Abstract
N6-methyladenosine (m6A) is the most prevalent internal (non-cap) modification present in the messenger RNA (mRNA) of all higher eukaryotes1,2. Although essential to cell viability and development3–5, the exact role of m6A modification remains to be determined. The recent discovery of two m6A demethylases in mammalian cells highlighted the importance of m6A in basic biological functions and disease6–8. Here we show that m6A is selectively recognized by the human YTH domain family 2 (YTHDF2) protein to regulate mRNA degradation. We identified over 3,000 cellular RNA targets of YTHDF2, most of which are mRNAs, but which also include non-coding RNAs, with a conserved core motif of G(m6A)C. We further establish the role of YTHDF2 in RNA metabolism, showing that binding of YTHDF2 results in the localization of bound mRNA from the translatable pool to mRNA decay sites, such as processing bodies9. The C-terminal domain of YTHDF2 selectively binds to m6A-containing mRNA whereas the N-terminal domain is responsible for the localization of the YTHDF2-mRNA complex to cellular RNA decay sites. Our results indicate that the dynamic m6A modification is recognized by selective-binding proteins to affect the translation status and lifetime of mRNA.
Messenger RNA (mRNA) is central to the flow of genetic information. Regulatory elements (e.g. AU-rich element, iron-responsive element), in the form of short sequence or structural motif imprinted in mRNA, are known to control the time and location of translation and degradation processes10. Reversible and dynamic methylation of mRNA could add another layer of more sophisticated regulation to the primary sequence2,11. m6A, a prevalent internal modification in the messenger RNA of all eukaryotes, is post-transcriptionally installed by m6A methyltransferase (e.g., MT-A70, Fig. 1a) within the consensus sequence of G(m6A)C (70%) or A(m6A)C (30%)12. The loss of MT-A70 leads to apoptosis in human HeLa cells13, and significantly impairs development in Arabidopsis4 and in Drosophila5. Our recent discoveries of m6A demethylases FTO (fat mass and obesity-associated protein)7 and ALKBH58 demonstrate that this RNA methylation is reversible and may dynamically control mRNA metabolism. The recently revealed m6A transcriptomes (methylome) in human cells and mouse tissues showed m6A enrichments within long exons and around stop codon14,15, further suggesting fundamental regulatory roles of m6A. However, despite these progresses the exact function of m6A remains to be elucidated.
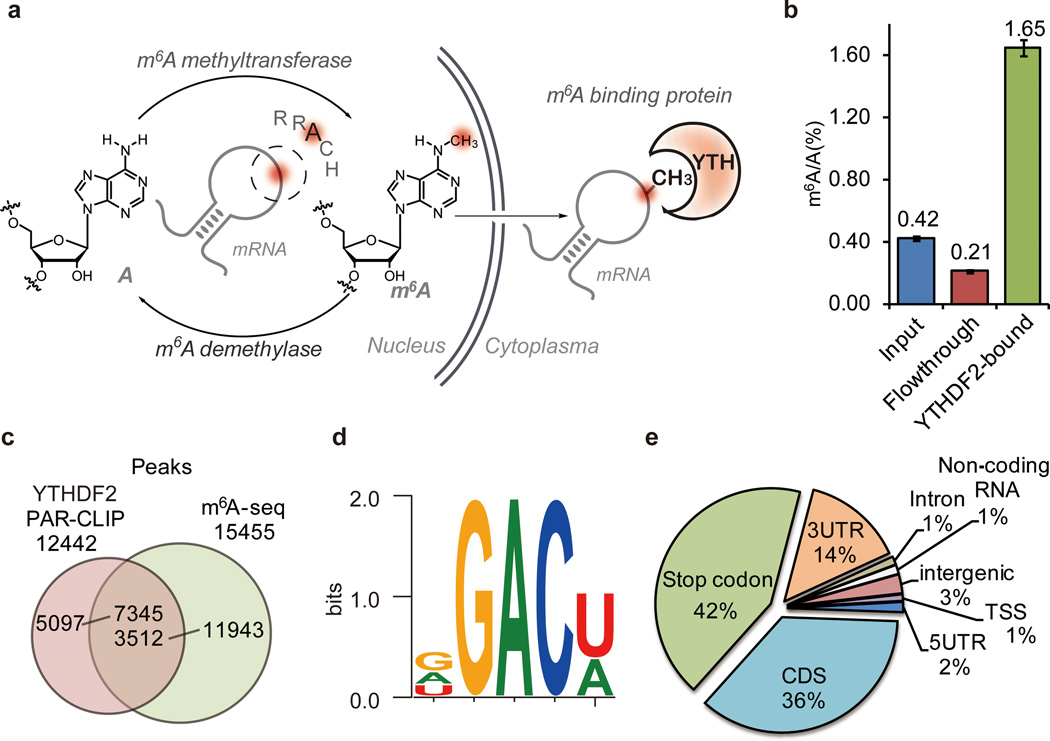
Figure 1. YTHDF2 selectively binds m6A-containing RNA.
a, Illustration of m6A methylatransferase, demethylase, and binding proteins. RRACH is the extended m6A consensus motif, where R is G or A and H is not G. b, LC-MS/MS showing m6A enrichment in GST-YTHDF2-bound mRNA while depleted in the flow-through portion. Error bars, mean ±s.t.d., n = 2, technical replicates. c, Overlap of peaks identified through YTHDF2-based PAR-CLIP and the m6A-seq peaks in the same cell line. d, Binding motif identified by MEME with PAR-CLIP peaks (p = 3.0 e−46, 381 sites were found under this motif out of top 1000 scored peaks). e, Pie chart depicting the region distribution of YTHDF2-binding sites identified by PAR-CLIP, TTS (200 bp window from the transcription starting site), stop codon (400 bp window centered on stop codon).
While methyltransferase may serve as the “writer” and demethylases (FTO and ALKBH5) act as the “eraser” of m6A on mRNA, potential m6A-selective-binding proteins could represent the “reader” of the m6A modification and exert regulatory functions through selective recognition of methylated RNA. Here, we show that the YTH-domain family member 2 (YTHDF2), initially found in pull-down experiments using m6A-containing RNA probes14, selectively binds m6A-methylated mRNA and controls RNA decay in a methylation-dependent manner .
The YTH domain family is widespread in eukaryotes and known to bind single-stranded RNA (ssRNA) with the conserved YTH domain (>60% identity) located at the C-terminus16,17. In addition to previously reported YTHDF2 and YTHDF314, we also discovered YTHDF1 as another m6A-selective binding protein by using methylated RNA bait containing the known consensus sites of G(m6A)C and A(m6A)C versus unmethylated control (Extended Data Fig. 1a). Further, highly purified poly(A)-tailed RNAs were incubated with recombinant GST-tagged YTHDF1–3 and then separated by GST-affinity column. By using a previously reported LC-MS/MS method7,8, we found that the m6A-containing RNAs were greatly enriched in the YTHDF-bound portion and diminished in the flow-through portion (Fig. 1b, Extended Data Fig. 1b). Gel shift assay revealed that YTHDF2 exhibits a 16-fold higher binding affinity to methylated probe compared to the unmethylated one as well as a slight preference to the consensus sequence (Extended Data Fig. 1c–d). This protein was selected for subsequent characterization since it exhibits a high selectivity to m6A, and was thought to be associated with human longevity18.
We next applied two independent methods to identify RNAs that are the binding partners of YTHDF2: i) photoactivatable ribounucleoside crosslinking and immunoprecipitation (PAR-CLIP)19 to locate the binding sites of YTHDF2; ii) sequencing profiling of the RNA of immuno-purified ribonucleoprotein complex (RNP) (RIP-seq)20 to extract cellular YTHDF2-RNA complexes. Approximately ten thousand crosslinked clusters covering 3,251 genes were identified in PAR-CLIP (Extended Data Fig. 2a–b). Most are mRNA but also 1% belongs to non-coding RNA. Among 2,536 transcripts identified in RIP-seq, 50% overlap with PAR-CLIP targets (Extended Data Fig. 2b). We also performed m6A-seq for the poly(A)-tailed RNA from the same HeLa cell line and found that 59% (7,345, out of 12,442) of the PAR-CLIP peaks of YTHDF2 overlap with m6A peaks (Fig. 1c). As shown in Fig. 1d, the conserved motif revealed from the top 1,000 scored clusters matches the m6A consensus sequence of RRACH12,14, which strongly supports the binding of m6A by YTHDF2 inside cells (see more motifs in Extended Data Fig. 2c–e). Coinciding with the previously reported pattern of m6A peaks14,15, YTHDF2 PAR-CLIP peaks showed enrichment near the stop codon and in long exons (Extended Data Fig. 2f–h). YTHDF2 predominantly targets the stop codon region, the 3’ untranslated region (3UTR), and the coding region (CDS) (Fig. 1e), suggesting that YTHDF2 may play a role in mRNA stability and/or translation.
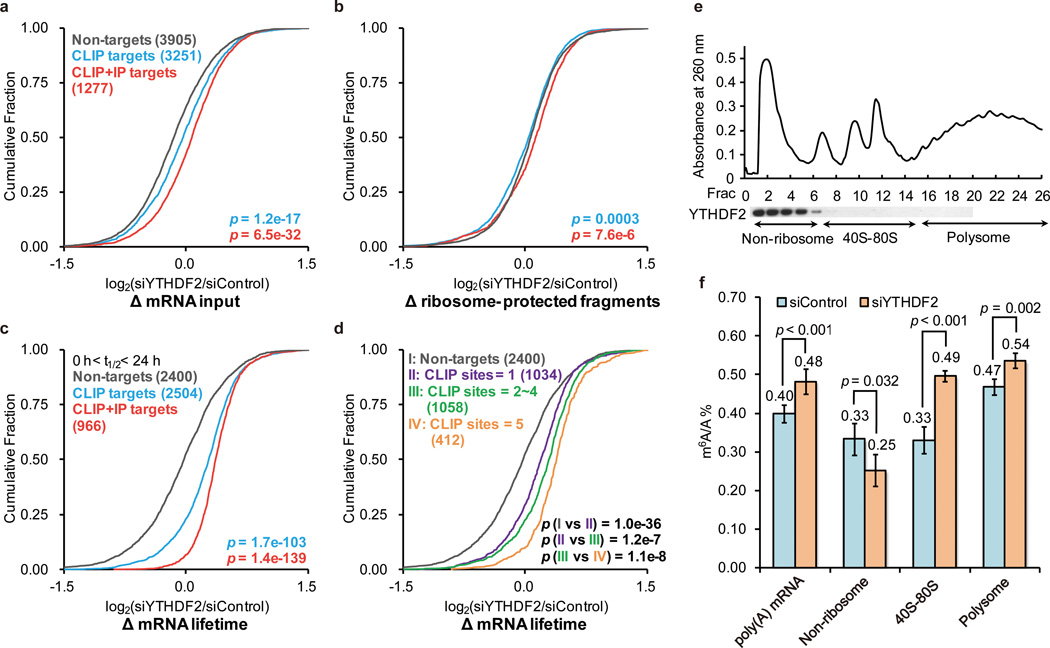
To dissect the role of YTHDF2 we employed ribosome profiling to assess the ribosome loading of each mRNA represented as ribosome-protected reads21,22. HeLa cells that were treated with YTHDF2 siRNA (Extended Data Fig. 3a) as well as siRNA control were subsequently subjected to ribosome profiling with mRNA-seq performed on the same sample. Transcripts present (RPKM > 1, reads per kilobase, per million reads) in both ribosome profiling and mRNA-seq samples were analyzed. These transcripts were then categorized as YTHDF2 PAR-CLIP targets (3,251), common targets of PAR-CLIP and RIP (1,277), and non-targets (3,905, absent from PAR-CLIP and RIP). A significant increase of input mRNA reads for YTHDF2 targets was observed in the YTHDF2 knockdown sample compared to the control (p < 0.001, Mann-Whitney U test), without a noticeable change for non-targets (Fig. 2a). However, compared with the increase in mRNA level, the differences in the ribosome-protected fraction in the knockdown sample compared to the control were small (Fig. 2b). Thus, YTHDF2 knockdown led to apparently reduced translation efficiency of its targets as a result of accumulation of non-translating mRNA (Extended Data Fig. 3b), suggesting the primary role of YTHDF2 in RNA degradation.
Figure 2. YTHDF2 destabilizes its cognate mRNAs.
a–d, Cumulative distribution of mRNA input (a), ribosome-protected fragments (b), and mRNA lifetime log2 fold changes (Δ, c) between siYTHDF2 (YTHDF2 knockdown) and siControl (knockdown control) for non-targets (grey), PAR-CLIP targets (blue), and PAR CLIP-RIP common targets (red). The mRNA lifetime log2 fold changes were further grouped and analyzed based on the number of CLIP sites on each transcript (d). The increased binding of YTHDF2 on its target transcript correlates with reduced mRNA lifetime. P values were calculated using two-sided Mann-Whitney or Kruskal-Wallis test (rank-sum test for the comparison of two or multiple samples, respectively). Detailed statistics were presented in Extended Data Fig. 3c. e, Western-blotting of flag-tagged YTHDF2 on each fraction of 10–50% sucrose gradient showing that YTHDF2 does not associate with ribosome. The fractions were grouped to non-ribosome mRNPs, 40–80S, and polysome. f, Quantification of the m6A/A ratio of the total mRNA, non-ribosome portion, 40–80S, and polysome by LC-MS/MS. Noticeable increases of the m6A/A ratio of the total mRNA, mRNA from 40–80S, and mRNA from polysome were observed in the siYTHDF2 sample compared to control after 48 h. A reduced m6A/A ratio of mRNA isolated from the non-ribosome portion was observed in the same experiment. P values were determined using two-sided Student’s t-test for paired samples. Error bars, mean ± s.t.d, for poly(A)-tailed total mRNA input, n = 10 (five biological replicates × two technical replicates), and for the rest, n = 4 (two biological replicates × two technical replicates).
Next, we performed RNA lifetime profiling by collecting and analyzing RNA-seq data on YTHDF2 knockdown and control samples obtained at different time points after transcription inhibition with actinomycin D. Indeed, YTHDF2 knockdown led to prolonged (~30% in average) lifetimes of its mRNA targets in comparison with non-targets (Fig. 2c). Interestingly, we found that as the number of binding sites increase the stabilization of the RNA targets caused by YTHDF2 knockdown also increase significantly23: more than 4 sites > 2~4 sites > 1 sites (Fig. 2d and Extended Data Fig. 3c, Kruskal-Wallis test, p <0.0001); however, transcripts grouped according to binding region show similar fold change indistinguishable in statistical test (Extended Data Fig.3c–d).
Three pools of mRNAs exist in cytoplasm as defined by their engagement in translation24,25 (Fig. 2e): non-ribosome mRNPs (mRNA-protein particles, with sedimentation coefficients of 20–35S in sucrose gradient), translatable mRNA pool associated with ribosomal subunits (40–80S), and actively translating polysome (>80S). YTHDF2 was observed to be present in non-ribosome fraction (Fig. 2e). After YTHDF2 knockdown, a 21% increase of the m6A/A ratio of the total mRNA was observed (Fig. 2f), confirming that the presence of YTHDF2 destabilizes the m6A-containing mRNA. YTHDF2 could affect localizing m6A-containing mRNA from a translatable pool to mRNPs. If so, the amount of methylated mRNA should decrease in mRNPs and increase in the translatable pool upon YTHDF2 knockdown. Indeed, after YTHDF2 knockdown, the m6A/A ratio of mRNA isolated from mRNPs showed a 24% decrease and the ratio from the translatable pool demonstrated a 46% increase (Fig. 2f). We also observed a 14% increase of the m6A/A ratio of mRNA isolated from polysome after YTHDF2 knockdown (Fig. 2f), although it is worth noting that this model provided no prediction of the behavior of polysome since the ribosome-loading number per transcript depends on the availability of both mRNA and free ribosomes. It should be also noted that the observed m6A/A ratio change does not appear to be resulted from the protein level change of methyltransferase and demethylase as detected by western blotting (Extended Data Fig. 3e).
Three YTHDF2-targeted RNAs were selected for further validation: the SON mRNA has multiple CLIP peaks in CDS, the CREBBP mRNA has CLIP peaks at 3’UTR, and a non-coding RNA PLAC2 (Extended Data Fig. 4a–d). As detected by gene-specific RT-PCR, after 48 h YTHDF2 knockdown, all three RNA transcripts increased by more than 60% with prolonged lifetime; both SON and CREBBP showed redistribution from non-ribosome mRNP to translatable pool (Extended Data Fig. 4e–n). Furthermore, knockdown of the known m6A methyltransferase MT-A70 led to noticeably reduced binding of YTHDF2 to its targets and increased stability of the targets similar to that of the YTHDF2 knockdown (Extended Data Fig. 5).
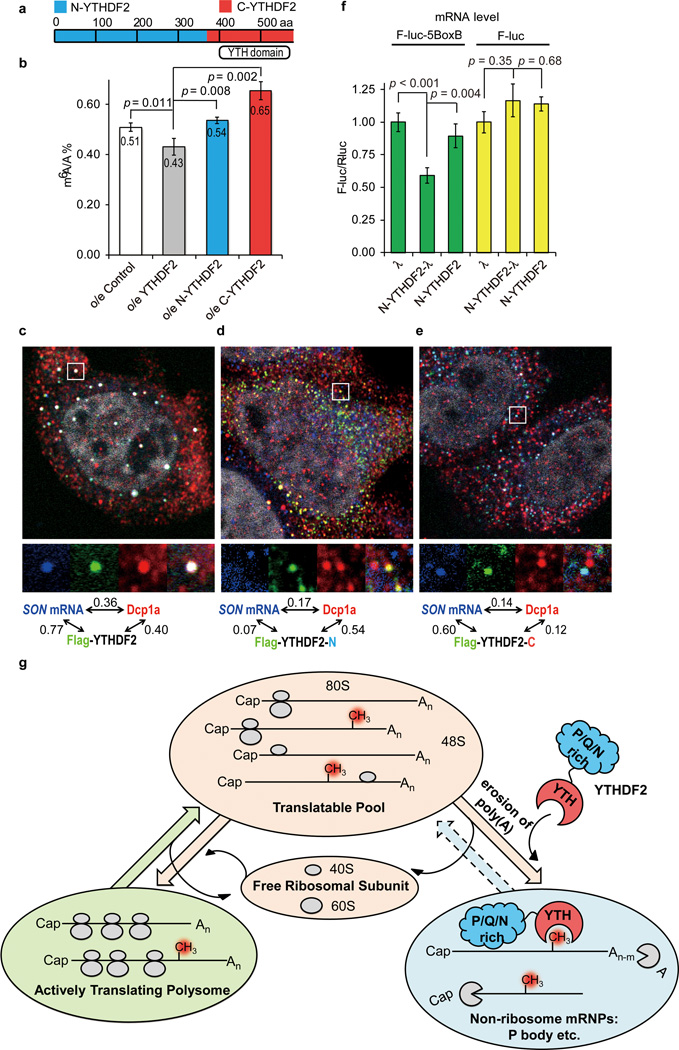
To gain mechanistic understanding of the YTHDF2-mRNA interaction, we analyzed the cellular distribution of YTHDF2 and found that YTHDF2 co-localizes with three markers (DCP1a, GW182 and DDX6) of processing bodies (P bodies) in cytoplasm, where mRNA decay occurs (Extended Data Fig. 6a–j)9,26. YTHDF2 is composed of a C-terminal RNA-binding domain (C-YTHDF2) and a P/Q/N rich N-terminus (N-YTHDF2, Fig. 3a and Extended Data Fig. 6k)27,28. While over-expression of YTHDF2 led to a reduced m6A/A ratio of the total mRNA, over-expression of either N-YTHDF2 or C-YTHDF2 yielded an increased m6A/A ratio (Fig. 3b), indicating that both domains are required for the YTHDF2-mediated mRNA decay. The in vitro pull-down experiment further showed that purified C-YTHDF2 is able to enrich m6A-containing mRNA from total mRNA (Extended Data Fig. 6l). The spatial distribution of the SON mRNA relative to YTHDF2 and N- and C-YTHDF2 truncates were examined by fluorescence in situ hybridization (FISH) and fluorescence immunostaining in HeLa cells (Fig. 3c–e). The location of the SON mRNA showed a strong correlation with that of the full-length YTHDF2 (Fig. 3c) and C-YTHDF2 (Fig. 3e). In contrast, a much lower correlation was observed for the SON mRNA with N-YTHDF2 (Fig. 3d). In addition, the full-length YTHDF2 and N-YTHDF2 co-localized with DCP1a, but to a much less extent for C-YTHDF2, thereby indicating the role of N-YTHDF2 in P-body localization. Furthermore, the over-expression of C-YTHDF2 led to a reduced co-localization of the SON mRNA with DCP1a (Fig. 3e).
Figure 3. YTHDF2 affects SON mRNA localization in processing body (P-body).
a, Schematic of the domain architecture (aa stands for amino acids) of YTHDF2, N-terminal of YTHDF2 (N-YTHDF2, aa 1–389, blue) and C-terminal of YTHDF2 (C-YTHDF2, aa 390-end, red). b, Over-expression of full-length YTHDF2 led to reduced levels of m6A after 24 h, while over-expression of N-YTHDF2 or C-YTHDF2 increased the m6A/A ratio of the total mRNA. P values were determined using two-sided Student’s t-test for paired samples. Error bars, mean ± s.t.d., n = 4 (two biological replicates × two technical replicates). c–e, Fluorescence in situ hybridization of SON mRNA and fluorescence immunostaining of DCP1a (P-body marker), flag-tagged YTHDF2 (c), flag-tagged C-YTHDF2, (d) and flag-tagged N-YTHDF2 (e). Full-length YTHDF2 and C-YTHDF2 co-localize with SON mRNA (bearing m6A) while the full-length YTHDF2 significantly increases the P-body localization of SON mRNA compared to N-YTHDF2 and C-YTDF2. The numbers shown above figures are Pearson correlation coefficients of each channel pair with the scale of the magnified region (white frame) set as 2 µm × 2 µm. f, Tethering N-YTHDF2-λ to a mRNA reporter F-luc-5BoxB led to a ~40% reduction of the reporter mRNA level compared to tethering N-YTHDF2 or λ alone (green) and controls without BoxB (F-luc, yellow). P values were determined using two-sided Student’s t-test for paired samples. Error bars, mean ± s.t.d., n = 6 (F-luc-5BoxB) or 3 (F-luc). g, A proposed model of m6A-dependent mRNA degradation mediated through YTHDF2. The three states of mRNAs in cytoplasm are defined by their engagement with ribosome using the sedimentation coefficient range in sucrose gradient: >80S for actively translating polysome; 40–80S for translatable pool; 20–35S for non-ribosome mRNPs.
In further support of this mechanism, N-YTHDF2 was fused with λ peptide (N-YTHDF2-λ) which recognizes Box B RNA with a high affinity in a tether reporter assay29,30. Tethering N-YTHDF2-λ to F-luc-5BoxB (five Box B sequence was inserted into the 3UTR of the mRNA reporter) led to a significantly reduced mRNA level (Fig. 3f) and shortening (40%) of its lifetime compared with tethering controls of N-YTHDF2 or λ alone (Extended Data Fig. 7a–e). The reporter mRNA bound by N-YTHDF2-λ possesses shorter poly(A) tail length in comparison with unbound portion, although a significant change of the deadenylation rate was not observed(Extended Data Fig. 7f–l). Together with the observation that YTHDF2 co-localizes with both deadenylation and decapping enzyme complexes (Extended Data Fig. 6), we propose a model (Fig. 3g) that consists of: (1) C-YTHDF2 selectively recognizes m6A-containing mRNA less engaged with translation; (2) this binding of YTHDF2 to methylated mRNA happens in parallel or at a later stage of deadenylation; (3) N-YTHDF2 localizes the YTHDF2-m6A-mRNA complex to more specialized mRNA decay machineries (P bodies etc.) for committed degradation.
Functional clustering of YTHDF2 targets versus non-targets revealed that the main functions of YTHDF2-mediated RNA processing are gene expression (molecular function) as well as cell death and survival (cellular function, Extended Data Fig. 8a–d). After 72 hours of YTHDF2 knockdown, the viability of HeLa cells reduced by 50% (Extended Data Fig. 8e–f), indicating that the YTHDF2-mediated RNA processing could have biological significance.
In summary, we present here a transcriptome-wide identification of YTHDF2-RNA interaction and a mechanistic model for m6A function mediated by this m6A-binding protein, as the first functional demonstration of a m6A reader protein. We show that YTHDF2 alters the distribution of the cytoplasmic states of several thousand m6A-containing mRNA. This present work demonstrates that reversible m6A deposition could dynamically tune the stability and localization of the target RNAs through m6A “readers”.
Methods
Plasmid construction and protein expression
Recombinant YTHDF1–3 were cloned from commercial cDNA clones (Open Biosystems) into vector pGEX-4T-1. The primers used for subcloning (from 5’ to 3’; F stands for forward primer; R stands for reverse primer) are listed below:
| GST-YTHDF1-F: | CGATCGAATTCATGTCGGCCACCAGCG |
| GST-YTHDF1-R: | CCATACTCGAGTCATTGTTTGTTTCGACTCTGCC |
| GST-YTHDF2-F: | CGTACGGATCCATGTCAGATTCCTACTTACCCAG |
| GST-YTHDF2-R: | CGATGCTCGAGTCATTTCCCACGACCTTGACG |
| GST-YTHDF3-F: | CGTACGGATCCATGTCAGCCACTAGCGTG |
| GST-YTHDF3-R: | CGTAGCTCGAGTCATTGTTTGTTTCTATTTCTCTCCCTAC |
The resulting clones were transfected into the E. coli strain BL21 and expression was induced at 16°C with 1 mM IPTG for 20 h. The pellet collected from 2 L bacteria culture was then lysed in 30 mL PBS-L solution (50 mM NaH2PO4, 150 mM NaCl, pH 7.2, 1 mM PMSF, 1 mM DTT, 1 mM EDTA, 0.1% (v/v) Triton X-100) and sonicated for 10 min. After removing cell debris by centrifuge at 12 krpm for 30 min, the supernatant were loaded to GST superflow cartridge (QIAGEN, 5 mL) and gradiently eluted by using PBS-EW (50 mM NaH2PO4, 150 mM NaCl, pH 7.2,1 mM DTT, 1 mM EDTA) as buffer A and TNGT (50 mM Tris, pH 8.0, 150 mM NaCl, 50 mM red, GSH, 0.05 % Triton-x-100) as buffer B. The crude products were further purified by gel-filtration chromatography in GF buffer (10 mM Tris, pH 7.5, 200 mM NaCl, 3 mM DTT and 5% glycerol). The yield was around 1–2 mg per liter of bacterial culture.
Flag-tagged YTHDF2 was cloned into vector pcDNA 3.0 (BamHI, XhoI; forward primer: CGTACGGATCCATGGATTACAAGGACGACGATGACAAGATGTCGGCCAGCAGCC; reverse primer: CGATGCTCGAGTCATTTCCCACGACCTTGACG). Flag-tagged YTHDF2 N-terminal was made by mutating E384 (GAA) to a stop codon (TAA) with Stratagenes QuickChange II site-directed mutagenesis kit (pcDNA-flag-Y2N, forward primer: CTGGATCTACTCCTTCATAACCCCACCCAGTGTTG; reverse primer: CAACACTGG GTGGGGTTATGAAGGAGTAGATCCAG). Flag-tagged YTHDF2 C-terminal was made by cloning amino acids from E384 to the end into vector pcDNA 3.0 (BamHI, XhoI, forward primer: CGTACGGATCCATGGATTACAAGGACGACGATGACAAGGAACCCCACCC AGTGTT; reverse primer: CGATGCTCGAGTCATTTCCCACGACCTTGACG). Plasmids with high purity for mammalian cell transfection were prepared with a Maxiprep kit (QIAGEN).
Tether reporter: pmirGlo Dual luciferase expression vector (Promega) was used to construct the tether reporter which contains firefly luciferase (F-luc) as the primary reporter and Renilla luciferase (R-luc) acting as a control reporter for normalization. F-luc-5BoxB mRNA reporter was obtained by inserting five Box B sequence (5BoxB) into the 3UTR of F-luc (SacI and XhoI, the resulting plasmid was named as pmirGlo-5BoxB;). The 5BoxB sequence29 (see below) was PCR amplified from PRL-5BoxB plasmid which was kindly provided by Prof. Witold Filipowi at Friedrich Miescher Institute for Biomedical Research (forward primer: CGATACGAGCTCTTCCCTAAGTCCAACTACCAAAC; reverse primer: CTATGGCTCGAGATAATATCCTCGATAGGGCCC; sequencing primer: GAC GAGGTG CCTAAAGA)31.
TTCCCTAAGTCCAACTACTAAACTGGGGATTCCTGGGCCCTGAAGAAGGGCCCCTCGACTAAGTCCAACTACTAAACTGGGCCCTGAAGAAGGGCCCATATAGGGCCCTGAAGAAGGGCCCTATCGAGGATATTATCTCGACTAAGTCCAACTACTAAACTGGGCCCTGAAGAAGGGCCCATATAGGGCCCTGAAGAAGGGCCCTATCGAGGATATTATCTCGAG
In order to study the decay kinetics of F-luc-5BoxB, another reporter plasmid (pmirGlo-Ptight-5BoxB) was constructed by replacing the original human phosphoglycerate kinase promoter of F-luc with Ptight promoter (restriction sites: ApaI and BglII). Ptight promoter was PCR amplified from pTRE-Tight vector (Clontech; forward primer: CGTACAGATCTCGAGTTTACTCCCTATCAGT; reverse primer: CTGTAGGGCCCT TCTTAATGTTTTTGGCATCTTCCATCTCCAGGCGATCTGACG; sequencing primer: AGCGGTGCGTACAATTAAGG). The resulting plasmid (pmirGlo-Ptight) was subjected to a second round of subcloning by inserting 5BoxB into the 3UTR of F-luc (restriction sites: XbaI and SbfI) to generate pmirGlo-Ptight-5BoxB (forward primer: CGATACTCTAGATTCCCTAAGTCCAACTACCAAAC; reverse primer: CTATGGCC TGCAGGATAATATCCTCGATAGGGCCC; sequencing primer: GACGAGGTG CCTAA AGA).
Tether effecter: λ peptide sequence (MDAQTRRRERRAEKQAQWKAAN) was fused to the C-terminal of N-YTHDF2 by subcloning N-YTHDF2 to pcDNA 3.0 with forward primer containing flag-tag sequence and reverse primer containing λ peptide sequence (pcDNA-flag-Y2Nλ, BamHI, XhoI; forward primer: GATACGGATCCATGG ATTACAAGGACGACGATGACAAGATGTCGGCCAGCAGCC; reverse primer: TAT GGCTCGAGTCAGTTTGCAGCTTTCCATTGAGCTTGTTTCTCAGCGCGACGCTCACGTCGTCGTGTTTGTGCGTCCATACCTGAAGGAGTAGATCCAGAACC). The λ peptide control was designed with a flag tag at N-terminal and a GGS spacer (pcDNA-flag-λ). The primer pair that contains flag-tagged λ peptide and sticky restriction enzyme sites (BamHI, XhoI) was annealed and directly ligated to digested pcDNA 3.0 (forward primer: GATCCATGGATTACAAGGACGACGATGACAAGGGTGGTAGCATGGACGCACAAACACGACGACGTGAGCGTCGCGCTGAGAAACAAGCTCAATGGAAAGCTGCAAACTAAC; reverse primer: GAGTTAGTTTGCAGCTTTCCATTGAGCTTGTTTCTCAGC GCGACGCTCACGTCGTCGTGTTTGTGCGTCCATGCTACCACCCTTGTCATCGTCGTCCTTGTAATCCATG).
EMSA (Electrophoretic Mobility Shift Assay / Gel shift assay)
The RNA probe was synthesized by a previously reported method with the sequence of 5’-AUGGGCCGUUCAUCUGCUAAAAGGXCUGCUUUUGGGGCUUGU-3’(X = A or m6A). After the synthesis, the RNA probe was labeled in a reaction mixture of 2 µL RNA probe (1 µM), 5 µL 5×T4 PNK buffer A (Fermentas), 1 µL T4 PNK (Fermentas), 1 µL32P-ATP and 41 µL RNase-free water (final RNA concentration 40 nM) at 37°C for 1 hour. The mixture was then purified by RNase-free micro bio-spin columns with bio-gel P30 in Tris buffer (BioRad 732–6250) to remove hot ATP and other small molecules. To the elute, 2.5 µL 20 × SSC (Promega) buffer was added. The mixture was heated to 65°C for 10 min to denature the RNA probe, and then slowly cooled down to room temperature. GST-YTHDF1–3 were diluted to concentration series of 200 nmol, 1 µM, 5 µM, 20 µM and 100 µM (or other indicated concentrations) in binding buffer (10 mM HEPES, pH 8.0, 50 mM KCl, 1mM EDTA, 0.05% Triton-X-100, 5% glycerol, 10 µg/mL Salmon DNA, 1 mM DTT and 40 U/mL RNasin). Before loading to each well, 1 µL RNA probe (4 nM final concentration) and 1 µL protein (20 nM, 100 nM, 500 nM, 2 µM, or 10 µM final concentration) were added and the solution was incubated on ice for 30 min. The entire 10 µL RNA-protein mixture was loaded to the gel (Novex 4~20% TBE gel) and run at 4 °C for 90 min at 90 V. Quantification of each band was carried out by using a storage phosphor screen (K-Screen; Fuji film) and Bio-Rad Molecular Imager FX in combination with Quantity One software (Bio-Rad). The Kd (dissociation constant) was calculated with nonlinear curve fitting (Function Hyperbl) of Origin 8 software with y = P1×x/(P2+x), where y is the ratio of [RNA-protein]/[free RNA]+[RNA-protein], x is the concentration of the protein, and P2 is Kd.
Mammalian cell culture, siRNA knockdown (KD), and plasmid transfection
Human HeLa cell line used in this study was purchased from ATCC (CCL-2) and grown in DMEM (Gibco, 11965) media supplemented with 10% FBS and 1% 100×Pen Strep (Gibco, 15140). HeLa Tet-off cell line was purchased from Clontech (631156) and grown in DMEM (Gibco, 11965) media supplemented with 10% FBS (Tet system approved, Clontech, 631101), 1% 100×Pen Strep (Gibco, 15140) and 200 µg/mL G418 (Clontech, 631308). YTHDF2 siRNA was ordered from QIAGEN as custom synthesis which targets 5’-AAGGACGTTCCCAATAGCCAA-3’ near the N terminal of CDS. MT-A70 siRNA was ordered from QIAGEN: 5’-CGTCAGTATCTTGGGCAAGTT-3’. Transfection was achieved by using Lipofectamine RNAiMAX (Invitrogen) for siRNA, and Lipofectamine 2000 for single type of plasmid or Lipofectamine LTX Plus (Invitrogen) for co-transfection of two or multiple types of plasmids (tethering assay) following the manufacturer’s protocols.
RNA isolation
mRNA isolation for LC/MSMS: Total RNA was isolated from wild-type or transiently transfected cells with TRIZOL Reagent (Invitrogen). mRNA was extracted using PolyATtract® mRNA Isolation Systems IV (Promega) followed by further removal of contaminated rRNA by using RiboMinus Transcriptome Isolation Kit (Invitrogen). mRNA concentration was measured by NanoDrop. Total RNA isolation for RT-PCR: following the instruction of RNeasy kit (QIAGEN) in addition to DNase I digestion step. Ethanol precipitation: to the RNA solution being purified or concentrated, 1/10 volume of 3 M NaOAc, pH 5.5, 1 µL glycogen (10 mg/mL) and 2.7 volume of 100% ethanol were added, stored at −80 °C for 1 h to overnight, and then centrifuged at 13 krpm for 15 min. After the supernatant was removed, the pellet was washed twice by using 1 mL 75% ethanol, and dissolved in the appropriate amount of RNase-free water as indicated.
In vitro pull down
0.8 µg mRNA (save 0.2 µg from the same sample as input) and YTHDF1–3 or C-YTHDF2 (final concentration 500 nM) were diluted into 200 µL IPP buffer (150 mM NaCl, 0.1% NP-40, 10 mM Tris, pH 7.4, 40 U/mL RNase inhibitor, 0.5 mM DTT), and the solution was mixed with rotation at 4 °C for 2 h. For YTHDF1–3, 10 µL GST-affinity magnetic beads (Pierce) were used for each sample after being washed four times with 200 µL IPP buffer for each wash. For C-YTHDF2, 20 µL Dynabeads® His-Tag Isolation & Pulldown beads (Invitrogen) were used after being washed four times with 200 µL IPP buffer for each wash. The beads were then re-suspended in 50 µL IPP buffer. The protein-RNA mixture was combined with GST or His6 beads and kept rotating for another 2 h at 4 °C. The aqueous phase was collected, recovered by ethanol precipitation, dissolved in 15 µL water, and saved as the flowthrough. The beads were washed four times with 300 µL IPP buffer each time. 0.4 mL trizol reagent was added to the beads and further purified according to manufacturer’s instruction. The purified fraction was dissolved in 15 µL water, and saved as YTHDF-bound. LC-MS/MS was used to measure the level of m6A in each sample of input, flowthrough, and YTHDF-bound.
LC-MS/MS7,8
200–300 ng of mRNA was digested by nuclease P1 (2 U) in 25 µl of buffer containing 25 mM of NaCl, and 2.5 mM of ZnCl2 at 37 °C for 2 h, followed by the addition of NH4HCO3 (1 M, 3 µl) and alkaline phosphatase (0.5 U). After an additional incubation at 37 °C for 2 h, the sample was diluted to 50 µL and filtered (0.22 µm pore size, 4 mm diameter, Millipore), and 5 µl of the solution was injected into LC-MS/MS. Nucleosides were separated by reverse phase ultra-performance liquid chromatography on a C18 column with on-line mass spectrometry detection using Agilent 6410 QQQ triple-quadrupole LC mass spectrometer in positive electrospray ionization mode. The nucleosides were quantified by using the nucleoside to base ion mass transitions of 282 to 150 (m6A), and 268 to 136 (A). Quantification was performed in comparison with the standard curve obtained from pure nucleoside standards running on the same batch of samples. The ratio of m6A to A was calculated based on the calibrated concentrations.
m6A profiling
Total RNA was isolated from HeLa cells with TRIZOL reagent. Poly(A)+ RNA was further enriched from total RNA by using FastTrack MAG Maxi mRNA isolation kit (Invitrogen). In particularly, an additional DNase I digestion step was applied to all the samples to avoid DNA contamination. RNA fragmentation, m6A-seq, and library preparation were performed according to the previous protocol developed by Dominissini et al14. The experiment was conducted in two biological replicates (Extended Data Table 1).
RIP-seq
The procedure was adapted from the previous report20. 60 million HeLa cells were collected (three 15 cm plates, after 24 h transfection of flag-tagged YTHDF2) by cell lifter (Corning Incorporated), pelleted by centrifuge for 5 min at 1 krcf and washed once with cold PBS (6 mL). The cell pellet was re-suspended with 2 volumes of lysis buffer (150 mM KCl, 10 mM HEPES pH 7.6, 2 mM EDTA, 0.5% NP-40, 0.5 mM DTT, 1:100 protease inhibitor cocktail, 400 U/mL RNase inhibitor; one plate with ~200 µL cell pellet and ~400 µL lysis buffer), pipetted up and down several times, and then the mRNP lysate was incubated on ice for 5 min and shock-frozen at −80°C with liquid nitrogen. The mRNP lysate was thawed on ice and centrifuged at 15 krcf for 15 min to clear the lysate. The lysate was further cleared by filtering through a 0.22 µm membrane syringe. 50 µL cell lysate was saved as input, mixed with 1 mL trizol. The anti-flag M2 magnetic beads (Sigma, 20 µL per mL lysate, ~30 µL to each sample) was washed with a 600 µL NT2 buffer (200 mM NaCl, 50 mM HEPES pH 7.6, 2 mM EDTA, 0.05% NP-40, 0.5 mM DTT, 200 U/mL RNase inhibitor) four times and then re-suspended in 800 µL ice-cold NT2 buffer. Cell lysate was mixed with M2 beads; the tube was flicked several times to mix the contents and then rotated continuously at 4 °C for 4 hours. The beads were collected, washed eight times with 1 mL ice-cold NT2 buffer. 5 packed beads volumes (~150 µL = 30 µL×5) of elution solution which was 500 ng/µL 3×Flag peptide (Sigma) in NT2 buffer were added to each sample, and the mixture was rotated at 4°C for 2 hours to elute. The supernatant was mixed with 1 mL trizol and saved as IP. RNA recovered from input was further subjected to mRNA purification by either Poly(A) selection (replicate 1, FastTrack MAG Maxi mRNA isolation kit, invitrogen) or rRNA removal (replicate 2, RiboMinus Eukaryote Kit v2, Ambion). Input mRNA and IP with 150–200 ng RNA of each sample were used to generate the library using TruSeq stranded mRNA sample preparation kit (Illumina).
PAR-CLIP
We followed the protocol described by Hafner et al. with the following modifications32. Sample preparation: Five 15 cm plates of HeLa cells were seeded at Day 1 18:00. At Day 2 10:00, the HeLa cells were transfected with flag-tagged YTHDF2 plasmid at 80% confluency. After six hours, the media was changed and 200 µM 4SU was added. At Day 3 10:00, the media was aspirated, and the cells were washed once with 5 mL ice-cold PBS for each plate. The plates were kept on ice, and the crosslink was carried by 0.15 J/cm2 Ultraviolet light. 2 mL PBS was added and the cells were collected by cell lifter.
Library construction: The final recovered RNA sample was further cleaned by RNA Clean & Concentrator (Zymo Research) before library construction by Tru-seq small RNA sample preparation kit (Illumina).
Mild enzyme digestion33: The first round of T1 digest was carried out under 0.2 U/µL for 15 min instead of 1 U/µL for 15 min. The second round of T1 digest was conducted under 10 U/µL for 8 min instead of 50 U/µL for 15 min.
Ribosome and polysome profiling
The procedure was adapted from the previous report22. Eight 15 cm plates of HeLa cells were prepared for 48 h knockdown (siControl, siYTHDF2, four plates each). Before collection, cycloheximide (CHX) was added to the media at 100 µg/mL for 7 min. The media was removed, and the cells were collected by cell lifter with 5 mL cold PBS with CHX (100 µg/mL). The cell suspension was spun at 1.6 krpm for 2 min and the cell pellet was washed once by 5 mL PBS-CHX per plate. 1 mL lysis buffer (10 mM Tris, pH 7.4, 150 mM KCl, 5 mM MgCl2, 100 µg/mL CHX, 0.5% Triton-X-100, freshly add 1:100 protease inhibitor, 40 U/mL SUPERasin) was added to suspend the cells and then kept on ice for 15 min with occasional pipetting and rotating. After centrifuge at 14 krpm for 15 min, the supernatant (~1.2 mL) was collected and tested O.D. at 260 nm (150–200 O.D./mL). To the lysate, 8 µL DNase Turbo was added. The lysate was then split by the ratio of 1:4 (Portion I: Portion II). 4 µL Super RNasin was added to Portion I. 40 µL MNase buffer and 3 µL MNase (6000 gel units, NEB) was added to Portion II. Both portions were kept at room temperature for 15 min, and then 8 µL SUPERasin was added to Portion II to stop the reaction. Portion I was saved and mixed with 1 mL Trizol to purify input mRNA. Portion II was used for ribosome profiling.
Ribosome profiling: a 10/50% w/v sucrose gradient was prepared in a lysis buffer without Triton-X-100. Portion II was loaded onto the sucrose gradient and centrifuged at 4 °C for 4 h at 27.5 krpm. The sample was then fractioned and analyzed by Gradient Station (BioCamp) equipped with ECONO Uv monitor (BioRad) and fraction collector (FC203B, Gilson). The fractions corresponding to 80S monosome (not 40S or 60S) were collected, combined, and mixed with an equal volume of Trizol to purify the RNA. The RNA pellet was dissolved in 30 µL water, mixed with 30 µL 2X TBE-urea loading buffer (Invitrogen), and separated on a 10% TBE-urea gel. A 21 nt and a 42 nt ssRNA oligo were used as size markers, and the gel band between 21 and 42 nt was cut. The gel was passed through a needle hole to break the gel, and 600 µL extraction buffer (300 mM NaOAc, pH 5.5, 1 mM EDTA, 0.1 U/mL RNasin) was added. The gel slurry was heated at 65°C for 10min with shaking, and then filtered through 1 mL QIAGEN filter. RNA was concentrated by ethanol precipitation and finally dissolved in 10 µL of RNase-free water.
Input mRNA: The input RNA was first purified by Trizol and the input mRNA was then separated by PolyATract. The resulting mRNA was concentrated by ethanol precipitation and dissolved in 10 µL of RNase-free water. The mRNA was fragmented by RNA fragmentation kit (Ambion). The reaction was diluted to 20 µL and cleaned up by micro Bio-Spin 30 column (cut-off: 20 bp; exchange buffer to Tris).
Library construction: The end structures of the RNA fragments of ribosome profiling and mRNA input were repaired by T4 PNK: (1) 3’ de-phosphorylation: RNA (20 µL) was mixed with 2.5 µL PNK buffer and 1 µL T4 PNK, and kept at 37°C for 1 h; (2) 5’-phosphorylation: to the reaction mixture, 1 µL 10 mM ATP and 1 µL extra T4 PNK were added, and the mixture was kept at 37 °C for 30 min. The RNA was purified by 500 µL Trizol reagent, and finally dissolved in 10 µL water. The library was constructed by Tru-seq small RNA sample preparation kit (Illumina). The sequencing data obtained from ribosome profiling (portion II) were denoted as ribosome-protected fragments and that from RNA input (portion I) as mRNA input. Translation efficiency was defined as the ratio of ribosome-protected fragments and mRNA input, which reflected the relative occupancy of 80S ribosome per mRNA species.
Polysome profiling: sample preparation and sucrose gradient were the same as those of the ribosome profiling procedure except eliminating MNase digestion. The fractions resulting from sucrose gradient were used for western blotting or pooled to isolate total RNA for RT-PCR and mRNA for LC-MS/MS test of m6A/A ratio.
RNA-seq for mRNA lifetime
Two 10 cm plates of HeLa cells were transfected with YTHDF2 siRNA or control siRNA at 30% confluency. After 6 hours, each 10 cm plate was re-seeded into three 6 cm plates, and each plate was controlled to afford the same amount of cells. After 48 hours, actinomycin D was added to 5 µg/mL at 6 hours, 3 hours, and 0 hours before tripsinization collection. The total RNA was purified by RNeasy kit (QIAGEN). Before construction of the library with Tru-seq mRNA sample preparation kit (Illumina), ERCC RNA spike-in control (ambion) was added to each sample (0.1 µL per sample). Two biological replicates were generated: (1) in replicate 1, RNA spike-in control was added proportional to cell numbers; (2) in replicate 2, RNA spike-in control was added proportional to total RNA. Although data obtained from the two sets showed systematic shift, they led to consistent conclusion that YTHDF2 knockdown leads to prolonged lifetime of its RNA targets (Extended Data Fig. 9).
Data analysis of seq-data
General pre-processing of reads: All samples were sequenced by illumine Hiseq2000 with single end 100-bp read length. For libraries that generated from small RNA (PAR-CLIP and ribosome profiling), the adapters was trimmed by using FASTX-Toolkit34. The deep sequencing data were mapped to Human genome version hg19 by Tophat version 2.035 without any gaps and allowed for at most two mismatches. RIP and Ribosome profiling were analyzed by DESeq36 to generate RPKM (reads per kilobase, per million reads). mRNA lifetime data were analyzed by Cuffdiff version 2.037 to calculate RPKM.
Data analysis for each experiment: (1) for m6A profiling, the m6A-enriched regions in each m6A-IP sample were extracted by using the model-based analysis of ChIP-seq (MACS) peak-calling algorithm38, with the corresponding m6A-Input sample serving as the input control. For each library, the enriched peaks with p < 1e-5 were used for further analysis; (2) for RIP, enrichment fold was calculated as log2(IP/input); (3) PAR-CLIP data were analyzed by PARalyzerv1.1 with default settings39; (4) for ribosome profiling, only genes with RPKM>1 were used for analysis and the change fold was calculated as log2(siYTHDF2/siControl); (5) for mRNA lifetime profiling: RKPM were converted to attomole by linear-fitting of the RNA spike-in.
The degradation rate of RNA k was estimated by
where t is transcription inhibition time (h), At and A0 represent mRNA quantity (attomole) at time t and time 0. Two k values were calculated: time 3 h versus time 0 h, and time 6 h versus time 0 h. The final lifetime was calculated by using the average of k3h and k6h.
Integrative data analysis and statistics: PAR-CLIP targets were defined as reproducible gene targets among three biological replicates (3,251). RIP targets (2528) were genes with log2(IP/input) >1. The overlap of PAR-CLIP and RIP targets were defined as CLIP+IP targets (1,277). And non-targets (3,905) should meet the conditions: (1) complementary set of PAR-CLIP targets; (2) RIP enrichment fold <0. For the comparison of PAR-CLIP and m6A peaks, at least 1 bp overlap was applied as the criteria of overlap peaks. Two biological replicates were conducted for ribosome profiling and mRNA lifetime profiling, respectively. And genes with sufficient expression level (RPKM>1) were subjected to further analysis. The change fold that used in the main text is the average of the two log2(siYTHDF2/siControl) values. Nonparametric Mann-Whitney U test (Wilcoxon rank-sum test, two sided, significance level = 0.05) was applied in ribosome profiling data analysis as previous reported22. For the analysis of cell viability (Extended Data Fig.8e), RPF of ribosome profiling data were analyzed by Cuffidff version 2.0 for differential expression test, and the genes that differentially expressed (p<0.05) were subjected to Ingeuity Pathway Analysis (IPA, Ingenuity System). RPF was chosen since it may better reflect the translation status of each gene.
Data accession: All the raw data and processed files have been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo). m6A profiling data are accessible under GSE 46705 (GSM1135030 and GSM1135031 are input samples while GSM1135032 and GSM1135033 are IP samples). All other data are accessible under GSE 49339.
RT-PCR
Real-time PCR (RT-PCR) was performed to assess the relative abundance of mRNA. All RNA templates used for RT-PCR were pre-treated with on column DNase I in the purification step. The RT-PCR primers were designed to span exon-exon junctions in order to further eliminate the amplification of genomic DNA and unspliced mRNA. When the examined gene had more than one isoform, only exon-exon junctions shared by all isoforms were selected to evaluate the overall expression of that gene. RT-PCR was performed by using Platinum one-step kit (Invitrogen) with 200–400 ng total RNA template or 10–20 ng mRNA template. HPRT1 was used as an internal control because: (1) HPRT1 mRNA did not have m6A peak from m6A profiling data; (2) HPRT1 mRNA was not bound by YTHDF2 from the PAR-CLIP and RIP sequencing data; (3) HPRT1 showed relative invariant expression upon YTHDF2 knockdown from the RNA-seq data; (4) HPRT1 was a house-keeping gene.
| YTHDF2: | TAGCCAACTGCGACACATTC; | CACGACCTTGACGTTCCTTT. |
| SON: | TGACAGATTTGGATAAGGCTCA; | GCTCCTCCTGACTTTTTAGCAA. |
| CREBBP: | CTCAGCTGTGACCTCATGGA; | AGGTCGTAGTCCTCGCACAC. |
| PLAC2: | AAGCGCTACCACATCAAGGT; | CCTCCAACCCAGACTACCTG. |
| LDLR: | GCTACCCCTCGAGACAGATG; | CACTGTCCGAAGCCTGTTCT. |
| HPRT1: | TGACACTGGCAAAACAATGCA; | GGTCCTTTTCACCAGCAAGCT. |
| F-luc or F-luc-5BoxB: | CACCTTCGTGACTTCCCATT; | TGACTGAATCGGACACAAGC. |
| R-luc: | GTAACGCTGCCTCCAGCTAC; | CCAAGCGGTGAGGTACTTGT. |
A combination of knockdown/over-expression/RIP/RT-PCR experiments was conducted to evaluable the occupancy change of YTHDF2 on its RNA targets after MT-A70 (METTL3) knockdown (Extended Data Fig. 5). Two 15 cm plates of HeLa cells were transfected with siControl or siMETTL3 siRNA. After 10 hours, the cells were re-seeded. After 14 hours, the cells were further transfected with flag-tagged YTHDF2 plasmid, and collected after another 24 h (in total 48 h knockdown of METTL3, 24 h o/e of flag-YTHDF2). Anti-flag beads were used to separate YTHDF2-bound portion (IP) from unbound portion (flowthrough) as described in the RIP section.
Fluorescence microscopy
Fluorescent immuno-staining: the protocol of Kedersha et al26 was followed. The cells were grown in an 8-well chamber (Lab-Tek). After treatment indicated in each experiment, the cells were washed once in PBS and then fixed in 4% paraformaldehyde in PBST (PBS with 0.05% Tween-20; prepared by mixing paraformalydehyde with PBST, heat at 60 °C until clear, pH~7.5) at r.t. for 15 min under rotating The fixing solution was removed, and −20 °C chilled methanol was immediately added to each chamber and incubated for 10 min at room temperature. The cells were rinsed once in PBS and incubated with blocking solution (10% FBS with PBST) for 1 hour at room temperature under rotation. After that, the blocking solution was replaced with primary antibody (diluted by fold indicated in Antibodies section in blocking solution) and incubated for 1 hour at r.t. (or overnight at 4 °C). After being washed 4 times with PBST (300 µL, 5–10 min for each wash), secondary antibody (1:300 dilution in PBST) was added to the mixture and incubated at r.t. for 1 hour. After washing 4 times with PBST (300 µL, 5–10 min for each wash), anti-fade reagent (slowfade, invitrogen) was added to mount the slides.
FISH in conjugation with Fluorescent immuno-staining: Stellaris FISH probe with Quasar 570 was used according to the manufacturer’s instructions. After the washing step, the sample preparation proceeded to the blocking step of the previous paragraph in the presence of 40 U/mL of RNase inhibitor. Secondary antibodies were Alexa 488 and Alexa 647 conjugates.
Image capture and analysis: The images were captured by Leica SP5 II STED-CW super-resolution laser scanning confocal microscope, analyzed by ImageJ. The colocalization was quantified by JAcoP (ImageJ plug-in) and the Pearson coefficients in main text Figure 3 were gained under Costes’ automatic threshold40.
Protein co-IP
HeLa cells expressing flag-tagged YTHDF2, N-YTHDF2, C-YTHDF2 or pcDNA3.0 blank vector were (three 15 cm plates for each) collected by cell lifter, and pelleted by centrifuge at 1 kpm for 5 min. The cell pellet was re-suspended with 2 volumes of lysis buffer (the same as the one used in RIP), and incubated on ice for 10 min. To remove the cell debris, the lysate solution was centrifuged at 15 krpm for 15 min at 4 °C, and the resulting supernatant was passed through a 0.22 µm membrane syringe filter. While 50 µL of cell lysate was saved as Input, the rest was incubated with the anti-flag M2 magnetic beads (Sigma) in ice-cold NT2 buffer (the same as the one used in RIP) for 4 h at 4 °C. Afterwards, the beads was subject to extensive wash with 8 × 1 mL portions of ice-cold NT2 buffer, followed by incubation with the elution solution containing 3×flag peptide (0.5 mg/mL in NT2 buffer, Sigma) at 4 °C for another 2 h. The eluted samples, saved as IP, were analyzed by western blotting. For IP samples, each lane was loaded with 2 µg IP portion; and the input lane were loaded with 10 µg Input portion which corresponded to ~1% of overall input.
Tether assay
Basic setting: 100 ng reporter plasmid (pmirGlo or pmirGlo-5BoxB) and 500 ng effecter plasmid (pcDNA-flag-λ, pcDNA-flag-Y2Nλ, or pcDNA-flag-Y2N) were used to transfect the HeLa cells in each well of six-well plate at 60~80% confluency. After 6 hours, each well was re-seeded into 96-well plate (1:20) and 12-well plate (1:2). After 24 hours, the cells in 96-well plate were assayed by Dual-Glo Luciferase Assay Systems (Promega). Firefly luciferase (F-luc) activity was normalized by Renilla luciferase (R-luc) to evaluate the translation of reporter. And samples in 12-well plate were processed to extract total RNA (DNase I digested), followed by RT-PCR quantification. The amount of F-luc mRNA was also normalized by that of R-luc mRNA.
RNA IP: Two 15 cm HeLa cells were transfected with 1 µg pmirGlo-5BoxB reporter and 5 µg pcDNA-flag-Y2Nλ effecter plasmids for each plate. After 24 hours, the samples were processed as described in RIP section. The recovered RNA from Input, IP and FT portions were used in poly(A) tail assay.
RNA decay: 200 ng reporter plasmid (pmirGlo-Ptight-5BoxB) and 1 µg effecter plasmid (pcDNA-flag-λ, pcDNA-flag-Y2Nλ, or pcDNA-flag-Y2N) were used for each 6 cm plate to transfect the HeLa Tet-off cell line (Clontech) in the presence of 400 ng doxycycline (Dox, Clontech). The transcription of F-luc5BoxB was under repression at this stage. After 18 hours, the cells in each 6 cm plate were washed twice with PBS, trypsinized, and washed twice with Dox-free media, then splitted to four equal portions and re-seeded to 12-well plate in Dox-free media. After 4 h pulse transcription of F-luc5BoxB, Dox were added to 400 ng in each well. The first time point (t = 0 h) was taken as after 20 minutes41, then 2h, 4h and 6h. Total RNA extracted from each sample were used for RT-PCR analysis and Poly(A) tail length assay.
Poly(A) tail length assay
Poly(A) tail length assay was performed by using Poly(A) Tail-Length Assay kit (Affymetrix) as previously reported7. The protocol of the manufacture (Extended Data Fig.7f–l) was followed, with 30 cycles of two-step PCR at the last step, and then visualized on 10% non-denaturing TBE gel. The forward primer of F-luc-5BoxB is 5’-CCGCTGAGCAATAACTAGCA-3’, and the gene-specific reverse primer is 5’-TGCAATTGTTGTTGTTAACTTGTTT-3’. The forward primer of CREBBP mRNA is 5’-GTCTTGGGCAATCCAGATGT-3’, and the gene-specific reverse primer is 5’-TTTGAATCCAAGTAGTTTTACCATC -3’.
Antibodies
The antibodies used in this study were listed below in the format of name (application; catalog; supplier; dilution fold): Rabbit anti-YTHDF1 (Western; ab99080; Abcam; 1000). Rabbit anti-YTHDF3 (Western; ab103328; Abcam; 1000). Mouse anti-Flag HRP conjugate (Western; A5892; Sigma; 5000). Rabbit anti-MT-A70 (Western; 15073-1-AP; Proteintech Group; 3000). Rabbit anti-FTO(Western; 5325-1; Epitomics; 10000). Goat anti-GAPDH HRP conjugate (Western; A00192; GeneScript; 15000). Rabbit anti-DCP2 (Western; Ab28658; Abcam; 1000). Rabbit anti-m6A (m6A-seq; 202003; Synaptic Systems; 4 µg/per seq). Rat anti-Flag (IF; 637304; Biolegend; 300). Mouse anti-DCP1a (IF; WH0055802M6; Sigma; 300). Mouse anti-GW182 (4B6) (IF; ab70522; Abcam; 100). Rabbit anti-DDX6 (IF; a300–461A; Bethyl Lab; 250). Anti-HuR (IF; WH0001994M2; Sigma; 50). Goat anti-eIF3 (N-20) (IF; sc-16377; Santa Cruz Biotech; 100). Mouse anti-CNOT7 (IF; sc-101009; Stanta Cruz Biotech; 100). Goat anti-PAN2 (C-20) (IF; sc-82110; Santa Cruz Biotech.; 100). Anti-PARN (IF; ab27778; Abcam; 100). Donkey anti-rat Alexa 488 (IF; A21208; Molecular Probes; 300). Goat anti-rabbit Alexa 647 (IF; A21446; Molecular Probes; 300). Goat anti-mouse Alexa 647 (IF; A21236; Molecular Probes; 300). Donkey anti-goat Alexa 647 (IF; A21447; Molecular Probes; 300).
Supplementary Material
Acknowledgements
This work is supported by National Institutes of Health GM071440 (C.H.) and EUREKA GM088599 (T.P. and C.H). The Mass Spectrometry Facility of the University of Chicago is funded by National Science Foundation (CHE-1048528). We thank Drs. A. E. Kulozik, W. Filipowicz, and J. A. Steitz for generously providing the sequence and plasmids of the tether reporter. We also thank S. F. Reichard for editing the manuscript.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions C. H. conceived the project. X. W. designed and performed most experiments. Z. L. and X. W. performed data analyses of high-throughput sequencing data. A. G. assisted with the experiments. Y. Y. and D. H. conducted the experimental and data analysis part of m6A profiling, respectively. Y. F. performed the RNA-affinity pull-down experiment of YTHDF1 and YTHDF3. M. P. and G. J. provided valuable discussions. G. H. and B. R. performed high throughput sequencing. Q. D. assisted in m6A synthesis. X. W. and C. H. interpreted the results and wrote the manuscript with input from T. P.
RNA sequencing data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE49339 and the processed results were presented as Supplementary Table 1.
The authors declare no competing financial interests.
References
- 1.Tuck MT. The formation of internal 6-methyladenine residues in eucaryotic messenger RNA. Int. J. Biochem. 1992;24:379–386. doi: 10.1016/0020-711x(92)90028-y. [DOI] [PubMed] [Google Scholar]
- 2.Jia G, Fu Y, He C. Reversible RNA adenosine methylation in biological regulation. Trends Genet. 2013;29:108–115. doi: 10.1016/j.tig.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–4518. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hongay CF, Orr-Weaver TL. Drosophila inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2012;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandekar T. RNA Motifs and Regulatory Elements. 2 ed. Berlin Heidelberg: Springer-Verlag; 2002. pp. 1–11. [Google Scholar]
- 11.He C. Grand challenge commentary: RNA epigenetics? Nature Chem. Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 12.Wei CM, Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977;16:1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- 13.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 14.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 15.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoilov P, Rafalska I, Stamm S. YTH: a new domain in nuclear proteins. Trends Biochem. Sci. 2002;27:495–497. doi: 10.1016/s0968-0004(02)02189-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. The YTH domain is a novel RNA binding domain. J. Biol. Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardelli M, et al. A polymorphism of the YTHDF2 gene (1p35) located in an Alu-rich genomic domain is associated with human longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:547–556. doi: 10.1093/gerona/61.6.547. [DOI] [PubMed] [Google Scholar]
- 19.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peritz T, et al. Immunoprecipitation of mRNA-protein complexes. Nat. Protoc. 2006;1:577–580. doi: 10.1038/nprot.2006.82. [DOI] [PubMed] [Google Scholar]
- 21.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336:233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee N, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang R, Brown CY, Morris DR. mRNA Formation and Function. New York: Academic press; 1997. Chapter 16 Analysis of ribosome loading onto mRNA species: implications for translational control. [Google Scholar]
- 25.Shenton D, et al. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 2006;281:29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- 26.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 27.Reijns MA, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. J. Cell Sci. 2008;121:2463–2472. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato M, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gehring NH, Neu-Yilik G, Schell T, Hentze MW, Kulozik AE. Y14 and hUpf3b form an NMD-activating complex. Mol. Cell. 2003;11:939–949. doi: 10.1016/s1097-2765(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 30.Behm-Ansmant I, et al. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafner M, et al. PAR-CliP--a method to identify transcriptome-wide the binding sites of RNA binding proteins. J. Vis. Exp. 2010;(41) doi: 10.3791/2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishore S, et al. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nature Methods. 2011;8:559–564. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- 34.Pearson WR, Wood T, Zhang Z, Miller W. Comparison of DNA sequences with protein sequences. Genomics. 1997;46:24–36. doi: 10.1006/geno.1997.4995. [DOI] [PubMed] [Google Scholar]
- 35.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1011. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corcoran DL, et al. PARalyzer: definition of RNA binding sites from PAR-CLIP short-read sequence data. Genome Biol. 2011;12:R79. doi: 10.1186/gb-2011-12-8-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 41.Clement SL, Lykke-Andersen J. A tethering approach to study proteins that activate mRNA turnover in human cells. Methods Mol. Biol. 2008;419:121–133. doi: 10.1007/978-1-59745-033-1_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.