Abstract
Voltage-gated calcium (CaV) channels catalyze rapid, highly selective influx of Ca2+ into cells despite 70-fold higher extracellular concentration of Na+. How CaV channels solve this fundamental biophysical problem remains unclear. Here we report physiological and crystallographic analyses of a calcium selectivity filter constructed in the homotetrameric bacterial NaV channel NaVAb. Our results reveal interactions of hydrated Ca2+ with two high-affinity Ca2+-binding sites followed by a third lower-affinity site that would coordinate Ca2+ as it moves inward. At the selectivity filter entry, Site 1 is formed by four carboxyl side-chains, which play a critical role in determining Ca2+ selectivity. Four carboxyls plus four backbone carbonyls form Site 2, which is targeted by the blocking cations, Cd2+ and Mn2+, with single occupancy. The lower-affinity Site 3 is formed by four backbone carbonyls alone, which mediate exit into the central cavity. This pore architecture suggests a conduction pathway involving transitions between two main states with one or two hydrated Ca2+ ions bound in the selectivity filter and supports a “knock-off” mechanism of ion permeation through a stepwise-binding process. The multi-ion selectivity filter of our CaVAb model establishes a structural framework for understanding mechanisms of ion selectivity and conductance by vertebrate CaV channels.
Ca2+ ions flow through voltage-gated Ca2+ (CaV) channels at a rate of ~106 ions/s, yet Na+ conductance is >500-fold lower1. Such high-fidelity, high-throughput CaV channel performance is important to regulate intracellular processes such as contraction, secretion, neurotransmission, and gene expression in many different cell types2. Because the extracellular concentration of Na+ is 70-fold higher than Ca2+, these essential biological functions require CaV channels to be highly selective for Ca2+ in preference to Na+, even though Ca2+ and Na+ have nearly identical diameter (~2 Å). Ion selectivity of CaV channels is proposed to result from high-affinity binding of Ca2+, which prevents Na+ permeation. Fast Ca2+ flux through CaV channels is thought to utilize a ‘knock-off’ mechanism in which electrostatic repulsion between Ca2+ ions within the selectivity filter overcomes tight binding of a single Ca2+ ion1,3–8. Most of these mechanisms require a multi-ion pore, yet extensive mutational analyses of ion selectivity and cation block of vertebrate CaV channels support a single high-affinity Ca2+ binding site1,9–14.
CaV channels contain a single ion-selective pore in the center of four homologous domains2. The central pore is lined by the S5 and S6 transmembrane helices and the intervening “P-loop” from each domain in a four-fold pseudosymmetric arrangement. The four voltage-sensing modules composed of S1–S4 transmembrane helices are symmetrically arranged around the central pore. CaV channels are members of the voltage-gated ion channel superfamily and are closely related to voltage-gated Na+ (NaV) channels. The structures of three homotetrameric bacterial NaV channels open the way to elucidating the structural basis for ion selectivity and conductance of vertebrate NaV and CaV channels15–17, which likely evolved from the bacterial NaChBac family and retained similar structures and functions (Supplementary Fig. 1)18–20. Interestingly, mutation of three amino acid residues in the selectivity filter of NaChBac is sufficient to confer Ca2+ selectivity21. We have introduced analogous mutations into the bacterial NaV channel NaVAb to create CaVAb and carried out electrophysiological and X-ray crystallographic analyses to determine the relative permeability of Ca2+ and define ion-binding sites in the selectivity filter. Our systematic analyses of CaVAb and intermediate derivatives provide structural and mechanistic insights into Ca2+ binding and ion permeation and suggest a conductance mechanism involving two energetically similar ion occupancy states with one or two hydrated Ca2+ ions bound.
Structure and function of CaVAb
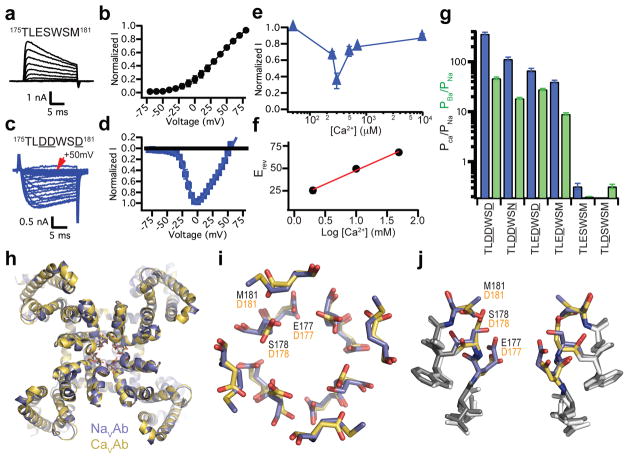
NaVAb channels have four identical pore motifs (175TLESWSM181) that form the ion selectivity filter15. The side chains of E177 form a high-field-strength site (SiteHFS) at the outer end of the filter, while two additional potential Na+ coordination sites, SiteCEN and SiteIN, are formed by the backbone carbonyls of L176 and T17515. To create CaVAb, E177, S178, and M181 were substituted with Asp, resulting in a mutant with the pore motif 175TLDDWSD181 (underlined letters indicate mutated residues). CaVAb was expressed in Trichopulsia ni cells (Hi5) and analyzed by whole-cell voltage clamp to determine its ion selectivity. In contrast to NaVAb, which does not conduct extracellular Ca2+ ions but carries outward Na+ current (Fig. 1a, b), CaVAb conducts inward Ca2+ current in a voltage-dependent manner (Fig. 1c, d). Complete titration curves for Ca2+ in the presence of Ba2+ as the balancing divalent cation (see Methods) revealed inhibition of Ba2+ current by low concentrations of Ca2+ followed by increases in Ca2+ current at higher Ca2+ concentrations (Fig. 1e). These results demonstrate the anomalous mole fraction effect characteristic of vertebrate CaV channels. Comparable experiments with Na+ as the balancing cation were not possible because of the instability of the Hi5 cells in solutions with low divalent cation concentrations. The reversal potential for Ca2+ current under bi-ionic conditions closely follows the expectation for a highly Ca2+-selective conductance (30.6 ± 2.3 mV/decade, Fig. 1f; Supplementary Fig. 2), and CaVAb selects Ca2+ 382-fold over Na+ under our standard recording conditions, yielding >10,000-fold PCa:PNa range for these constructs (Fig. 1g). Intermediate CaVAb derivatives with single and double Asp substitutions had progressive increases in Ca2+ selectivity (Fig. 1g; Supplementary Fig. 2), as observed for NaChBac21. The 175TLDDWSN181 mutant has an Asn residue in place of the final Asp, as observed in one domain of mammalian CaV channels (Supplementary Fig. 1), and it still favors Ca2+ over Na+ by more than 100-fold (Fig. 1g).
Figure 1. Structure and function of the CaVAb channel.
a, b, Outward Na+ current conducted by NaVAb with 10 mM extracellular Ca2+ and 140 mM intracellular Na+. Holding potential, −100 mV; 20-ms, 10-mV step depolarizations. c, d, Voltage-dependent conductance of inward Ca2+ current by CaVAb under the same conditions. 20-ms, 5-mV step depolarizations. e, Biphasic anomalous mole fraction effect of increasing Ca2+ as indicated, with Ba2+ as the balancing divalent cation: 10 mM Ba2+ with 0 to 0.5 mM Ca2+, 9.3 mM Ba2+ with 0.7 mM Ca2+, and 0 mM Ba2+ with 10 mM Ca2+ (n = 4–10). f. Reversal potential (Erev) versus Ca2+ concentration. g, Relative permeability of CaVAb and its derivatives as measured from bi-ionic reversal potentials. PCa/PNa, blue; PBa/PNa, green (n=5–22). h, Cartoon representation of the overall structure of CaVAb (yellow) superimposed with NaVAb (slate). i, j, Top and side views of the superimposed selectivity filters of CaVAb (yellow) and NaVAb (slate) in stick representation. The three original NavAb residues (black) and substituted CavAb residues (orange) are indicated. Errors bars in a–g are standard error of the mean.
We have crystallized and determined the structure of CaVAb and its derivatives by molecular replacement using the NaVAb structure (PDB code 3RVY) as the search template (Supplementary Table 1). The overall structure of CaVAb is very similar to that of NaVAb, with an RMSD of 0.4 Å (Fig. 1h). However, the electrostatic potential at the outer entry to the selectivity filter is more negative for CaVAb than for NaVAb (Supplementary Fig. 3). The three negatively charged Asp residues introduced at the selectivity filter of CaVAb create a wide, short, electronegatively lined pore (6 Å diameter, 10 Å length) with no significant alteration in backbone structure with respect to NaVAb (Fig. 1i, j, Supplementary Fig. 4). Thus, Ca2+ selectivity of CaVAb is mainly determined by the side chains of the amino acids at the selectivity filter.
Ca2+-binding sites in the permeation pathway
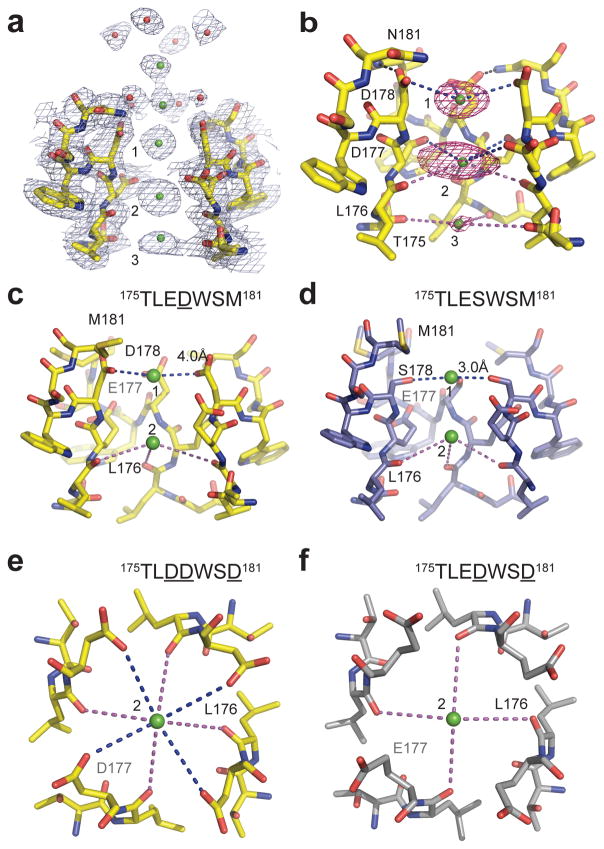
The 3.2 Å resolution structure of the mutant 175TLDDWSN181 in the presence of 10 mM Ca2+ reveals electron densities in the selectivity filter consistent with three Ca2+ ions aligned on the central axis (Fig. 2a). In the outer vestibule leading to the selectivity filter, there are two additional less-intense on-axis peaks associated with weaker surrounding densities. To confirm the identity of the bound ions, we collected X-ray diffraction data at 1.75 Å wavelength and calculated the F+ca-F−ca anomalous difference map. Two strong peaks followed by a weaker peak on the intracellular side were found in the selectivity filter along the ion-conduction pathway, verifying three binding sites for Ca2+ (Fig. 2b). We name these Site 1, Site 2, and Site 3 from the extracellular to the intracellular side.
Figure 2. Ca2+ binding sites in and near the selectivity filter of NaVAb, CaVAb, and their derivatives.
a, Electron density at the selectivity filter of 175TLDDWSN181 (also see Supplementary Fig. 4). The 2Fo−Fc electron density map (contoured at 2σ) of select residues in the selectivity filter with two diagonally opposed subunits shown in sticks, the Ca2+ ions along the ion pathway in green spheres and water molecules in red spheres. b, Densities at Ca2+ binding Site 1 and 2 from the anomalous difference Fourier map (3σ) calculated from the diffraction data of a 175TLDDWSN181 mutant crystal soaked in the presence of 5 mM Ca2+ and collected at 1.75 Å wavelength. The distances between Ca2+ and oxygen atoms (dashed lines) are about 4.0 Å at Site 1 (blue lines), 4.4 Å at Site 2 (blue and magenta lines), and 5.0 Å (magenta line) at Site 3. For clarity, the subunit closest to the viewer is not shown. c, d, A comparison between 175TLEDWSM181 and 175TLESWSM181 (NaVAb) highlighting the importance of Site 1 for Ca2+ selectivity. e, f, A comparison between 175TLDDWSD181 (CaVAb) and 175TLEDWSD181 highlighting the role of Site 2 in fine tuning Ca2+ selectivity. All structures were determined in the presence of 15 mM Ca2+.
The Ca2+ ion at Site 1 is predominantly coordinated by the carboxyl groups of D178 (SiteEX in NaVAb), which define a plane at the selectivity filter entrance on the extracellular side of the bound Ca2+ ion (Fig. 2b). The distance between the carboxyl oxygen and Ca2+ is about 4.0 Å. This distance suggests that the ion binds at this site in a hydrated form because the ionic diameter of Ca2+ is 2.28 Å, too small to interact with the carboxylate anions directly but appropriate for interaction through bound water molecules. Further into the pore, the four acidic side chains of D177 (SiteHFS in NaVAb) are located along the wall of the selectivity filter rather than projecting into the lumen, thereby also allowing the binding of a fully hydrated Ca2+ ion (Fig. 2b). Different from Site 1, this central Ca2+ binding site (Site 2) is surrounded by a box of 4 carboxylate oxygen atoms from D177 above and 4 backbone carbonyl oxygen atoms from L176 below (SiteCEN in NaVAb), with oxygen-Ca2+ distances of 4.5 Å and 4.2 Å, respectively (Fig. 2b). At the intracellular side of the pore, the third Ca2+ binding site (Site 3) is composed of one plane of four carbonyls from T175 (SiteIN in NaVAb), which point inward to the lumen (Fig. 2b). Here the Ca2+ ion lies nearly on the same plane as T175 carbonyls. The chemical environment of Site 3 hints at a lower affinity, consistent with its role in exit of Ca2+ from the selectivity filter into the central cavity. Throughout the selectivity filter, the oxygen-Ca2+ coordination distances are in the range of 4.0 – 5.0 Å, suggesting that the bound Ca2+ ion is continuously stabilized in a fully hydrated state when it passes through the pore. We observed diffuse electron density and in favorable cases discrete water molecules surrounding the bound Ca2+, consistent with the presence of an inner shell of bound waters of hydration (Supplementary Fig. 5).
Although the anomalous difference map did not resolve clear peaks at the outer vestibule beyond the selectivity filter, we interpret the two on-axis 2Fo-Fc densities above the three Ca2+ sites as two additional Ca2+ ions poised to enter the pore (Fig. 2a). This assignment is supported by the surrounding eight islets of density, which likely represent eight stabilized water molecules. Just as at Site 2 in the selectivity filter, these eight water molecules appear to serve as a square antiprism cage coordinating a hydrated Ca2+ ion at the center (Fig. 2a). The second Ca2+ ion located at the bottom of this cage is ~4.5 Å away from the four carboxyl oxygen atoms of D178, suggesting that part of its second hydration shell is replaced by D178 before the ion enters the selectivity filter. The selectivity filter, therefore, appears to select Ca2+ at its mouth by recognizing the Ca2+-hydration complex and conduct Ca2+ by fitting the Ca2+-hydration complex into the pore. Because Ca2+ is more electropositive than Na+, it should bind more tightly in the ion selectivity filter of CaVAb, providing a mechanistic basis for the block of Na+ permeation by Ca2+ at low Ca2+ concentration and preferential permeation of Ca2+ at higher Ca2+ concentration (Supplementary Discussion).
Functional roles of key selectivity filter residues
Measurements of bi-ionic reversal potentials revealed that the relative permeability of different CaVAb intermediate constructs for Ca2+ follows the order of CavAb (175TLDDWSD181) > 175TLDDWSN181 > 175TLEDWSD181 > 175TLEDWSM181 > NaVAb (175TLESWSM181) > 175TLDSWSM181 (Fig. 1g; Supplementary Fig. 2). A comparison of the Ca2+ selectivity ratios between 175TLEDWSM181 and 175TLESWSM181 (NaVAb) shows that substitution of S178 with Asp is sufficient to convert the selectivity from Na+ to Ca2+ with >100-fold change in PCa:PNa (Fig. 1g). Placement of the Asp carboxyl side chain at this position allows formation of the first hydrated Ca2+ binding site in the selectivity filter (Fig. 2c; Supplementary Fig. 6). By contrast, S178 in NaVAb binds Ca2+ directly by displacing its hydration shell, which blocks conductance of both Na+ and Ca2+ (Fig. 2d). Therefore, formation of Site 1 for binding hydrated Ca2+ is both necessary and sufficient for conferring Ca2+ selectivity over Na+ to NaVAb.
The Ca2+ selectivity ratio of CaVAb (175TLDDWSD181) is 5.5-fold higher than 175TLEDWSD181 (Fig. 1g). This functional difference reflects a role of Site 2 in adjusting Ca2+ selectivity. Different from the side chains of D177 in CaVAb (175TLDDWSD181), which interact with the Ca2+ ion (Fig. 2e), the carboxyl group of E177 in 175TLEDWSD181 swings away from the selectivity filter and forms a hydrogen bond with D181 and the main-chain nitrogen atoms of S180 (Fig. 2f, Supplementary Fig. 7). Site 2 in 175TLEDWSD181, therefore, is exclusively formed by the four carbonyl oxygen atoms of L176, which conceivably leads to a lower Ca2+ binding affinity and a decreased Ca2+ selectivity. This comparison highlights both the importance of Site 2 in supporting high Ca2+ selectivity and the critical role of the backbone carbonyl groups of L176 in constructing this ion-binding site.
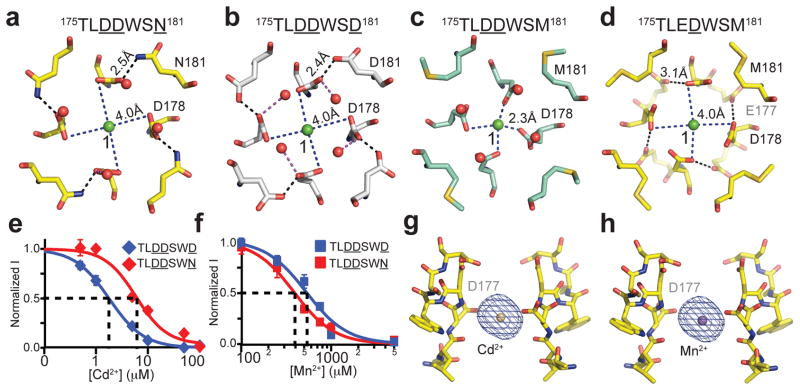
Distinct from D177 and D178, the N181 residue of 175TLDDWSN181 lies outside the ion-conducting pore and is not directly involved in Ca2+ ion coordination. In close proximity to the carboxyl groups of D178, which form a ring that lines the perimeter of the pore entryway, the side chain of N181 embraces the perimeter of the D178 ring by donating a hydrogen bond to its side chain carboxyls (Fig. 3a). Such a structural arrangement is also found in CaVAb (175TLDDWSD181) (Fig. 3b), although the more electronegative environment created by the extra negatively charge residue, D181, likely attracts Ca2+ more strongly and confers a 4- to 5-fold higher degree of Ca2+ selectivity to CaVAb (175TLDDWSD181) in comparison to 175TLDDWSN181 (Fig. 1g and Supplementary Fig. 3).
Figure 3. Ion binding and block of CaVAb and its derivatives.
a, b, Top view of Site 1 with a hydrated Ca2+ ion coordinated by D178 with the help of N181 and D181 in 175TLDDWSN181 and 175TLDDWSD181 (CaVAb), respectively. c, Binding of a dehydrated Ca2+ ion at Site 1 in the nonconductive 175TLDDWSM181 mutant. d, Coordination of a hydrated Ca2+ ion at the Site 1 of the 175TLEDWSM181 mutant. Despite the absence of a polar residue at amino acid 181, E177 in 175TLEDWSM181 is able to hold D178 in place to allow the binding of a hydrated Ca2+ ion. e, f, Block of Ca2+ conductance by the indicated concentrations of Cd2+ and Mn2+. 175TLDDWSD181: IC50(Cd2+), 1.7±0.04 μM; IC50(Mn2+, 526±22 μM. 175TLDDWSN181: IC50(Cd2+), 5.9±0.4 μM; IC50(Mn2+), 388±7 μM. Error bars are standard error of the mean. g, h, Side view of the Cd2+ and Mn2+ binding sites in the selectivity filter of CaVAb. The anomalous difference Fourier map densities (blue mesh, contoured at 5σ) of the bound blocking ions are calculated using diffraction data collected at 1.75 Å wavelength. For clarity, the residues forms the selectivity filter of the closest subunit to the viewer is removed.
175TLDDWSM181, which has the hydrophobic residue M181 packed next to the D178 ring, is the only CaVAb intermediate that does not conduct Ca2+ (Supplementary Fig. 2). The crystal structure of this mutant reveals a blocking Ca2+ ion tightly bound at Site 1 in a dehydrated state with an oxygen-ion distance of 2.3 Å (Fig. 3c). Superposition analysis shows few structural differences between 175TLDDWSM181 and 175TLDDWSN181, except for the side chain of D178, which is fixed by N181 in 175TLDDWSN but unconstrained in 175TLDDWSM181 (Fig. 3a, c). This comparison indicates that N181 in 175TLDDWSN181 and D181 in CaVAb play a critical role in engaging D178 and allowing the reversible binding of the Ca2+-H2O hydration complex for active Ca2+ conductance. Although the subtle difference in Ca2+ selectivity between 175TLEDWSD181 and 175TLEDWSM181 seems to argue against this conclusion (Fig. 1g), E177 in 175TLEDWSM181 actually plays a structural role equivalent to that of N181 in 175TLDDWSN181 — by pointing away from the selectivity filter lumen, E177 forms a carboxylate-carboxylate pair with D178 and holds it in a conduction-competent position (Fig. 3d, Supplementary Fig. 8).
Block of NaVAb and CaVAb channels by divalent cations
Cd2+, Mn2+ and other inorganic cations are effective blockers of CaV channels1. Block of Ca2+ conductance of CaVAb by Cd2+ and Mn2+ gives Ki values of 1.78 μM for Cd2+ and 526 μM for Mn2+ (Fig. 3e, f, blue). Cd2+ has lower affinity and Mn2+ has higher affinity for block of 175TLDDWSN181 (Fig. 3e, f, red). Crystals with bound Cd2+ and Mn2+ were obtained by soaking CaVAb crystals in a cryo-solution containing these heavy metal ions, and the anomalous difference map was calculated from a dataset collected at 1.75Å wavelength. The structures show that both Cd2+ and Mn2+ bind in the selectivity filter at the central site (Site 2), which is coordinated by the side chains of the four D177 residues and the carbonyl groups of L176 (Fig. 3g, h). Locked at this site, these blocking ions would inhibit the Ca2+ current by competitively binding to the high affinity site required for Ca2+ permeation. Another important common feature of the two blocking complexes of CaVAb is the block of permeation by binding of a single divalent cation within the selectivity filter, which supports the hypothesis that at least two divalent cation binding sites must be located close enough to induce repulsive interactions and allow divalent cation conductance by a knock-off mechanism. Because they are smaller than Ca2+, the bound Cd2+ (d=2.18 Å) and Mn2+ (d=1.94 Å) must interact with the selectivity filter through bound waters of hydration, and electron density consistent with bound waters of hydration is observed in our structures (Supplementary Fig. 5).
Ion binding at the Ca2+ selectivity filter
To assess the properties of the three Ca2+ binding sites in the selectivity filter of 175TLDDWSN181, we titrated the concentration of Ca2+ in the cryo-solution and calculated the anomalous difference maps. At low Ca2+ concentration, two strong peaks of approximately equal intensity are found at Site 1 and Site 2 (Supplementary Fig. 9). As the Ca2+ concentration is raised, the electron density of Site 2 is substantially enhanced, but the peak intensity is reduced at Site 1 and remains low at Site 3 (Supplementary Fig. 9). These results imply that the central site has the highest affinity, whereas Site 3 is the weakest. It is likely that this titration pattern reflects independent binding of Ca2+ to Sites 1, 2, and 3 located in different individual molecules of CaVAb at low Ca2+ concentration, whereas increasing concentrations of Ca2+ saturate Site 2 in most or all individual CaVAb molecules and reduce or eliminate binding at Sites 1 and 3 by repulsion. Importantly, the two flanking sites have lower affinity than the central site, as proposed in the “stepwise binding model” of CaV channel permeation7. In this model, the presence of flanking sites of intermediate affinity facilitates the movement of Ca2+ into and out of a central high-affinity site, which can result in high ion conductance, even in the limiting case where there is no repulsion between bound ions.
Consistent with a high binding affinity, Ca2+ binds at Site 2 with its first hydration shell waters coordinated with eight oxygen atoms from the channel (Fig. 2b, Supplementary Fig. 5). In contrast, Ca2+ at Site 1 is mainly stabilized by one plane of four carboxyl groups from D178. The distance between the Ca2+ ion at Site 1 and the carboxyl group of D177 at Site 2 is about 5.5Å –6Å. As the Ca2+ ion moves inward, this distance will be reduced enough for D177 to form a stable coordination with the moving Ca2+ ion. This spatial configuration suggests that the two sites are separated by a low energy barrier. The differences of negative charge between D178 and the carbonyls of T175 and the differences in the geometry of their interactions with Ca2+ provide a plausible explanation for the higher Ca2+ binding affinity at Site 1 than Site 3.
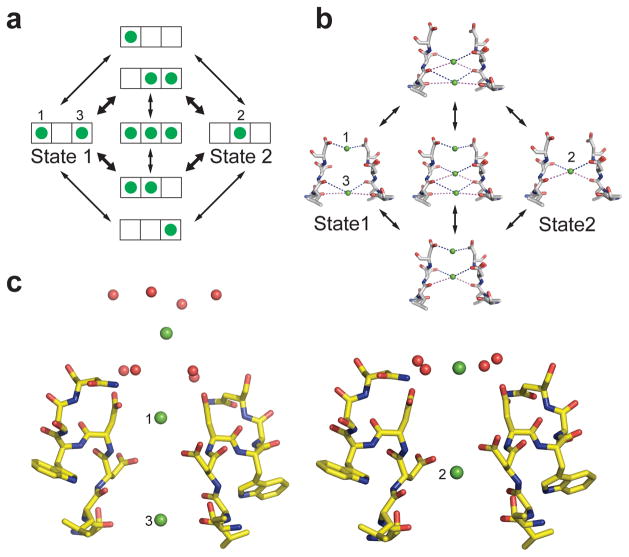
Ion permeation mechanism
The three Ca2+-binding sites in the selectivity filter of 176LDDWSN181 are separated by a distance of about 4.5 Å, which would result in substantial electrostatic repulsive interactions between bound ions. As in the case of the KcsA channel22, it is energetically unfavorable for Ca2+ ions to occupy adjacent sites simultaneously. This leads directly to our hypothesis of two interchangeable functional states of the selectivity filter in the crystal structure (Fig. 4a, b). In State 1, Ca2+ ions occupy Site 1 and Site 3. In State 2, a single Ca2+ ion occupies Site 2. These two states might be further coupled with one of the two Ca2+ ions at the outer vestibule ready to enter the pore (Fig. 4c). The transition between these two states occurs either when Ca2+ jumps from position 1 or 3 to position 2 or a third ion enters on one side of the filter, causing an ion to move into position 2. It is likely that our crystal structures reflect a mixed population of CaVAb molecules in which only Site 2 is occupied by Ca2+ plus CaVAb molecules in which Site 1 and/or Site 3 are occupied. Because of the high concentration of Ca2+ in the extracellular solution, Ca2+ will prefer to enter Site 1 and the weak binding of Ca2+ to Site 3 will force loss of Ca2+ into the low Ca2+ concentration in the cytosol. This generates a unidirectional flux of Ca2+ into the cell (Fig. 4c). The three-ion occupied state would be manifest only when the external Ca2+ concentration is increased enough that the flux reaches a limiting value3. The presence of the lower affinity Site 3 flanking the central cavity would further accelerate the flux of ions by allowing stepwise binding with relatively low chemical potential energy barriers7. The combination of ionic repulsion between Ca2+ ions bound at these sites and their stepwise change in binding affinity work together to allow rapid conductance in spite of the intrinsic high affinity for Ca2+ binding.
Figure 4. Catalytic cycle for Ca2+ conductance by CaVAb.
a, An ionic occupancy state diagram of CaVAb showing two proposed low energy states and the potential transitions that connect them. Each state of the selectivity filter is represented by a three-box rectangle with Sites 1 – 3 going from left to right. Green circles represent Ca2+ ions. Note that transitions in the inner circle potentially lead to ion repulsion, which might facilitate conduction. These transitions in the inner circle are more probable than those in the outer circle, as denoted by the bold arrows. b, The structural basis of the ionic occupancy states depicted in the inner circle of the state diagram shown on the left. The clockwise cycle represents a path for inward flux of Ca2+ ions through the selectivity filter. c, Coupling of extracellular Ca2+ binding sites and the three sites within the selectivity filter in the two proposed ionic occupancy states. When two Ca2+ ions bind to position 1 and 3 in the filter, the entryway Ca2+ ion is placed furthest from the pore (left). When one Ca2+ ion binds to position 2 within the filter, the ion outside the filter is pulled closer to the pore (right).
The mechanism underlying the dramatic difference in selectivity for Ca2+ over Na+ in CaVAb versus NaVAb is different from the mechanisms responsible for selectivity of K+ over Na+ and for Ca2+ block revealed by high-resolution structural studies of the NaK channel (Supplementary Discussion)23–25. Biophysical modeling of Ca2+ permeation in vertebrate CaV channels has led to multiple proposed mechanisms, most of which involve two or more Ca2+ binding sites, yet only a single high affinity site that is required for both permeation and Ca2+ block was identified by mutagenesis and physiological analyses1. Our results with CaVAb channels resolve this apparent discrepancy by showing that multiple Ca 2+ binding sites are necessary for permeation, but only Site 2 binds divalent cations with sufficient affinity for block. Our results indicate that Ca2+ is conducted as a hydrated cation (Supplementary Discussion and Supplementary Fig. 5), consistent with the large estimated functional diameter of vertebrate CaV channels of 6Å26. Detailed structure-function studies of vertebrate CaV channels show that mutations of the four residues equivalent to E177 have distinct effects on Ca2+ conductance and block, implying that domain-specific interactions with Ca2+ have evolved in vertebrate four-domain CaV channels 10,11,27–29. Vertebrate CaV channels might share similar molecular mechanisms for Ca2+ permeation and selectivity despite their pseudosymmetric four-domain configuration.
METHODS
Protein expression and purification
The pFastBac-FLAG-NaVAb (I217C) was used as the genetic background for CaVAb constructs was previously described15,16. CaVAb and its derivatives, 175TLDDWSN181, 175TLEDWSD181, 175TLEDWSM181, and 175TLDSWSM181 were generated via site-directed mutagenesis using QuickChange (Stratagene). Recombinant baculovirus were produced using the Bac-to-Bac system (Invitrogen), and T. ni insect cell were infected for large-scale protein purification. Cells were harvest 72 h post-infection and re-suspended in 50 mM Tris-HCl pH=8.0, 200 mM NaCl (Buffer A) supplemented with protease inhibitors and DNase. After sonication, digitonin (EMD Biosciences) was added to 1% and solubilization was carried out for 1–2 h at 4°C. Clarified supernatant was then incubated with anti-Flag M2-agarose resin (Sigma) for 1–2 h at 4 °C with gentle mixing. Flag-resin was washed with ten column volumes of buffer B (buffer A supplemented with 0.12 % digitonin) and eluted with buffer B supplemented with 0.1 mg/ml Flag peptide. The eluent was concentrated and then passed over a Superdex 200 column (GE Healthcare) in 10 mM Tris-HCl pH=8.0, 100 mM NaCl and 0.12 % digitonin. The peak fractions were concentrated using a Vivaspin 30K centrifugal device.
Crystallization and data collection
CaVAb and its derivatives were concentrated to ~20mg ml−1 and reconstituted into DMPC:CHAPSO (Anatrace) bicelles according to standard protocols30,31. The protein-bicelle preparation and a well solution containing 1.8–2.0 M ammonium sulfate, 100 mM Na-citrate pH=5.0 was mixed with a 1:1 ratio and set up in a hanging-drop vapour-diffusion format. The Ca2+- derivative crystals were obtained by soaking CaVAb and other mutant crystals in a cryo-protection solution (0.1M Na-acetate pH=5.0, 26% glucose and 2.0 M ammonium sulfate) containing the indicated concentrations of Ca2+ for 40–60 min at 4 °C. The Cd2+ and Mn2+ derivatives were obtained by soaking CaVAb in the presence of 100 mM Cd2+ or Mn2+, respectively. Crystals were then plunged into liquid nitrogen and maintained at 100 K during all data collection procedures.
All anomalous diffraction datasets were collected at 1.75 Å with the same synchrotron radiation source (Advanced Light Source, BL8.2.1). To optimize the anomalous signal, the datasets were collected by using the “inverse beam strategy” with the wedge size of 5°.
Structure determination, refinement, and analyses
X-ray diffraction data were integrated and scaled with the HKL2000 package32 and further processed with the CCP4 package33. The structure of CaVAb and its derivatives were solved by molecular replacement by using an individual subunit of the NaVAb structure (PDB code 3RVY) as the search template. The datasets were processed in C2 space group and there are four molecules in one asymmetric unit. We choose the I222 space group to process the datasets for initial structural determination, but we found that the bound ions are slightly off-center with respect to the axis of the pore. Therefore, to better interpret the coordination of Ca2+, Cd2+, and Mn2+, we solved the structures in the C2 space group. Crystallography and NMR System software 34 was used for refinement of coordinates and B-factors. Final models were obtained after several cycles of refinement with REFMAC35 and PHENIX36 and manual re-building using program COOT37. The geometries of the final structural models of CaVAb and its derivatives were verified using PROCHECK38. The divalent cations were identified by anomalous difference Fourier maps calculated using data collected at wavelengths of 1.75 Å for Ca2+, Cd2+ and Mn2+. Detailed crystallographic data and refinement statics for all the constructs are shown in Table S1. All structural figures were prepared with the PyMol software39.
Electrophysiology
NavAb-WT expressed by infection of insect cells (High5) activates at very negative potentials V1/2 ~ −98 mV) and shows a strong, late use-dependent phase of slow inactivation. Mutation N49K shifts the activation curve ~75 mV to more positive potentials and abolishes the use-dependent inactivation40. All NavAb/CavAb constructs used were made on the background of N49K mutation and showed good expression, allowing measurement of ionic currents 24–48 h post-infection.
Whole-cell currents were recorded using an Axopatch 200 amplifier (Molecular Devices, Sunnyvale, CA) with glass micropipettes (2–5 MΩ). Capacitance was subtracted and 80–90% of series resistance was compensated using internal amplifier circuitry. For reversal potential measurements, the intracellular pipette solution contained (in mM): 100 NaF, 10 NaCl, 20 HEPES-Na, 10 EGTA, pH 7.4 (adjusted with NaOH, [Na+]Total = 146 mM). Extracellular solution contained in (mM) 10 CaCl2, 140 NMDG-methanesulfonate, 20 HEPES, (pH 7.4, adjusted with Ca(OH)2, [Ca2+]Total = 12 mM). For Ba2+ reversal potential measurements, BaCl2 replaced CaCl2. Current-voltage (I–V) relationships were recorded in response to steps to voltages ranging from −100 to +70 mV in 5 or 10 mV increments from a holding potential of −100 mV. Pulses were generated and currents were recorded using Pulse software controlling an Instrutech ITC18 interface (HEKA, Great Neck, NY). Data were analyzed using Igor Pro 6.2 (WaveMetrics, Lake Oswego, OR). Sample sizes were chosen to give SEM values of less than 10 % of peak values based on prior experimental experience.
Relative permeability values were calculated as described41. The permeability ratio was calculated as:
where F, R, T, and Erev are Faraday constant, gas constant, absolute temperature, and reversal potential, respectively. ax, denotes the activity of the external divalent ion, x, (Ca2+ or Ba2+) and aNa, the activity of intracellular sodium. The calculated activity coefficients were γCa = 0.33, γBa = 0.30, γNa = 0.74. All potentials were corrected for the experimentally determined liquid junction potential.
For anomalous mole fraction and blocking experiments, the divalent (Ca2+, Cd2+, and Mn2+) was diluted in 10 mM BaCl2, 140 mM NMDG-methanesulfonate, and 10 mM HEPES and perfused for 2–3 min before recording a current-voltage curve. The peak value of the I–V curve was measured and normalized to the peak value without the divalent cation.
Supplementary Material
Acknowledgments
We are grateful to the beamline staff at the Advanced Light Source (BL8.2.1 and BL8.2.2) for their assistance during data collection. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number R01NS015751 (W.A.C.), the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under award number R01HL112808 (W.A.C. and N.Z.), and a National Research Service Award from training grant T32GM008268 (T.M.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Howard Hughes Medical Institute (N. Z.).
Footnotes
Author contributions
L.T., T.M.G.D., J.P., T.S., N.Z., and W.A.C. designed the experiments. J.P. initiated the experimental work. L.T. conducted the protein purification, crystallization, and diffraction experiments. L.T., J.P., and N.Z. determined and analyzed the structures of the apo and cation bound forms of CaVAb and the intermediate CaVAb constructs. T.M.G.D. and T.S. performed physiological studies of CaVAb and related constructs. G.M. and T.M.H made the constructs and performed the preliminary data collection. All authors interpreted the structures in light of the physiological data. L.T., N.Z., and W.A.C. wrote the manuscript with input from all co-authors. W. A. C. and N. Z. are co-senior authors.
Coordinates and structure factors have been deposited in the Protein Data Bank under accession codes: 4MS2 (TLDDWSN, 15 mM Ca2+), 4MTF(TLDDWSN, 0.5 mM Ca2+), 4MTG (TLDDWSN, 2.5 mM Ca2+), 4MTO (TLDDWSN, 5 mM Ca2+), 4MVM (TLDDWSN, 10 mM Ca2+), 4MVO (TLDDWSN, 15 mM Ca2+), 4MVQ (TLDDWSD, 15 mM Ca2+), 4MVR (TLDDWSD, 100 mM Mn2+), 4MVS (TLDDWSD, 100 mM Cd2+), 4MVZ (TLEDWSD, 15 mM Ca2+), 4MW3 (TLDDWSM, 15 mM Ca2+), 4MVU (TLEDWSM, 15 mM Ca2+), 4MW8 (NavAb, 15 mM Ca2+).
The authors declare no competing financial interests.
References
- 1.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almers W, McCleskey EW. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almers W, McCleskey EW, Palade PT. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong CM, Neyton J. Ion permeation through calcium channels. A one-site model. Ann N Y Acad Sci. 1991;635:18–25. doi: 10.1111/j.1749-6632.1991.tb36477.x. [DOI] [PubMed] [Google Scholar]
- 7.Dang TX, McCleskey EW. Ion channel selectivity through stepwise changes in binding affinity. J Gen Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopin KV, Obejero-Paz CA, Jones SW. Evaluation of a two-site, three-barrier model for permeation in Ca(V)3.1 (alpha1G) T-type calcium channels: Ca2+, Ba2+, Mg2+, and Na+ J Membr Biol. 2010;235:131–143. doi: 10.1007/s00232-010-9264-3. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann SH, Terlau H, Stuhmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- 10.Ellinor PT, Yang J, Sather WA, Zhang JF, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Morii T, Sun LX, Imoto K, Mori Y. Structural determinants of ion selectivity in brain calcium channel. FEBS Lett. 1993;318:145–148. doi: 10.1016/0014-5793(93)80009-j. [DOI] [PubMed] [Google Scholar]
- 13.Cibulsky SM, Sather WA. The EEEE locus is the sole high-affinity Ca2+ binding structure in the pore of a voltage-gated Ca2+ channel: block by Ca2+ entering from the intracellular pore entrance. J Gen Physiol. 2000;116:349–362. doi: 10.1085/jgp.116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloues RK, Cibulsky SM, Sather WA. Ion interactions in the high-affinity binding locus of a voltage-gated Ca2+ channel. J Gen Physiol. 2000;116:569–586. doi: 10.1085/jgp.116.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 19.Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 20.Koishi R, et al. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. doi: 10.1074/jbc.M313100200. [DOI] [PubMed] [Google Scholar]
- 21.Yue L, Navarro B, Ren D, Ramos A, Clapham DE. The cation selectivity filter of the bacterial sodium channel, NaChBac. J Gen Physiol. 2002;120:845–853. doi: 10.1085/jgp.20028699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 23.Alam A, Jiang Y. Structural analysis of ion selectivity in the NaK channel. Nat Struct Mol Biol. 2009;16:35–41. doi: 10.1038/nsmb.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam A, Shi N, Jiang Y. Structural insight into Ca2+ specificity in tetrameric cation channels. Proc Natl Acad Sci U S A. 2007;104:15334–15339. doi: 10.1073/pnas.0707324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derebe MG, et al. Tuning the ion selectivity of tetrameric cation channels by changing the number of ion binding sites. Proc Natl Acad Sci U S A. 2011;108:598–602. doi: 10.1073/pnas.1013636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCleskey EW, Almers W. The Ca channel in skeletal muscle is a large pore. Proc Natl Acad Sci U S A. 1985;82:7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XH, Tsien RW. Aspartate substitutions establish the concerted action of P-region glutamates in repeats I and III in forming the protonation site of L-type Ca2+ channels. J Biol Chem. 1997;272:30002–30008. doi: 10.1074/jbc.272.48.30002. [DOI] [PubMed] [Google Scholar]
- 28.Cibulsky SM, Sather WA. Control of ion conduction in L-type Ca2+ channels by the concerted action of S5–6 regions. Biophys J. 2003;84:1709–1719. doi: 10.1016/S0006-3495(03)74979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson AV, Sather WA. Nonglutamate pore residues in ion selection and conduction in voltage-gated Ca2+ channels. Biophys J. 1999;77:2575–2589. doi: 10.1016/s0006-3495(99)77092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faham S, Bowie JU. Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J Mol Biol. 2002;316:1–6. doi: 10.1006/jmbi.2001.5295. [DOI] [PubMed] [Google Scholar]
- 31.Faham S, Boulting GL, Massey EA, Yohannan S, Yang D, Bowie JU. Crystallization of bacteriorhodopsin from bicelle formulations at room temperature. Protein Sci. 2005;14:836–840. doi: 10.1110/ps.041167605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 33.ccp4. The CCP4 suite: programs for protein crystallography. Acta crystallographica Section D, Biological crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 34.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta crystallographica Section D, Biological Crystallography. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 35.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta crystallographica Section D, Biological crystallography. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 36.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica Section D, Biological crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta crystallographica Section D, Biological crystallography. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 38.Laskowski RA, Moss DS, Thornton JM. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- 39.DeLano WL. PyMOL molecular viewer(V12r3pre) 2002 ( http://www.pymol.org)
- 40.Gamal El-Din TM, Martinez GQ, Payandeh J, Scheuer T, Catterall WA. A gating charge intereaaction required for late slow inactivation of the bacterial sodium channel NavAb. J Gen Physiol. 2013;142:181–190. doi: 10.1085/jgp.201311012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue L, Navarro B, Ren D, Ramos A, Clapham DE. The cation selectivity filter of the bacterial sodium channel, NaChBac. J Gen Physiol. 2002;120:845–853. doi: 10.1085/jgp.20028699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.