Abstract
Purpose
Our objective was to examine the associations of physical activity in different life domains with peak femoral neck strength relative to load in adult women. Composite indices of femoral neck strength integrate body size with femoral neck size and bone mineral density to gauge bone strength relative to load during a fall, and are inversely associated with incident fracture risk.
Methods
Participants were 1919 pre- and early perimenopausal women from the Study of Women’s Health Across the Nation. Composite indices of femoral neck strength relative to load in three failure modes (compression, bending, and impact) were created from hip DXA scans and body size. Usual physical activity within the past year was assessed with the Kaiser Physical Activity Survey in four domains: sport, home, active living, and work. We used multiple linear regression to examine the associations.
Results
Greater physical activity in each of the four domains was independently associated with higher composite indices, adjusted for age, menopausal transition stage, race/ethnicity, SWAN study site, smoking status, smoking pack-years, alcohol consumption level, current use of supplementary calcium, current use of supplementary vitamin D, current use of bone-adverse medications, prior use of any sex steroid hormone pills or patch, prior use of depo-provera injections, history of hyperthyroidism, history of previous adult fracture, and employment status: standardized effect sizes ranged from 0.04 (p<0.05) to 0.20 (p<0.0001).
Conclusions
Physical activity in each domain examined was associated with higher peak femoral neck strength relative to load in pre- and early perimenopausal women.
Keywords: Physical Activity, Peak Bone Strength, Composite Strength Indices, Femoral Neck Strength Relative to Load, Active Living
INTRODUCTION
Osteoporosis-related fractures can have major negative consequences in older women, including decreased mobility, loss of independence, incident depression, and reduced quality of life, and may even increase the risk of death. Osteoporosis-related fractures constitute a major public health concern in the United States [1] and worldwide [2,3]. Hence there is an urgent need to identify potentially modifiable causes of osteoporosis, or low bone strength, in women.
Bone mass in postmenopausal women is a function of both peak bone mass acquired prior to the menopause transition and the rate of bone loss during and after the transition [4]; thus, low peak bone mass in premenopause is a major and potentially modifiable risk factor for osteoporotic fracture in later years [5,6]. Our objective was therefore to examine associations between physical activity level (potentially modifiable) and peak bone strength in pre- and early perimenopausal women who have not yet experienced menopause-related declines in bone mineral density (BMD) and thus have peak bone mass or close to it [7].
Although areal BMD measured by dual-energy x-ray absorption (DXA) has been widely used for the assessment of bone strength, it explains only a modest proportion of fracture risk [8,9]. In many cases, BMD is a poor surrogate for bone strength (i.e., its ability to resist fracture). For example, although BMD is higher or similar in Caucasian women than in Asian women [10,11], hip fracture risk is higher (not lower) in Caucasians [12]. In addition, higher hip fracture risk associated with type 2 diabetes is not consistent with the higher (not lower) BMD in type 2 diabetics than in non-diabetics [13]. These discordances between BMD and hip fracture risk may reflect differences between the groups in bone size (which influences bone strength independent of BMD) [14,15] and body size (which determines the load that bone is exposed to in a fall) [16].
Composite indices of femoral neck strength integrate bone size and body size with femoral neck BMD to gauge strength relative to load (impact forces) borne during a fall [17]. These indices are inversely associated with incident fractures [17–20], and unlike BMD, are consistent with fracture risk differences across race/ethnicity groups [21], and between diabetes and non-diabetes [22]. Furthermore, unlike BMD, the composite indices can predict fracture risk in middle-aged women without requiring race/ethnicity information [20]. These findings suggest that the femoral neck composite strength indices are better measures of a woman’s ability to resist fracture than is BMD.
While several studies have examined the role of physical activity in explaining variation in peak bone mass, and one study examined physical activity in relation to femoral neck composite strength indices in young boys and girls [23], no previous study has examined physical activity as a determinant of peak femoral neck strength relative to load in adult women. Physical activity appears to increase BMD and bone size [24,25], and decrease body weight [26]; their combined effect on femoral neck strength relative to load is not immediately apparent. Of particular relevance to women, most studies of physical activity and peak bone mass have focused on sports and regular leisure-time exercise, but women may obtain more physical activity while they are engaged in household chores and family care roles and may be less likely to participate in leisure-time physical activity on a regular basis than men [27,28]. We therefore designed this study to examine the cross sectional-associations of self-reported physical activity in four domains; sport, home, active living, and work, with the femoral neck composite strength indices among women who were at peak bone mass or close to it. We hypothesized that women who reported higher levels of physical activity in each domain will have higher levels of peak femoral neck strength relative to load.
METHODS
Study Participants
We analyzed data from the baseline visit of the Study of Women’s Health Across the Nation (SWAN), a multisite study of the menopausal transition in a community-based sample of 3302 women from five ethnic/racial backgrounds in the United States: Caucasian, African-American, Chinese, Japanese, and Hispanic. The eligibility criteria, described in detail elsewhere [29], included age 42–53 years, intact uterus and at least one intact ovary, not currently using sex-steroid hormones, at least one menses in the three months before screening, and self- identification as a member of one of the five eligible ethnic/racial backgrounds. Participants were enrolled in 1996 – 1997 at seven clinical sites in the following areas: Boston, Chicago, Detroit, Pittsburgh, Los Angeles, Newark and Oakland. The Chicago and Newark sites did not perform BMD measurement, and did not contribute to the SWAN bone cohort. Each of the other five sites enrolled Caucasians, and also enrolled women from another ethnic group: African American in Boston, Detroit, and Pittsburgh, Chinese in Oakland, and Japanese in Los Angeles. These five sites together enrolled total of 2,413 women into the bone study cohort, 46 of whom did not obtain hip BMD because they weighted more than 136 kg (the maximum allowed on the DXA scans). As a part of the SWAN Hip Strength Across the Menopausal Transition, femoral neck size measurements on archived hip DXA scans were made on 1,940 women in the SWAN bone cohort who had hip DXA scans at the baseline visit and at least two follow up visits by 2006–2007. Twenty one of these women were missing an important covariate such as body height (needed to calculate two of the three strength indices and body mass index (BMI), n=16), employment status (n=2) or alcohol consumption (n=3), leaving an analytic sample of 1,919 women (960 Caucasian, 502 African American, 220 Chinese, and 237 Japanese). The SWAN and sub-study protocols were approved by the Institutional Review Board at each site, and all participants gave written informed consent.
Measurements
Measurements of DXA scans were acquired with Hologic QDR 2000 (Pittsburgh and Oakland) or QDR 4500 scanners (Boston, Detroit, and Los Angeles) and OsteoDyne’s Hip Positioner System. The DXA quality control protocols in SWAN have been previously described [30]. The projected (areal) BMD in the femoral neck was recorded, and two femoral neck dimensions were measured using the region of interest (ROI) window, which was repositioned and resized by the DXA operator so that a side of the ROI window spanned the geometric measures of interest. Then the pixel locations of relevant window corners were recorded, and used to calculate the relevant distances in millimeters, using pixel dimensions provided by the manufacturer, Hologic, Inc. Femoral neck axis length (FNAL) is the distance along the femoral neck axis from the lateral margin of the base of the greater trochanter to the apex of the femoral head. Femoral neck width (FNW) is the smallest thickness of the femoral neck along any line perpendicular to the femoral neck axis (Fig. 1). Composite indices of femoral neck strength relative to load during a fall were created as follows [17]:
Fig. 1. Femoral neck size measurements and formulae to compute composite femoral neck strength indices.
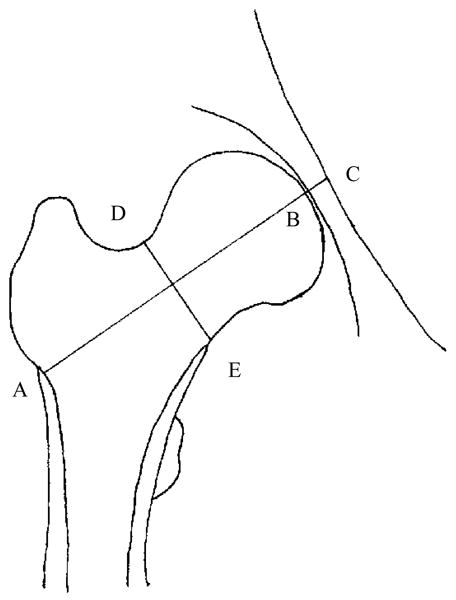
AB is the femoral neck axis length (FNAL): the distance from the base of the greater trochanter to the apex of the femoral head. DE is the femoral neck width (FNW): the smallest thickness of the femoral neck along any line perpendicular to the femoral neck axis. C is where the femoral neck axis meets the inner pelvic rim.
Compression strength index (CSI) = BMD*FNW/weight
Bending strength index (BSI) = BMD*(FNW)2 /(FNAL*weight)
Impact strength index (ISI) = BMD*FNW*FNAL /(height*weight)
All three indices were recorded in units of g/kg-m with BMD measured in g/cm2, FNW and FNAL in cm, weight in kg, and height in meter. CSI and BSI were scaled by 100 to obtain values in units of g/kg-m. CSI reflects the ability of the femoral neck to withstand an axial compressive load, BSI reflects its ability to withstand bending forces, and ISI reflects the ability of the femoral neck to absorb the energy of impact in a fall from standing height. To examine reproducibility, 20 women volunteers were scanned twice on the same day with repositioning, and femoral neck BMD, FNW, and FNAL were measured twice. Intraclass correlation coefficients for the three indices were all greater than 0.98 [21].
Physical activity was assessed with an adapted version of the Kaiser Physical Activity Survey (KPAS), which is based on the Baecke questionnaire [31]. This survey has established test-retest reliability and validity against activity records, accelerometer recordings, and maximal oxygen consumption in women [32]. The version of the KPAS used in SWAN had minor changes in a few KPAS response categories and some household activities were grouped by intensity [27]. This self-report instrument grades physical activity in four domains: sport, home, daily routine (termed active living), and work (among those employed). Scores representing the average responses to domain-specific questions range from 1 (low) to 5 (high) for each domain. The sport activity score was created from answers to questions about: a) frequency of sports/exercise/exertion causing sweating, b) self-rated level of participation in recreational activity compared to other women of same age, and c) frequency, duration, and intensity of the two most frequent sports/physical activities over the previous year. The five home activity questions asked about frequency of: a) child or dependent adult care, b) meal preparation and clean up, c) light chores such as dusting, d) moderate chores such as vacuuming, and e) heavy chores such as home repair. The two ‘active living’ questions asked about average hours per day: a) walking or biking for transportation, and b) television watching, each on a scale of 1–5; the latter was reverse-scored. The nine work activity questions assessed time spent: a) sitting, b) standing, c) walking, d) lifting loads greater than 15 lb, e) stooping/bending, f) moving heavy equipment, and g) sweating from exertion. The work score also included self-rated physical activity level at work compared with others, and work activity intensity, based on occupational census code [27]. We assigned a work activity score of 1 for those who were not employed, and added scores across the four domains to create a total physical activity score (range, 4 to 20). In sensitivity analyses, we also examined a total activity score that did not include work activity (range, 3 to 15).
Standardized interview and self-reported questionnaires were used to obtain the following covariate information: age (years), race/ethnicity (Caucasian, African-American, Chinese, Japanese), menopause transition stage (premenopausal [regular menses], early peri- menopausal [menses within 3 months but menses less predictable]), smoking status (never smoking, ex-smoker, or current), smoking pack-years (less than or equal to 10 years, greater than 10 years but less than or equal to 30 years, or greater than 30 years), alcohol categories (abstainer, infrequent: greater than zero but less than or equal to 1 drink per week, light-to-moderate: greater than 1 but less than 7 drinks per week, heavy: greater than 7 drinks per week), current use of supplementary calcium (none vs. any), current use of supplementary vitamin D (none vs. any), current use of bone-adverse medications (oral steroid or anti-epileptics: none vs. any), prior use of any sex steroid hormone pills or patch (none vs. any), prior use of depo-provera injections, history of hyperthyroidism (yes vs. no), history of previous adult fracture (yes vs. no), and employment status (yes vs. no). Height and weight were measured with a fixed stadiometer and a digital scale with the participants wearing light clothing and no shoes. BMI was calculated as weight in kilo-grams divided by the square of height in meters.
Statistical Analysis
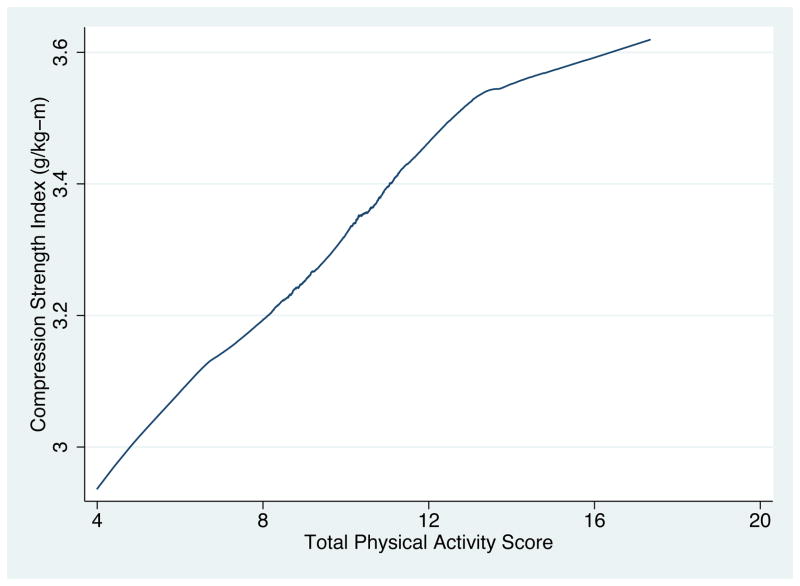
Loess plots of the composite strength indices as a function of physical activity score (see Fig 2, for example) suggested that the physical activity scores could be used as continuous, linear predictors of the strength indices. We used separate multiple linear regressions to examine the associations between each femoral neck strength index (continuous) and physical activity in each domain (continuous), and between each strength index and total score of physical activity across domains (continuous), adjusted for age (continuous), menopausal transition stage, race/ethnicity, SWAN study site, smoking status, smoking pack-years, alcohol consumption level, current use of supplementary calcium, current use of supplementary vitamin D, current use of bone-adverse medications, prior use of any sex steroid hormone pills or patch, prior use of depo-provera injections, history of hyperthyroidism, history of previous adult fracture, and employment status (only in work activity and total score). Use of osteoporosis medications (bisphosphonates, selective estrogen receptor modulators, calcitonin, parathyroid hormone, or vitamin D in pharmacological doses) at baseline was reported by only one participant, and therefore not included in the model.
Fig. 2.
Loess plot of Compression Strength Index as a function of Total Physical Activity Score
Initial models did not include controls for BMI, because changes in BMI represent one potential pathway by which physical activity might influence bone strength [33]. In the next step, we added controls for BMI by adding BMI as a continuous (linear) term, plus a squared (quadratic) term to allow for possible higher-order associations, plus multiplicative interaction terms between BMI and race/ethnicity because of the large race/ethnicity differences in BMI. Finally we ran regressions with all four domains of physical activity together in the same model to examine the independent influence of each domain on bone strength. All statistical tests were performed 2 sided using the Statistics Analysis System Version 9.2 (SAS Institute Inc, Cary, North Carolina, U.S.A.) and the STATA Version 12.1 (StataCorp LP, College Station, Texas, U.S.A.).
RESULTS
The study sample was similar to the full SWAN bone cohort with respect to all major characteristics (Table 1). The average age of study participants was 45.9 years, 50.0% were Caucasian, 26.2% were African American, 11.5% were Chinese, and 12.4% were Japanese. Mean physical activity scores (range, 1–5) were between 2.0 and 2.7 in each of the four activity domains, median scores were between 1.9 and 2.8, and there was substantial variability in each activity domain (standard deviations 0.8 to 1.0; skew 0.11 to 0.80). Physical activity scores were not highly correlated across domains; the highest correlation was between sport activity score and active living score (r=0.30). Mean total physical activity score was 9.8 (on a scale of 4 to 20).
Table 1.
Characteristics of study participants compared with the SWAN bone cohort at baseline
| Characteristics | Study Sample (N=1919) | SWAN bone cohort (N=2413) |
|---|---|---|
| Age (year) | 45.9±2.7 | 45.8±2.7 |
| Race | ||
| Caucasian | 960 (50.0%) | 1196 (49.6%) |
| African American | 502 (26.2%) | 686 (28.4%) |
| Chinese | 220 (11.5%) | 250 (10.4%) |
| Japanese | 237 (12.4%) | 281 (11.7%) |
| Body Mass Index (kg/m2) | 27.5±6.9 | 28.0±7.5 |
| Menopause status | ||
| Premenopausal | 1085 (56.5%) | 1288 (54.1%) |
| Early perimenopausal | 834(43.5%) | 1094(45.9%) |
| Smoking status | ||
| Current | 283 (14.7%) | 393 (16.4%) |
| Ex-smoker | 485 (25.3%) | 619 (25.9%) |
| Never smoked* | 1151 (60.0%) | 1383 (57.8%) |
| Smoking (Pack-year) | ||
| 0 | 1246 (64.9%) | 1526 (63.2%) |
| ≤10 years | 304 (15.8%) | 377 (15.6%) |
| >10 ≤ 30 years | 292 (15.2%) | 389 (16.1%) |
| ≥30 years | 77 (4.0%) | 121 (5.0%) |
| Alcohol consumption | ||
| Abstainer | 990 (51.6%) | 1244 (51.8%) |
| Infrequent | 179 (9.3%) | 220 (9.2%) |
| Light to moderate | 489 (25.5%) | 607 (25.3%) |
| Heavy | 261 (13.6%) | 332 (13.8%) |
| Use of supplementary calcium | 862 (45.0%) | 1066 (44.5%) |
| Use of supplementary Vitamin D | 741 (38.6%) | 917 (38.2%) |
| Use of bone-adverse medications** | 44 (2.3%) | 56 (2.3%) |
| Prior use of any sex steroid hormones | 127 (6.6%) | 179 (7.4%) |
| Prior use of depo-provera injections | 14 (0.7%) | 20 (0.8%) |
| History of hyperthyroidism | 70 (3.7%) | 89 (3.7%) |
| History of previous adult fracture | 352 (18.3%) | 457 (18.9%) |
| Employment Status | 1581 (82.4%) | 1977 (82.0%) |
| Femoral neck bone mineral density (g/cm2) | 0.84±0.13 | 0.85±0.14**** |
| Compression strength index (g/kg-m) | 3.31±0.64 | - |
| Bending strength index (g/kg-m) | 1.02±0.22 | - |
| Impact strength index (g/kg-m) | 0.18±0.04 | - |
| Sport Activity Score | 2.71±1.02 | 2.69±1.03 |
| Home Activity Score | 2.69±0.83 | 2.70±0.85 |
| Active Living Score | 2.36±0.78 | 2.36±0.79 |
| Work Activity Score*** | 1.99±0.80 | 2.00±0.81 |
| Total Physical Activity Score | 9.76±1.95 | 9.75±2.00 |
Note: For continuous variables, mean and standard deviation are shown. For categorical variables, number and percentage are shown.
13 women who were missingsmoking status answered 0 for pack years, so we assigned them to the never-smoked group.
Bone-adverse medications included oral steroid and anti-epileptics. At baseline, no one took chemotherapy or aromatase-inhibitors.
Unemployed women were assigned 1 for the work activity score.
There were a total of 2413 women in five SWAN bone sites, but only 2328 women had femoral neck bone scans at baseline.
Adjusted for covariates other than BMI, higher levels of physical activity in each of the four domains was significantly associated with higher levels of each of three composite indices of femoral neck strength relative to load (Table 2). Each standard deviation (SD) increment in physical activity level in the sport and active living domains was associated with between 0.16 and 0.20 SD increments in the strength indices (p<0.0001), while every SD increment in physical activity level in the home and work activity domains was associated with between 0.04 and 0.06 SD increments in strength indices (p<0.05). In addition, total score of physical activity across domains was also positively and strongly associated with each of the three composite strength indices (Table 2): standardized effect sizes 0.19 to 0.22 (p<0.0001).
Table 2.
Adjusted1 associations2 of domain-specific physical activity level with femoral neck composite strength indices
| Compression Strength Index | Bending Strength Index | Impact Strength Index | |
|---|---|---|---|
| Sport | 0.19**** | 0.16**** | 0.20**** |
| Active Living | 0.19**** | 0.17**** | 0.19**** |
| Home | 0.05* | 0.04* | 0.06** |
| Work | 0.05* | 0.05* | 0.05* |
| Total Score | 0.22**** | 0.19**** | 0.22**** |
| After additional adjustment for body mass index | |||
| Sport | 0.08**** | 0.07**** | 0.09**** |
| Active Living | 0.05** | 0.06** | 0.05** |
| Home | 0.05*** | 0.05* | 0.06*** |
| Work | 0.06** | 0.06** | 0.06** |
| Total Score | 0.11**** | 0.10**** | 0.11**** |
| With additional adjustment for the other three domains of physical activity | |||
| Sport | 0.07**** | 0.06** | 0.07**** |
| Active Living | 0.03* | 0.05* | 0.03 |
| Home | 0.04* | 0.03 | 0,04** |
| Work | 0.05** | 0.05* | 0.05* |
Adjusted for age, menopause transition stage, race/ethnicity, SWAN study site, smoking status, smoking pack-years, alcoholconsumption level, use of supplementary calcium, use of supplementary vitamin D, use of bone -adverse medications, prioruse of any sex steroid hormones, prior use of depo-provera injections, history of hyperthyroidism, history of previous adult fracture, and employment status (only in work activity and total score).
Units: Strength index standard deviation (SD) per SD increment in activity score
p<0.05,
p<0.01,
p<0.001,
p<0.0001
Additional adjustment for BMI attenuated the magnitudes of the associations with sport activity and active living, but did not attenuate associations with home activity or work activity. With the BMI adjustment, each SD increment in total physical activity score was associated with between 0.10 and 0.11 SD increments in the strength indices (p<0.0001) (Table 2). In a sensitivity analysis that excluded work activity from the total activity score (because 17.6 % of women were unemployed) associations were similarly strong: standardized effect sizes remained between 0.09 and 0.10 (data not shown).
Finally, when we included all four domains of physical activity in the same model, and adjusted for all covariates including BMI, higher level of physical activity in each of the four domains was independently associated with higher values of two or more of the composite strength indices (Table 2). Adjusting for the other domains of activity did not substantially attenuate the magnitudes of the associations between activity level in each domain and the composite indices.
DISCUSSION
As hypothesized, greater physical activity in each of the four domains examined: sport, home, active living, and work, was independently associated with greater femoral neck strength relative to load in a sample of pre- and early perimenopausal women. In addition, total physical activity score across the domains was also strongly, positively associated with femoral neck strength relative to load. Previous studies found that each SD increment in the composite indices of femoral neck strength was associated with ~40% relative decrement in the rate (hazard) of fracture at any site in women going through the menopause transition [20], and ~60% relative decrement in the risk of hip fracture over 10 years in postmenopausal women [17]. If the activity-related differences in composite indices seen in this study lead to similar fracture risk differences, then each SD increment in total activity across domains would be associated with ~10% relative reduction in fracture hazard in women going through the menopause transition and ~17% relative reduction in 10-year hip fracture risk in postmenopausal women.
Taken together with an earlier study that showed that femoral neck strength relative to load does not change substantially before the menopause transition [34], these findings suggest that physical activity that occurs as a part of daily routine such as at home, at work, and simply being active in life can positively influence peak femoral neck strength relative to load in women, and lower their risk for osteoporosis and fragility fractures later in life. They also highlight the importance of expanding our focus beyond only sports and leisure-time exercise to include other domains of daily life where women can be physically active, and gain health benefits. Although independent, beneficial bone strength associations were seen in each activity domain, effect sizes were larger for sport and for active living than for home and work activity. This could be a reflection of the lower intensity of usual physical activity at home and at work. In addition, our instrument may not have captured more rigorous activity at home, making it difficult to discern stronger effects that might truly exist for those who performed more physically demanding tasks at home.
A previous cross sectional study using the full SWAN cohort found positive associations between BMD and sport and home activity, but did not find associations between BMD and either work activity or active living [27]. Our study, which examined femoral neck strength relative to load found positive associations with each domain of physical activity. The importance of assessing bone strength relative to load has been demonstrated in multiple cohorts [17–22,35–37]. Since physical activity can affect bone strength relative to load in ways other than by affecting BMD, both by influencing bone size [24,25] and by affecting body weight (which determines load) [26], it is not enough to look at BMD in isolation.
Controlling for BMI attenuated the effect sizes for sport activity and active living, suggesting that at least some of the association of physical activity with bone strength relative to load is due to physical activity effects on body weight. However, controlling for BMI did not attenuate effect sizes for work and home activity, suggesting that the usual level of physical activity at work and home in our sample may not have influenced body weight.
The limitations of our study must be acknowledged. First, this is a cross sectional study that does not allow us to make causal inferences. Second, we did not measure life-long patterns of physical activity, which are reported to affect peak bone mass [38–40]. Third, the study sample is limited to women, and the results may not generalize to men. Fourth, the composite indices are structural measures based on macroscopic measurements from DXA scans and ignore microscopic features such as differences in the quality of cancellous mineralization and microarchitecture, both of which are important determinants of bone strength [21,41,42].
Despite these limitations, our study has several strengths, including the multi-site, nature and size of the study sample, assessment of physical activity in multiple domains, and assessment of bone strength relative to load (impact forces in a fall). It is the first study to examine physical activity as a determinant of peak bone strength relative to load in women, and demonstrate the independent effects of physical activity in multiple domains of daily life, not limited to sports and leisure-time exercise. Since peak bone mass in women before they go through the menopause transition is a major predictor of osteoporosis in later life [43], physical activity in young and midlife may prevent late-life fracture and its associated morbidities.
In summary, greater physical activity in each of the domains of sport, home, active living, and work was independently associated with greater femoral neck strength relative to load in pre- and early perimenopausal women. Being physically active in all domains of life may be an important way in which women can prevent osteoporotic fractures in later life.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The Hip Strength Through the Menopausal Transition has grant support from the NIA (AG026463). Takahiro Mori was supported by the VA Advanced Fellowship Program in Geriatrics and the VA Greater Los Angeles Geriatric Research Education and Clinical Center. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, VA or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. Journal of Bone and Mineral Research. 2007;22 (3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17 (12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nature reviews Rheumatology. 2010;6 (2):99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17 (3):337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 5.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ (Clinical research ed) 1991;303 (6808):961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone. 1996;19 (1):9–12. doi: 10.1016/8756-3282(96)00102-0. [DOI] [PubMed] [Google Scholar]
- 7.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS, Karlamangla AS. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN) Journal of Bone and Mineral Research. 2012;27 (1):111–118. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV. Identification of high-risk individuals for hip fracture: a 14-year prospective study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2005;20 (11):1921–1928. doi: 10.1359/jbmr.050520. [DOI] [PubMed] [Google Scholar]
- 9.Waterloo S, Nguyen T, Ahmed LA, Center JR, Morseth B, Nguyen ND, Eisman JA, Sogaard AJ, Emaus N. Important risk factors and attributable risk of vertebral fractures in the population-based Tromso study. BMC musculoskeletal disorders. 2012;13:163. doi: 10.1186/1471-2474-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2005;20 (2):185–194. doi: 10.1359/jbmr.041007. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein JS, Lee M-LT, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang M-H, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. Journal of Clinical Endocrinology & Metabolism. 2002;87 (7):3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 12.Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, Yun H, Zhang J, Delzell ES. Recent trends in hip fracture rates by race/ethnicity among older US adults. Journal of Bone and Mineral Research. 2012 doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18 (4):427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 14.Alonso CG, Curiel M, Carranza F, Cano R, Perez A. Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Multicenter Project for Research in Osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11 (8):714. [PubMed] [Google Scholar]
- 15.Faulkner KG, Cummings SR, Black D, Palermo L, Glüer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. Journal of bone and mineral research. 2009;8 (10):1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 16.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does Obesity Really Make the Femur Stronger? BMD, Geometry, and Fracture Incidence in the Women’s Health Initiative-Observational Study. Journal of Bone and Mineral Research. 2009;24 (8):1369–1379. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15 (1):62–70. doi: 10.1007/s00198-003-1513-1. [DOI] [PubMed] [Google Scholar]
- 18.Yu N, Liu YJ, Pei Y, Zhang L, Lei S, Kothari NR, Li DY, Papasian CJ, Hamilton J, Cai JQ, Deng HW. Evaluation of compressive strength index of the femoral neck in Caucasians and chinese. Calcified tissue international. 2010;87 (4):324–332. doi: 10.1007/s00223-010-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li GW, Chang SX, Xu Z, Chen Y, Bao H, Shi X. Prediction of hip osteoporotic fractures from composite indices of femoral neck strength. Skeletal radiology. 2012 doi: 10.1007/s00256-012-1473-7. [DOI] [PubMed] [Google Scholar]
- 20.Ishii S, Greendale GA, Cauley JA, Crandall CJ, Huang M-H, Danielson ME, Karlamangla AS. Fracture Risk Assessment without Race/Ethnicity Information. Journal of Clinical Endocrinology & Metabolism. 2012 doi: 10.1210/jc.2012-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 doi: 10.1007/s00198-011-1723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii S, Cauley JA, Crandall CJ, Srikanthan P, Greendale GA, Huang MH, Danielson ME, Karlamangla AS. Diabetes and Femoral Neck Strength: Findings from The Hip Strength Across the Menopausal Transition Study. The Journal of clinical endocrinology and metabolism. 2011 doi: 10.1210/jc.2011-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardinha LB, Baptista F, Ekelund U. Objectively measured physical activity and bone strength in 9-year-old boys and girls. Pediatrics. 2008;122 (3):e728–736. doi: 10.1542/peds.2007-2573. [DOI] [PubMed] [Google Scholar]
- 24.Uusi-Rasi K, Sievänen H, Vuori I, Pasanen M, Heinonen A, Oja P. Associations of physical activity and calcium intake with bone mass and size in healthy women at different ages. Journal of Bone and Mineral Research. 1998;13 (1):133–142. doi: 10.1359/jbmr.1998.13.1.133. [DOI] [PubMed] [Google Scholar]
- 25.Uusi-Rasi K, Sievänen H, Pasanen M, Beck TJ, Kannus P. Influence of calcium intake and physical activity on proximal femur bone mass and structure among pre-and postmenopausal women. A 10-year prospective study. Calcif Tissue Int. 2008;82 (3):171–181. doi: 10.1007/s00223-008-9105-x. [DOI] [PubMed] [Google Scholar]
- 26.Langsetmo L, Hitchcock CL, Kingwell EJ, Davison KS, Berger C, Forsmo S, Zhou W, Kreiger N, Prior JC. Physical activity, body mass index and bone mineral density-associations in a prospective population-based cohort of women and men: the Canadian Multicentre Osteoporosis Study (CaMos) Bone. 2012;50 (1):401–408. doi: 10.1016/j.bone.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greendale GA, Huang MH, Wang Y, Finkelstein JS, Danielson ME, Sternfeld B. Sport and home physical activity are independently associated with bone density. Medicine and science in sports and exercise. 2003;35 (3):506–512. doi: 10.1249/01.mss.0000056725.64347.c9. [DOI] [PubMed] [Google Scholar]
- 28.Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39 (8):1423. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 29.Sowers MCS, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Menopause: biology and pathobiology. Academic; San Diego: 2000. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community based cohort study of women and the menopausal transition; pp. 175–188. [Google Scholar]
- 30.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. The Journal of clinical endocrinology and metabolism. 2008;93 (3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. The American journal of clinical nutrition. 1982;36 (5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32 (7):1327. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 33.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16 (11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 34.Ishii S, Cauley J, Greendale G, Crandall C, Huang M, Danielson M, Karlamangla A. Trajectories of femoral neck strength in relation to the final menstrual period in a multi-ethnic cohort. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 doi: 10.1007/s00198-013-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faulkner KG, Wacker W, Barden H, Simonelli C, Burke P, Ragi S, Del Rio L. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporosis international. 2006;17 (4):593–599. doi: 10.1007/s00198-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 36.Dufour A, Roberts B, Broe K, Kiel D, Bouxsein M, Hannan M. The factor-of-risk biomechanical approach predicts hip fracture in men and women: the Framingham Study. Osteoporosis International. 2012;23 (2):513–520. doi: 10.1007/s00198-011-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie W, Pahlavan P, Tsang J, Lix L. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporosis international. 2009;20 (10):1767–1774. doi: 10.1007/s00198-009-0874-5. [DOI] [PubMed] [Google Scholar]
- 38.Greendale GA, Barrett-Connor E, Edelstein S, Ingles S, Haile R. Lifetime leisure exercise and osteoporosis. The Rancho Bernardo study. American journal of epidemiology. 1995;141 (10):951–959. doi: 10.1093/oxfordjournals.aje.a117362. [DOI] [PubMed] [Google Scholar]
- 39.Micklesfield L, Rosenberg L, Cooper D, Hoffman M, Kalla A, Stander I, Lambert E. Bone mineral density and lifetime physical activity in South African women. Calcified tissue international. 2003;73 (5):463–469. doi: 10.1007/s00223-002-2129-8. [DOI] [PubMed] [Google Scholar]
- 40.Ulrich CM, Georgiou CC, Gillis DE, Snow CM. Lifetime physical activity is associated with bone mineral density in premenopausal women. Journal of women’s health / the official publication of the Society for the Advancement of Women’s Health Research. 1999;8 (3):365–375. doi: 10.1089/jwh.1999.8.365. [DOI] [PubMed] [Google Scholar]
- 41.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34 (5):783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Cauley J, Danielson M, Gregg E, Vogt M, Zmuda J, Bauer D. Calcaneal ultrasound attenuation in older African-American and Caucasian-American women. Osteoporosis international. 1997;7 (2):100–104. doi: 10.1007/BF01623683. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez C, Beaupre G, Carter D. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporosis international. 2003;14 (10):843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]