Introduction
Depression is a prevalent condition in pregnancy affecting up to 13% of women (1). Untreated antenatal depression is associated with poor self-care during pregnancy, risk of post-partum depression, risk of impaired maternal-infant attachment, and delays in infant development when it persists into the post-partum period (2, 3). Available treatments for depressive disorders include psychotherapeutic interventions and antidepressant medications such as selective serotonin inhibitors (SSRIs) and tricyclic antidepressants (TCAs). Although psychotherapy may be a reasonable treatment option for mild to moderate depression, antidepressants are often required for the effective treatment of severe maternal depression (4, 5). Recent estimates of antidepressant exposure among pregnant women range from 3% to 13% (6, 7).
Preterm birth (PTB) and low birth weight (LBW) occur at national rates of 12.2% and 8.2%, respectively (8). Several studies over the past two decades have attempted to characterize the relationship between antidepressant use in pregnancy and risk of adverse birth outcomes (9). However, in general, the observational studies published to date have provided inconsistent and sometimes conflicting findings on the relationship between antidepressant exposure and LBW and PTB. Differences in study design (prospective, retrospective), patient populations (patients recruited from mental health settings, patients identified from registries), comparator groups (non-depressed or depressed controls), and sample sizes make it difficult to interpret the variability of findings. Many studies are also limited in their ability to adequately control for important potential confounding variables such as smoking, substance abuse, medical conditions (such as pregnancy induced hypertension and gestation diabetes), and depression severity—all of which have been found to be independently associated with adverse birth outcomes (10, 11).
A recent meta-analysis has shown that although antidepressant use in pregnancy was not associated with spontaneous abortion, exposure was significantly associated with both preterm delivery and low birth weight (12). The goal of this meta-analysis is to add to the literature by further examining this association (e.g. reproductive outcomes of depressed women who are treated with antidepressants of any type during pregnancy), adding eight studies published after June 2010 (the end of the above systematic review). We also examine how the quality and study design of these previous studies influence the outcomes reported in this meta-analysis via sensitivity analyses.
Methods
Search strategy for identification of studies
We searched for English and non-English language articles via MEDLINE, CINAHL, PyschINFO, and reference lists of review papers. The electronic search included studies from the respectively databases’ start dates and ended on December 1, 2012. We used the following keywords and their combinations: antidepressant, selective serotonin reuptake inhibitor, SSRI, pregnancy, antenatal, prenatal, birthweight, birth weight, preterm, prematurity, gestational age, fetal growth restriction, intrauterine growth restriction, and small-for-gestational age. Published English-language and non-English language studies were included in this meta-analysis if they provided the relative risk or adequate data for the calculation of an effect size as an odds ratio between antidepressant use and an adverse birth outcome (i.e. LBW or PTB). Included studies could be either prospective or retrospective. Studies were excluded if they lacked the outcomes of interest. Authors H.H. and S.C. contacted authors of studies that reported outcomes of interest as continuous variables for information to calculate effect sizes as odds ratios.
Data extraction
Adverse birth outcomes
Two authors (H.H. and S.C.) reviewed all studies. A standardized eligibility and quality of study coding sheet were designed a priori (13). Of the 222 published studies reviewed, 28 met the inclusion criteria. Fifteen studies on LBW (<2500g) and 28 studies on PTB (<37 weeks gestational age) were included in this meta-analysis (Table 1).
Table 1.
Characteristics of studies included in the meta-analysis
| Trial | Year | Exposure | Controls | Trial design | Sample size | RR (95% CI) | RR (95% CI) | Quality Score | Controlled for Depression Severity |
|---|---|---|---|---|---|---|---|---|---|
| LBW | PTB | ||||||||
| Grzeskowiak (30) | 2012 | SSRI | Dep | retrospective | 1787 | 2.26 (1.31–3.91) | 2.68 (1.83–3.93) | 8 | No |
| El Marroun (31) | 2012 | SSRI | non-Dep | prospective | 7126 | 1.65 (0.77–3.56) | 2.14 (1.08–4.25) | 9 | No |
| Klieger-Grossmann (32) | 2012 | escitalopram | non-Dep | prospective | 425 | 4.51 (1.43–18.69) | 2.21 (0.92–5.68) | 7 | No |
| Nordeng (33) | 2012 | various AD | mixed | prospective | 62204 | 0.62 (0.33–1.16) | 1.21 (0.87–1.69) | 11 | Yes |
| Yonkers (34) | 2012 | SSRI | non-Dep | prospective | 2432 | 1.62 (1–2.5) | 9 | Yes | |
| Colvin (35) | 2011 | SSRI | non-Dep | retrospective | 96698 | 1.4 (1.25–1.56) | 1.43 (1.24–1.65) | 8 | No |
| Latendresse (36) | 2011 | SSRI | Mixed | retrospective | 100 | 11.7 (2.2–60.7) | 7 | Yes | |
| Roca (25) | 2011 | SSRI | non-Dep | retrospective | 252 | 1.37 (0.46–3.81) | 3.44 (1.30–9.11) | 7 | No |
| Einarson (37) | 2010 | various AD | non-Dep | retrospective | 1856 | 1.7 (1.18–2.45) | 6 | No | |
| Lewis (38) | 2010 | SSRI or SNRI | mixed | prospective | 54 | 8.33 (1.11–62.67) | 4.52 (0.47–43.41) | 9 | Yes |
| Reis (39) | 2010 | SSRI | Mixed | retrospective | 1068177 | 1.13 (0.97–1.31) | 1.45 (1.31–1.63) | 6 | No |
| Lund (40) | 2009 | SSRI | non-Dep | prospective | 52099 | 0.63 (0.15–2.67) | 2.02 (1.29–3.16) | 7 | No |
| Toh (41) | 2009 | SSRI | mixed | retrospective | 5796 | 1.27 (0.59–2.76) | 8 | No | |
| Wisner (23) | 2009 | SSRI | non-Dep | prospective | 179 | 5.43 (1.98–14.84) | 9 | No | |
| Maschi (42) | 2008 | various AD | Mixed | prospective | 1400 | 1.18 (0.53–2.41) | 2.31 (1.14–4.63) | 3 | No |
| Davis (43) | 2007 | SSRI | Mixed | retrospective | 50710 | 1.45 (1.25–1.68) | 3 | No | |
| Lennestal (44) | 2007 | SNRI | Mixed | retrospective | 860215 | 1.12 (0.74–1.68) | 1.6 (1.19–2.15) | 8 | No |
| Pearson (45) | 2007 | various AD | non-Dep | retrospective | 252 | 1.07 (0.4–2.67) | 4 | No | |
| Suri (46) | 2007 | various AD | Dep | prospective | 71 | 3.5 (0.4–165.11) | 11 | Yes | |
| Djulus (47) | 2006 | mirtazapine | non-Dep | prospective | 208 | 5.43 (1.11–51.83) | 7 | No | |
| Wen (48) | 2006 | SSRI | Mixed | retrospective | 4850 | 1.58 (1.19–2.11) | 1.57 (1.28–1.92) | 7 | No |
| Sivojelezova (49) | 2005 | citalopram | non-Dep | prospective | 264 | 2.31 (0.71–8.71) | 6 | No | |
| Kallen (50) | 2004 | various AD | Mixed | prospective | 563656 | 1.98 (1.55–2.52) | 1.96 (1.60–2.41) | 9 | No |
| Casper (51) | 2003 | SSRI | Dep | prospective | 44 | 0.4 (0.005–33.99) | 11 | Yes | |
| Simon (52) | 2002 | SSRI | Dep | retrospective | 370 | 2.73 (0.92–8.09) | 4.38 (1.57–12.22) | 8 | No |
| Ericson (53) | 1999 | various AD | Mixed | retrospective | 281728 | 1.32 (0.96–1.80) | 1.43 (1.14–1.8) | 4, 6 | No |
| Chambers (22) | 1996 | fluoxetine | non-Dep | prospective | 290 | 2.65 (0.98–6.89) | 3 | No | |
| Pastuszak (54) | 1993 | fluoxetine | non-Dep | prospective | 256 | 0.85 (0.22–3.09) | 2 | No |
Methodologic quality assessment
H.H. and S.C. rated each of the studies independently and assigned a quality score to each of the studies selected for this meta-analysis according to guidelines described by Downs and Black (14). We used a consensus approach and resolved differences in scoring prior to assigning a final quality score. The quality measure was based on the following indicators: whether characteristics of patients were clearly described, whether measures of antidepressant exposure were reliable and valid, the degree of adjustment for multiple potential confounding variables in analyses, whether measurement and adjustment for depression severity was made, the study representativeness of the potential population, and sample size. The total quality scores ranged from 0–13.
Analysis
The association between antidepressant exposure in the antenatal period and adverse birth outcome was examined using relative risks (RRs). To do this, we considered odds ratios (ORs) as surrogates for RRs because when outcomes undergoing study are relatively uncommon, the relative odds approximate RRs (2). Each study’s RR was weighted according to the inverse of its variance using random-effects models in order to calculate a pooled RR. Ninety-five percent confidence intervals (95% CIs) were calculated for each study result and for the pooled estimates. Statistical analyses were performed using Comprehensive Meta-analysis version 2.2 (Biostat, Englewood, New Jersey).
Heterogeneity of effect size was assessed using the Cochran Q χ2 statistic (P≤.10) and the I2 statistic, which indicates the percentage of variation in the effect size estimate attributable to heterogeneity rather than sampling error (15). Random-effects models were used in all analyses because the Q statistic and the I2 statistic indicated substantial heterogeneity of effect size in the primary analyses examining the association between antidepressant exposure and each adverse birth outcome. Random-effects meta-regression analyses and moderator analyses were conducted to determine whether four study characteristics could explain variability across studies: (1) methodological quality of studies; (2) drug type (SSRI vs. other or mixed); (3) control status (depressed, mixed, or non-depressed); and (4) study design (prospective vs. retrospective). “Leave-one-out” analyses were conducted by iteratively deleting each study and calculating the resulting effect size (16).
Publication bias was assessed visually using a funnel plot and quantitatively using a regression procedure to measure funnel plot asymmetry (17). The trim-and-fill method by Duval and Tweedie (18, 19) was used to adjust for potential publication bias. This method assesses asymmetry in the funnel plot, imputes the number of suspected missing studies, and recalculates the adjusted pooled effect size estimate. The adjusted result can be used as a sensitivity analysis to indicate the extent to which publication bias may affect the pooled estimate (2, 20).
Results
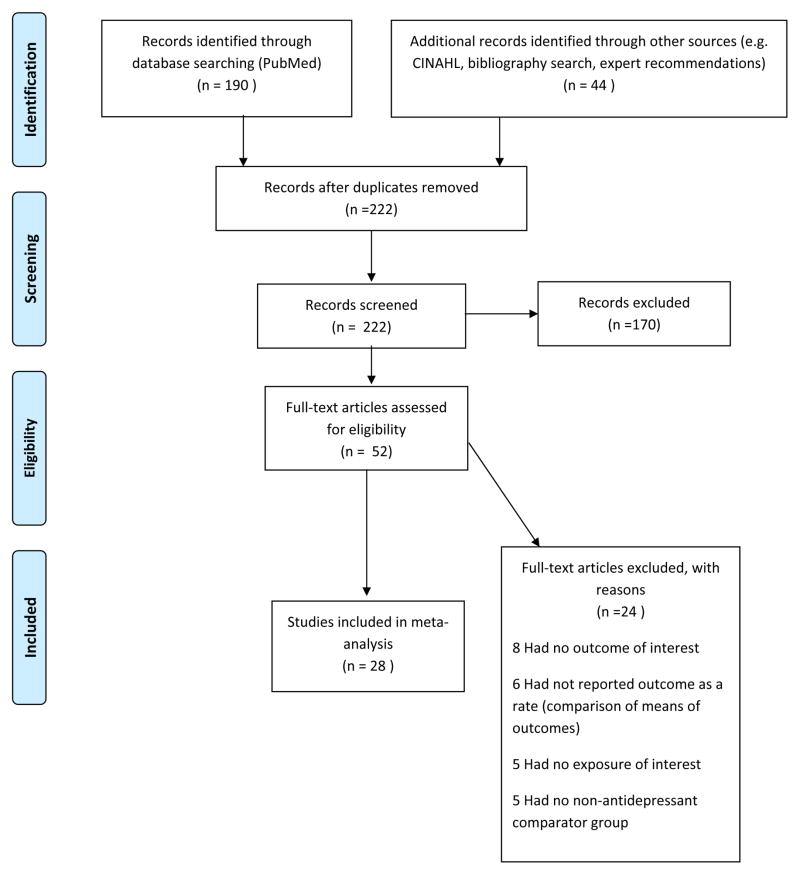
The retrieval and selection strategy is shown in Figure 1. Of the 222 citations found to meet the initial search criteria, 52 full-text articles were assessed for eligibility, and 28 articles were ultimately included in this analysis. Table 1 provides the characteristics of these studies. Further information was requested from 12 authors (of 16 studies) of whom six authors responded with two of these authors providing data allowing two additional studies to be included in the meta-analysis.
Figure 1.
Identification of independent studies for inclusion in the meta-analysis (from PRISMA flow diagram guidelines)
Association between antidepressant use in pregnancy and adverse birth outcomes
Low birth weight
Fifteen studies evaluated the association between antenatal antidepressant use and LBW with RRs ranging from 0.62 to 8.33 (Table 2). Using the random-effects model, antenatal antidepressant exposure was significantly associated with LBW (RR= 1.44, 95% CI: 1.21–1.70). Nine of the studies found no significant association. Significant heterogeneity across studies was noted (Q14= 37.1; P=.001; I2 = 62%).
Table 2.
Effect of Antenatal Antidepressant Exposure on Outcomes of Low Birth Weight and Preterm Birth
| Outcome | No. of Studies | Relative Risk|| % (95% CI) | P Value | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Qdf Within | P Value | % Variance Explained | ||||
| Low Birth Weight | 15 | 1.44 (1.21–1.70) | <.001 | 37.114 | .001 | 62 |
| Preterm Birth | 28 | 1.69 (1.52–1.88) | <.001 | 49.427 | .005 | 45 |
Abbreviations: No. indicates number; CI, confidence interval; df, degrees of freedom.
Pooled effect size estimated using the random-effects model.
Preterm birth
Twenty-eight studies evaluated the association between antenatal antidepressant exposure and PTB with RRs ranging from 0.40 to 11.70 (Table 2). Using the random-effects model, antenatal antidepressant exposure was significantly associated with PTB (RR: 1.69, 95% CI: 1.52–1.88). Nine of the studies found no significant association. Significant heterogeneity across studies was noted (Q27=49.4; P=.005; I2 = 45%).
Moderators of Outcome
Moderator analyses were conducted to explore sources of heterogeneity (Table 3). In LBW studies, although the omnibus test was not statistically significant, studies that used a depressed control group without antidepressant exposure yielded larger pooled RRs than studies that used mixed controls (Q1=4.30, P=.038). Similarly, PTB studies that used a depressed control group without antidepressant exposure yielded larger RRs than studies that used either mixed controls (Q1=10.45, P=.001) or non-depressed controls (Q1=4.35, P=.037). In PTB studies, heterogeneity among studies was reduced by the addition of the control group moderator (depressed: Q3= 2.01; P=.57; I2 = 0%; mixed: Q10= 17.42; P=.07; I2 = 43%; non-depressed: Q2= 18.17; P=.11; I2 = 34%). Drug type, study design, control for depression severity, and study quality were not significant moderators of LBW or PTB.
Table 3.
Moderators of Effect of Antidepressant Exposure on Outcomes of Low Birth Weight and Preterm Birth
| Moderator | No. of Studies | Within Group
|
Effect of Moderator | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Relative Risk|| % (95% CI) | P Value | Heterogeneity | |||||||
|
| |||||||||
| Qdf Within | P Value | % Variance Explained | Qdf Between | P Value | % Variance Explained | ||||
| Low Birth Weight | |||||||||
| Drug Type | |||||||||
| SSRIs | 9 | 1.48 (1.22–1.79) | <.001 | 17.98 | .02 | 55 | 0.31 | .57 | 1 |
|
|
|||||||||
| Other/mixed antidepressants | 6 | 1.31 (0.90–1.90) | .16 | 18.45 | .003 | 73 | |||
|
| |||||||||
| Study Design | |||||||||
| Retrospective | 8 | 1.36 (1.18–1.57) | <.001 | 12.77 | .08 | 45 | 0.21 | .66 | 1 |
|
|
|||||||||
| Prospective | 7 | 1.54 (0.92–2.58) | .10 | 19.66 | .003 | 69 | |||
|
| |||||||||
| Control Group§ | |||||||||
| Depressed | 2 | 2.35 (1.44–3.83) | .001 | 0.11 | .76 | 0 | |||
|
|
|||||||||
| Mixed | 8 | 1.32 (1.03–1.68) | .03 | 25.87 | .001 | 73 | 4.32 | .12 | 12 |
|
|
|||||||||
| Non-Depressed | 5 | 1.53 (1.06–2.20) | .02 | 5.64 | .23 | 29 | |||
|
| |||||||||
| Control for Depression Severity | |||||||||
| Yes | 2 | 1.89 (0.15–23.5) | .62 | 5.81 | .02 | 83 | 0.01 | .85 | 0 |
|
|
|||||||||
| No | 13 | 1.47 (1.26–1.72) | <.001 | 27.812 | .006 | 57 | |||
|
| |||||||||
| Preterm Birth | |||||||||
| Drug Type | |||||||||
| SSRIs | 18 | 1.74 (1.52–2.00) | <.001 | 35.117 | .006 | 52 | 0.41 | .55 | 1 |
|
|
|||||||||
| Other/mixed antidepressants | 10 | 1.63 (1.38–1.93) | <.001 | 13.59 | .14 | 33 | |||
|
| |||||||||
| Study Design | |||||||||
| Retrospective | 13 | 1.59 (1.42–1.78) | <.001 | 24.212 | .02 | 50 | 2.61 | .11 | 5 |
|
|
|||||||||
| Prospective | 15 | 1.91 (1.57–2.32) | <.001 | 18.114 | .20 | 23 | |||
|
| |||||||||
| Control Group‡ | |||||||||
| Depressed | 4 | 2.85 (2.00–4.07) | <.001 | 2.03 | .57 | 0 | |||
|
|
|||||||||
| Mixed | 11 | 1.55 (1.39–1.73) | <.001 | 17.410 | .07 | 43 | 11.52 | .003 | 23 |
|
|
|||||||||
| Non-Depressed | 13 | 1.84 (1.50–2.27) | <.001 | 18.212 | .11 | 34 | |||
|
| |||||||||
| Control for Depression Severity | |||||||||
| Yes | 6 | 1.90 (1.07–3.38) | .03 | 10.15 | .07 | 50 | 0.11 | .70 | 0 |
|
|
|||||||||
| No | 22 | 1.70 (1.53–1.89) | <.001 | 39.021 | .01 | 46 | |||
Abbreviations: No. indicates number; CI, confidence interval; df, degrees of freedom.
Pooled effect size estimated using the random-effects model.
Pairwise effect of moderator: depressed vs. mixed, Q1=4.3, P=.04; depressed vs. non-depressed, Q1=1.9, P=.17; mixed vs. non-depressed, Q1=0.4, P=.51.
Pairwise effect of moderator: depressed vs. mixed, Q1=10.4, P=.001; depressed vs. non-depressed, Q1=4.4, P=.04; mixed vs. non-depressed, Q1=2.1, P=.14.
Leave-One-Out Analyses
Sensitivity analyses revealed that no single study unduly influenced the pool risk ratio estimates of the association between antenatal antidepressant exposure and LBW and PTB.
Publication bias
In PTB studies, visual inspection of the funnel plot in which each study’s effect size (as measured by log RR) was plotted against the standard error and showed marked asymmetry, suggesting that studies with negative findings may not have been published; evidence of possible publication bias was confirmed using the regression intercept approach (17) (P=.001). As shown in Table 4, the trim-and-fill adjusted RRs for PTB, while generally lower than the unadjusted RRs, are robust to the effects of publication bias. There was no evidence of publication bias for LBW studies.
Table 4.
Comparison of Unadjusted Pooled Relative Risks and Trim-and-Fill Adjusted Pooled Relative Risks
| Control Group | No. of Studies | Unadjusted Pooled RR (95% CI)† | Number of Missing Studies | Trim-and-Fill Adjusted Pooled RR (95% CI)‡ |
|---|---|---|---|---|
| Overall | 28 | 1.69 (1.52 to 1.88) | 7 | 1.62 (1.44 to 1.82) |
| Depressed | 4 | 2.85 (2.00 to 4.07) | 0 | 2.85 (2.00 to 4.07) |
| Mixed | 11 | 1.55 (1.40 to 1.73) | 2 | 1.53 (1.36 to 1.74) |
| Non-Depressed | 13 | 1.84 (1.50 to 2.27) | 4 | 1.63 (1.29 to 2.05) |
RR indicates relative risk; CI, confidence interval.
Using random effects models.
Using random-random effects trim-and-fill models.
Discussion
This systematic review found that antidepressant exposure during pregnancy was associated with significant increased risks of LBW and PTB. A prior meta-analysis by Lattimore (2005) in which nine studies were included also examined this relationship and showed a non-significant increase in risk for PTB ( OR: 1.85, 95% CI: 0.79–4.29), but a stronger association for an increase in risk for LBW (OR: 3.64, 95% CI: 1.01–13.08) (21). One explanation for the differences found between our study and the Lattimore study is that the inclusion criteria used in each study differed (we included all studies with the outcomes of interest—both prospective and retrospective—while the Lattimore study included only prospective studies). A more recent meta-analysis by Ross et. al, that reviewed studies completed through 2010 also confirmed the existence of a statistically significant relationship between antidepressant exposure in pregnancy and both LBW and PTB (12). However, Ross and colleagues emphasized that the differences found between women exposed to antidepressants versus those not exposed on gestational age (approximately three days shorter) and birth weight (approximately 75 grams lower) were small and of questionable clinical significance.
There is some evidence that the length of exposure or timing of exposure during certain trimesters may influence antidepressants’ effects on fetal development and subsequent birth outcomes. An early study by Chambers and colleagues found that late fluoxetine exposure was associated with PTB and LBW compared with earlier exposure (22). Other work suggests that the timing of (23) or duration of (24) antidepressant exposure influences the risk of these outcomes. Findings from a recent study by Wisner and colleagues also suggested that the timing of exposure may affect birth outcomes (23). They found that mothers taking an antidepressant throughout pregnancy were more likely to have PTB infants than those exposed partially or not at all during pregnancy. On the other hand, a study by Oberlander and colleagues that used propensity score matching on population-based data of pregnant women showed that longer antidepressant exposure duration during pregnancy and not timing of exposure was associated with LBW (24). Antidepressant dosing has also been implicated as a factor in affecting adverse birth outcomes. For instance, a recent study that examined antidepressant dosing found that pregnant women exposed to high doses of antidepressants were five-fold more likely to have PTBs than those who were exposed to low-medium doses (25).
These results must be tempered by results of a recent meta-analysis that found that the illness of depression was also associated with risk of LBW and PTB (2). Moreover, Wisner and colleagues have shown that both persistent depressive symptoms throughout pregnancy as well as antidepressant exposure were independent risk factors for LBW and PTB (23). Tapering antidepressants in pregnant women with histories of depression has also been shown to be associated with a significantly higher risk of relapse compared to women remaining on antidepressant treatment (26). Lack of depression treatment in pregnancy increases the likelihood that depression will continue into the postpartum period with attendant suffering of the mother and possible complications in maternal-infant bonding, delayed developmental milestones, and subsequent behavioral problems (27).
The decision to initiate or remain on antidepressant treatment in pregnant women should be based on risk-benefit ratio and should occur in the context of shared decision making between the patient and her physician. It is certainly reasonable in many women, given concerns about both depression and SSRI use being linked to adverse birth outcomes, to initiate treatment with an evidence-based psychotherapy such as interpersonal therapy or cognitive behavioral therapy and potentially adding an antidepressant for non-response. However, the highest risk of depression during pregnancy is in low-income populations which often have the greatest barriers to finding psychotherapeutic services due to limitations in insurance coverage for mental health issues. There are also limitations in being able to pay out-of-pocket costs since co-pays are generally higher for mental health services. Lastly, low-income patients face a multitude of difficulties in attending mental health visits including taking time off from work, obtaining childcare services, and transportation costs.
Strengths of this study include the development of a coding sheet for inclusion and methodological quality a priori. We also aimed to characterize the quality of studies based on their ability to control important confounding factors such as the severity of depression, smoking, and alcohol use which all affect birth outcomes. We were able to extend the findings of our colleagues Ross et. al, (12) by including eight additional studies that have been published since 2011.
The main limitation of our study is exclusion of studies based on our selection criteria. For instance, studies in which only the means of birth weight or gestational age were provided were not included in our study if authors did not reply to our request for additional data (14 studies were excluded). Furthermore, the included studies varied widely in design, type of population, control group, and methods. Most importantly, few studies were able to control for all potential confounding factors that are associated with the exposure (antidepressant use) and events (PTB and LBW). Pregnant women with depression have significantly more pregnancy-related somatic symptoms (28) which likely lead to more physician visits, are more likely to take over-the-counter and allopathic medicines for these somatic symptoms, have more comorbid medical illnesses preceding pregnancy such as hypertension (29), and have higher rates of smoking, higher BMIs and use of illicit substances. Moreover, women with greater depression severity and persistence of depression are more likely to receive antidepressant treatment (confounding by indication) and few studies controlled for severity or persistence of depression. More prospective epidemiologic studies that control for all these potential confounding factors as well as severity of depression are needed to better describe the strength of association between antenatal antidepressant exposure and PTB and LBW.
Conclusions
Antidepressant use during pregnancy may significantly increase the risk for preterm birth and low birth weight. Our finding highlights the need for a careful examination of the risk-benefit ratio when considering the initiation or maintenance of antidepressant therapy in pregnant women with depression.
Acknowledgments
Funding/Support:
The research was supported by the following grant from the Health Services Division of NIMH: T32 MH20021-14 (principal investigator: Wayne Katon, MD).
Footnotes
Conflict of Interest Notification:
Drs. Huang, Coleman, Bridge, and Katon have no potential conflicts of interest to disclose.
Dr. Yonkers discloses royalties from Up-To-Date.
Additional Contributions:
We thank KeriLee Horan for her thoughtful review of the manuscript, for which no compensation was received.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 2.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry. 2010 Oct 1;67(10):1012–24. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development. 2010;33(1):1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonkers K, Vigod S, Ross L. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstetrics and Gynecology. 2011 Apr;117(4):961–77. doi: 10.1097/AOG.0b013e31821187a7. [DOI] [PubMed] [Google Scholar]
- 5.APA. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, Third Edition. 2010 [cited 10/21/2012]; Available from: http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485.
- 6.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. American Journal of Obstetrics and Gynecology. 2004;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. American Journal of Obstetrics and Gynecology. 2007;196(6):e1–5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 9.Udechuku A, Nguyen T, Hill R, Szego K. Antidepressants in pregnancy: a systematic review. Australian New Zealand Journal of Psychiatry. 2010;44(11):978–96. doi: 10.3109/00048674.2010.507543. [DOI] [PubMed] [Google Scholar]
- 10.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics and Gynecology. 2000;182(2):465–72. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstetrics and Gynecology. 2009 Sep;114(3):703–13. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross Le GSML, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: A systematic review and meta-analysis. JAMA Psychiatry. 2013;70(4):436–43. doi: 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- 13.Lipsey M, Wilson D. Practical Meta-Analysis. Thousand Oaks, California: Sage Publications; 2001. [Google Scholar]
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal Epidemiology Community Health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Greenhouse J, Iyengar S. In: Sensitivity analyses and diagnostics. HMC, LVH, editors. New York, New York: Russell Sage Foundation; 1994. [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000 Jun;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000 Jun 10;320(7249):1574–7. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007 Nov 10;26(25):4544–62. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 21.Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Jr, Vazquez DM. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis. Journal of Perinatology. 2005;25(9):595–604. doi: 10.1038/sj.jp.7211352. [DOI] [PubMed] [Google Scholar]
- 22.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. New England Journal of Medicine. 1996;335(14):1010–5. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 23.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. American Journal of Psychiatry. 2009;166(5):557–66. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. British Journal of Psychiatry. 2008;192(5):338–43. doi: 10.1192/bjp.bp.107.037101. [DOI] [PubMed] [Google Scholar]
- 25.Roca A, Garcia-Esteve L, Imaz ML, Torres A, Hernandez S, Botet F, et al. Obstetrical and neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitors: the relevance of dose. Journal of Affective Disorders. 2011;135(1–3):208–15. doi: 10.1016/j.jad.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006 Feb 1;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 27.Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, et al. Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(12):1367–74. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Kelly RH, Russo J, Katon W. Somatic complaints among pregnant women cared for in obstetrics: normal pregnancy or depressive and anxiety symptom amplification revisited. General Hospital Psychiatry. 2001 May-Jun;23(3):107–13. doi: 10.1016/s0163-8343(01)00129-3. [DOI] [PubMed] [Google Scholar]
- 29.Katon WJ, Russo JE, Melville JL, Katon JG, Gavin AR. Depression in pregnancy is associated with preexisting but not pregnancy-induced hypertension. General Hospital Psychiatry. 2012;34(1):9–16. doi: 10.1016/j.genhosppsych.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grzeskowiak LE, Gilbert AL, Morrison JL. Neonatal outcomes after late-gestation exposure to selective serotonin reuptake inhibitors. Journal of Clinical Psychopharmacology. 2012 Oct;32(5):615–21. doi: 10.1097/JCP.0b013e31826686bc. [DOI] [PubMed] [Google Scholar]
- 31.El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Archives of General Psychiatry. 2012 Jul;69(7):706–14. doi: 10.1001/archgenpsychiatry.2011.2333. [DOI] [PubMed] [Google Scholar]
- 32.Klieger-Grossmann C, Weitzner B, Panchaud A, Pistelli A, Einarson T, Koren G, et al. Pregnancy outcomes following use of escitalopram. The Journal of Clinical Pharmacology. 2012 May 1;52(5):766–70. doi: 10.1177/0091270011405524. [DOI] [PubMed] [Google Scholar]
- 33.Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. Journal of Clinical Psychopharmacology. 2012 Apr;32(2):186–94. doi: 10.1097/JCP.0b013e3182490eaf. [DOI] [PubMed] [Google Scholar]
- 34.Yonkers KA, Norwitz ER, Smith MV, Lockwood CJ, Gotman N, Luchansky E, et al. Depression and serotonin reuptake inhibitor treatment as risk factors for preterm birth. Epidemiology. 2012 Sep;23(5):677–85. doi: 10.1097/EDE.0b013e31825838e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colvin L, Slack-Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol. 2011 Mar;91(3):142–52. doi: 10.1002/bdra.20773. [DOI] [PubMed] [Google Scholar]
- 36.Latendresse G, Ruiz RJ. Maternal corticotropin-releasing hormone and the use of selective serotonin reuptake inhibitors independently predict the occurrence of preterm birth. Journal of Midwifery Womens Health. 2011;56(2):118–26. doi: 10.1111/j.1542-2011.2010.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Einarson A, Choi J, Einarson TR, Koren G. Adverse effects of antidepressant use in pregnancy: an evaluation of fetal growth and preterm birth. Depression Anxiety. 2010;27(1):35–8. doi: 10.1002/da.20598. [DOI] [PubMed] [Google Scholar]
- 38.Lewis AJ, Galbally M, Opie G, Buist A. Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Australian New Zealand Journal of Psychiatry. 2010;44(5):482–7. doi: 10.3109/00048670903559593. [DOI] [PubMed] [Google Scholar]
- 39.Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological Medicine. 2010;40(10):1723–33. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- 40.Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Archives of Pediatric Adolescent Medicine. 2009 Oct 1;163(10):949–54. doi: 10.1001/archpediatrics.2009.164. [DOI] [PubMed] [Google Scholar]
- 41.Toh S, Mitchell AA, Louik C, Werler MM, Chambers CD, Hernandez-Diaz S. Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. Journal of Clinical Psychopharmacology. 2009;29(6):555–60. doi: 10.1097/JCP.0b013e3181bf344c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maschi S, Clavenna A, Campi R, Schiavetti B, Bernat M, Bonati M. Neonatal outcome following pregnancy exposure to antidepressants: a prospective controlled cohort study. BJOG. 2008;115(2):283–9. doi: 10.1111/j.1471-0528.2007.01518.x. [DOI] [PubMed] [Google Scholar]
- 43.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiology and Drug Safety. 2007;16(10):1086–94. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 44.Lennestal R, Kallen B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. Journal of Clinical Psychopharmacology. 2007;27(6):607–13. doi: 10.1097/jcp.0b013e31815ac4d2. [DOI] [PubMed] [Google Scholar]
- 45.Pearson KH, Nonacs RM, Viguera AC, Heller VL, Petrillo LF, Brandes M, et al. Birth outcomes following prenatal exposure to antidepressants. Journal of Clinical Psychiatry. 2007;68(8):1284–9. doi: 10.4088/jcp.v68n0817. [DOI] [PubMed] [Google Scholar]
- 46.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. American Journal of Psychiatry. 2007;164(8):1206–13. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 47.Djulus J, Koren G, Einarson TR, Wilton L, Shakir S, Diav-Citrin O, et al. Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. Journal of Clinical Psychiatry. 2006;67(8):1280–4. doi: 10.4088/jcp.v67n0817. [DOI] [PubMed] [Google Scholar]
- 48.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961–6. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Sivojelezova A, Shuhaiber S, Sarkissian L, Einarson A, Koren G. Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. American Journal of Obstetrics & Gynecology. 2005 Dec;193(6):2004–9. doi: 10.1016/j.ajog.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. JAMA Pediatrics. 2004 Apr;158(4):312–6. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 51.Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. Journal of Pediatrics. 2003;142(4):402–8. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 52.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. American Journal of Psychiatry. 2002;159(12):2055–61. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 53.Ericson A, Kallen B, Wiholm B. Delivery outcome after the use of antidepressants in early pregnancy. European Journal of Clinical Pharmacology. 1999 Sep;55(7):503–8. doi: 10.1007/s002280050664. [DOI] [PubMed] [Google Scholar]
- 54.Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) JAMA. 1993;269(17):2246–8. [PubMed] [Google Scholar]