We also explored whether TFIIIC generated a positioned nucleosome at the downstream end of box B (Fig. 4). The footprint of TFIIIC at box B (dark gray box) and the TFIIIC-dependent nucleosomal protection on chromatin between boxes A and B (gray ellipse) were again seen. A hypersensitive site was also noted on the distal side of box B (marked with an asterisk), but no positioned nucleosome could be ascertained downstream of box B (compare lanes 9 to 11 with 13 to 15). The chromatin structure in the region upstream of box A was also separately examined: no positioned nucleosomes could be detected (data not shown).
The footprinting analysis also showed that the TFIIIC-positioned nucleosome lies between two sites of enhanced MNase cleavage (bp +47 and +193 define the sharp boundaries of protection) but does not display the 10-bp periodicity of nuclease accessibility that is indicative of rotational positioning. It is clear, however, that the chromatin structure generated by the S-190 extract does encroach on DNA sequence normally occupied by the τB domain of TFIIIC. This is most evident in Fig. 4, where the extent of protection both upstream and downstream of box B is reduced in chromatin compared to naked DNA.
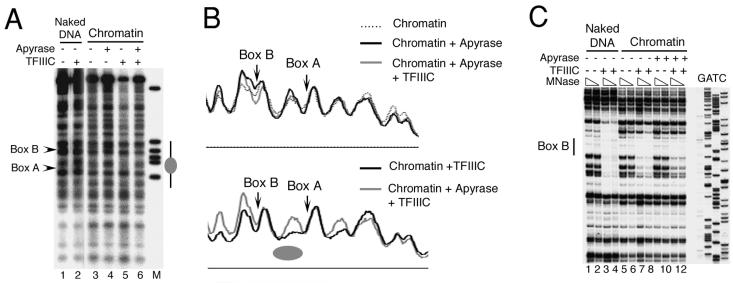
ATP dependence of chromatin remodeling and transcription in the presence of TFIIIC.
Several ATP-dependent remodeling activities for pol II genes in the S-190 extract have been reported elsewhere (50). Therefore, we examined the ATP dependence of TFIIIC-mediated nucleosome positioning and the concomitant activation of U6 transcription (Fig. 5 and 6). Following 4.5 h of chromatin assembly, ATP in the assembly mix was hydrolyzed with apyrase and both IEL and footprinting approaches were used for the structure analysis of the resultant chromatin. (Complete ATP hydrolysis under the conditions used was confirmed in a separate assay using both α- and γ-[32P]ATP as tracer for analysis by thin-layer chromatography.) The IEL-MNase digestion analysis of chromatin in Fig. 5A and B shows the effect of apyrase on nucleosome positioning. When TFIIIC was added to chromatin after assembly and apyrase treatment (lane 6, Fig. 5A), the cleavage profile was indistinguishable (top panel, Fig. 5B) from those of assembled chromatin (lane 3, Fig. 5A) and apyrase-treated chromatin (lane 4, Fig. 5A). This differs markedly from a parallel reaction in which apyrase was not added to chromatin prior to TFIIIC (lane 5, Fig. 5A), and a nucleosome-size protection was observed between box A and box B (gray ellipse, bottom panel, Fig. 5B). A higher-resolution footprinting analysis was carried out in order to determine whether TFIIIC binds to chromatin in the absence of ATP (Fig. 5C). The footprint of TFIIIC over box B in apyrase-treated chromatin was discernible but less complete than in the presence of ATP (compare lanes 11 and 12 with lanes 7 and 8). The extent of the TFIIIC-protected box B region was also shorter at the upstream end in apyrase-treated chromatin (compare lanes 12 and 8; also Fig. 3A and C), possibly reflecting additional encroachment of nucleosomal structure in the absence of chromatin remodeling. We conclude that the TFIIIC-dependent nucleosome positioning is an ATP-dependent process.
FIG. 5.
ATP requirement for chromatin remodeling. (A) Chromatin assembly, probed by MNase digestion and IEL, was carried out for 4.5 h, followed by treatment with apyrase for 15 min and incubation with TFIIIC (2.5:1 molar excess over DNA) for an additional 20 to 30 min, as specified above each lane. A gray ellipse marks the position of a nucleosome between boxes A and B in lane 5. (B) Aligned density profiles of lanes in panel A. Top panel: comparison of lanes 3, 4, and 6. Bottom panel: comparison of lanes 5 and 6. (C) TFIIIC binds chromatin in the absence of ATP. MNase footprinting analysis over box B is shown. Chromatin was assembled for 4.5 h (lanes 5 to 12), treated with apyrase for 15 min (lanes 9 to 12), and incubated with an eightfold molar excess of TFIIIC (lanes 7, 8, 11, and 12) prior to digestion with two concentrations of MNase. A parallel digestion of naked DNA without (lanes 1 and 2) or with (lanes 3 and 4) TFIIIC is also shown. The black vertical bar shows the location of box B. The 3′ end of the primer extension probe was 113 bp away from the 3′ end of box B, complementary to the top strand. GATC shows the sequencing reaction lanes.
To determine whether TFIIIC binding to chromatin in the absence of ATP-dependent remodeling could contribute to the high activation of chromatin transcription (Fig. 1), we removed ATP from assembled chromatin by gel filtration (48). Passage of the assembly mix (after 4.5 h of assembly) through a Sephadex G-25 column resulted in an ∼2.5-fold dilution but did not disrupt the chromatin, as judged by MNase digestion ladders (data not shown). Monitoring by thin-layer chromatography showed that the column also removed ATP completely from the assembly mix (data not shown). This ATP-depleted chromatin was used for transcription either with or without addition of TFIIIC. The scheme of this experiment is shown in Fig. 6A with the corresponding samples analyzed in Fig. 6B. Its salient features are that TFIIIC is added late in the reaction sequence (samples 6 to 8) or omitted entirely (samples 3 to 5) and that ATP is removed (samples 3, 4, 6, and 7) but subsequently readded (samples 4 and 7). Control samples 1 and 2 monitor naked DNA transcription without and with TFIIIC, respectively (as in Fig. 1C). Results are presented in Fig. 6B and quantified in Fig. 6C. Chromatin was fully repressed in the absence of TFIIIC (Fig. 6B, lanes 3 to 5). Addition of TFIIIC to preassembled chromatin still secured a 46-fold activation (lane 8), relative to the corresponding repressed chromatin (lane 5), and a 4.9-fold increase over naked DNA transcription with or without TFIIIC (average of lanes 1 and 2). The manipulations involved in removing ATP still allowed a 34-fold activation by TFIIIC in the presence of readded ATP relative to the corresponding repressed chromatin (compare lanes 7 and 4); the ∼25%-lower activation relative to lane 8 could be due to a partial loss of chromatin or to its dilution during gel filtration. TFIIIC also secured some restoration of transcription in the absence of an ATP resupply (lane 6): an ∼13-fold activation relative to the corresponding repressed chromatin (lane 3), to a level slightly exceeding (1.3-fold) transcription of naked DNA (lanes 1 and 2).
To summarize, results presented in this study suggest that the reported requirement of TFIIIC for transcription of the yeast U6 snRNA gene in chromatin is associated with a remodeling of chromatin structure after TFIIIC invades the repressive chromatin structure to bind its sites. Chromatin remodeling correlates with a high level of activation of the U6 snRNA gene. The relevance of these results to a possible in vivo mechanism is discussed below.
DISCUSSION
Nucleosome assembly is not necessarily repressive for transcription. The folding of DNA over a single positioned nucleosome or an array of positioned nucleosomes can facilitate the activation of genes by juxtapositioning regulatory elements that would otherwise be widely separated (53, 59, 62, 76). The association of positioned nucleosomes with transcription of pol III genes has been analyzed extensively, particularly in the context of 5S rRNA synthesis (reviewed in references 67 and 69). High-level transcription of the human U6 snRNA gene in vitro was associated with chromatin structure upstream of the transcriptional start (57). A positioned nucleosome in the upstream region between the distal sequence element (DSE) and the proximal promoter element (PSE) was shown subsequently to mediate the cooperative binding of the Oct-1 and SNAPc transcription factors (76). Our study of the yeast U6 gene has shown positioning of a nucleosome downstream of the transcriptional initiation site, between boxes A and B.
A positioned nucleosome and transcription of the yeast U6 snRNA gene.
The box A and box B elements and TFIIIC are essential for SNR6 transcription in crude transcription systems and in the context of chromatin in vitro or in vivo (10, 12, 17, 36). The optimal spacing between the box A and box B elements, which is accommodated by an unseen ∼10-nm linker between the τA and τB domains of TFIIIC (55), is 30 to 60 bp (4). Rather than increasing transcription, shortening the distance between SNR6 box A and box B diminished transcription in vivo and in crude transcription systems in vitro: an 84-bp deletion (corresponding to approximately one superhelical turn around a nucleosome) decreased transcription twofold, and each 42-bp half of this deletion made matters significantly worse (12). These observations led to the proposal that TFIIIC-mediated derepression of transcription in chromatin may involve placement of a nucleosome between box A and box B, bringing these elements within optimal range for simultaneous binding by TFIIIC (9, 12).
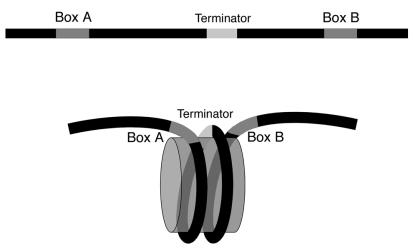
A schematic representation of the summary of structural analysis presented in this study is given in Fig. 7. Translational positioning of a nucleosome in the presence of TFIIIC to wrap bp ∼+50 to ∼+190 over a histone octamer effectively reduces the linear separation between boxes A and B. This positioning aligns the terminator and the two boxes in space and may help to stabilize the TFIIIC-DNA complex further. It is noteworthy that the 10-bp T:A stretch of the SNR6 terminator lies near the nucleosomal dyad axis. Poly(dT):poly(dA) stretches (T tracts) are relatively stiff (3) and preferentially occupy the unbent ends of the nucleosome core particle (34, 52). However, incorporation of even longer T tracts into nucleosomes is not without precedent (18; K. Luger, personal communication). We note that the resolution of the footprinting gels did not allow detection of a footprint over the box A promoter element by TFIIIC in chromatin. This may partly reflect the fact that the SNR6 box A sequence is suboptimal (10) and that, even with optimal box A elements and spacings to box B, the box A footprint is often partial or difficult to detect in the absence of TFIIIB (cf. reference 27). That TFIIIC does interact with box A in chromatin is indicated by levels of transcription well above that of naked DNA, since it is TFIIIC binding to the box A element that specifies the placement of TFIIIB. It is also conceivable that the nucleosome between boxes A and B circumvents the necessity for box A by bringing box B and the TATA box into proximity for direct deposition of TFIIIB.
FIG. 7.
Nucleosome positioning reduces the gap between the SNR6 gene's boxes A and B.
A mechanism of SNR6 transcription in vivo?
It has been shown recently that the HMG1-related chromatin proteins Nhp6A and Nhp6B, which are 96% similar and functionally redundant, play an important role in SNR6 transcription in vivo. Briefly, S. cerevisiae harboring deletions in both Nhp6A and Nhp6B (nhp6ΔΔ) is viable but temperature sensitive. This temperature sensitivity is suppressed by multicopy plasmids harboring the SNR6 gene, and synthetic lethality is generated when nhp6ΔΔ is combined with a 42-bp deletion between the SNR6 terminator and box B. Nhp6 also stimulates SNR6 transcription in vitro (29, 33, 37). Nhp6A/B bends DNA by at least 90° (2, 74), suggesting that three to four molecules of Nhp6 would be sufficient to spool DNA 360°, bringing box A and box B into greater proximity for simultaneous occupancy by TFIIIC (29). However, such a role for Nhp6 and nucleosome positioning are not necessarily mutually exclusive (29). Nhp6 is intimately associated with nucleosomal DNA structure, complicating any interpretation of its function in SNR6 transcription in isolation of nucleosomal structure effects in vivo. Nhp6 binds to nucleosomes as a component of the yeast FACT complex, generating a conformational change that does not require NTP hydrolysis (13, 51). Deletion of both Nhp6 genes is also synthetically lethal with mutations in either of two common subunits of the ATP-dependent remodeling complexes RSC and SWI/SNF, and a moderately salt-resistant complex between Nhp6A and the RSC remodeling complex has been documented (58). Given the absence of functional communication between box A and box B in vitro in the context of transcription with purified TFIIIC, TFIIIB, and pol III, an additional box A-box B separation-reducing mechanism must account for the viability of yeast lacking Nhp6. Clearly, that mechanism is much more sensitive to a 42-bp (approximately half a nucleosome superhelical turn) deletion between box B and the SNR6 terminator in the absence of Nhp6 and may involve a nucleosome positioned between boxes A and B. We have shown that TFIIIC is indeed capable of mediating the placement of a nucleosome between box A and box B in vitro.
The role for a nucleosome between box A and box B in vivo, however, remains unclear. In vivo chromatin footprinting either demonstrated a subnucleosomal-size zone of protection between the terminator and box B (17) or could not assess the presence of a nucleosome between boxes A and B due to the paucity of MNase cleavage sites between these elements (36). There is a caveat in interpreting these chromatin footprinting experiments, since they reflect the result of TFIIIB assembly and concomitant transcription and not the process of assembly itself. The presence of TFIIIC on actively transcribed tRNA genes appears to be low in contrast to that of TFIIIB (21), and observations of the presence of TFIIIC at the SNR6 box B element by chromatin footprinting differ markedly between studies (interpretable either as artifactual TFIIIC binding or as dissociation during lysis) (17, 36). The continuous presence of a DNA binding factor is required for maintaining the induced, remodeled structure (46), and given the ability of pol III to displace a nucleosome during transcription (56), it would be surprising to find a nucleosome-size protection within pol III-transcribed DNA sequence in the above studies (17, 36). Whether the approximately half-nucleosome-size protection observed by Gerlach et al. (17) downstream of the SNR6 terminator represents partial displacement of histones due to transcription or occupancy by some other component of chromatin remains uncertain.
Transcriptional activation of the yeast SNR6 gene and chromatin remodeling.
Our experiments on the yeast U6 gene showed that, in the presence of the S-190 extract, TFIIIC-dependent nucleosome positioning was largely dependent on the presence of ATP (Fig. 6A), implicating one or more of the ATP-dependent remodeling complexes of the S-190 extract in this process. TFIIIC-dependent activation of transcription was also greatly reduced by the removal of ATP and was substantially restored by the addition of ATP with TFIIIC (Fig. 6C) but not by acetyl S-coenzyme A (data not shown), suggesting that histone acetylation is not a major component of this TFIIIC-dependent transcriptional activation. Yeast TFIIIC, unlike its human counterpart, does not contain a histone acetyltransferase activity in any of its subunits (20, 30, 44).
The accessibility of box B to TFIIIC in chromatin in the absence of ATP (Fig. 5C) and the ∼50- to 100-fold total activation over that of repressed chromatin due to TFIIIC are properties also exhibited by strong activators of pol II genes (38, 47-49), which are known to invade repressive chromatin structures (45, 48). Some transcriptional activators can recruit a chromatin remodeling complex in a gene-specific manner (75). Tandem affinity purification of yeast protein complexes (14) implies an association of TFIIIC with the ATP-dependent ISW2 and RSC nucleosome remodeling complexes as well as the Nhp6A/B subunits of the ATP-independent yeast FACT complex (13). Homologs of yeast remodeling complexes are found in humans and in Drosophila (65). This suggests the possibility that a remodeling complex in the S-190 extract could interact with yeast TFIIIC. If TFIIIC works like an activator of the SNR6 gene, it could recruit a specific remodeling complex (located in the S-190 extract) to the gene and the combined action of both could produce the observed chromatin derepression and transcriptional activation. In this context, a genome-wide analysis of the location of the RSC nucleosome remodeling complex in yeast might suggest that the RSC associated with numerous tRNA genes is recruited by the pol III machinery itself (42). Needless to say, such possibilities will have to be tested by analysis of the homologous (yeast) chromatin remodeling systems. It should also be possible to generate transcriptional activation without direct interaction through the combined but independent action of chromatin remodeling and TFIIIC binding.
Acknowledgments
We thank E. Peter Geiduschek for insightful comments and suggestions and sustained editorial help. We also thank J. T. Kadonaga for a helpful evaluation of the manuscript.
Financial support from the Council for Scientific and Industrial Research (CSIR), Government of India, and the Fogarty International Research Center, NIH (grant R03 TW001322), is gratefully acknowledged. S.S. is a recipient of a CSIR Senior Research Fellowship.
REFERENCES
- 1.Aalfs, J. D., and R. E. Kingston. 2000. What does ‘chromatin remodeling' mean? Trends Biochem. Sci. 25:548-555. [DOI] [PubMed] [Google Scholar]
- 2.Allain, F. H., Y. M. Yen, J. E. Masse, P. Schultze, T. Dieckmann, R. C. Johnson, and J. Feigon. 1999. Solution structure of the HMG protein NHP6A and its interaction with DNA reveals the structural determinants for non-sequence-specific binding. EMBO J. 18:2563-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allewell, N. 1988. Why does DNA bend? Trends Biochem. Sci. 13:193-195. [DOI] [PubMed] [Google Scholar]
- 4.Baker, R. E., S. Camier, A. Sentenac, and B. D. Hall. 1987. Gene size differentially affects the binding of yeast transcription factor tau to two intragenic regions. Proc. Natl. Acad. Sci. USA 84:8768-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, P. B., and W. Horz. 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71:247-273. [DOI] [PubMed] [Google Scholar]
- 6.Brow, D. A., and C. Guthrie. 1988. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature 334:213-219. [DOI] [PubMed] [Google Scholar]
- 7.Brow, D. A., and C. Guthrie. 1990. Transcription of a yeast U6 snRNA gene requires a polymerase III promoter element in a novel position. Genes Dev. 4:1345-1356. [DOI] [PubMed] [Google Scholar]
- 8.Bulger, M., and J. T. Kadonaga. 1994. Biochemical reconstitution of chromatin with physiological nucleosome spacing. Methods Mol. Genet. 5:241-261. [Google Scholar]
- 9.Burnol, A.-F., F. Margottin, J. Huet, G. Almouzni, M.-N. Prioleau, M. Mechali, and A. Sentenac. 1993. TFIIIC relieves repression of U6 snRNA transcription by chromatin. Nature 362:475-477. [DOI] [PubMed] [Google Scholar]
- 10.Burnol, A.-F., F. Margottin, P. Schultz, M.-C. Marsolier, P. Oudet, and A. Sentenac. 1993. Basal promoter and enhancer element of yeast U6 snRNA gene. J. Mol. Biol. 233:644-658. [DOI] [PubMed] [Google Scholar]
- 11.Cairns, B. R. 1998. Chromatin remodeling machines: similar motors, ulterior motives. Trends Biochem. Sci. 23:20-25. [DOI] [PubMed] [Google Scholar]
- 12.Eschenlauer, J. B., M. W. Kaiser, V. L. Gerlach, and D. A. Brow. 1993. Architecture of a yeast U6 RNA gene promoter. Mol. Cell. Biol. 13:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formosa, T., P. Eriksson, J. Wittmeyer, J. Ginn, Y. Yu, and D. J. Stillman. 2001. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 20:3606-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin, A.-C., M. Bosche, R. Krause, et. al. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 15.Geiduschek, E. P., and G. A. Kassavetis. 2001. The RNA polymerase III transcription apparatus. J. Mol. Biol. 310:1-26. [DOI] [PubMed] [Google Scholar]
- 16.George, P. C., and J. T. Kadonaga. 1996. Primer-extension analysis of RNA, p. 133-139. In P. A. Krieg (ed.), A laboratory guide to RNA: isolation, analysis and synthesis. Wiley-Liss, Inc., New York, N.Y.
- 17.Gerlach, V. L., S. K. Whitehall, E. P. Geiduschek, and D. A. Brow. 1995. TFIIIB placement on a yeast U6 RNA gene in vivo is directed primarily by TFIIIC rather than by sequence-specific DNA contacts. Mol. Cell. Biol. 15:1455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes, J. J., S. Dimitrov, and A. P. Wolffe. 1994. Physical and chemical analysis of the dynamics of nucleosome and chromatin structure. Chemtracts Biochem. Mol. Biol. 5:269-290. [Google Scholar]
- 19.Howe, L., and J. Ausio. 1998. Nucleosome translational position, not histone acetylation, determines TFIIIA binding to nucleosomal Xenopus laevis 5S rRNA genes. Mol. Cell. Biol. 18:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh, Y.-J., T. K. Kundu, Z. Wang, R. Kovelman, and R. G. Roeder. 1999. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Mol. Cell. Biol. 19:7697-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huibregtse, J. M., and D. R. Engelke. 1989. Genomic footprinting of a yeast tRNA gene reveals stable complexes over the 5′-flanking regions. Mol. Cell. Biol. 9:3244-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joazeiro, C. A. P., G. A. Kassavetis, and E. P. Geiduschek. 1994. Identical components of yeast transcription factor IIIB are required and sufficient for transcription of TATA box-containing and TATA-less genes. Mol. Cell. Biol. 14:2798-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadonaga, J. T. 1998. Eukaryotic transcription: an interlaced network of transcription factors and chromatin modifying machines. Cell 92:307-313. [DOI] [PubMed] [Google Scholar]
- 24.Kassabov, S. R., B. Zhang, J. Persinger, and B. Bartholomew. 2003. SWI/SNF unwraps, slides, and rewraps the nucleosome. Mol. Cell 11:391-403. [DOI] [PubMed] [Google Scholar]
- 25.Kassavetis, G. A., B. Bartholomew, J. A. Blanco, T. E. Johnson, and E. P. Geiduschek. 1991. Two essential components of the Saccharomyces cerevisiae transcription factor TFIIIB: transcription and DNA-binding properties. Proc. Natl. Acad. Sci. USA 88:7308-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassavetis, G. A., A. K. Kumar, E. Ramirez, and E. P. Geiduschek. 1998. Functional and structural organization of Brf, the TFIIB-related component of the RNA polymerase III transcription initiation complex. Mol. Cell. Biol. 18:5587-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassavetis, G. A., D. L. Riggs, R. Negri, L. H. Nguyen, and E. P. Geiduschek. 1989. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol. Cell. Biol. 9:2551-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingston, R. E., and G. J. Narlikar. 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13:2339-2352. [DOI] [PubMed] [Google Scholar]
- 29.Kruppa, M., R. D. Moir, D. Kolodrubetz, and I. M. Willis. 2001. Nhp6, an HMG1 protein, functions in SNR6 transcription by RNA polymerase III in S. cerevisiae. Mol. Cell 7:309-318. [DOI] [PubMed] [Google Scholar]
- 30.Kundu, T. K., Z. Wang, and R. G. Roeder. 1999. Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Mol. Cell. Biol. 19:1605-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkel, G. R. 1991. RNA polymerase III transcription of genes that lack internal control regions. Biochim. Biophys. Acta 1088:1-9. [DOI] [PubMed] [Google Scholar]
- 32.Lemon, B., and R. Tjian. 2000. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14:2551-2569. [DOI] [PubMed] [Google Scholar]
- 33.Lopez, S., M. Livingstone-Zatchej, S. Jourdain, F. Thoma, A. Sentenac, and M.-C. Marsolier. 2001. High-mobility-group proteins NHP6A and NHP6B participate in activation of the RNA polymerase III SNR6 gene. Mol. Cell. Biol. 21:3096-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luger, K., A. W. Mader, R. K. Richmond, D. F. Sargent, and T. J. Richmond. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389:251-260. [DOI] [PubMed] [Google Scholar]
- 35.Margottin, F., G. Dujardin, M. Girard, J. M. Egly, J. Huet, and A. Sentenac. 1991. Participation of the TATA factor in transcription of the yeast U6 gene by RNA polymerase C. Science 251:424-426. [DOI] [PubMed] [Google Scholar]
- 36.Marsolier, M.-C., S. Tanaka, M. Livingstone-Zatchej, M. Grunstein, F. Thoma, and A. Sentenac. 1995. Reciprocal interferences between nucleosomal organization and transcriptional activity of the yeast SNR6 gene. Genes Dev. 9:410-422. [DOI] [PubMed] [Google Scholar]
- 37.Martin, M. P., V. L. Gerlach, and D. A. Brow. 2001. A novel upstream RNA polymerase III promoter element becomes essential when the chromatin structure of the yeast U6 RNA gene is altered. Mol. Cell. Biol. 21:6429-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuguchi, G., T. Tsukiyama, J. Wisniewski, and C. Wu. 1997. Role of nucleosome remodeling factor NURF in transcriptional activation of chromatin. Mol. Cell 1:141-150. [DOI] [PubMed] [Google Scholar]
- 39.Mizuguchi, G., A. Vassilev, T. Tsukiyama, Y. Nakatani, and C. Wu. 2001. ATP-dependent nucleosome remodeling and histone hyperacetylation synergistically facilitate transcription of chromatin. J. Biol. Chem. 276:14773-14783. [DOI] [PubMed] [Google Scholar]
- 40.Moenne, A., S. Camier, G. Anderson, F. Margottin, J. Beggs, and A. Sentenac. 1990. The U6 gene of Saccharomyces cerevisiae is transcribed by RNA polymerase C (III) in vivo and in vitro. EMBO J. 9:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narlikar, G. J., H.-Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 42.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2002. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes Dev. 16:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orphanides, G., and D. Reinberg. 2000. RNA polymerase II elongation through chromatin. Nature 407:471-475. [DOI] [PubMed] [Google Scholar]
- 44.Paule, M. R., and R. J. White. 2000. Transcription by RNA polymerases I and III. Nucleic Acids Res. 28:1283-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pazin, M. J., and J. T. Kadonaga. 1997. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell 88:737-740. [DOI] [PubMed] [Google Scholar]
- 46.Pazin, M. J., P. Bhargava, E. P. Geiduschek, and J. T. Kadonaga. 1997. Nucleosome mobility and the maintenance of nucleosome positioning. Science 276:809-812. [DOI] [PubMed] [Google Scholar]
- 47.Pazin, M. J., J. W. Hermann, and J. T. Kadonaga. 1998. Promoter structure and transcriptional activation with chromatin templates assembled in vitro. J. Biol. Chem. 273:34653-34660. [DOI] [PubMed] [Google Scholar]
- 48.Pazin, M. J., R. T. Kamakaka, and J. T. Kadonaga. 1994. ATP-dependent nucleosome configuration and transcriptional activation from preassembled chromatin templates. Science 266:2007-2011. [DOI] [PubMed] [Google Scholar]
- 49.Pazin, M. J., P. L. Sheridan, K. Cannon, Z. Cao, J. G. Keck, J. T. Kadonaga, and K. A. Jones. 1996. NF-kappa B-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 10:37-49. [DOI] [PubMed] [Google Scholar]
- 50.Robinson, K. M., and J. T. Kadonaga. 1998. The use of chromatin templates to recreate transcriptional regulatory phenomena in vitro. Biochim. Biophys. Acta 1378:M1-M6. [DOI] [PubMed] [Google Scholar]
- 51.Ruone, S., A. R. Rhoades, and T. Formosa. 2003. Multiple Nhp6 molecules are required to recruit Spt16-Pob3 to form yFACT complexes and to reorganize nucleosomes. J. Biol. Chem. 278:45288-45295. [DOI] [PubMed] [Google Scholar]
- 52.Satchwell, S. C., H. R. Drew, and A. A. Travers. 1986. Sequence periodicities in chicken nucleosome core DNA. J. Mol. Biol. 191:659-675. [DOI] [PubMed] [Google Scholar]
- 53.Schild, C., F.-X. Claret, W. Wahli, and A. P. Wolffe. 1993. A nucleosome-dependent static loop potentiates estrogen-regulated transcription from the Xenopus vitellogenin B1 promoter in vitro. EMBO J. 12:423-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schramm, L., and N. Hernandez. 2002. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 16:2593-2620. [DOI] [PubMed] [Google Scholar]
- 55.Schultz, P., N. Marzuoki, C. Marck, A. Ruet, P. Oudet, and A. Sentenac. 1989. The two DNA-binding domains of yeast transcription factor tau as observed by scanning transmission electron microscopy. EMBO J. 8:3815-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Studitsky, V. M., G. A. Kassavetis, E. P. Geiduschek, and G. Felsenfeld. 1997. Mechanism of transcription through the nucleosome by eukaryotic RNA polymerase. Science 278:1960-1963. [DOI] [PubMed] [Google Scholar]
- 57.Stunkel, W., I. Kober, and K. H. Seifart. 1997. A nucleosome positioned in the distal promoter region activates transcription of the human U6 gene. Mol. Cell. Biol. 17:4397-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szerlong, H., A. Saha, and B. R. Cairns. 2003. The nuclear actin-related proteins Arp7 and Arp9: a dimeric module that cooperates with architectural proteins for chromatin remodeling. EMBO J. 22:3175-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas, G. H., and S. C. Elgin. 1988. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 7:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tse, C., T. Sera, A. P. Wolffe, and J. C. Hansen. 1998. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol. Cell. Biol. 18:4629-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyler, J. K., and J. T. Kadonaga. 1999. The “dark side” of chromatin remodeling: repressive effects on transcription. Cell 99:443-446. [DOI] [PubMed] [Google Scholar]
- 62.Urnov, F. D., and A. P. Wolffe. 2001. An array of positioned nucleosomes potentiates thyroid hormone receptor action in vivo. J. Biol. Chem. 276:19753-19761. [DOI] [PubMed] [Google Scholar]
- 63.Urnov, F. D., and A. P. Wolffe. 2001. Chromatin remodeling and transcriptional activation: the cast (in order of appearance). Oncogene 20:2991-3006. [DOI] [PubMed] [Google Scholar]
- 64.Varga-Weisz, P. 2001. ATP dependent chromatin remodeling factors: nucleosome shufflers with many missions. Oncogene 20:3076-3085. [DOI] [PubMed] [Google Scholar]
- 65.Vignali, M., A. H. Hassan, K. E. Neely, and J. L. Workman. 2000. ATP-dependent chromatin remodeling complexes. Mol. Cell. Biol. 20:1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitolo, J. M., C. Thiriet, and J. J. Hayes. 2000. The H3-H4 N-terminal tail domains are the primary mediators of transcription factor IIIA access to 5S DNA within a nucleosome. Mol. Cell. Biol. 20:2167-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White, R. J. 1998. RNA polymerase III transcription, 2nd ed. Springer-Verlag, New York, N.Y.
- 68.White, R. J. 2002. RNA polymerase III transcription, 3rd ed. Landes Bioscience, Georgetown, Tex.
- 69.Wolffe, A. P. 1994. Nucleosome positioning and modification: chromatin structures that potentiate transcription. Trends Biochem. Sci. 19:240-244. [DOI] [PubMed] [Google Scholar]
- 70.Wolffe, A. P. 1999. Chromatin: structure and function, 3rd ed. Academic Press, San Diego, Calif.
- 71.Wolffe, A. P. 2001. Chromatin remodeling: why it is important in cancer. Oncogene 20:2988-2990. [DOI] [PubMed] [Google Scholar]
- 72.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-557. [DOI] [PubMed] [Google Scholar]
- 73.Wu, C. 1980. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Nature 286:854-860. [DOI] [PubMed] [Google Scholar]
- 74.Yen, Y. M., B. Wong, and R. C. Johnson. 1998. Determinants of DNA binding and bending by the Saccharomyces cerevisiae high mobility group protein NHP6A that are important for its biological activities: role of the unique N terminus and putative intercalating methionine. J. Biol. Chem. 273:4424-4435. [DOI] [PubMed] [Google Scholar]
- 75.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao, X., P. S. Pendergrast, and S. Hernandez. 2001. A positioned nucleosome on the human U6 promoter allows recruitment of SNAPc by the Oct-1 POU domain. Mol. Cell 7:539-549. [DOI] [PubMed] [Google Scholar]
- 77.Zhu, Z., and D. J. Thiele. 1996. A specialized nucleosome modulates transcription factor access to a C. glabrata metal responsive promoter. Cell 87:459-470. [DOI] [PubMed] [Google Scholar]