Abstract
Rationale
The interaction between ethanol (EtOH) and anxiety plays an integral role in the development and maintenance of alcoholism. Many medications in pre-clinical or clinical trials for the treatment of alcoholism share anxiolytic properties. However, these drugs typically have untoward side effects, such as sedation or impairment of motor function that may limit their clinical use. We have recently demonstrated that BRL 37344 (BRL), a selective β3-adrenoceptor agonist, enhances a discrete population of GABAergic synapses in the basolateral amygdala (BLA) that mediates feed-forward inhibition from lateral paracapsular (LPC) GABAergic interneurons onto BLA pyramidal cells. Behavioral studies revealed that intra-BLA infusion of BRL significantly reduced measures of unconditioned anxiety-like behavior without locomotor depressant effects.
Objectives
The present studies tested the effect of BRL (0.1, 0.5, or 1.0μg/side) on EtOH self-administration using an intermittent access (IA) home cage two-bottle choice procedure, and limited access operant responding for EtOH or sucrose.
Results
Intra-BLA infusion of BRL did not reduce homecage, intermittent EtOH self-administration. However, using an operant procedure that permits the discrete assessment of appetitive (seeking) and consummatory measures of EtOH self-administration, BRL reduced measures of EtOH and sucrose seeking, but selectively reduced operant responding for EtOH during extinction probe trials. BRL had no effect on consummatory behaviors for EtOH or sucrose.
Conclusions
Together, these data suggest that intra-BLA infusion of BRL significantly reduces motivation to seek EtOH and provide initial evidence that β3-ARs and LPC GABAergic synapses may represent promising targets for the development of novel pharmacotherapies for the treatment of alcoholism.
Keywords: alcoholism, anxiety, GABA, negative reinforcement, norepinephrine
INTRODUCTION
Anxiety and stress play an integral role in the etiology of addiction (Koob 2011). Additionally, alcohol dependent individuals with a comorbid anxiety disorder are more prone to relapse compared to dependent individuals who are not diagnosed with an anxiety disorder (Kushner et al. 2005). A number of neuromodulators that reduce anxiety-like behaviors are efficacious in reducing ethanol (EtOH) intake in rodents and EtOH craving in humans (Myrick et al. 2008), and many drugs used in alcohol dependent individuals to maintain abstinence or to treat EtOH withdrawal also reduce anxiety (Fox et al. 2012). Recent evidence supports a role for the noradrenergic system, which is activated during acute stress and/or anxiety, in reducing EtOH drinking in rodent models and reducing craving/relapse in alcohol dependent individuals (Verplaetse et al. 2011; Fox et al. 2012). Interestingly, several recent studies by us and others have shown that β3 adrenoceptor (AR) agonists decrease a range of anxiety-like behaviors in rodents (Stemmelin et al. 2008; Silberman et al. 2010), and peripherally, β3 adrenoceptor expression is upregulated following chronic EtOH intake in monkeys (Cheng et al. 2010). Together, these data suggest that β3-ARs may play a role in regulating anxiety-like behaviors and they are also susceptible to alterations by long-term EtOH exposure. However, the effects of β3-AR activation on EtOH drinking-related behaviors are not known.
The basolateral amygdala (BLA) has been well characterized for its role in mediating anxiety-like behaviors. Human and animal studies have shown that increases in BLA excitability are associated with an increase in anxiety-like behaviors, whereas BLA inactivation reduces anxiety measures in rodents (reviewed in Menard and Treit 1999). In accord, the BLA receives dense noradrenergic projections, primarily from the locus coeruleus, and norepinephrine levels rise in the BLA in response to stress or anxiety. The excitability of glutamatergic pyramidal projection neurons, the primary cell type in the BLA, is regulated by two distinct populations of GABAergic cells: local circuit interneurons (which serve as the primary source of feedback inhibition onto BLA pyramidal neurons) and lateral paracapsular cells (LPCs) (Marowsky et al. 2005). LPCs are densely clustered along the external capsule border of the BLA and provide the major source of feed-forward inhibition onto BLA pyramidal neurons. Our recent work in rats has shown that β3-AR activation selectively enhances LPC GABAergic synapses in the BLA with no effects on local inhibition or glutamatergic excitation. In addition, intra-BLA infusion of a selective β3-AR agonist significantly reduced measures of anxiety-like behavior without any motor depressant effects (Silberman et al. 2010).
Given the evidence that intra-BLA β3-AR activation can reduce anxiety-like behaviors and prior findings that many anxiolytic drugs are effective in reducing EtOH intake, the following experiments were conducted to assess the effect of BLA microinfusion of a β3-AR agonist (BRL 37344; BRL) on appetitive and consummatory EtOH drinking behaviors in adult male rats. Two separate EtOH exposure paradigms were used. In the first study, home cage EtOH drinking was assessed using an intermittent access, two-bottle choice paradigm (modified from Wise (1973)) that engenders pharmacologically relevant blood EtOH concentrations (BECs) in rodents (Simms et al. 2008; Chappell et al. 2013). Additionally, in two separate cohorts of rats, EtOH intake/seeking and sucrose intake/seeking were measured using an operant conditioning paradigm. Previous studies have shown that EtOH consumption and motivation to seek EtOH are distinct, uncorrelated processes (Samson and Czachowski, 2003). These measures can be procedurally separated using an operant paradigm requiring rats to complete a discrete number of lever presses for uninterrupted access to an EtOH or sucrose solution (Samson et al. 1998; Samson and Czachowski 2003). Consummatory behavior, measured by EtOH intake, is thought to reflect an unconditioned behavioral process. In contrast, appetitive behaviors, measured by lever responding, are thought to reflect learned processes that relate to motivation to seek EtOH (Samson et al. 1998). Both consummatory and appetitive behaviors are critical aspects of alcohol use disorders, and thus a model in which each component can be studied separately is critical for advancing our understanding of the neurocircuitry underlying motivation for, and consumption of, EtOH. We hypothesized in the current studies that intra-BLA β3-AR activation would reduce EtOH drinking and/or motivation to seek EtOH.
EXPERIMENTAL PROCEDURES
Subjects
Adult male Long-Evans rats (Harlan Laboratories, Indianapolis, IN USA) weighing approximately 200g upon arrival (aged approximately 5 weeks) were used in the following studies. Rats were given one week to acclimate to the laboratory and handling procedures before experiments were initiated. Rats were singly housed for the duration of all experiments and maintained on a 12-hour light/dark cycle (lights on 7:00A.M. to 7:00 P.M.). Rats had ad libitum food and water access in their home cages for the duration of the studies. Care of animals and experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011) and the Wake Forest University School of Medicine Animal Care and Use Committee.
Home Cage Two-Bottle Choice Intermittent Access Drinking Procedure
After one week of acclimation to the laboratory, one cohort of rats began home cage EtOH drinking following an intermittent access (IA), two-bottle choice design (N = 17; (Chappell and Weiner 2008). Using this model, rodents were given two bottles in their home cage containing 20% EtOH and water, respectively, on Mondays, Wednesdays, and Fridays. Water and EtOH consumption were measured daily after 30 minute and 24 hour (daily) access to EtOH. An EtOH preference ratio (EtOH drank/total fluid intake) was calculated at each time point. Data from our lab have previously shown that BECs at the 30min time point in this model (in rats showing similar intake levels) approximate 40mg/dl (Chappell et al., 2013). Water and EtOH were given in graduated drinking tubes (MED Associates, St. Albans, VT, USA), and the position of the bottles was alternated on each drinking day to control for potential side preferences. Following 3.5 weeks of baseline drinking, rats were implanted with bilateral intra-BLA cannulae (described below). Following a four day recovery period, rats resumed the IA, two-bottle choice procedure. During week five of drinking, bilateral microinjections of 0.9% saline were given to test the patency of the cannulae. For pharmacologic testing of the β3-AR agonist BRL 37344 (BRL) effects on home cage drinking and EtOH preference, a randomized Latin square design was implemented in which each rat received one bilateral microinjection weekly (each Wednesday) of 0.9% saline or BRL (0.1, 0.5, or 1.0 μg/side; Sigma-Aldrich Co., St. Louis, MO, USA) for a total of five microinjections. Additionally, each rat was given a sham microinjection once weekly (each Monday) and they were allowed EtOH access without an injection once weekly (each Friday) to maintain the intermittent access design. Rats were given ad libitum access to food throughout the drinking paradigm and were weighed daily.
Operant Conditioning Drinking Paradigm
A modified sucrose-fading procedure was used to train two separate cohorts of rats to respond on a lever for 10% EtOH or 3% sucrose (Samson 1986; Samson et al. 1999). These concentrations of EtOH and sucrose were chosen because they have been shown to produce similar levels of operant responding (Samson et al. 1998). All operant conditioning sessions were conducted in chambers housed in sound-attenuating enclosures (MED Associates). Each operant conditioning chamber contained one retractable lever, one retractable sipper tube, and a house light. Each chamber was also equipped with a fan for ventilation and additional noise to attenuate extraneous sounds outside of the chamber. The operant conditioning chambers were interfaced to a PC for online monitoring of lever presses and sipper licks achieved by each rat and data acquisition using MED-PC software (MED Associates).
Operant conditioning sessions were conducted Monday-Friday (between 0700–1000 am). Training was tailored to shape rats’ responding from an initial fixed ratio 1 (FR1) schedule and systematically increased to a response requirement of 16 (RR16). RR16 requires that each subject must emit 16 lever presses before being presented with the reinforcer. Subjects are then allowed 20 minutes of uninterrupted access to a sipper tube containing 10% EtOH or 3% sucrose (Samson et al. 1998; Samson et al. 1999). Each subject had 10 minutes to complete the RR16; however, on microinjection days, if RR16 was not achieved, the sipper was automatically extended to assess drug effects on consummatory measures. For EtOH drinking rats, training began with a solution of 10% sucrose only, and the sucrose concentration was gradually decreased as the EtOH concentration simultaneously increased, from 2% to 10% EtOH, over a three week period. Each respective EtOH/sucrose solution was presented for at least two consecutive operant sessions. Rats that were trained to respond for sucrose only were trained to lever press for decreasing concentrations of sucrose, from 10% to 3%, over a two week period. Baseline operant responding and intake was assessed for ten days and five days, respectively, in EtOH and sucrose rats, before an extinction probe trial was conducted. The extinction probe trial was carried out in the same manner as the self-administration sessions, except that the sipper tube was never extended and the dependent measure was the total number of lever presses completed during the 20 min session. Lever pressing during extinction probe trials may be interpreted as a measure of appetitive “EtOH seeking-like” behavior (Samson et al. 2003). Following establishment of stable drinking behavior and one extinction trial, rats were implanted with bilateral intra-BLA cannulae (described below). Operant conditioning sessions resumed two days following surgery. One week post-surgery, an additional five sessions were conducted to assess baseline responding and intake post-surgery. Upon confirmation that responding returned to pre-surgery baseline levels, a randomized Latin square design was implemented for bilateral intra-BLA administration of BRL (0.1, 0.5, or 1.0 μg/side) or 0.9% saline. Twice per week (Tuesdays and Fridays) for two weeks, microinjections were given five minutes prior to placement in the operant chamber. Operant conditioning sessions without microinjections were conducted on Mondays, Wednesdays, and Thursdays. Dependent variables included the latency to first lever press, time to complete lever response requirement from the start of the session, latency to first lick, and EtOH/sucrose intake. After testing the effect of BRL on operant responding and intake, the middle dose of BRL (0.5 μg/side) was tested for its effect on responding during an extinction probe trial. On these days, each rat received BRL (0.5 μg/side) or 0.9% saline one week apart, given five minutes prior to an extinction trial. Rats were allowed ad libitum access to water and rat chow in their home cage for the duration of the experiments. Blood EtOH concentrations (BECs) were measured from blood samples acquired from the lateral tail vein immediately following completion of a drinking session. These BEC samples were collected after the establishment of baseline responding and drinking on a RR16 schedule before surgeries were conducted (week 6). BECs were determined using a commercially available alcohol dehydrogenase/NADH enzymatic assay kit (Diagnostic Chemicals, Oxford, CT). N = 8 for the EtOH-drinking cohort and N = 7 the sucrose-drinking cohort, as one rat failed to complete the response requirement in two trials in the sucrose cohort.
Intra-BLA Cannulae Surgeries and Microinjection Procedure
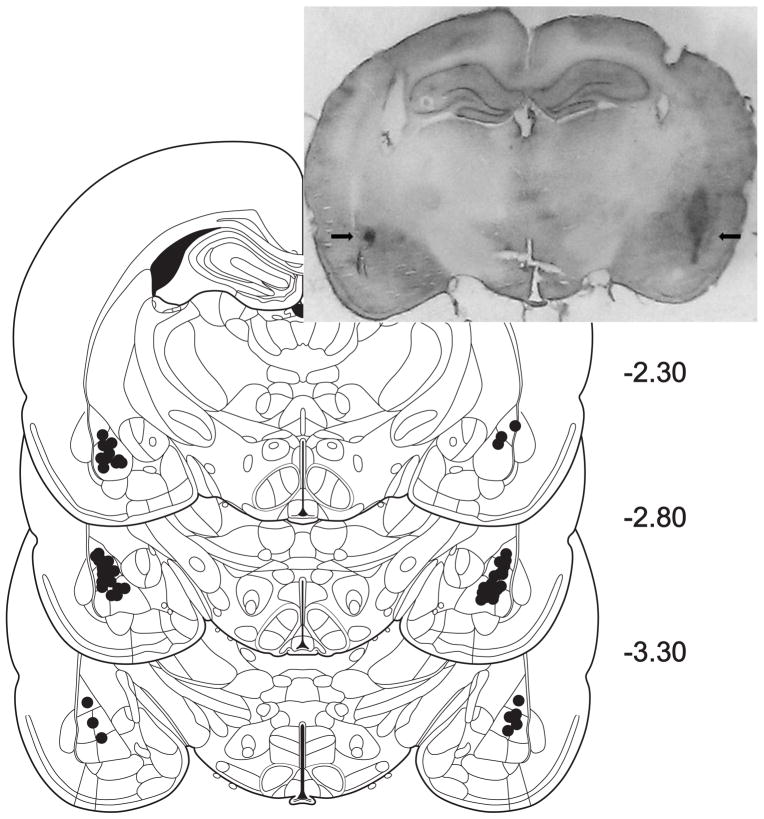
Surgery for intra-BLA placement of bilateral cannulae was conducted after subjects reached stable responding and drinking during training for the drinking paradigms detailed above, and each rat weighed at least 300g. Microinjection procedures were conducted in accordance with previous studies (McCool and Chappell 2007; Lack et al. 2008; Silberman et al. 2010). Subjects were surgically implanted with bilateral guide cannulae (26 gauge) targeted to terminate 1 mm above the BLA injection site (2.8 mm posterior to bregma, 8.2 mm ventral to bregma, and 5.0 mm lateral to the midline) and allowed to recover at least two days before habituation to the microinjection procedure and resuming behavioral testing. Prior to the BRL microinjections, the habituation procedure was repeated, though at this time subjects received an injection of vehicle (0.9% saline; 33 gauge injector cannulae) in a volume of 0.5 μl per hemisphere over 60 seconds to test the patency of the cannula. Injection needles were left in place for 30 seconds after injection and each subject remained in the holding tubs for an additional 3–4 minutes. Rats were given homecage access to EtOH or began their operant conditioning session five minutes following the microinjections. Upon completion of studies, rodents were perfused with buffered saline, followed by 10% formalin in saline. Brains were removed and stored in 10% formalin for at least one week, after which they were sliced coronally at 90 μm thickness using a freezing microtome. Slices were stained with cresyl violet and examined using a light microscope for verification of cannulae placement (for placements, see Fig. 1). Rats were included in behavioral analyses if both cannulae appropriately terminated in the BLA.
Fig. 1.
Bilateral intra-BLA cannulae placements for BRL microinjections. Numbers on the right indicate the anterior-to-posterior position of each coronal brain section, relative to Bregma. This figure was modified from Paxinos & Watson (1997). Inset: Representative photomicrograph illustrating cannulae placements within the BLA (black arrows).
Statistical Analyses
For home cage, two-bottle choice EtOH self administration data, a one-way repeated measures ANOVA (factor: treatment) was conducted to assess the effect of BRL on EtOH intake. Water and EtOH intake were also recorded on Mondays and Fridays, but these data were not included in the statistical analyses. Student-Neumann-Keuls (SNK) post-hoc effects were interpreted when appropriate. Appetitive and consummatory behaviors measured in the operant conditioning paradigm were also analyzed using a one-way repeated measures ANOVA (factor: treatment), with SNK post-hoc effects interpreted when appropriate. If the data were not normally distributed, the non-parametric Friedman Repeated Measures Analysis of Variance on Ranks test was employed. For lever press data gathered during extinction trials, paired t-tests were conducted. t-tests were also conducted to compare level of responding for sucrose and EtOH between cohorts. Data were analyzed and graphs were generated using SigmaPlot 11.0 and GraphPad Prism4 software. The significance level for all analyses was set at p < 0.05.
RESULTS
Intra-BLA Administration of BRL Reduces Home Cage EtOH Drinking
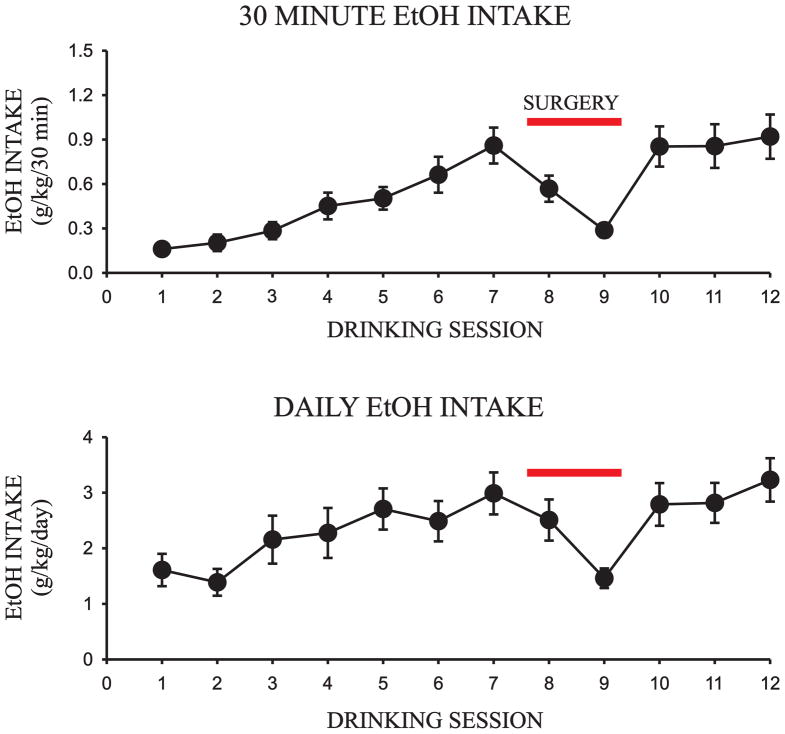
Intermittent access to 20% EtOH presented in the home cage has been shown to result in relatively high EtOH consumption in outbred rat strains without the use of food or water restriction (Wise 1973; Simms et al. 2008; Chappell et al. 2013). Over the four week drinking acclimation period, rats steadily increased their 30 minute and daily EtOH intake from session one (30 minute intake: 0.16 ± 0.02 g/kg; daily intake: 1.61 ± 0.29 g/kg; Fig. 1) to session 12 (30 minute intake: 0.92 ± 0.15 g/kg; daily intake: 3.23 ± 0.39 g/kg; Fig. 1). All rats remained healthy throughout the home cage drinking procedure, gaining 179.1 ± 4.9g throughout the four week drinking acclimation period, and an additional 68.9 ± 3.1g during the four week microinjection phase of the experiment (N = 17).
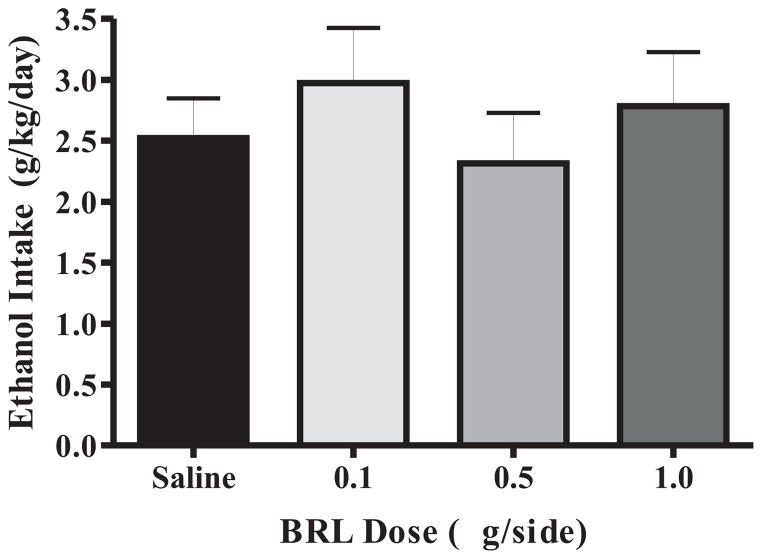
The goal of the first study was to assess whether intra-BLA activation of β3-ARs by BRL would reduce home cage EtOH intake. Each rat received one drug or saline microinjection weekly (0.9% saline; 0.1, 0.5, 1.0 μg/side BRL; Wednesdays). A one-way RM ANOVA showed no significant effect of any dose of BRL compared to saline on EtOH intake at 30 minutes or 24 hours (Fig. 3). A one-way ANOVA for EtOH preference at 30 minutes indicated that 1.0 μg/side BRL significantly increased EtOH preference relative to saline (F(3,59) = 3.626, p < 0.05). BRL had no effect on EtOH preference over the 24h period. Two rats were excluded from analysis due to improper cannula placements (N = 15).
Fig. 3.
EtOH intake at 30 minutes in the two-bottle, intermittent access home cage paradigm is unaffected by BRL (N = 15).
Intra-BLA Administration of BRL Reduces Operant Responding for EtOH
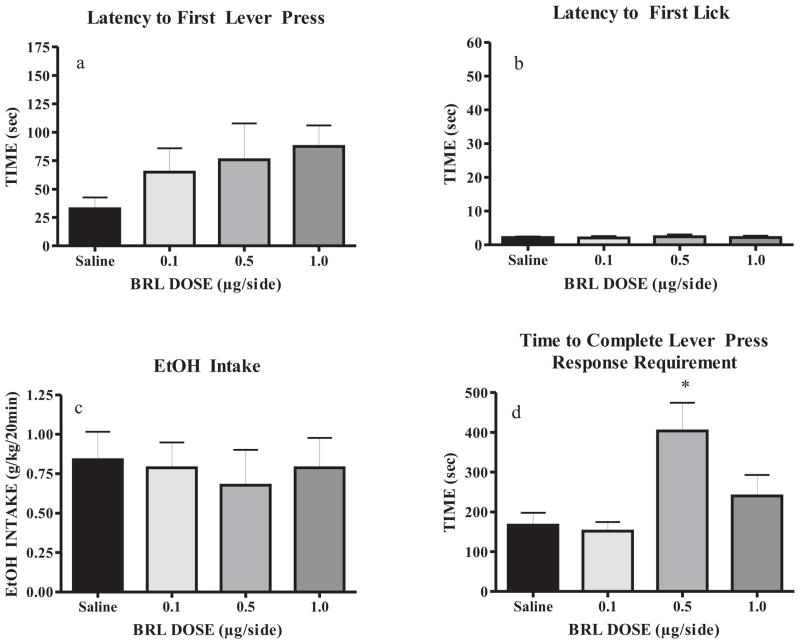
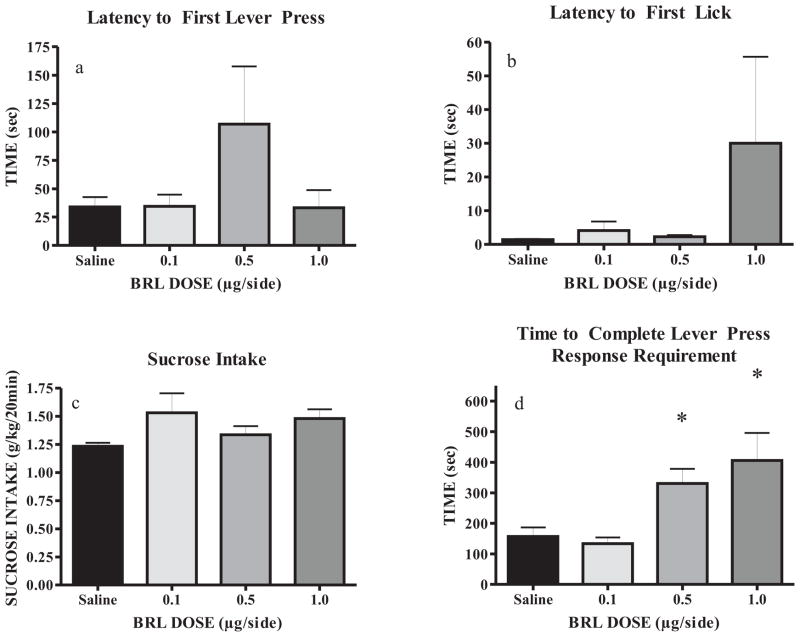
The operant self-administration paradigm used in the current studies was implemented to procedurally separate consummatory and appetitive EtOH drinking-related behaviors. All rats remained healthy throughout the study, gaining an average of 179.0 ± 6.9g throughout training and prior to surgery, and another 69.9 ± 4.4g post-surgery and through the microinjection procedures. Upon achieving stable baseline drinking and prior to microinjection studies, BECs achieved during the 20 min EtOH access period reached 69.5 (±13.1) mg/dl. Bilateral microinjections were given to each rodent (0.9% saline, 0.1, 0.5, and 1.0 BRL μg/side) on Tuesday and Friday for two weeks, and rats were placed into the operant chamber five minutes after drug administration. A repeated measures one-way ANOVA found no significant effect of BRL compared to saline on the latency to the first lever press or latency to first lick (Fig. 4a and 4b), thus suggesting that BRL did not acutely impair locomotion or task-specific memory. Additionally, EtOH intake (g/kg) over the 20 min session was not affected by BRL administration (Fig. 4c). However, a main effect of drug treatment was observed for the time taken to complete the response requirement of 16 lever presses (F(3,31) = 1.012, p < 0.01; Fig. 4d). Post-hoc SNK tests showed that administration of the 0.5 μg/side dose of BRL significantly increased the appetitive measure of total time to complete the lever response requirement for access to EtOH (p < 0.05), resulting in more than twice the amount of time taken to complete the response requirement compared to saline. It should be noted that three rats failed to complete the RR16, but the sipper tube was automatically extended after 10min. Neither the 0.1 nor the 1.0 μg/side dose of BRL affected the time to complete the lever response requirement. These data suggest that intra-BLA administration of BRL (0.5 μg/side) reduces appetitive drive for EtOH seeking without altering subsequent consummatory behavior.
Fig. 4.
BRL (0.5 μg/side) significantly increases appetitive drive for EtOH by increasing time required to complete the response requirement (panel d), without affecting locomotion or EtOH intake (panels a–c) (N = 8). *p < 0.01 vs. saline
Intra-BLA Administration of BRL Reduces Extinction Responding for EtOH
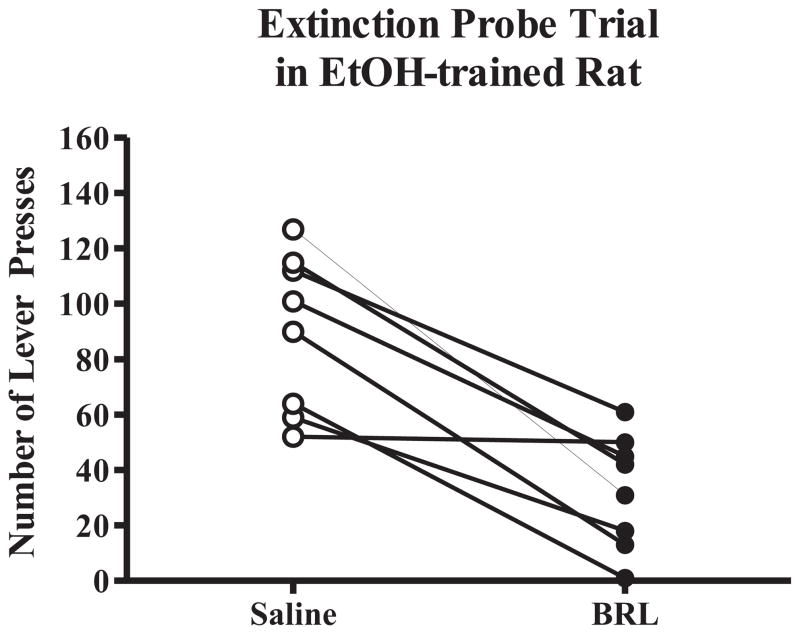
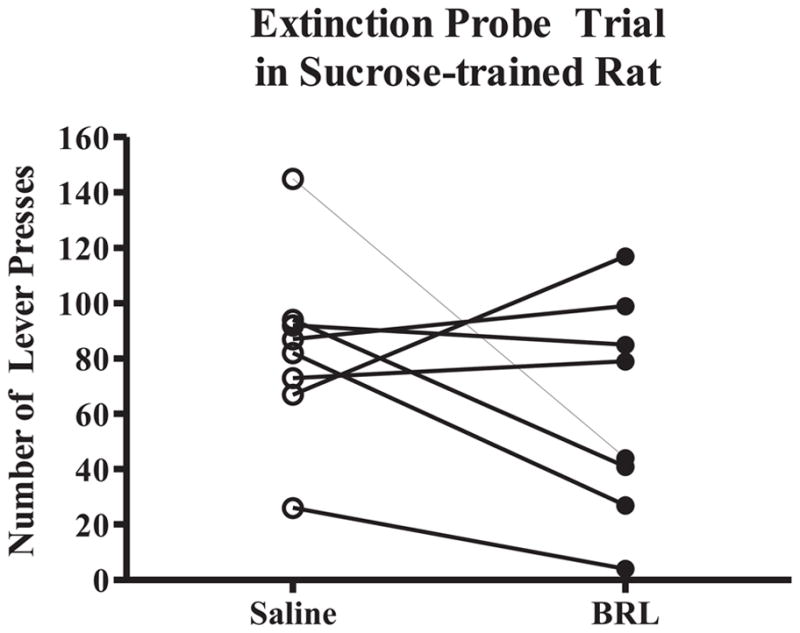
Extinction probe trials were also conducted to test appetitive responding for EtOH after microinjections of saline or the middle dose of BRL (0.5 μg/side) that reduced appetitive responding for EtOH during an operant self-administration session. Total lever presses were recorded for the 20 minute session in the operant chamber, during which time the sipper was never extended. The data revealed a significant decrease in total lever presses following intra-BLA administration of BRL compared to total lever presses following intra-BLA saline (t = 4.677, p < 0.01; Fig. 5). Following BRL, rats made an average of 32.6 (± 7.73) lever presses as compared to an average of 90.0 (± 10.1) lever presses following saline microinjection. Thus, decreased motivation to seek EtOH following administration of BRL is supported by an increased amount of time required to complete the response requirement for access to EtOH, and significantly reduced appetitive responding during the extinction trial.
Fig. 5.
BRL (0.5 μg/side) significantly reduced appetitive responding during the extinction probe trial compared to saline (N = 8). *p < 0.01 vs. saline
Intra-BLA Administration of BRL Reduces Operant Responding for Sucrose
A separate cohort of rats was trained in the operant paradigm for 20 minute access to 3% sucrose. Previous studies have shown that 10% EtOH and 3% sucrose engender similar levels of lever responding, and thus have similar reinforcing efficacy (Samson et al. 1998). Indeed, in comparing the two cohorts used in these studies on extinction responding prior to surgery/microinjections and following the establishment of baseline operant responding, t-tests showed no difference between the EtOH and sucrose cohorts on total number of lever presses or latency to the first lever press (data not shown).
Prior to microinjections, baseline sucrose intake was stable and reached an average of 1.47 ± 0.04 g/kg for the 20 minute access period. As detailed above, bilateral microinjections were given to each rodent (0.9% saline, 0.1, 0.5, and 1.0 BRL μg/side) each Tuesday and Friday for two weeks, and rats were placed into the operant conditioning chamber five minutes after drug administration. One rat was removed from analyses of operant parameters for failure to complete the response requirement following two separate doses of BRL (0.5 and 1.0 μg/side). A repeated measures one-way ANOVA revealed that data for the latency to the first lever press and latency to the first lick were not normally distributed; therefore, a Friedman Repeated Measures Analysis of Variance on Ranks was conducted, showing no significant effect of BRL on these dependent measures (Figs. 6a and 6b). BRL administration also did not affect total sucrose intake Fig. 6c). However, as observed with EtOH drinking rats, BRL administration significantly increased the total time taken to complete the response requirement (F(3, 27) = 8.181, p < 0.01; Fig. 6d). Post-hoc SNK tests revealed that administration of both 0.5 and 1.0 μg/side dose of BRL increased the total time taken to complete the response requirement compared to saline (ps < 0.05). Taken together, these data suggest that intra-BLA infusion of the β3-AR agonist BRL can reduce a measure of appetitive sucrose seeking behavior.
Fig. 6.
BRL (0.5 and 1.0 μg/side) significantly increased the total time taken to complete the response requirement for sucrose (panel d), without affecting locomotion or sucrose intake (panels a–c; N = 7). *p < 0.05 vs. saline
Intra-BLA Administration of BRL Does Not Reduce Extinction Responding for Sucrose
Extinction probe trials were also conducted to test appetitive responding for sucrose after microinjections of saline or the middle dose of BRL (0.5 μg/side) that reduced appetitive responding for EtOH during an operant conditioning test session and extinction. Total lever presses were recorded for the 20 minute session in the operant conditioning chamber. In contrast to EtOH-trained rats, the total number of lever presses was not reduced following intra-BLA administration of BRL compared to total lever presses following intra-BLA saline (Fig. 7).
Fig. 7.
BRL (0.5 μg/side) did not significantly (n.s.) reduce appetitive responding during the extinction probe trial compared to saline (N = 8)
DISCUSSION
We recently demonstrated that activation of β3-ARs selectively potentiates feed-forward GABAergic synapses from LPC interneurons onto BLA pyramidal cells in rat brain slices (Silberman et al. 2010). We also found that intra-BLA infusion of BRL, a β3-AR agonist, can decrease anxiety-like behaviors, suggesting that enhancement of LPC inhibition can reduce BLA pyramidal cell excitability enough to induce anxiolysis. In this study, we used BRL to examine the effects of in vivo enhancement of LPC GABAergic synapses on appetitive and consummatory EtOH drinking-related behaviors. Our results suggest that BRL-mediated potentiation of LPC inhibition preferentially reduces appetitive, rather than consummatory, EtOH drinking-related behaviors. In an operant model that procedurally separates appetitive and consummatory behaviors, BLA microinfusion of BRL significantly reduced appetitive measures with no significant effect on consummatory behaviors. Intra-BLA infusion of BRL also reduced some, but not all, appetitive measures of operant sucrose self-administration. Together, these data provide the first evidence that LPC GABAergic synapses may play an important role in appetitive responding for access to a reinforcer, presumably via an inhibition of BLA pyramidal cell excitability.
The finding that BRL selectively decreased appetitive or motivational elements of EtOH and sucrose self-administration, with minimal effects on consummatory measures, is not unprecedented. Microinjection of raclopride, a dopamine D2 receptor antagonist, into the nucleus accumbens core similarly reduces appetitive responding for EtOH without affecting EtOH intake (Samson and Chappell 2004). In addition, both naltrexone and acamprosate, drugs approved for the treatment of alcohol dependence, reduce responding for both EtOH and sucrose using an operant procedure similar to that used in the current studies (Czachowski and Delory 2009). Sabino et al. (2011) also showed that increasing motivation to seek EtOH on a PR schedule of reinforcement by administration of a sigma receptor agonist similarly increased motivation to seek sucrose and saccharin (Sabino et al. 2011). Importantly, intra-BLA activation of β3-ARs reduced EtOH seeking behaviors without having a depressant effect on locomotor behavior or a general anhedonia-like effect, as approach times to the lever and the sipper tube were not reduced by BRL whether EtOH or sucrose was the available reinforcer. In fact, systemic administration of β3-AR agonists has been shown to have anti-anxiety and anti-depressant-like effects in the forced swim test and social interaction test when given either acutely or chronically, without affecting locomotion (Consoli et al. 2007; Tamburella et al. 2010). Moreover, we recently found that intra-BLA infusion of BRL reduced measures of unconditioned anxiety-like behavior in Long Evans rats without any detectable impairment in locomotor function (Silberman et al. 2010).
It is noteworthy that BRL reduced the time to complete the lever press response requirement for both EtOH and sucrose responding, but in the extinction probe trials, BRL only reduced lever presses in EtOH-trained rats. This finding cannot be attributed to a difference in the reinforcing strength of the two reinforcers as there were no differences in baseline extinction responding for 10% EtOH and 3% sucrose. However, separate processes may govern motivation to respond when a reinforcer is available vs. motivated behavior in the absence of the reinforcer. Motivation to respond for EtOH in an operant task varies as a function of the schedule of reinforcement (e.g, fixed ratio vs. variable interval; Hay et al. 2013). Also, other studies have noted no drug-induced change in alcohol self-administration behavior on an operant schedule, but a significant suppression of EtOH extinction responding without any effect on either measure for sucrose (Colombo et al. 2003). Alternatively, these findings may reflect the fact that sucrose is a natural reinforcer, with taste representing the primary cue that reinforces its consumption. In contrast, EtOH is not a natural reinforcer but rather is thought to become a reinforcer only as a result of subjective experience with the interoceptive effects of this drug (Samson et al. 2000). It is possible that LPC inhibition of BLA pyramidal neurons may play a greater role in regulating appetitive behaviors that require repeated exposure to the reinforcer, such as those associated with drugs of abuse, like EtOH. The selectivity of BRL’s effects on appetitive vs. consummatory behaviors is also consistent with our evidence that the primary effect of this drug within the BLA is to enhance LPC-mediated feed-forward inhibition onto BLA pyramidal neurons (Silberman et al. 2010). β3-AR selective concentrations of BRL potentiated LPC, but not local GABAergic synapses onto BLA pyramidal neurons without any effects on glutamatergic excitation or intrinsic excitability of BLA neurons themselves. It is generally well established that distinct, albeit overlapping, circuits underlie appetitive and consummatory behaviors (Kelley et al. 2005). Moreover, previous studies using the operant model employed here have noted no correlation between EtOH intake and extinction responding (Chappell and Weiner 2008); again, supporting the notion that divergent neurobiological mechanisms govern motivation to seek EtOH versus consumption when access is no longer contingent upon responding. There is compelling evidence that the BLA (Tye et al. 2008), and the excitatory projection from the BLA to the nucleus accumbens, play an integral role in appetitive, seeking-like responding (Koob 2009; Belujon and Grace 2011). For example, a recent optogenetic study found that the selective activation of BLA glutamatergic efferents to the nucleus accumbens (NAc) was sufficient to serve as a reinforcer in an operant paradigm, and that inhibition of this excitatory drive from the BLA to the NAc decreased appetitive responding (Stuber et al. 2011). Similarly, Ambroggi et al. (2008) showed that activation of the BLA projections to the NAc were required for enhancement of dopamine in the NAc in response to reward-related cues. Our data are consistent with the idea that intra-BLA administration of BRL decreases BLA excitability via a potentiation of LPC-mediated inhibition of pyramidal cells in this region. This would result in a reduction in glutamatergic drive to the NAc, and thus, reduced motivated behavioral responding for reinforcers.
It should also be noted that the dose-dependence of BRL effects were clearly biphasic. In the operant procedure, only the 0.5 μg/side dose reduced the appetitive measure of time to complete the lever press requirement. The biphasic nature of these effects may well be explained by the fact that, while BRL is a selective agonist at β3-ARs, it does have some affinity for β1 and β2 receptors (Oriowo et al. 1996; Clouse et al. 2007). Since activation of β1/2 receptors in the BLA is primarily excitatory, due to a strong enhancement of glutamatergic excitation of BLA pyramidal neurons (Abraham et al. 2008; Liebmann et al. 2009), it is possible that the highest dose of BRL that we tested increased excitability in the BLA appetitive circuitry. Also of note in the EtOH homecage self-administration procedure, the high dose of BRL (1.0 μg/side) increased the EtOH preference ratio at 30min without affecting EtOH intake. It is important to note that this dose did not affect EtOH intake or motivation to gain access to EtOH in the operant paradigm, though this dose significantly decreased motivation to seek sucrose reinforcement. It will be of interest in future studies to determine if combining a β3-AR agonist with a selective β 1/2-AR antagonist may prove more effective in reducing ethanol seeking behaviors.
To date, the α1-AR has been the adrenergic receptor most studied in the context of EtOH drinking and EtOH seeking (Walker et al. 2008; Rasmussen et al. 2009; Verplaetse et al. 2011). For instance, IP administration of the α1-AR antagonist prazosin was shown to decrease EtOH drinking (consummatory behavior) in alcohol-preferring P rats (Rasmussen et al. 2009; Verplaetse et al. 2011), and EtOH seeking (appetitive behavior) in P rats and Wistar rats (Walker et al. 2008; Verplaetse et al. 2011). To date, relatively little research has been conducted regarding the expression and function of β3-ARs in the CNS, as the expression of these receptors was initially thought to be primarily localized in peripheral tissues (e.g., brown adipose tissue). However, a few reports have shown that β3-AR mRNA is expressed in human (Rodriguez et al. 1995), mouse, and rat brain (Summers et al. 1995; Claustre et al. 2008), and electrophysiological data from our lab suggest that, within the BLA, functional β3-ARs are restricted to LPC GABAergic synapses (Silberman et al. 2008). Many receptor systems targeted for the treatment of neuropsychiatric disorders fail, in part, because of their widespread CNS expression and deleterious side-effect profiles. The relatively restricted expression of β3-ARs in the CNS, coupled with the absence of locomotor impairment associated with systemic or intra-BLA administration of β3-AR agonists, suggest that these receptors, and LPC synapses, may represent promising targets for future pharmacotherapies directed at addictive and anxiety disorders.
One final issue that bears comment relates to recent findings from our lab that rearing history can have a profound and enduring effect on EtOH drinking behaviors and anxiety measures in male Long Evans rats. Rats socially isolated during adolescence exhibit long-lasting increases in appetitive and consummatory measures of EtOH drinking (Chappell et al. 2013; McCool and Chappell 2009) relative to rats that were group-housed during this critical adolescent period. Adolescent social isolation also results in increases in anxiety-like behavior that endure for at least four months, even when the behavior of these subjects is compared to adolescent group-housed animals that were then isolated in adulthood (Yorgason et al. 2013). Importantly, we have also discovered that rats procured from our commercial supplier (Harlan, IN) as adults exhibit an anxiety-like and EtOH drinking phenotype similar to that of rats that have been isolated during adolescence (Chappell et al. 2013). Although subjects used in these experiments were somewhat younger than the adult rats used in our prior study (appr. 7 vs. 10 weeks old), subjects were singly housed for the duration of these studies and other ongoing studies in our lab suggest that even rats of this age do exhibit relatively high levels of anxiety-like behavior. Systemic (Consoli et al. 2007; Silberman et al. 2010; Tamburella et al. 2010) and intra-BLA infusion (Silberman et al. 2010) of β3-AR agonists reduce a broad range of anxiety-like measures and many drugs with anti-anxiety like effects are effective in reducing EtOH self-administration, particularly in EtOH dependent subjects with increased anxiety-like behavior (Funk et al. 2006; Gilpin et al. 2008). Therefore, future studies will be needed to determine if BRL is particularly effective at suppressing appetitive responding for EtOH in subjects that exhibit high levels of anxiety-like behavior (e.g. socially isolated vs. group housed rats; EtOH dependent vs. non-dependent rats). Such findings would suggest that, while BRL likely inhibits ethanol seeking behavior at least in part by decreased BLA activation of accumbal circuitry, other BLA circuits that regulate anxiety-like behaviors (eg. BLA to central amygdala), may also contribute to the inhibition of seeking behaviors, particularly in subjects with elevated anxiety-like behavior.
In summary, the results of these studies demonstrate that intra-BLA microinfusion of a β3-AR agonist results in a robust, dose-dependent, and relatively selective, inhibition of appetitive EtOH drinking-related behaviors in male Long Evans rats. Given that BRL selectively increases LPC-mediated feed-forward inhibition of BLA pyramidal cells, our findings provide the first evidence that enhancement of LPC GABAergic synapses may be sufficient to regulate appetitive circuitry in the mammalian CNS. The relatively sparse expression of β3-ARs in the brain, coupled with the absence of locomotor side effects and reduced anxiety-like behaviors associated with β3-AR agonists, suggest that these receptors, and LPC synapses, may represent promising targets for the development of novel treatments for alcohol dependence, particularly in individuals who also suffer from anxiety disorders.
Fig. 2.
Escalation of EtOH intake in the two-bottle, intermittent access home cage paradigm over the first four weeks of the procedure (12 sessions; N = 17). a) 30 minute EtOH intake (g/kg); b) Daily EtOH intake (g/kg)
Table 1.
EtOH preference ratio in the two-bottle, intermittent access homecage paradigm at 30 minutes and 24 hours. Unexpectedly, 1.0 μg/side BRL significantly increased EtOH preference at 30 minutes.
| Saline | 0.1 μg/side | 0.5 μg/side | 1.0 μg/side | |
|---|---|---|---|---|
| 30 minutes | 0.53 ± 0.06 | 0.68 ± 0.07 | 0.67 ± 0.04 | 0.84 ± 0.03* |
| 24 hours | 0.27 ± 0.05 | 0.30 ± 0.04 | 0.25 ± 0.05 | 0.28 ± 0.05 |
p<0.05 vs. saline
Acknowledgments
This work was supported by National Institutes of Health Grants AA 21099, AA 17056, AA 17531, and AA 10422 and T32 AA 7565.
Supported by AA 21099, AA 17531, and 5 T32 AA 7565-18.
References
- Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH, Li H. beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res. 2008;1209:65–73. doi: 10.1016/j.brainres.2008.02.082. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male long evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Weiner JL. Relationship between ethanol’s acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res. 2008;32:2088–2099. doi: 10.1111/j.1530-0277.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Grant KA, Han QH, Daunais JB, Friedman DP, Masutani S, Little WC, Cheng CP. Up-regulation and functional effect of cardiac beta3-adrenoreceptors in alcoholic monkeys. Alcohol Clin Exp Res. 2010;34:1171–1181. doi: 10.1111/j.1530-0277.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claustre Y, Leonetti M, Santucci V, Bougault I, Desvignes C, Rouquier L, Aubin N, Keane P, Busch S, Chen Y, Palejwala V, Tocci M, Yamdagni P, Didier M, Avenet P, Le Fur G, Oury-Donat F, Scatton B, Steinberg R. Effects of the beta3-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: relevance to its antidepressant/anxiolytic-like profile. Neuroscience. 2008;156:353–364. doi: 10.1016/j.neuroscience.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Clouse AK, Riedel E, Hieble JP, Westfall TD. The effects and selectivity of beta-adrenoceptor agonists in rat myometrium and urinary bladder. Eur J Pharmacol. 2007;573:184–189. doi: 10.1016/j.ejphar.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology (Berl) 2003;167:221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Consoli D, Leggio GM, Mazzola C, Micale V, Drago F. Behavioral effects of the beta3 adrenoceptor agonist SR58611A: is it the putative prototype of a new class of antidepressant/anxiolytic drugs? Eur J Pharmacol. 2007;573:139–147. doi: 10.1016/j.ejphar.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RA, Jennings JH, Zitzman DL, Hodge CW, Robinson DL. Specific and Nonspecific Effects of Naltrexone on Goal-Directed and Habitual Models of Alcohol Seeking and Drinking. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit Disorder. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29:1432–1443. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann L, Karst H, Joels M. Effects of corticosterone and the beta-agonist isoproterenol on glutamate receptor-mediated synaptic currents in the rat basolateral amygdala. Eur J Neurosci. 2009;30:800–807. doi: 10.1111/j.1460-9568.2009.06882.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell A. Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is ‘state’ dependent. Behav Brain Res. 2007;178:70–81. doi: 10.1016/j.bbr.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriowo MA, Chapman H, Kirkham DM, Sennitt MV, Ruffolo RR, Jr, Cawthorne MA. The selectivity in vitro of the stereoisomers of the beta-3 adrenoceptor agonist BRL 37344. J Pharmacol Exp Ther. 1996;277:22–27. [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Carillon C, Coquerel A, Le Fur G, Ferrara P, Caput D, Shire D. Evidence for the presence of beta 3-adrenergic receptor mRNA in the human brain. Brain Res Mol Brain Res. 1995;29:369–375. doi: 10.1016/0169-328x(94)00274-i. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell AM. Effects of raclopride in the core of the nucleus accumbens on ethanol seeking and consumption: the use of extinction trials to measure seeking. Alcohol Clin Exp Res. 2004;28:544–549. doi: 10.1097/01.alc.0000121649.81642.3f. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Chappell A, Legg B. Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol. 2003;31:77–86. doi: 10.1016/j.alcohol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res. 2000;24:766–773. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology. 2010;35:1886–1896. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Francon D, Alonso R, Stahl SM, Keane P, Avenet P, Scatton B, le Fur G, Griebel G. Stimulation of the beta3-Adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116:2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburella A, Micale V, Leggio GM, Drago F. The beta3 adrenoceptor agonist, amibegron (SR58611A) counteracts stress-induced behavioral and neurochemical changes. Eur Neuropsychopharmacol. 2010;20:704–713. doi: 10.1016/j.euroneuro.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of Prazosin, an alpha(1) -Adrenergic Receptor Antagonist, on the Seeking and Intake of Alcohol and Sucrose in Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–31. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]