Abstract
Voltage-gated sodium (NaV) channels are essential for initiating and propagating action potentials in the brain. More than 800 mutations in genes encoding neuronal NaV channels including SCN1A and SCN2A have been associated with human epilepsy. Only one epilepsy-associated mutation has been identified in SCN3A encoding the NaV1.3 neuronal sodium channel. We performed a genetic screen of pediatric patients with focal epilepsy of unknown cause and identified four novel SCN3A missense variants: R357Q, D766N, E1111K and M1323V. We determined the functional consequences of these variants along with the previously reported K354Q mutation using heterologously expressed human NaV1.3. Functional defects were heterogeneous among the variants. The most severely affected was R357Q, which had significantly smaller current density and slower activation than the wild-type (WT) channel as well as depolarized voltage dependences of activation and inactivation. Also notable was E1111K, which evoked a significantly greater level of persistent sodium current than WT channels. Interestingly, a common feature shared by all variant channels was increased current activation in response to depolarizing voltage ramps revealing a functional property consistent with conferring neuronal hyper-excitability. Discovery of a common biophysical defect among variants identified in unrelated pediatric epilepsy patients suggests that SCN3A may contribute to neuronal hyperexcitability and epilepsy.
Keywords: SCN3A, sodium channel, epilepsy, persistent current, ramp currents
INTRODUCTION
Voltage-gated sodium (NaV) channels are essential for initiating and propagating action potentials in the brain. The channels exist in a native protein complex composed of a pore-forming α subunit and two smaller accessory β subunits (Catterall, 1988; Isom et al., 1994). More than 800 sodium channel mutations, mostly in SCN1A and a few in SCN2A, have been associated with epilepsy (Lossin, 2009). SCN3A is clustered with SCN1A and SCN2A on human chromosome 2q24, but only one mutation in SCN3A, encoding NaV 1.3, is known to be associated with epilepsy (Holland et al., 2008). The involvement of NaV1.3 in epilepsy is supported by studies showing that SCN3A mRNA is expressed at higher levels in human CA4 hilar cells in the epileptic hippocampus (Whitaker et al., 2001) and in rat neurons of CA1–CA3 and in the dentate granule cell layer after the induction of status epilepticus (Bartolomei et al., 1997; Aronica et al., 2001).
NaV1.3 channels possess several properties that may cause neuronal hyper-responsiveness. For example, NaV1.3 channels recover from inactivation rapidly and sustain high-frequency firing (Cummins et al., 2001), activate during slow ramp depolarizations, and produce persistent sodium current (Chen et al., 2000; Cummins and Waxman, 1997; Sun et al., 2007; Estacion et al., 2010). The NaV1.3 epilepsy-associated mutation K354Q was previously demonstrated to enhance persistent current and ramp current (Holland et al., 2008; Estacion et al., 2010). Several studies indicate that NaV persistent current participates in spontaneous neuronal firing in a variety of cell types including hippocampal neurons (Agrawal et al., 2001; Kearney et al., 2001) and subicular neurons isolated from patients with temporal lobe epilepsy (Vreugdenhil et al., 2004). Moreover, several epilepsy-associated NaV1.1 mutations are known to enhance persistent current (Lossin et al., 2002; Rhodes et al., 2004; Spampanato et al., 2004; Kahlig et al., 2008).
We performed a genetic screen of pediatric patients with cryptogenic focal epilepsy and identified four novel SCN3A missense variants: R357Q, D766N, E1111K and M1323V. Electrophysiological studies revealed a diversity of functional defects, but interestingly, all mutant channels exhibited abnormal current activation during a slow depolarizing voltage ramp. This common defect among the novel alleles could explain neuronal hyperexcitability.
MATERIALS AND METHODS
Study subjects
Subjects were ascertained from the St. Louis Children’s Hospital Pediatric Epilepsy Center and the Cincinnati Children’s Hospital Comprehensive Epilepsy Center. Diagnoses were classified as focal epilepsy of unknown cause according to the International League Against Epilepsy guidelines (Berg et al., 2010). Additional inclusion criteria used at the Cincinnati Children’s Hospital Comprehensive Epilepsy Center were: i) treatment with carbamazepine or oxcarbamazepine; and ii) have classifiable treatment response (as defined in Holland et al, 2007). Research was approved by the local institutional review boards and informed consent was obtained from the parent or guardian of each individual. Additional clinical details for the four subjects with novel SCN3A variants are provided below.
Individual 1 presented with complex focal seizures with secondary generalization at age 5. Seizures were all <2 minutes in duration and were well controlled on carbamazepine with only a few breakthrough seizures. At age 10 he had been seizure free for 2 years and was successfully weaned off carbamazepine. He also has mild speech delay and attention deficit hyperactivity disorder (ADHD), but was otherwise healthy. He has a cousin with febrile seizures and no additional family history of epilepsy.
Individual 2 presented at 22 months of age with complex focal seizures characterized by staring an unresponsiveness lasting less than 1 minute. He had rare seizures but became seizure-free on carbamazepine, which discontinued at 10 years of age after a 3-year period of seizure freedom. His initial EEG demonstrated midline occipital epileptiform discharges and subsequent EEGs were abnormal because of mild slowing of the background activity. He was diagnosed with a non-verbal learning disability after testing demonstrated a borderline full scale IQ (78) with impairment in non-verbal skills but normal verbal skills.
Individual 3 is an 8 year old girl whose seizures began at 2 days of age. Seizures consisted of clonic movements involving the left arm that corresponded to rhythmic right central discharges on the EEG. She was treated with phenobarbital and had excellent seizure control until age 3 months when she had a single breakthrough seizure also involving the left arm with unresponsiveness. At the time, her phenobarbital level was 15, and she responded to an increase in medication with no further seizure activity. At age 2 years, the EEG was repeated and was normal and she was successfully weaned off phenobarbital. Birth history was unremarkable and development was normal. She has a paternal aunt who had seizures that began at 1 month of age and lasted until 11 months.
Individual 4 had two simple febrile seizures at 16 months of age. One month later the individual had an unprovoked generalized tonic clonic seizure lasting 45 minutes which was followed by postictal eye deviation to the left and tremulousness of the right arm. At that time oxcarbazepine was initiated. Over the following months the individual had several seizures characterized by loss of awareness and right sided jerking and one with left sided jerking. The dose of oxcarbazepine was progressively increased over a 3 month period until the individual became seizure-free and oxcarbazepine was stopped at 5 years of age. Multiple EEGs were normal; however no EEGs were done prior to the initiation for treatment. The individual’s mother had surgery for intractable epilepsy (pathology unknown) and the paternal grandfather also had epilepsy (details unknown).
SCN3A Screening
Genomic DNA was extracted from peripheral blood and the 26 coding exons of SCN3A were amplified by PCR using previously described primers (Weiss et al., 2003). Exons were screened for variants by sequencing as previously described (Holland et al., 2008) or by heteroduplex analysis using conformation-sensitive gel electrophoresis followed by sequencing as previously described (Escayg et al., 2000). Nucleotide and amino acid residue numeration correspond to the major SCN3A isoform that includes the exon 5 adult (5A) and exon 12v1 (646 bp) splice variants (Genbank NM_001081676.1 (Thimmapaya et al., 2005). NINDS Neurologically Normal Control Panels (Coriell NDPT006 and NDPT009; n = 184) were screened for variants R357Q, D766N, M1323V and E1111K to determine population allele frequencies. For some variants, we screened an additional panel of 111 subjects who were >60 years of age without personal or family history of neurological disease (Rainier et al., 2006). Screening of this same control panel was previously reported for variant K354Q (Holland et al., 2008). DNA from family members of study subjects was not available for screening.
Cell Culture
All electrophysiology experiments were conducted using tsA201 cells (HEK-293 cells stably transfected with SV40 large T antigen) grown at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (ATLANTA Biologicals, Norcross, GA, USA), 2 mM L-glutamine, and penicillin (50 units/ml)-streptomycin (50 µg/ml). Only cells from passage number <13 were used. Unless otherwise stated, all tissue culture media was obtained from Life Technologies, Inc. (Grand Island, NY, USA).
Plasmids and Cell Transfection
A plasmid encoding the major splice isoform of human NaV1.3, with the exon 5 adult (5A) and exon 12v1 (646 bp) splice variants (GenBank NM_001081676.1), was previously described (Wang et al., 2010). Full-length NaV1.3 was propagated in STBL2 cells at 30°C (Invitrogen) and the open reading frame of all plasmid preparations was fully sequenced prior to transfection. Variants were introduced by site-directed mutagenesis. Plasmids encoding the human NaV channel accessory subunits β1 or β2 in vectors containing the marker genes CD8 (pCD8-IRES-β1) or GFP (pGFP-IRES-β2) were also used. Expression of NaV1.3, β1 and β2 subunits was achieved by transient transfection (2 µg of total cDNA: SCN3A, β1, β2 mass ratio was 10:1:1) using Superfect Transfection Reagent (QIAGEN, Valenceia, CA, USA). Cells were incubated for 48 hr as described above after transfection before their use in electrophysiology experiments. Transfected cells were dissociated by brief exposure to trypsin/EDTA, resuspended in supplemented DMEM medium, plated on glass coverslips and allowed to recover for ~2 hrs at 37°C in 5% CO2. Polystyrene microbeads precoated with anti-CD8 antibody (Dynabeads M-450 CD 8, Dynal, Great Neck, NY, USA) were added and only cells positive for both CD8 antigen (i.e., β1 expression) and GFP fluorescence (i.e., β2 expression) were studied.
Electrophysiology
Coverslips were placed into a recording chamber on the stage of an inverted microscope with epifluorescence capability and allowed to equilibrate for 10 min in bath solution before starting the experiments. Bath solution contained (in mM): 145 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES (N-(2-hydroxyethyl)piperazine-N′-2-ethanosulphonic acid), pH 7.35, 310 mOsm/kg. The composition of the pipette solution was (in mM): 10 NaF, 110 CsF, 20 CsCl, 2 EGTA (ethyleneglycol-bis-(β-aminoethylether), 10 HEPES, pH 7.35, 310 mOsm/kg. Osmolarity and pH values were adjusted with sucrose and NaOH, respectively. As a reference electrode, a 2% agar-bridge with composition similar to the bath solution was utilized. Junction potentials were zeroed with the filled pipette in the bath solution. Unless otherwise stated, all chemicals were obtained from (Sigma-Aldrich, St. Louis, MO, USA).
Patch pipettes were pulled from thin-wall borosilicate glass (World Precision Instruments, Inc., Sarasota, FL, USA) with a multistage P-97 Flaming-Brown micropipette puller (Sutter Instruments Co., San Rafael, CA, USA) and fire-polished with a Micro Forge MF 830 (Narashige, Japan). After heat polishing, the resistance of the patch pipettes was 1–3 MΩ in standard bath and pipette solutions. Once whole-cell recording mode was achieved the access resistance averaged 2.5 ± 0.1 MΩ. Whole-cell sodium currents were recorded as previously described (Lossin et al., 2002; Vanoye et al., 2006) at room temperature (20–23°C) in the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) using an Axopatch 200B series amplifier (Molecular Devices Corp. Sunnyvale, CA, USA). Cells were allowed to equilibrate for 10 min after establishment of the whole-cell configuration before currents were recorded. Pulse generation was done with Clampex 8.1 (Molecular Devices Corp., Sunnyvale, CA) and whole-cell currents acquired at 20 kHz and filtered at 5 kHz. Linear leak and residual capacitance artifacts were subtracted out using the P/4 method. To reduce voltage errors, 80–90% series resistance and prediction compensation were applied. Cells were excluded from analysis if the predicted voltage error exceeded 3 mV or the current at the holding potential (−120 mV) was >5% of the peak current.
Peak currents were measured using 20 ms pulses between −80 and +50 mV every 5 s from a holding potential of −120 mV. The peak current was normalized for cell capacitance and plotted against voltage to generate peak current density–voltage relationships. Whole-cell conductance (G) was calculated from the peak current amplitude using the formula: G = I / (V − Erev) where G is the conductance, I is the peak inward current, V is the membrane potential step, and Erev is the estimated Na+ reversal potential. Conductance data were normalized by maximum conductance recorded between −80 and +20 mV. The normalized G-V curves were fit with the Boltzmann function: G = 1 / (1 + exp[(V − V½) / k] to determine the voltage for half-maximal channel activation (V½) and slope factor (k). The voltage-dependence of channel availability was assessed following a 100 msec pre-pulse to various potentials followed by 20 ms pulse to −10 mV. The normalized current was plotted against the voltage and the steady-state channel availability curves were fit with Boltzmann functions (I/Imax = 1 / (1 + exp[(V − V½) / k]) to determine the voltage for half-maximal channel inactivation (V½) and slope factor (k). Recovery from fast inactivation was determined using a two-pulse protocol. A 100 ms pre-pulse to −10 mV was followed by a variable amount of time for channel recovery at −120 mV and a 10 ms test pulse to −10 mV. The peak current from the test pulse was normalized to the peak current from the pre-pulse and plotted against the recovery period. Data were fit with the two exponential function, I/Imax= Af × [1 − exp (−t/τf)]+ As × [1 − exp (−t/τs)], where τf and τs denote time constants (fast and slow components, respectively), and Af and As represent the fast and slow fractional amplitudes.
Persistent current was evaluated by 200 msec depolarizations to −10 mV first in the absence and then in the presence of 500 nM tetrodotoxin (TTX; Sigma, St. Louis, MO). The average steady-state persistent current for each cell was measured during the final 10 ms of five 200 ms voltage steps to −10 mV. The voltage steps were elicited from a holding potential of −120 mV with an inter-pulse duration of 10 s. Percent late current was normalized to peak TTX-sensitive Na+ channel current and plotted as % persistent current.
Ramp current was assessed by changing the membrane potential from −120 mV to +40 mV over 800 ms (0.2 mV/ms). For each cell the average of 5 ramp pulses with an inter-pulse duration of 10 s were recorded before and after TTX (500 nM) addition. The TTX-sensitive ramp currents were then obtained by digital subtraction of the average sodium currents recorded before and after TTX. The inward charge (pC) for each cell was calculated between the voltage at which the Na+ currents activated and the voltage where the currents reversed polarity, and normalized to the peak current measured during a pulse test to −10 mV.
Data Analysis
Data were collected for each experimental condition from at least 3 transient transfections and analyzed and plotted using a combination of Clampfit 8.1 (Molecular Devices Corp.), Excel 2002 (Microsoft, Seattle, WA, USA), SigmaPlot 10 (Systat Software, Inc., San Jose, CA, USA) and OriginPro 7.0 (OriginLab, Northampton, MA, USA) software. Results are represented as means ± SEM, and number of cells used for each experimental condition is given in the Figure legends or Table 2. Current values were normalized to membrane capacitance. In some figures, the SE bars are smaller than the data symbols.
Table 2.
Biophysical properties of wild-type and mutant NaV1.3 channels co-expressed with β1 and β2 subunits.
| Current density (−10 mV) |
Voltage- dependence of activation |
Voltage-dependence of fast inactivation1 |
Recovery from fast inactivation2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pA/pF | n | V1/2 (mV) |
k | n | V1/2 (mV) |
k | n | τ1 (ms) | τ2 (ms) | n | |
| WT | −374.6 ± 32.6 | 29 | −21.0 ± 0.9 | 6.8 ± 0.2 | 29 | −71.5 ± 0.4 | 6.6 ± 0.1 | 23 | 3.5 ± 0.2 [94.8 ± 0.8%] |
196.5 ± 42.0 [5.2 ± 0.8%] |
10 |
| K354Q | −349.6 ± 28.8 | 28 | −20.9 ± 1.2 | 6.8 ± 0.2 | 28 | −73.1 ± 1.0 | 6.7 ± 0.2 | 19 | 4.2 ± 0.4 [95.5 ± 4.5%] |
208.0 ± 61.1 [4.5 ± 1.0%] |
6 |
| R357Q | −252.8 ± 51.6* | 14 | −17.4 ± 1.5** | 7.0 ± 0.3 | 14 | −68.1 ± 1.0‡‡ | 6.5 ± 0.2 | 11 | 3.3 ± 0.2 [95.5 ± 1.6%] |
298.3 ± 141.0 [4.4 ± 0.6%] |
5 |
| D766N | −443.0 ± 64.8 | 14 | −19.7 ± 1.9 | 6.9 ± 0.3 | 14 | −69.2 ± 1.5 | 6.8 ± 0.1 | 13 | 3.6 ± 0.3 [95.6 ± 0.6%] |
306.4 ± 108.1 [4.4 ± 0.6%] |
8 |
| E1111K | −461.3 ± 69.3 | 15 | −20.3 ± 1.3 | 6.8 ± 0.3 | 15 | −70.8 ± 0.7 | 6.5 ± 0.1 | 14 | 4.0 ± 0.3 [95.5 ± 0.6%] |
423.2 ± 253.8 [4.5 ± 0.6%] |
6 |
| M1323V | −312.4 ± 50.3 | 13 | −16.9 ± 1.2‡ | 7.3 ± 0.2 | 13 | −68.8 ± 0.6† | 6.7 ± 0.2 | 10 | 3.1 ± 0.4 [89.1 ± 2.8%]††† |
52.2 ± 13.2†† [10.9 ± 2.8%]† |
5 |
Steady-state inactivation determined following a 100 ms prepulse to various potentials followed by 20 ms pulse to −10 mV;
Recovery from fast inactivation determined following a 100 ms prepulse to −10 mV;
P = 0.045;
P = 0.036;
P = <0.001;
P = 0.013;
P = 0.002;
P = 0.028;
P = 0.041 (mutant vs wild-type using t-test).
Differences between the wild-type channel and each mutant channel were assessed by Student's t test using SigmaStat 2.03 (Systat Software, Inc.) with p < 0.05 considered statistically significant.
RESULTS
We identified four novel SCN3A missense variants (R357Q, D766N, E1111K, M1323V) in a screen of 179 pediatric patients with focal epilepsy who were SCN1A-mutation negative. None of the variants were detected in up to 590 chromosomes from control subjects (Table 1). Three of the variants (R357Q, D766N, M1323V) were not detected by the NHLBI Exome Sequencing Project (Exome Variant Server, 2013) in 4300 EuropeanAmerican or 2203 AfricanAmerican subjects. One heterozygous E1111K carrier was reported in this database with a minor allele frequency = 0.0001 (EuropeanAmerican). The observed frequency of singleton missense variants in our clinical population of (4/179) was 3-fold greater than observed in the general population (46/6503) (Exome Variant Server, 2013). In a concurrent screen of 75 pediatric patients with generalized epilepsy we did not identify any singleton missense variants in SCN3A. The clinical features associated with these SCN3A variants are summarized in Table 1.
Table 1.
Clinical features in four individuals with cryptogenic focal epilepsy.
| Individual | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Seizure Type(s) | Focal | Focal | Focal | Febrile seizures and Focal |
| Age at Onset | 4 years | 22 months | Neonatal | 16 months |
| EEG | Occipital and temporal epileptiform discharges | Occipital epileptiform discharges | Normal | Normal |
| MRI | Normal | small focus of increased signal in left subcortical frontal white matter | Normal | Normal except for low lying cerebellar tonsils |
| Developmental Delay | Yes | Mild | No | No |
| Amino Acid Variant | R357Q | D766N | E1111K | M1323V |
| Location in NaV1.3 (see Fig.1) | D1 S5-S6 | D2 S2 | D2-D3 loop | D3 S5-S6 |
| Family History | Yes | No | Yes | Yes |
| Ethnicity | European American | African American | European American | European American |
| Heterozygote Frequency in Neurologically Normal Controls | 0/295 | 0/184 | 0/295 | 0/184 |
| Heterozygote Frequency in Exome VariantServer – European American | 0/4299 | 0/4299 | 1/4299 | 0/4299 |
| Heterozygote Frequency in Exome VariantServer – African American | 0/2203 | 0/2203 | 0/2203 | 0/2203 |
The locations of the variants in the NaV1.3 protein and the evolutionary conservation of the variant residues are illustrated in Fig. 1. Amino acids K354 and R357 are located in the pore region of domain 1 (Fig. 1A). Both of these residues are invariant in other vertebrate NaV1.3 orthologs, and are highly conserved in other human sodium channel genes (Fig. 1B). D766 lies at the intracellular face of a transmembrane alpha helix and exhibits a high degree of evolutionary conservation (Fig. 1B). E1111 is located in the domain II/III cytoplasmic linker and is evolutionarily invariant in other vertebrate NaV1.3 orthologs and in the other human brain sodium channel genes (Fig. 1B). M1323 is located in the extracellular linker between transmembrane segment 5 and the pore region of domain 3. M1323 is evolutionarily invariant through birds and reptiles, while fish have conservative substitutions. Among the other human sodium channel genes, a methionine is present at this position in the most closely related SCN2A gene, while this position is not conserved in more distantly related sodium channel genes. We utilized two bioinformatic algorithms, Provean and SIFT, to predict the consequences of these NaV1.3 variants (Kumar et al, 2009; Choi et al 2012). Both programs predict R354Q, R357Q, D766N and E1111K to have deleterious effects on protein function, while M1323V is predicted to be benign (Table S1). To experimentally determine whether the novel SCN3A variants cause channel dysfunction, we performed electrophysiological studies of heterologously expressed NaV1.3 channels.
Figure 1. Location and evolutionary conservation of NaV1.3 variants.
(A) Predicted transmembrane topology of NaV1.3 depicting the location of the variants (●) characterized in this study. The grey rectangle behind the depiction represents the plasma membrane. The S4 segments are illustrated as black cylinders with plus (+) signs. (B) Multiple alignment of human homologs and species orthologs of NaV1.3 (Clustal Omega; Sievers et al, 2011). The variant amino acids are shaded to highlight conservation at the variant positions.
Biophysical properties of wild-type human NaV1.3 channels
We first examined the functional properties of wild-type (WT) NaV1.3 to obtain a control activity profile under our expression and recording conditions. Figure 2 illustrates the biophysical properties of WT human NaV1.3 co-expressed with both β1 and β2 accessory subunits in tsA201 cells. Rapidly activating and inactivating voltage-dependent inward whole-cell currents were observed in response to depolarizing test pulses from a holding potential of −120 mV (Fig. 2A). Inward currents observed at potentials more depolarized than −50 mV peaked between −10 and 0 mV (Fig. 2B). Wild-type NaV1.3 channels are fast activating as illustrated by the voltage dependence of time-to-peak current plotted in Fig. 2C. The channels inactivated rapidly with a bi-exponential time course. Inactivation time constants and corresponding fractional amplitudes for the fast and slow components are shown in Figs. 2D and 2E. Voltage dependence of activation and fast inactivation (Fig. 2F), and recovery from fast inactivation were also examined (Fig. 2G, Table 2). NaV1.3 recovery from fast inactivation following a 100 ms inactivating pulse to −10 mV was distributed into two populations, with the majority of channels (Af = 95 ± 3%) recovering within a few milliseconds (τf = 3.5 ± 0.2 ms) and a minor population (AS = 5 ± 1%) recovering more slowly (τs = 196.5 ± 42.0 ms, n = 11; Fig. 2G, Table 2).
Figure 2. Biophysical properties of heterologously expressed human NaV1.3.
(A) Averaged whole-cell currents recorded from cells transiently coexpressing NaV1.3 and both β1 and β2 subunits (n = 29). (B) Current-voltage relationships measured from NaV1.3-expressing cells (n = 29). (C) Voltage dependence of time to peak current (n = 29). (D) Voltage dependence of inactivation time constants (fast, ●; slow, ○; n = 25–27). (E) Fractional amplitudes for inactivation time constants (fast, ■; slow, □; n = 25–27). (F) Superimposed steady-state channel availability (○) and conductance-voltage relationship (●) for NaV1.3 currents (n = 29 and 23, respectively). Solid lines represent data fitted with Boltzmann functions. (G) Time course of recovery from fast inactivation (holding potential was −120 mV). Line represents data fitted with a two-exponential function (n = 10). Biophysical fit parameters are given in Table 2.
Biophysical properties of mutant NaV1.3 channels associated with pediatric epilepsy
We determined the functional properties of the four novel epilepsy-associated NaV1.3 variants, and included the previously identified K354Q mutation (Holland et al., 2008; Estacion et al., 2010) in our analyses for comparison. Figure 3 shows the average whole-cell currents obtained from tsA201 cells transiently expressing K354Q, R357Q, D766N, E1111K or M1323V channels. Figure 3F illustrates the average current density-voltage relationships measured from cells transiently expressing the variant channels with the average WT-NaV1.3 currents illustrated as a gray line. Expression of R357Q yielded sodium current density smaller than the wild type channel (Table 2), but all other NaV1.3 variants exhibited current densities that were not significantly different from WT channels.
Figure 3. Whole-cell currents recorded from heterologously expressed NaV1.3 channels.
Average whole-cell currents recorded from tsA201 cells transiently coexpressing mutant NaV1.3 channels plus β1 and β2 accessory subunits. (A) K354Q (∀), n = 28; (B) R357Q (ν), n = 14; (C) D766N (X), n = 14; (D) E1111K (M), n = 15 and (E) M1323V (8), n = 13. (F) Current-voltage relationships measured from mutant NaV1.3-expressing cells. The gray solid line illustrates the data from cells expressing WT channels (Fig. 1A).
All variant channels exhibited fast activating and inactivating kinetics in response to depolarizing test pulses (Tables S2 and S3). Only R357Q affected NaV1.3 activation kinetics with time-to-peak values ~10% longer than WT (Table S2). Entry into fast inactivation was slowed by both R357Q and M1323V, but by different mechanisms. Whereas R357Q increased the fast time constant (τF) for inactivation at certain voltages, M1323V increased both the proportion of the slow component of inactivation and increased the fast time constant at various test potentials (Table S3). Both R357Q and M1323V exhibited depolarized voltage-dependence of activation and inactivation when compared to the WT channel (Table 2). However, the voltage sensitivity (slope factor, k) for both activation and inactivation were not different from WT-NaV1.3. The K354Q, D766N and E1111K variants did not affect the voltage-dependence of activation or inactivation (Table 2). Recovery from inactivation was faster for M1323V but the other variants were similar to WT channels (Table 2).
We also determined channel availability of WT and mutant NaV1.3 channels during repetitive stimulation (use-dependence) with trains of 100 depolarizing voltage steps to −10 mV at various frequencies. Only K354Q and E1111K channels exhibited altered channel availability, an effect only observed at the highest stimulation frequency (100 Hz, Fig. S1). These mutant channels had lower channel availability than WT NaV1.3 (WT = 70.2 ± 1.3 %, K354Q = 62.8 ± 2.8 %, E1111K = 64.4 ± 2.6 %).
Persistent currents in WT and variant NaV1.3 channels
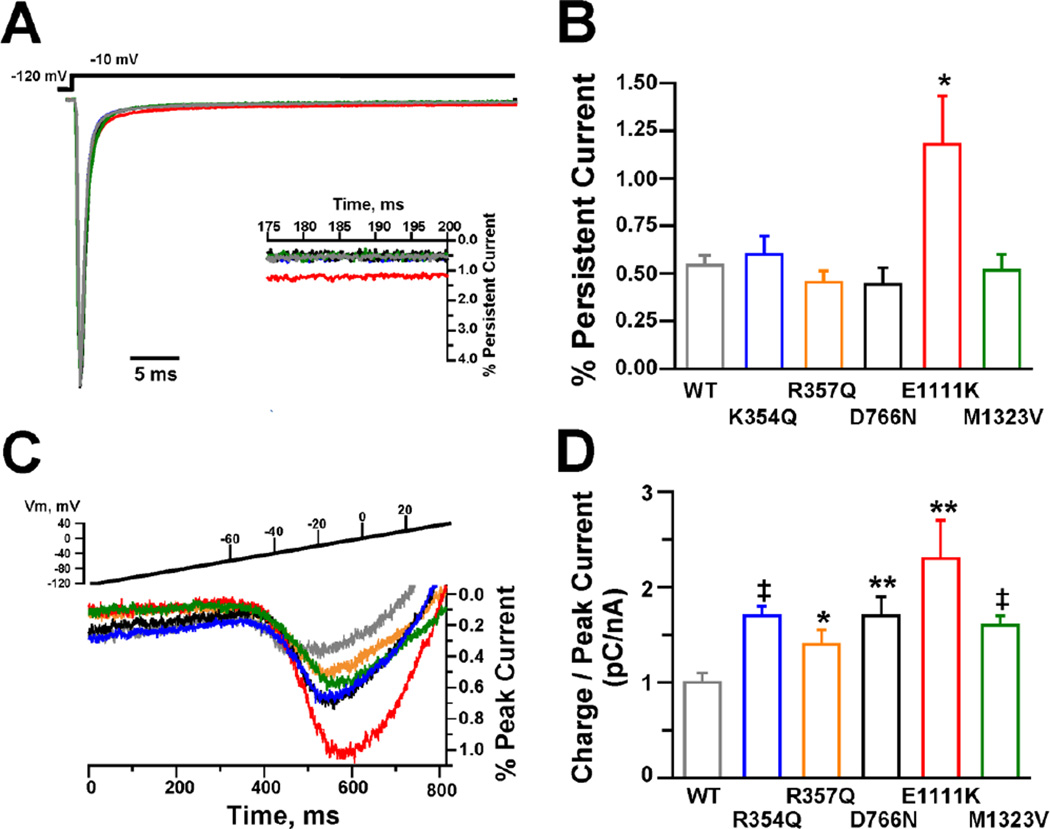
Many epilepsy-associated mutant NaV channels exhibit impaired inactivation resulting in increased persistent sodium current (Lossin et al., 2002; Rhodes et al., 2004; Spampanato et al., 2004; Kahlig et al., 2008; Holland et al., 2008). Because a larger persistent current could accentuate subthreshold depolarizations and facilitate action potential generation leading to neuronal hyperexcitability, we compared this property between WT and variant NaV1.3 channels. Figure 4A illustrates TTX-sensitive whole-cell currents (normalized to peak transient current) recorded from cells transiently expressing WT or variant NaV1.3 channels. Only E1111K (red traces) displayed a persistent current larger than WT channels (Fig. 4B). Persistent current generated by E1111K was more than 2-fold greater than WT channels measured as a percentage of peak current (E1111K = 1.2 ± 0.2%, n = 5; WT = 0.5 ± 0.1%, n = 8; p = 0.009) and absolute current density (E1111K = 4.9 ± 2.1 pA/pF, n = 5; WT = 1.9 ± 0.5 pA/pF, n = 8; p = 0.024).
Figure 4. Persistent and ramp current for NaV1.3 channels.
(A) Average TTX-sensitive persistent currents normalized to the peak sodium current. Inset shows an expanded y-axis to highlight the increased persistent current of E1111K compared to WT. (B) Magnitude of persistent current as a percent of peak current for WT (n = 8), K354Q (n = 5), R357Q (n = 5), D766N (n = 6), E1111K (n = 5) and M1323V (n = 7). Asterisk indicates significance at p < 0.01. (C) Average TTX-sensitive ramp currents during a 0.2 mV/ms voltage ramp from a holding potential of −120 mV and expressed a percent of peak current. (D) Average TTX-sensitive inward charge movement calculated from ramp currents and normalized to the TTX-sensitive peak current generated by a voltage step to −10 mV to account for variation in channel expression (WT, n = 5; K354Q, n = 5; R357Q, n = 6; D766N, n = 6; E1111K, n = 6 and M1323V, n = 7). * p = 0.021; ** p ≤ 0.02; ‡ p ≤ 0.002.
Ramp currents in WT and variant NaV1.3 channels
Because aberrant activation of inward currents during ramp depolarizations can aid in neuronal hyperexcitability, we examined the effects of depolarizing voltage ramps from −120 mV to +40 mV over 800 ms (0.2 mV/ms) on the SCN3A variants. Figure 4C illustrates the average TTX-sensitive ramp currents expressed as percent of peak current recorded from cells expressing WT or variant NaV1.3 channels. Ramp current was quantified as the total conducted charge (pC) for each cell by integrating the TTX-sensitive inward current between the voltage at which the Na+ currents first activated and the voltage where the currents reversed polarity. Total charge was normalized to the peak current measured during a pulse test to −10 mV to correct for variation in channel expression levels between cells. Figure 4D demonstrates that all mutant channels conduct significantly more inward charge than WT channels.
DISCUSSION
Among the myriad epilepsy-associated NaV mutations described to date, only one SCN3A mutation has been reported previously in a patient with focal epilepsy (Holland et al., 2008; Lossin, 2009). In this study we report four new SCN3A variants that were identified in pediatric focal epilepsy. We further demonstrated that all four novel SCN3A variants confer significant functional defects to the encoded NaV1.3 sodium channel suggesting these as putative epilepsy-associated mutations. Two functional properties of the variant NaV1.3 channels deserve in-depth discussion – persistent current and ramp current.
Persistent currents in wild-type and mutant NaV1.3 channels
Several studies support the notion that persistent sodium currents can drive intrinsic neuronal excitability (Alzheimer et al., 1993; Baker et al., 2003). Moreover, several epilepsy-associated NaV1.1 mutations can enhance persistent current (Lossin et al., 2002; Rhodes et al., 2004; Spampanato et al., 2004; Kahlig et al., 2008; Holland et al., 2008), and transgenic mice expressing mutant NaV1.2 channels generating increased persistent current have a severe epilepsy (Kearney et al., 2001). However, most of the novel SCN3A variants we report here exhibited levels of persistent sodium current similar to the WT channel. By contrast, E1111K channels displayed greater levels of persistent current than WT channels. The residue at position 1111 is located within the DII-DIII linker and is conserved among many species (e.g., rodent, dog, cow, zebrafish) and several isoforms (NaV1.1, NaV1.2, NaV1.5 [D instead of E] and NaV1.6). The variant occurs several amino acids (~50) C-terminal from a region identified for ankyrin-G interaction (LeMaillet et al., 2003) and CK2 phosphorylation, which regulates that interaction (Brechet et al., 2008). It is conceivable that this charge reversal variant could alter the interaction of ankyrin-G with NaV1.3 and affect inactivation state stability. Interestingly, ankyrin-G co-expression has been demonstrated to reduce persistent current generated by NaV1.6 (Shirahata et al., 2006).
Unlike previous studies, the K354Q mutation did not generate persistent currents larger than WT-NaV1.3 under our recording conditions. The previous characterizations of the K354Q mutation used human cardiac NaV1.5 or rat NaV1.3 to express this variant allele (Holland et al., 2008; Estacion et al., 2010) whereas we employed the human NaV1.3 channel. Therefore, the divergent effects may be due simply to the molecular context used for heterologous expression.
Importantly, we did not observe large persistent sodium currents in cells expressing WT human NaV1.3 in contrast to previous studies that reported values up to 10–15% of peak current. The NaV1.3 splice variant used in those studies (Chen et al., 2000; Sun et al., 2007) included exon 5N, which has a serine at position 208 (Gazina et al., 2010). Because our studies were conducted using the NaV1.3 exon 5A splice variant (aspartic acid at position 208), we tested whether this single amino acid substitution accounts for the different persistent current levels. Expression of the NaV1.3 5N variant in tsA201 cells generated TTX-sensitive persistent current similar to the 5A variant under our recording conditions (5A: 0.5 ± 0.1%, 1.9 ± 0.5 pA/pF, n = 8; 5N: 0.6 ± 0.1%, 1.5 ± 0.6 pA/pF, n = 5). Additionally, we did not observe a significant effect of using fluoride-free intracellular solution with or without nucleotides ATP and GTP (Chen et al., 2000; Cusdin et al., 2010; Estacion et al., 2010; Sun et al., 2007) on the level of TTX-sensitive persistent current recorded from cells expressing the NaV1.3 5A variant (1.7 ± 0.2%, n = 6). Our results along with other previous NaV1.3 studies using CHO cells as the expression system that describe negligible persistent current (Chen et al., 2000; Meadows et al., 2002) suggest that large persistent current is not an intrinsic property of the NaV1.3 channel. Large persistent current may be due to modulation by proteins such as β subunits [(Cusdin et al., 2010), but see (Meadows et al., 2002)] or G-proteins (Ma et al., 1994; Mantegazza et al., 2005).
Increased ramp currents in all mutant NaV1.3 channels
A common defect shared by all variant NaV1.3 channels was increased ramp currents. The larger ramp currents may represent a pattern of channel dysfunction capable of enhancing the response to subthreshold depolarizing inputs, leading to reduced firing threshold (Lampert et al., 2006). Similar to previous studies using rat NaV1.3 (Estacion et al., 2010) the K354Q mutation expressed in human NaV1.3 splice 5A variant (our data) generated ramp currents larger than the wild-type channel suggesting that this effect, unlike persistent current, is not dependent on animal species, expression system or recording conditions. Estacion and Waxman (2013) showed that NaV1.3 response to ramp stimuli includes an early component dependent on ramp rate and a second one that correlates with persistent current. Our data show that the mutant channel that generated the largest persistent current, E111K, was also the one with the largest response to ramp stimulus. However, the other mutant channels yielded larger ramp currents when compared to WT-NaV1.3 in the absence of larger persistent currents. This discrepancy could be due to the presence of fluoride in our internal solution which may affect persistent current levels (Jarecki et al, 2008).
Decreased channel availability in response to repetitive stimulation indicates a loss of channel function which may nullify some of the gain-of-function properties evoked by the mutations. However, only K354Q and E1111K channels exhibited a small decrease (6–5%) in channel availability when compared to WT-NaV1.3, detectable only at 100 Hz. Thus, lower NaV1.3 channel availability (i.e., loss of function) is not a prominent feature.
Three of the variants, R357Q, K354Q and M1323V, occur in the pore domain, specifically the S5-S6 linker (Fig. 1), which may alter channel gating by charge neutralization or allosteric effects (Estacion et al., 2010). The variant D766N is located at the intracellular end of the S2 segment in DII (Fig. 1). Segment 2 in voltage-gated channels has been implicated in voltage gating and may undergo conformational changes (Cha and Bezanilla, 1997; Ma et al., 2009; Seoh et al., 1996). The charge-neutralizing effect of this variant (D/N) could conceivably influence NaV1.3 gating by a related mechanism.
Genotype-phenotype Correlations
Although all variant channels we studied exhibited increased ramp current, the associated clinical phenotypes are somewhat different. Two variants with substantial functional defects were identified in individuals with more severe phenotypes. The individual heterozygous for R357Q exhibited developmental delay, and among all NaV1.3 mutant channels, R357Q channels were the most dysfunctional. Additionally, the individual who was heterozygous for E1111K (largest ramp current and the only mutant expressing persistent current) had neonatal seizures that remitted. Other channel properties, variation in other genes or other cellular factors may influence the severity or timing of the disease. It is interesting to note that CK2-regulated binding of NaV channels to ankyrin-G is required to immobilize the channels at the axonal initial segment early in development, but in mature neurons the diffusion barrier aids in immobilizing proteins that do not bind ankyrin-G (Brechet et al., 2008).
In summary, we analyzed four novel NaV1.3 variants identified in childhood epilepsy patients. All variant channels studied have increased ramp currents, which may reduce neuronal firing threshold and promote hyperexcitable networks, consistent with an epilepsy phenotype. Whether increased ramp current alone or in combination with other biophysical defects correlates with clinical phenotype severity are interesting possibilities. Although defining the mechanism by which NaV1.3 variants influence neuronal excitability will require additional studies, the identification of four additional SCN3A variants with a common biophysical defect provides further support for a contribution of NaV1.3 to childhood epilepsy.
Supplementary Material
Highlights.
Identified four novel SCN3A missense variants in pediatric focal epilepsy patients.
Variant characterization in heterologously expressed NaV1.3 revealed functional defects.
All variants exhibited increased current in response to depolarizing voltage ramps.
Increased ramp currents may be a common biophysical defect in SCN3A-associated epilepsy.
ACKNOWLEDGEMENTS
This work was supported by NIH grants NS032387 (A.L.G.) and NS053792 (J.A.K.). The authors would like to thank the NHLBI GO Exome Sequencing Project and its ongoing studies which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal N, Hamam BN, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in rat entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. doi: 10.1016/s0306-4522(00)00455-3. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal Gating of Na+ Channels As A Mechanism of Persistent Na+ Current in Pyramidal Neurons from Rat and Cat Sensorimotor Cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Yankaya B, Troost D, van Vliet EA, Lopes da Silva FH, Gorter JA. Induction of neonatal sodium channel II and III alpha-isoform mRNAs in neurons and microglia after status epilepticus in the rat hippocampus. Eur J Neurosci. 2001;13:1261–1266. doi: 10.1046/j.0953-816x.2001.01502.x. [DOI] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–382. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J Neurocytol. 1997;26:667–678. doi: 10.1023/a:1018549928277. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of voltage-sensitive sodium and calcium channels. Braz J Med Biol Res. 1988;21:1129–1144. [PubMed] [Google Scholar]
- Cha A, Bezanilla F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 1997;19:1127–1140. doi: 10.1016/s0896-6273(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Chen YH, Dale TJ, Romanos MA, Whitaker WR, Xie XM, Clare JJ. Cloning, distribution and functional analysis of the type III sodium channel from human brain. Eur J Neurosci. 2000;12:4281–4289. [PubMed] [Google Scholar]
- Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS ONE. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Aglieco F, Renganathan M, Herzog RI, Dib-Hajj SD, Waxman SG. Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci. 2001;21:5952–5961. doi: 10.1523/JNEUROSCI.21-16-05952.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusdin FS, Nietlispach D, Maman J, Dale TJ, Powell AJ, Clare JJ, Jackson AP. The sodium channel {beta}3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions. J Biol Chem. 2010;285:33404–33412. doi: 10.1074/jbc.M110.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Estacion M, Gasser A, Dib-Hajj SD, Waxman SG. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp Neurol. 2010;224:362–368. doi: 10.1016/j.expneurol.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Estacion M, Waxman SG. The response of Na(V)1.3 sodium channels to ramp stimuli: multiple components and mechanisms. J Neurophysiol. 2013;109:306–314. doi: 10.1152/jn.00438.2012. [DOI] [PubMed] [Google Scholar]
- Exome Variant Server. NHLBI GO Exome Sequencing Project (ESP) Seattle, WA: (URL: http://evs.gs.washington.edu/EVS/) [July, 2013] [Google Scholar]
- Gazina EV, Richards KL, Mokhtar MB, Thomas EA, Reid CA, Petrou S. Differential expression of exon 5 splice variants of sodium channel alpha subunit mRNAs in the developing mouse brain. Neuroscience. 2010;166:195–200. doi: 10.1016/j.neuroscience.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holland KD, Glauser TA. Response to carbamazepine in children with newly diagnosed partial onset epilepsy. Neurology. 2007;69:596–599. doi: 10.1212/01.wnl.0000267274.69619.f3. [DOI] [PubMed] [Google Scholar]
- Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, Glaaser IW, Kass RS, Meisler MH. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett. 2008;433:65–70. doi: 10.1016/j.neulet.2007.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Jarecki BW, Sheets PL, James O, Jackson JO, Cummins TR. Paroxysmal extreme pain disorder mutations within the D3/S4–S5 linker of Nav1.7 cause moderate destabilization of fast inactivation. J Physiol. 2008;586:4137–4153. doi: 10.1113/jphysiol.2008.154906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Rhodes TH, Pusch M, Freilinger T, Pereira-Monteiro JM, Ferrari MD, van den Maagdenberg AM, Dichgans M, George AL., Jr Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci USA. 2008;105:9799–9804. doi: 10.1073/pnas.0711717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JA, Plummer NW, Smith MR, Kapur J, Cummins TR, Waxman SG, Goldin AL, Meisler MH. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–317. doi: 10.1016/s0306-4522(00)00479-6. [DOI] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;47:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- Lampert A, Hains BC, Waxman SG. Upregulation of persistent and ramp sodium current in dorsal horn neurons after spinal cord injury. Exp Brain Res. 2006;174:660–666. doi: 10.1007/s00221-006-0511-x. [DOI] [PubMed] [Google Scholar]
- LeMaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain Dev. 2009;31:114–130. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CGAL, AL Molecular basis of an inherited epilepsy. Neuron. 2002;34:877–884. doi: 10.1016/s0896-6273(02)00714-6. [DOI] [PubMed] [Google Scholar]
- Ma JY, Li M, Catterall WA, Scheuer T. Modulation of brain Na+ channels by a G-protein-coupled pathway. Proc Natl Acad Sci USA. 1994;91:12351–12355. doi: 10.1073/pnas.91.25.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Kong J, Kallen RG. Studies of alpha-helicity and intersegmental interactions in voltage-gated Na+ channels: S2D4. PLoS ONE. 2009;4:e7674. doi: 10.1371/journal.pone.0007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Powell AJ, Clare JJ, Catterall WA, Scheuer T. Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits. J Neurosci. 2005;25:3341–3349. doi: 10.1523/JNEUROSCI.0104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Chen YH, Powell AJ, Clare JJ, Ragsdale DS. Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary beta 1, beta 2 and beta 3 subunits. Neuroscience. 2002;114:745–753. doi: 10.1016/s0306-4522(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Rainier S, Sher C, Reish O, Thomas D, Fink JK. De novo occurrence of novel SPG3A/atlastin mutation presenting as cerebral palsy. Arch Neurol. 2006;63:445–447. doi: 10.1001/archneur.63.3.445. [DOI] [PubMed] [Google Scholar]
- Rhodes TH, Lossin C, Vanoye CG, Wang DW, George AL., Jr Noninactivating voltage-gated sodium channels in severe myoclonic epilepsy of infancy. Proc Natl Acad Sci USA. 2004;101:11147–11152. doi: 10.1073/pnas.0402482101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins G. Fast, scalable generation of high quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7:539. doi: 10.1038/msb.2011.75. doi:10.1038/msb.2011.75 Epub 2011 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata E, Iwasaki H, Takagi M, Lin C, Bennett V, Okamura Y, Hayasaka K. Ankyrin-G regulates inactivation gating of the neuronal sodium channel, Nav1.6. J Neurophysiol. 2006;96:1347–1357. doi: 10.1152/jn.01264.2005. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Kearney JA, de Haan G, Mcewen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamazepine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia. 2007;48:774–782. doi: 10.1111/j.1528-1167.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Thimmapaya R, Neelands T, Niforatos W, vis-Taber RA, Choi W, Putman CB, Kroeger PE, Packer J, Gopalakrishnan M, Faltynek CR, Surowy CS, Scott VE. Distribution and functional characterization of human Nav1.3 splice variants. Eur J Neurosci. 2005;22:1–9. doi: 10.1111/j.1460-9568.2005.04155.x. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Lossin C, Rhodes TH, George AL., Jr Single-channel properties of human NaV1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J Gen Physiol. 2006;127:1–14. doi: 10.1085/jgp.200509373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–2778. doi: 10.1111/j.1460-9568.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- Wang DW, Mistry AM, Kahlig KM, Kearney JA, Xiang J, George AL., Jr Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front Pharmacol. 2010;1:144. doi: 10.3389/fphar.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, Reichert J, Buxbaum JD, Meisler MH. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Molecular Psychiatry. 2003;8:186–194. doi: 10.1038/sj.mp.4001241. [DOI] [PubMed] [Google Scholar]
- Whitaker WR, Faull RL, Dragunow M, Mee EW, Emson PC, Clare JJ. Changes in the mRNAs encoding voltage-gated sodium channel types II and III in human epileptic hippocampus. Neuroscience. 2001;106:275–285. doi: 10.1016/s0306-4522(01)00212-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.