Abstract
Addiction to nicotine and ability to quit smoking are influenced by genetic factors. We used functional genomic approaches (chromatin immunoprecipitation (ChIP) and whole genome sequencing) to identify CREB targets following chronic nicotine administration and withdrawal in rodents. fWe found that chronic nicotine and withdrawal differentially modulate CREB binding to the gene for Neuregulin 3 (NRG3). Quantitative analysis of saline, nicotine, and nicotine withdrawal in two biological replicates corroborate this finding, with NRG3 increases in both mRNA and protein following withdrawal from chronic nicotine treatment. To translate these data for human relevance, single nucleotide polymorphisms (SNPs) across NRG3 were examined for association with prospective smoking cessation among smokers of European ancestry treated with transdermal nicotine in two independent cohorts. Individual SNP and haplotype analysis support association of NRG3 SNPs and smoking cessation success. NRG3 is a neural-enriched member of the EGF family, and a specific ligand for the receptor tyrosine kinase ErbB4, which is also up-regulated following nicotine treatment and withdrawal. Mice with significantly reduced levels of NRG3 or pharmacological inhibition of ErbB4 show similar reductions in anxiety following nicotine withdrawal compared to control animals, suggesting a role for NRG3 in nicotine dependence. While the function of the SNP in NRG3 in humans is not known, these data suggest that Nrg3/ErbB4 signaling may be an important factor in nicotine dependence.
Keywords: mouse, human, single nucleotide polymorphism (SNP), ChIP-Seq, neuregulin 3 (NRG3), nicotine, smoking cessation
INTRODUCTION
Nicotine is the addictive compound in tobacco, exerting its biological effects through activation of the nicotinic acetylcholine receptor (nAChR). Though the acute effects of nicotine are exclusively mediated through these nAChRs, it is now becoming clear that continued drug use induces adaptive changes in the central nervous system that lead to drug dependence1. These long-term adaptations in cellular signaling mechanisms are likely part of the maintenance of drug dependence. Specifically, the long lasting nature of these behaviors and drug responses indicate that changes in gene expression may be necessary for their development and persistence.
One well-characterized protein responsible for regulating gene expression is the transcription factor cAMP response element binding protein (CREB). CREB is required for the rewarding aspects of nicotine as measured in a place-conditioning paradigm using CREBαΔ mice2, which have constitutive deletion of CREB, as well as in animals with dominant negative knock-down of CREB selectively in the nucleus accumbens3. Correlative evidence suggests that CREB may be required for behaviors manifested during nicotine withdrawal as well. CREB protein levels, phosphorylated CREB (pCREB)4, 5 and CREB-DNA binding5 are differentially modulated throughout the brain following nicotine withdrawal.
Nicotine withdrawal is characterized by both affective and cognitive symptoms and the role of hippocampus in these responses has been well characterized. Structural and functional alterations in hippocampus have been associated with nicotine withdrawal symptoms, cue reactivity, and quitting success in human neuroimaging studies6–10. Thus, to identify relevant CREB-target genes that are altered in the hippocampus following chronic nicotine and 24h withdrawal (WD), we performed chromatin immunoprecipitation analyses coupled to high-throughput sequencing (ChIP-Seq) and target expression analysis. A neural-enriched member of the EGF family, NRG3 was found to be up-regulated following nicotine treatment and withdrawal. We used these data to inform an exploratory human genetic analysis of prospective smoking cessation. We then evaluated potential mechanisms underlying propensity to relapse using genetic and pharmacological manipulation of NRG3 and its receptor ErbB4 in mice. These studies show that interruption of NRG3 signaling during chronic treatment with nicotine reduces withdrawal-induced anxiety, suggesting that aberrant NRG3/ErbB4 signaling may be an important factor in nicotine dependence.
METHODS
Animals
Male Nrg3ska mice were purchased from Jackson Laboratories (n=48; 6 weeks of age; 20–25g) These mice arose as a spontaneous mutation which was later identified as a microsatellite repeat within intron 7 of the Nrg3 gene; strain of origin is A/J. CREBαΔ mutant mice were backcrossed to the inbred mouse strains 129SvEv and C57BL/6 for >40 generations. For these experiments, WT and CREBαΔ mutants were F1 hybrids obtained from crossing mice heterozygous for the CREB mutation from each strain (n=164; 2–3 months of age; 20–30g). All mice were group housed and maintained on a 12 h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. For the NIH paradigm, mice were housed in groups of two. All experimental testing sessions were conducted between 9:00 A.M. and 3:00 P.M., with animals randomly assigned to treatment conditions and tested in counterbalanced order.
Drugs and Treatments
Osmotic Drug Delivery Groups
Nicotine tartrate (reported as free base weight; Sigma–Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline solution and infused through subcutaneous osmotic minipumps for 14 days (Model 2002, Alzet, Palo Alto, CA, USA). Mice were anesthetized with an isoflurane/oxygen vapor mixture (1–3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the wound. The wound was closed with 7 mm stainless steel wound clips (Reflex, Cellpoint Scientific, Gaithersburg, MD, USA). Osmotic minipump treatment groups: In all experiments, animals were implanted with osmotic minipumps to deliver chronic administration of either saline or nicotine (18 mg/kg/day). Chronic treatment with 18mg/kg/day of nicotine in mice yields plasma levels of approximately 0.3 μM, (based on nicotine free base molecular weight), a concentration similar to that observed in human smokers consuming an average of 17 cigarettes a day (plasma levels between 0.06–0.31 μM)11. Following two weeks of chronic saline or nicotine administration, one-half of the animals from each group received sham surgery, where the mice were anesthetized with an isoflurane/oxygen vapor mixture (1–3%), an incision was made above the pump at shoulder level, and the incision was then closed with 7 mm stainless steel wound clips. To initiate spontaneous withdrawal from nicotine treatment, the osmotic minipumps were physically removed from the other half of the animals similar to above. In all experiments, mice were sacrificed for tissue collection at 24 hours following surgery.
Injection Drug Delivery Groups
Afatinib (BIBW-2992; reported as salt weight; LC Laboratories, Woburn, MA) is an irreversible inhibitor at ErbB4 and ErbB2/4 receptors12, 13. Afatinib was dissolved in saline containing 16% ethanol and 100mM Captisol® (Captisol, La Jolla, CA), a modified beta-cyclodextrin that has a very low side-effect profile and is currently in use in clinical studies14. Ethanol at this concentration has been shown to have no locomotor behavioral effects15. Afatinib treated mice were injected once daily for 10 days prior to behavioral testing. Animals not receiving afatinib were instead injected with vehicle (saline containing 16% ethanol and 100mM Captisol®) for 10 days prior to behavioral testing.
Novelty-induced hypophagia (NIH) test
The NIH test is a well-validated model of anxiety-like behavior in mice that is sensitive to treatment with anxiolytic drugs16–25. One week prior to training and for the duration of the experiment, mice were housed in groups of two. Training consisted of daily sessions in which mice were exposed to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) in a clear plastic dish. Plastic dividers (dividing the standard mouse cage lengthwise) were placed inside each cage to separate the mice during the training and home cage testing periods. Mice were acclimated to the barriers for 1 h before placement of food. Food was placed in the cage for 15min and latency to consume was measured. By the 12th day, a baseline latency to approach and consume the food was reached such that there was <20% variability between mice. Following training, mice underwent their respective chronic treatments and were not exposed to behavioral testing during this time. Testing in the home cage (home day) and novel environment (novel day) occurred on the last 2 days of minipump viability. On Home Day, mice were presented with food and latency to consume as well as amount consumed were recorded. In those animals receiving chronic injections, the animals were injected 1h prior to testing in either the home or novel environment. Following Home Day testing, minipumps were surgically removed for the withdrawal groups and sham surgeries were performed on the chronic nicotine group as well as saline animals. Twenty-four hours later on Novel Test Day, animals were acclimated for 1h prior to testing in the novel environment. Mice were removed from the home cage and placed in an empty standard cage with no bedding. The cage was wiped with a cleanser (Pine Sol, 1:10 dilution) to emit a novel odor and placed in a white box with bright light illumination (2150lux). Latency to consume was recorded. Number of subjects in each treatment: Wildtype F1 – saline+vehicle (n=6), nicotine+vehicle (n=6), 24hWD+vehicle (n=6), 24hWD+10mg/kg Afatinib (n=7), and 24hWD+20mg/kg Afatinib (n=6); NRG3ska - saline (n=16), nicotine (n=16), 24hWD (n=16).
Marble-burying Test
The marble burying test is an anxiety model in mice, which possesses high predictive value to detect anxiolytic drugs 26. All mice were implanted with 14d osmotic minipumps filled with nicotine (18mg/kg/day) or 0.9% saline. During this chronic nicotine treatment, the F1 animals were also injected daily for 10 days with either vehicle or afatinib. Following chronic treatment, animals were tested in the marble-burying test 24, 27. One hour prior to testing, F1 mice were injected i.p. with vehicle or drug at the doses indicated and left to acclimate to the testing room. In the case of the NRG3ska animals, no injections were given. Then the mice were placed individually in small cages (26×20×14 cm), in which twenty marbles had been equally distributed on top of mouse bedding (5-cm deep), and a wire lid was placed on top of the cage. Mice were left undisturbed for 15 min, after which time the number of buried marbles (i.e., those covered by bedding three-quarters or more) was counted by a blind observer. Number of subjects in each treatment: Wildtype F1 –saline+vehicle (n=6), nicotine+vehicle (n=5), 24hWD+vehicle (n=6), 24hWD+10mg/kg Afatinib (n=7), and 24hWD+20mg/kg Afatinib (n=6); NRG3ska - saline (n=10), nicotine (n=10), 24hWD (n=10).
Locomotor Activity
Locomotor activity in response to chronic drug treatments (minipump and i.p. injection) was analyzed in a “home cage” activity monitoring system (MedAssociates, St. Albans, VT). The home cage (28.9 cm × 17.8 cm × 12 cm) was placed in a photo-beam frame (30 cm × 24 cm × 8 cm) with sensors arranged in an 8-beam array strip. For injection studies in F1 mice, mice chronically treated with drug were injected i.p. with vehicle or drug as indicated. One hour following drug administration, the mice were individually placed in the cages. No injections were given to the NRG3ska mice. Beam break data was monitored and recorded for 60 min. Number of subjects in each treatment: Wildtype F1 –saline+vehicle (n=6), nicotine+vehicle (n=6), 24hWD+vehicle (n=6), 24hWD+10mg/kg Afatinib (n=7), and 24hWD+20mg/kg Afatinib (n=6); NRG3ska - saline (n=4), nicotine (n=6), 24hWD (n=5).
ChIP-Seq library construction, sequencing, and peak calling
CREB ChIP was performed on chromatin isolated from the hippocampus of saline (n=3), nicotine (n=2), or 24hWD (n=3) treated mice. Immunoprecipitations were performed and ChIP-Seq libraries were prepared as previously described 28, 29. Libraries for each hippocampus sample were sequenced individually on an Illumina GAIIx. Reads for each individual ChIP-Seq library were mapped to the UCSC mm8 reference genome using Illumina’s Eland pipeline. Redundant reads were discarded within each replicate. For each condition (saline, nicotine, 24hWD), non-redundant reads from all 3 biological replicates were pooled into a single read set, and peak-calling was performed with HOMER v3.0 (5% FDR)30.
ChIP-Seq Differential Binding Analysis
To determine the extent of differential CREB binding between conditions genome-wide, we first merged the saline, nicotine and 24hWD HOMER peak calls to a common set of binding regions. At each region, the peak height was computed for each condition by extending reads to 108bp fragments and computing the maximum height of the stack height profile. Peak heights as a fraction of total reads were compared using Fisher’s exact test for nicotine vs. saline, and 24hWD vs. saline. P-values were corrected for multiple testing by Benjamini-Hochberg step-up method.
Chromatin Immunoprecipitation (ChIP)
ChIP was modified from a previously reported method 31. Chromatin was prepared from fresh tissue (n=6 mice per group). The hippocampus was dissected out, chopped, and immediately crosslinked in 1.1% formaldehyde/PBS for 10 minutes with constant agitation, then quenched with glycine to a final concentration of 0.125M. Tissue was homogenized using a dounce homogenizer (Kontes, 18×150mm tube reservoir; 8×220mm pestle shaft), followed by a spin at 1000g for 5 minutes. The nuclear pellet was re-suspended in cell lysis buffer then sonicated to an optimal 200bp using a Diagenode Bioruptor for 5 cycles (5 minutes; 30 sec on, 30 sec off). Immunoprecipitation with CREB (sc-186, Santa Cruz) and control IgG (sc-2025, Santa Cruz) was performed as previously described 32. CREB enrichment was determined by PCR using SYBR green with primers (underlined in sequence below; forward 5′-cacacacacatacacccgaag-3′ and reverse 5′-gtgagtcaaactccagcaagc-3′) that flanked the 2 CRE sites (bracketed and in bold in sequence below) within the NRG3 promoter.
accctgtccgcggcgactctacacacaaacacacacacacatacacccgaagccgcctggttgagtgcaagaaaacgcttgga gcagcggtcac[gacgtc]cgggacccctgtcgctatctcccatttttgctagcccgggtcggccaggc[agcgtca]ccgga gctgtttggtatcctctgcgtgctggtcggggtgcgccccggggtaccccgctcctgagcggcggggtagcttgctggagtttgactc
Primers flanking 18s were used as a control for the PCR reaction (forward 5′-cagaatgcccttggaagaga-3′ and reverse 5′-gggaaaccagaagaccaaca-3′).
qPCR
Quantitative rtPCR was performed as previously described33 on hippocampi from saline (n=6), nicotine (n=10), or 24hWD (n=11) animals. Briefly, RNA was isolated using the RNeasy Mini kit (Qiagen). cDNA was synthesized and SYBR-green QPCR reactions were assembled using Applied Biosystems 2X SYBR-Green master mix along with 300nM primers (final concentration). The mRNA levels of target genes were normalized to the housekeeping gene, TATA binding protein (TBP). Primer sequences are available upon request.
Western Blots
Protein analysis was performed as described previously34 on hippocampi from the following groups: saline (n=4–7), nicotine (n=5–9), and 24hWD (n=10) treated F1 wildtype mice; saline (n=9), nicotine (n=9), and 24hWD (n=10) treated CREBαΔ mutant mice; saline (n=6–7), nicotine (n=8), and 24hWD (n=7) treated Nrg3ska mice. Briefly, 15μg of protein from treated tissues were resolved in 10% SDS–PAGE and transferred to nitrocellulose membranes. Membranes were incubated with LI-COR blocking buffer (LI-COR, Lincoln, Nebraska) for 1h at RT prior to reacting overnight at 4 °C with primary antibodies (pCREB (1:1000, CREB phosphorylated at Ser-133, Cell Signaling Technology, Lake Placid, NY), NRG3 (1:1000, Abgent, San Diego CA), NRG1 (1:100, sc-28916, Santa Cruz Biotechnology, Santa Cruz, CA), ErbB4 (1:100, sc-283, Santa Cruz Biotechnology, Santa Cruz, CA) and beta-tubulin (1:2000, BD biosciences, San Jose, CA)). After washing in PBS-T, the blots were incubated in fluorescent secondary antibodies (1:20,000, LI-COR, Lincoln, Nebraska) in LI-COR blocking buffer for 1h at RT. Membranes were then washed and immunolabeling detection and densitometry measurements were performed using the LI-COR Odyssey System (LI-COR, Lincoln, Nebraska). Ratios of pCREB, NRG3 (77 kD band), NRG1 (83 kD), or ErbB4 to β-tubulin densities were calculated for each sample and analyzed across conditions.
Receptor Binding
Hippocampal tissues were homogenized in 50mM Tris HCl buffer, pH 7.4 at 24°C, and centrifuged twice at 35,000xg for 15min in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing [3H]Epibatidine (EB, PerkinElmer, Boston, MA) with or without competing drugs. Incubations were performed in Tris buffer at pH 7.4 for 2h at 24°C with [3H]EB. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass-fiber filters (Brandel, Gaithersburg, MD) that were prewet with 0.5% polyethyleneimine, and the filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding.
Behavioral and Molecular Data Analysis
Mouse data was analyzed using Graphpad Prism 5.0. Significance was determined using either 1-way or 2-way ANOVA followed by Bonferroni’s Multiple Comparison Test.
Exploratory Genetic Analysis of Smoking Cessation
To evaluate the potential translational relevance of the preclinical NRG3 findings, we examined NRG3 SNPs for associations with smoking cessation in clinical samples.
Discovery Cohort
Samples from 449 treatment-seeking European ancestry smokers ages 18–65 who smoked ≥ 10 cigarettes per day were assayed. Smokers participated in a clinical trial of transdermal nicotine and were enrolled from 2004–2008 at the University of Pennsylvania. The exclusion criteria were: DSM IV Axis I psychiatric or substance abuse disorder; current use of psychotropic medications; and pregnancy or lactation. 42% of participants were female and 35% were college graduates. The mean age was 44.8 years (sd = 10.5), and mean cigarettes smoked per day was 22.11 (sd = 9.03). The mean score on the Fagerström Test for Nicotine Dependence (FTND) was 5.27 (sd = 2.17). All participants received 8 weeks of transdermal nicotine and brief behavioral cessation counseling.
Self-reported smoking was assessed using the timeline follow-back procedure35, and biochemically verified with a carbon monoxide (CO) breath sample. The primary outcome was biochemically confirmed 7-day point-prevalence abstinence at the end of 8 weeks of nicotine patch treatment. As per convention36, participants who reported smoking within 7 days prior to the assessment (n = 213), failed to provide a CO sample (n = 28), or provided a CO > 10ppm (n = 15) were considered non-abstinent. Additional details of this clinical trial have been reported previously 37.
Based on the preclinical findings, we genotyped SNPs in the gene encoding neuregulin 3 (NRG3). Ninety-three multi-marker haplotype tagging SNPs were selected from Hapmap (www.hapmap.org) (all MAFs >0.2) across the 1.1 cM gene (Supplementary Table 1). Using Sequenom design tools, 4 panels from the 93 SNPs were assembled for multiplexed genotyping assays. Briefly, 10ng of genomic DNA was used for a multiplexed PCR to amplify up to 40 short genomic regions containing the targeted SNPs. Sequenom genotyping was conducted in 384-well format with 10% replicates to check assay concordance. The data were assembled using the Sequenom software package to track positive and negative controls, calculate quality control parameters such as Hardy-Weinberg equilibrium, and export genotype reports formatted for analysis. (See Supplementary Figure 1 for the linkage disequilibrium plot.)
The power analysis was conducted using PASS (Power and Sample Size, NCSS Software, Kaysville, UT). Discovery cohort analyses were powered to detect an odds ratio of 1.84 or greater for allelic frequencies of 0.2 or greater with 80% power and type I error rate (a) of 5%. Individual SNP associations with cessation were evaluated using logistic regression as a screening tool, with chi-squared tests based on likelihood ratio. Models were adjusted for age, sex, and nicotine dependence score. Odds ratios present the increase in risk per copy of the minor allele (coded 0, 1, 2). P-values in this initial cohort were not corrected for multiple testing because the analysis was conducted primarily for discovery purposes.
Pair-wise LD between all SNP markers in NRG3 was computed using Haploview38, with haplotype blocks defined by default criteria recommended by Gabriel et al.39 (Supplementary Figure 2). The single-SNP analysis informed which haplotype blocks would be examined following single SNP analysis. Ultimately, we included significant SNPs found at the 3′end of NRG3 (i.e., haplotype blocks 18, 19 & 20), as there was low LD between the significant SNPs located at the 5′ end of the gene. We used the EM algorithm40 to estimate haplotype frequencies, and haplotype-specific associations with cessation were tested using generalized linear models (GLM)41. This approach allowed us to assess the global significance between all haplotypes and outcome, and allowed us to include observations with some missing genotype information (failed read at one or more SNPs). Analysis relied on the haplo.stat program (haplo.glm, haplo.score, haplo.em; R version 2.7.2, http://www.R-project.org), with some specific contrasts made using the wald.test from the AOD package. Haplotypes were treated as categorical predictors, and had to exceed a 5% threshold; those under 5% were combined. For ease of interpretation, we also conducted haplotype-specific tests and estimated haplotype-specific odds ratios and confidence intervals using the common haplotype as the reference haplotype.
Replication Cohort
To confirm associations of SNPs identified as significant in the discovery cohort, we utilized an independent sample of participants in an open-label trial of nicotine patch versus nicotine nasal spray42. Criteria and clinical trial procedures were identical to those described above. Of the 334 participants in the nicotine patch condition (those in the spray condition were excluded from analysis), 215 were of European ancestry; of these, 174 were genotyped. Three participants had call rates of .70 or below on the SNPs examined in the discovery cohort and were excluded from analyses. As described in the Results below, to select SNPs from the discovery cohort findings to test in the replication cohort, we used a strict Bonferroni correction (p<0.0042); 6 subjects in the replication cohort had no result for the single SNP meeting this criterion in the discovery cohort (rs1896506), leaving a final sample of 165. 46 percent of participants were female and 50% were college graduates. The mean age was 46.8 years (sd = 11.9), and mean cigarettes smoked per day was 23.25 (sd = 9.12). The mean score on the Fagerström Test for Nicotine Dependence (FTND) was 5.50 (sd = 2.15).
RESULTS
Chronic Nicotine and 24h Withdrawal Increases pCREB in the Hippocampus
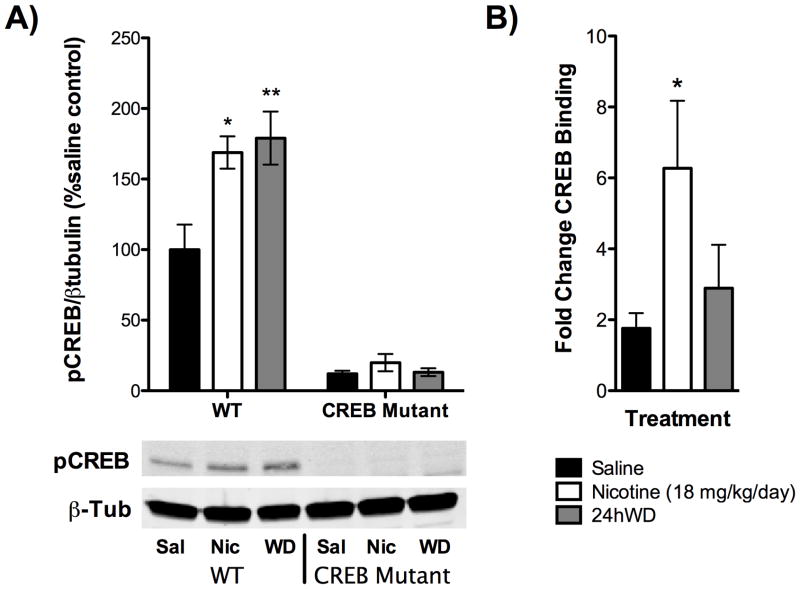
CREB is a highly conserved transcription factor that may require activation through phosphorylation following chronic nicotine and withdrawal4. Therefore, to determine if changes in CREB-phosphorylation are present in the hippocampus following chronic treatment with nicotine and/or 24hWD, we measured levels of phosphorylated-CREB in the hippocampus and observed that nicotine and 24hWD increase the amount of phosphorylated CREB (Fig 1A).
Figure 1. Chronic treatment with nicotine and/or 24hWD significantly increases phosphorylation of CREB in the hippocampus and increases CREB binding to the promoter for Nrg3.
Animals were treated in vivo with either saline, nicotine (18 mg/kg/day), or undergoing 24h withdrawal (WD) from nicotine. Hippocampal tissues from treated animals were used in A) Western blot analysis to evaluate phosphorylation levels of CREB as well as in B) ChIP analysis to evaluate binding to the promoter for Nrg3. A) Both chronic nicotine as well as 24h WD from chronic nicotine elicited significant increases in pCREB relative to saline in wildtype (WT) animals. No change was observed in CREB mutant mice, which exhibit a 90% reduction in total CREB levels (N=4–5). Representative blots are shown below panel A. B) CREB binding to Nrg3 was significantly increased following chronic treatment with nicotine (18 mg/kg/day) (N=6–7). *, p<0.05; **, p<0.01.
Chronic Nicotine and 24h Withdrawal Increases CREB Binding to DNA
CREB and its genomic targets represent an important pathway in the hippocampus that facilitates long-term plasticity43. The dynamic changes in CREB phosphorylation in the hippocampus following chronic nicotine and 24hWD led us to investigate potential CREB targets. To this end, we performed chromatin immunoprecipitation coupled to high-throughput sequencing (ChIP-Seq) and gene expression analysis on mouse hippocampus in saline, nicotine, and 24WD. Our results demonstrate that CREB-DNA binding was enriched in the nicotine and 24WD treatment groups, particularly in pathways implicated in cell-to-cell signaling and development (Ingenuity Pathway Analysis) (Table 1). For a complete list of CREB targets see Supplementary Tables 2, 3, and 4. We evaluated CREB binding in a biological replicate to one of the most highly enriched genes from our ChIP-Seq experiment, neuregulin 3 (NRG3). As shown in Fig 1B, CREB binding was significantly enriched at the NRG3 Cre site following chronic treatment with nicotine.
Table 1.
Top Networks Altered Following Chronic Nicotine and 24h Withdrawal
| Rank | Associated Network Functions | Score |
|---|---|---|
| 1 | Gene Expression, Cell-to-Cell Signaling and Interaction, Cellular Development | 25 |
| 2 | Cancer, Cell Cycle, Cell Death | 2 |
| 3 | Cell Death, Cell-to-Cell Signaling and Interaction, Cellular Development | 2 |
| 4 | Post-Tranlational Modification, Cellular Growth and Proliferation | 2 |
| 5 | Cell-to-Cell Signaling and Interaction, Carbohydrate Metabolism, Lipid Metabolism | 2 |
Chronic Nicotine and 24h Withdrawal Increases Neuregulin 3 in the Hippocampus
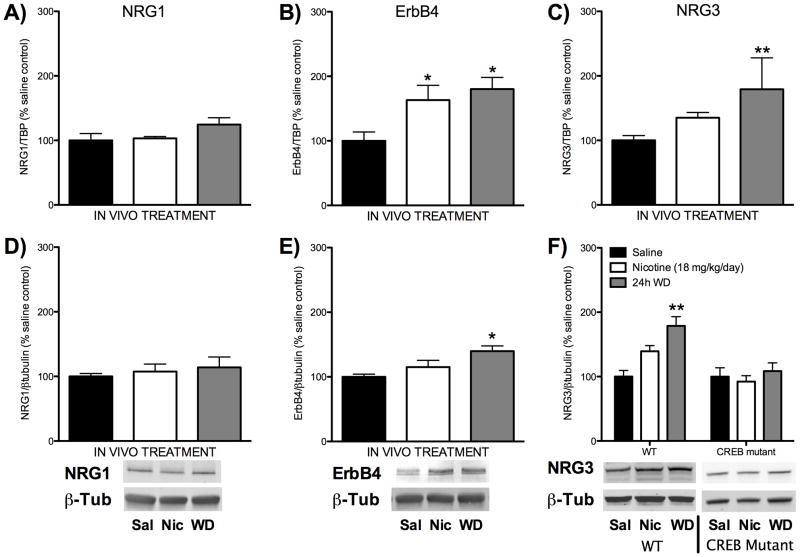
To determine if enrichment of CREB at the Nrg3 promoter had an impact on gene transcription, we evaluated mRNA levels of members of the Neuregulin-ErbB4 pathway following chronic nicotine and 24hWD. We found a significant increase in mRNA levels for NRG3, but not NRG1, following 24h WD from chronic nicotine (Fig 2A,C). Furthermore, we found a significant increase in mRNA levels for NRG3’s cognate receptor, ErbB4, following both chronic nicotine and 24hWD (Fig 2B). In addition, protein analysis showed significant increases in ErbB4 protein levels following chronic nicotine and 24hWD (Fig 2E). Furthermore, we observed increased protein levels of the CREB target gene, NRG3, following 24h WD from chronic nicotine, which was CREB dependent as shown by an absence of induction in CREBαΔ mutant mice (Fig 2F). Protein levels for NRG1 were not altered in any of the treatment groups (Fig 2D).
Figure 2. Chronic treatment with nicotine and/or 24hWD significantly increases neuregulin 3, but not neuregulin 1, in the hippocampus.
Animals were treated in vivo with either saline, nicotine (18 mg/kg/day), or undergoing 24h withdrawal (WD) from nicotine. Hippocampal tissues from treated animals were used in qPCR or Western blot experiments to evaluate mRNA or protein levels. As shown, chronic treatment with nicotine or 24hWD does not alter neuregulin 1 (NRG1) (A) mRNA or (D) protein levels in the hippocampus. In contrast, mRNA (B) and protein (E) levels of ErbB4 are increased following chronic treatment with nicotine and/or during 24hWD. Additionally, mRNA levels of the CREB target neuregulin 3 are increased following chronic nicotine treatment and 24hWD (C). Furthermore, protein levels of NRG3 (F) are increased following 24hWD in wildtype (WT), but not CREB mutant, mice. Representative blots are shown below each panel. *, p<0.05; **, p<0.01. A–C: N=6–11; D–F: 6–10.
Neuregulin 3 SNP Analysis During Smoking Abstinence
The preclinical findings were translated to a human genetic exploratory analysis of NRG3. Of the 449 participants genotyped in the discovery cohort, 114 (25.3%) were verified quitters and 335 had relapsed. Twelve SNPs in NRG3 exhibited nominal associations (p < 0.05) with smoking cessation (uncorrected for multiple testing) (Table 2; Supplementary Figure 3). These include one SNP (rs10883934) located before haplotype block 2; four SNPs (rs1896506, rs1649947, rs765833, and rs1649967) located between haplotype blocks 2 and 3; one in haplotype block 4 (rs1336287); two in haplotype block 18 (rs495978, rs540697); one in haplotype block 19 (rs19236560), and one between haplotype blocks 19 and 20 (rs2026495); and two in haplotype block 20 (rs4362091 and rs10787519). However, corrected for multiple testing using a stringent Bonferroni correction for all the SNPs tested, only the association between rs1896506 and 8-week smoking cessation would remain statistically significant (p < 0.004).
Table 2.
Allele Frequencies for NRG3 SNPs association with Smoking Cessation (p < 0.05) (not controlling for any covariates)
| SNP (allele) | Chromosome Location | Haplotype Block | MAF | Minor Allele | MAF (Relapse) | MAF (Quit) | OR (95% CI) | N | p-value |
|---|---|---|---|---|---|---|---|---|---|
| rs10883934 (A/C) | 83679476 | Before block 1 | 0.26 | C | 0.23 | 0.32 | 1.48 (1.08, 2.04) | 421 | 0.014 |
| rs1896506 (G/A) | 83864363 | b/w blocks 2–3 | 0.24 | A | 0.27 | 0.16 | 0.51 (0.35, 0.75) | 423 | 0.0004 |
| rs1649947 (G/T) | 83864945 | b/w blocks 2–3 | 0.22 | T | 0.25 | 0.16 | 0.57 (0.39, 0.83) | 425 | 0.002 |
| rs765833 (C/G) | 83870738 | b/w blocks 2–3 | 0.3 | G | 0.27 | 0.37 | 1.59 (1.16, 2.19) | 425 | 0.003 |
| rs1649967 (C/T) | 83897704 | b/w blocks 2–3 | 0.37 | T | 0.35 | 0.42 | 1.38 (1.02, 1.87) | 425 | 0.04 |
| rs1336287 (C/A) | 83943696 | 4 | 0.45 | A | 0.43 | 0.51 | 1.38 (1.02, 1.89) | 426 | 0.03 |
| rs495978 (G/A) | 84616939 | 18 | 0.34 | A | 0.31 | 0.4 | 1.46 (1.08, 1.96) | 435 | 0.01 |
| rs540697 (A/G) | 84622849 | 18 | 0.34 | G | 0.31 | 0.41 | 1.51 (1.12, 2.03) | 433 | 0.007 |
| rs1923560 (C/A) | 84675570 | 19 | 0.32 | A | 0.35 | 0.28 | 0.7 (0.51, 0.96) | 436 | 0.03 |
| rs2026495 (T/G) | 84678732 | b/w 19–20 | 0.39 | G | 0.42 | 0.34 | 0.71 (0.52, 0.95) | 436 | 0.02 |
| rs4362091 (G/A) | 84713964 | 20 | 0.3 | A | 0.32 | 0.24 | 0.67 (0.49, 0.93) | 437 | 0.02 |
| rs10787519 (T/C) | 84714762 | 20 | 0.31 | C | 0.34 | 0.26 | 0.67 (0.48, 0.92) | 438 | 0.01 |
In the haplotype analysis we focused on the significant SNPs located in haplotype blocks 18, 19, and 20 (rs495978, rs540697, rs2026495, rs1923560, rs4362091, and rs10787519). The number of participants with complete genotype data for all these SNPs was 399. This subsample did not differ significantly from the entire sample of 449 on any of the demographics or quit outcome parameters. Common haplotypes accounted for 92% of all haplotypes, with the most common one being A-G-T-C-G-T with a frequency of 30%. The overall regression analysis based on categorical haplotypes was significant (p = 0.0009). We tested the haplotype regression against a model containing only the controlling variables; the Wald chi-square test was also significant (p = 0.03). When each haplotype was compared to the most common haplotype (A-G-T-C-G-T), haplotype G-AG-A-A-C was found to have a significantly reduced probability of cessation at the end of treatment (OR = 0.56, 95% CI = (0.38, 0.82), p = 0.003). None of the other haplotypes were associated with quitting compared to the reference haplotype.
Of the 165 participants included in the replication cohort, 59 (35.8%) were verified abstinent at end of treatment and 106 were classed as non-abstinent. A logistic regression of verified 7-day point prevalence abstinence at end of treatment, treating rs1896506 genotype as dichotomous (0 = G/G, 1 = */A) and adjusting for age, sex, and nicotine dependence score, was performed. The effect of rs1896506 was significant (OR = 0.44, 95% C.I. = (0.22, 0.87); p = .019); no other predictors contributed significantly. When treated as categorical in the logistic model, the effect of rs1896506 genotype remained significant (p = .046). Eight-week quit rates by rs1896506 in the discovery and replication cohort are shown in Figure 3.
Figure 3. Smoking cessation with transdermal nicotine, by study and NRG3 rs1896506 genotype. A).
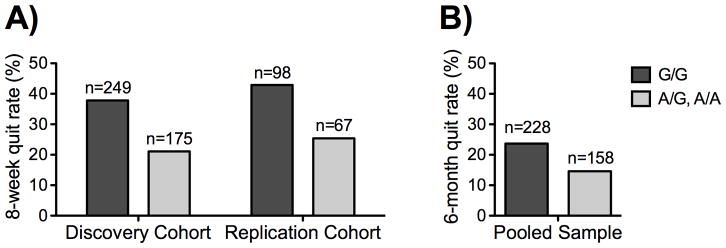
Eight-week quit rate was significantly lower in A/* carriers of the rs1896506 genotype in both the discovery cohort and the replication cohort. B) Six-month quit rate in the pooled sample of participants from both cohorts who received standard 8-week duration therapy was significantly lower in A/* carriers of the rs1896506 genotype. The number of subjects in each group is indicated above its respective bar.
Lastly, data from the two cohorts were pooled and logistic regressions of verified 7-day point prevalence abstinence at 8 weeks were carried out. The following predictors were included: rs1896506 genotype, age, sex, nicotine dependence score, and study. rs1896506 was a significant predictor, when treated as dichotomous (OR = 0.45, 95% C.I. = (0.31, 0.65), p < .001) or categorical (p < .001). Additionally, when the logistic regression was repeated in the pooled sample of participants from both cohorts who received standard 8-week duration therapy (n = 386) using 6-month abstinence as the outcome, there was a significant association of rs1896506 (dichotomous) with abstinence (OR = 0.54, 95% C.I. = (0.31, 0.94), p = .028).
The association of rs1896506 was not significant for pre-treatment nicotine dependence score or smoking rate (p values >.20); however, in treatment-seeking smokers, the variability in nicotine dependence is limited.
Evaluation of NRG3ska Mice: NRG3 Protein Levels and Regulation of Neuronal nAChRs by Chronic Nicotine and 24h Withdrawal
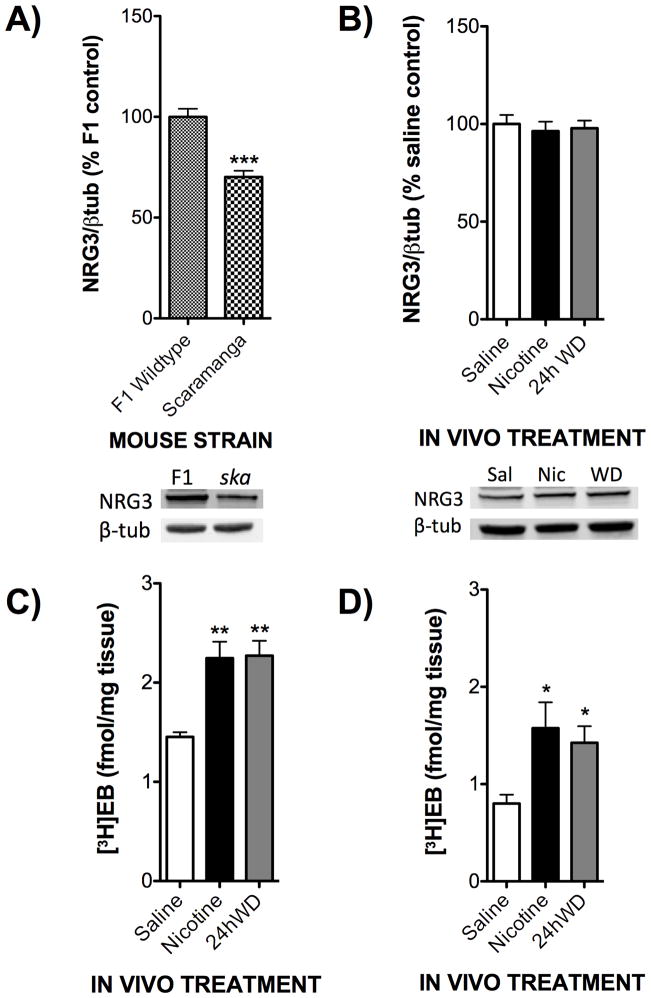
While the alterations in NRG3 following chronic nicotine and withdrawal in conjunction with the human relapse data suggest a role for this novel molecule in affecting smoking cessation, these data are correlational. Therefore, in order to evaluate more directly the contribution of NRG3 to nicotine withdrawal phenotypes that may influence smoking cessation in humans, we utilized the scaramanga (NRG3ska) mouse. This mouse line arose as a spontaneous mutation, which was later identified as corresponding to a microsatellite repeat in exon 7 of the Nrg3 gene, resulting in reduced expression of NRG344. Indeed, we confirmed this reduction of NRG3 protein level in the hippocampus of NRG3ska mice compared to the F1 wildtype mice utilized in these experiments (Fig 4A). However, following chronic treatment with nicotine or 24h WD, NRG3 protein is not upregulated in the NRG3ska mice compared to saline levels (Fig 4B). In order to evaluate whether this lack of effect by nicotine was due to a general lack of response to chronic nicotine administration in NRG3ska mice, we measured nicotinic receptor (nAChR) upregulation, a hallmark of chronic nicotine treatment45, 46. nAChRs were measured with radioactively labeled epibatidine, a nAChR ligand that binds with high affinity to heteromeric nAChRs, which are the specific type of nAChRs altered following chronic administration of nicotine47–49. As shown in Figure 4C, wildtype F1 mice display significantly greater [3H]epibatidine binding following chronic treatment with nicotine and 24hWD compared with their saline treated controls. Similarly, the NRG3ska mice also display this nAChR upregulation following chronic treatment with nicotine and 24hWD (Fig 4D), suggesting that the lack of NRG3 mRNA and protein induction by nicotine is not attributable to a general lack of response to chronic nicotine administration.
Figure 4. Chronic treatment with nicotine and withdrawal results in differential biochemical responses in the F1 wildtype and NRG3ska mice.
F1 hybrid and NRG3ska mice were treated in vivo with either saline, nicotine (18 mg/kg/day), or undergoing 24h withdrawal (WD) from nicotine. A) Hippocampal tissues from saline treated animals were used in Western blot analysis to evaluate basal levels of NRG3 in the two strains of mice. NRG3ska mice demonstrated a signficant reduction in NRG3 levels compared to F1 hybrids (N=8). Representative blots are shown below. B) Hippocampal tissues from NRG3ska mice treated with saline, nicotine, or 24h WD were used in Western blot analysis to evaluate treatment changes in NRG3 levels. NRG3ska mice did not show an induction of NRG3 following chronic nicotine or 24h WD (N=8). Representative blots are shown below. C) Hippocampal tissues from F1 hybrid mice treated with saline, nicotine, or 24h WD were used for homogenate-binding experiments with a saturating concentration of [3H]epibatidine ([3H]EB, 1.5 nM). F1 hybrid mice chronically treated with either nicotine or 24h WD displayed an increased density of nAChRs compared to saline controls (N=4). D) Hippocampal tissues from NRG3ska mice treated with saline, nicotine, or 24h WD were used for homogenate-binding experiments with a saturating concentration of [3H]epibatidine ([3H]EB, 1.5 nM). NRG3ska mice chronically treated with either nicotine or 24h WD displayed an increased density of nAChRs compared to saline controls (N=4). *, p<0.05; **, p<0.01; ***, p<0.005.
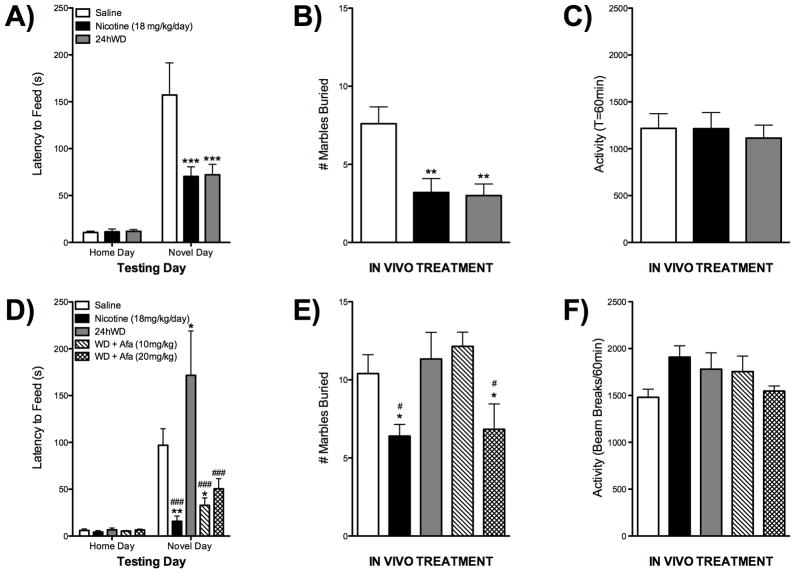
Neuregulin 3-ErbB4 Signaling is Required for Anxiogenic Responses to Nicotine Withdrawal in the Novelty-Induced Hypophagia Test and the Marble-Burying Test
Nicotine withdrawal in smokers is often characterized by an increase in anxiety50. To model this preclinically in the NRG3ska mice, we utilized the NIH test, which is a well-validated model for assessing anxiety in mice16–24. Of particular interest to the present study, the NIH test is unique in that both the anxiolytic effects of chronic nicotine as well as the anxiogenic effects of 24h withdrawal from chronic nicotine can be observed in the same test51. In this paradigm, F1 wildtype mice demonstrate an anxiolytic effect following chronic nicotine treatment compared to F1 control mice (Fig 5D). However, mice undergoing 24hWD exhibit an anxiogenic effect as shown by an increased latency to feed in a novel environment (Fig 5D). NRG3ska mice treated with chronic nicotine show similar reductions in anxiety following chronic nicotine compared to F1 wildtype mice, however they do not demonstrate any increases in anxiety during nicotine withdrawal (Fig 5A). Furthermore, afatinib, an irreversible inhibitor at ErbB4 and ErbB2/4 receptors12, 13, blocks the increased anxiety associated with nicotine withdrawal (Fig 5D). We then evaluated these treatments in a second measure of anxiety, the marble-burying test26, which is sensitive to nicotinic drugs24, 27, 52. As was observed in the NIH test, nicotine causes an anxiolytic effect in the marble-burying test in NRG3ska mice (Fig 5B) as well as in F1 hybrids (Fig 5E). Additionally, the increased anxiety seen following 24hWD is absent in mice with reduced levels of NRG3 (Fig 5B) as well as when the NRG3 receptor, ErbB4, is inhibited with chronic afatinib treatment (Fig 5E). None of these effects are attributable to alterations in locomotor activity, as there were no significant treatment effects in home cage locomotor activity measurements (Fig 5C,F).
Figure 5. Chronic treatment with nicotine and withdrawal results in differential behavioral responses in the F1 wildtype and NRG3ska mice.
NRG3ska mice were treated in vivo with either saline, nicotine (18 mg/kg/day), or undergoing 24h withdrawal (WD) from nicotine. F1 hybrid mice were treated in vivo with either saline, nicotine (18 mg/kg/day), or undergoing 24h withdrawal (WD) from nicotine; additionally, these mice also received daily injections of either vehicle or afatinib at the concentrations indicated for 10 days. A) NRG3ska mice treated with saline, nicotine, or 24h WD were tested in the NIH test. Mice chronically treated with either nicotine or 24h WD displayed a reduced latency to feed on novel test day compared to saline controls (N=16). B) NRG3ska mice treated with saline, nicotine, or 24h WD were tested in the marble-burying test. Mice chronically treated with either nicotine or 24h WD buried fewer marbles compared to saline controls (N=10). C) Locomotor activity was tested in NRG3ska mice treated with saline, nicotine, or 24h WD. No treatment effects were observed (N=4–6). D) F1 hybrid mice treated with saline, nicotine, 24h WD, or 24h WD + Afatinib were tested in the NIH test. Mice chronically treated with either nicotine or 24h WD + Afatinib displayed a reduced latency to feed on novel test day compared to the saline group or the 24hWD group (N=6–7). E) F1 hybrid mice treated with saline, nicotine, 24h WD, or 24h WD + Afatinib were tested in the marble-burying test. Mice chronically treated with either nicotine or 24h WD + 20mg/kg Afatinib buried fewer marbles compared to saline controls (N=5–7). F) Locomotor activity was tested in treated F1 Hybrid mice. No treatment effects were observed (N=4–6). Significant compared to vehicle control - **, p<0.01; ***, p<0.005. Significant compared to 24hWD - #, p<0.05; ###, p<0.005.
DISCUSSION
The translational utility of cross-species models is exemplified in the present study, which identifies a role for the NRG3/ErbB4 pathway in nicotine dependence. Our findings in the mouse demonstrate that a significant induction of NRG3 occurs during early withdrawal that is correlated with an increase in anxiety as measured in two different behavioral models. In mice with a spontaneous mutation in NRG3 (NRG3ska), levels of NRG3 are reduced, but nicotine or 24WD does not induce any upregulation of NRG3 as is seen in F1 WT mice. Of interest, the anxiety phenotype observed during 24WD is also absent in NRG3ska mice, suggesting a causal relationship between the protein changes and the behavior. Furthermore, when the NRG3 signaling pathway is perturbed by afatinib, an irreversible inhibitor at ErbB4 and ErbB2/4 receptors as well as EGFRs12, 13, the anxiety-like withdrawal phenotypes are abolished. These preclinical data support a causative role of NRG3-ErbB4 signaling in the anxiety effects of nicotine withdrawal.
While the mouse studies suggest that NRG3 alterations in smokers may impact relapse rates by modulating anxiety withdrawal symptoms, the specific impact of this pathway in humans and how NRG3 contributes to the increased smoking relapse rates among carriers of the NRG3 SNP is not known. Regulation of NRG3 in the hippocampus may have a significant effect in mediating synaptic plasticity in this brain region, which has been associated with affective and cognitive symptoms prominent during withdrawal6–8, 51. Identification of this candidate gene in the mouse nominated it for analysis of single nucleotide polymorphisms (SNPs) for association with prospective smoking cessation in two independent cohorts of smokers treated with transdermal nicotine. Some SNPs previously associated with cognition or psychosis were not included in the panel53–57 and it is possible that other unexamined SNPs may impact NRG3 expression and be more relevant to nicotine dependence. However, of the SNPs selected for this investigation, individual SNP and haplotype analysis suggest an association of SNPs and smoking cessation success. These findings, in conjunction with Loukola et al. who identify associations of ErbB4, the receptor for NRG3, in a different cohort of smokers, strongly implicate this pathway in the molecular mechanisms underlying nicotine dependence and smoking cessation.
Neuregulins are presynaptic epidermal growth factor (EGF)-like molecules, which are released via presynaptic cleavage. The neuregulin extracellular domain then signals postsynaptically through ErbB receptors, which are receptor tyrosine kinases. Activation of the ErbB receptor by any of the neuregulin family results in activation of downstream intracellular signaling pathways, including the AKT and JAK/STAT cascades58–60. While we observed no mRNA changes in NRG1, we found a significant increase in NRG3 mRNA and protein following 24hWD from chronic nicotine. NRG3 expression is found in the cerebellar nuclei, vestibular nuclei, olfactory nucleus, medial habenula, and hypothalamus, and expression is especially high in the hippocampus, cortex, and thalamus61, 62. Unlike many other neuregulins, NRG3 binds quite specifically to the ErbB4 receptor subtype61, which has been shown to have a critical role in activity-dependent excitatory synapse formation63.
Most studies have described the function of NRG3 in mammary development44; however, very little is known about the specific function of NRG3 in the brain. A limited number of gene association studies have suggested a role for NRG3 in schizophrenia53–56 and ADHD57. Its involvement in these disorders is particularly interesting in light of smoking rates among these co-morbid populations. For example, nicotine dependence is highly co-morbid in schizophrenia, where ~80% of the population smoke64, as well as in ADHD, where ~45% of the population smoke65.
The functional significance of the SNP in NRG3 replicated in the two cohorts is not known nor are the effects of NRG3-ErbB4 stimulation in an individual experiencing nicotine withdrawal. However, SNPs in the closely related gene, NRG1, predict α7 nAChR expression66, which has been suggested to potentially underlie some of the neurological deficits apparent in schizophrenia67. While the precise mechanism by which these NRG1 SNPs influence nAChRs is unknown, application of NRG1 in slice and cell culture preparations alters both nAChR expression as well as function68–70. Furthermore, behavioral studies in NRG1 transgenic mice that have reduced expression of NRG1 demonstrate a differential behavioral effect of nicotine compared to wildtypes71.
The present studies show that chronic treatment with nicotine elicits CREB-dependent changes in NRG3 mRNA and protein levels. Whereas other CREB targets may drive other aspects of nicotine withdrawal, we describe the mechanism by which nicotine induces CREB binding and activation of the NRG3 gene, resulting in its increased expression and subsequent activation of the ErbB4 receptor, which then drives nicotine withdrawal-induced anxiety. Our investigation into this pathway presents a novel target for therapeutic development of smoking cessation aids designed specifically to address nicotine withdrawal-induced anxiety, which has been shown to directly influence smoking relapse rates72–76.
In order to model withdrawal-associated anxiety in our evaluation of NRG3/ErbB4 signaling during 24hWD, we utilized two complementary models of anxiety in mice, the NIH test and the marble-burying test. The marble-burying test measures the ability of an aversive environment to induce an anxiety-like state, which results in an active response (burying). This is in contrast to the NIH test, where an aversive environment elicits a passive behavioral response (not approaching and consuming the food), which is indicative of heightened anxiety in the animal. Both ourselves24, 27, 51 and others52, 77–79 have utilized these behavioral paradigms to investigate the anxiety-related effects of nicotinic drugs. It is important to note that there may be some developmental effects associated with the polymorphism present in the NRG3ska mice; however, it is unlikely that these effects account for their anxiolytic phenotype following 24hWD from nicotine. Previous studies conducting extensive behavioral phenotyping of these mice demonstrated that while they exhibit reduced sociability, there are no significant effects on aggression, nest building, motor coordination, or learning and memory80, 81. Furthermore, these mice present with an anxiety-like and antidepressant-resistant phenotype in multiple behavioral measures80, 82, 83. This is supported by our data showing an increased baseline latency to approach the food on Novel Test Day in the saline treated NRG3ska mice compared with the saline treated F1 WT mice (Fig 5A,D). While chronic nicotine treatment results in anxiolytic responses in the NIH in both the F1 and NRG3ska, they exhibit differential responses to 24hWD. Though it is possible the blunted 24hWD phenotype in the NRG3ska mice may be due to developmental compensation, the more likely phenotype would have been an exacerbated anxiogenic phenotype during 24hWD. Furthermore, the 24hWD behavior in the NIH test is ablated in F1 WT mice chronically treated with an ErbB4 inhibitor (afatinib, Fig 5D), suggesting that modulation of this pathway in particular impacts nicotine withdrawal behaviors. This is further supported by the human data, where individuals possessing a SNP in the NRG3 gene have significantly higher relapse rates. It is important to replicate the human genetic association findings in independent samples, with larger populations and longer follow-up. In addition, the function of this SNP (and other SNPs in NRG3) is unknown, and in the case of a lack of functionality, this SNP may be in linkage disequilibrium with other functional variants that have gone undetected to date. However, despite these limitations, these translational findings in conjunction with those highlighted in Loukola et al, provide support for the NRG3-ErbB4 pathway in nicotine dependence.
Cigarette smoking constitutes a major health burden in the US with smoking accounting for 1 in 5 deaths each year in the U.S.84 Even with available FDA-approved treatments, most smokers fail, underscoring the need to develop novel medications72. Therefore, it is crucial to discover the cellular, molecular, neural, and behavioral basis of early nicotine abstinence effects that contribute to relapse. Methods used to identify these mechanisms can vary. Our approach was to focus on the targets of a transcription factor, CREB, which mediates long-term plasticity43 and has been shown to be required for nicotine conditioned place preference2, 3. Furthermore, studies examining CREB signaling following nicotine administration and/or withdrawal show induction of CREB phosphorylation in the reward pathway2,4, and our current findings demonstrate a significant effect of chronic nicotine and withdrawal on CREB phosphorylation in the hippocampus. Analysis of differentially induced CREB ChIP-Seq targets identified a number of genes associated with gene expression, cell-to-cell signaling, and cellular development. More specifically, these data highlighted enrichments in the neuregulin3/ErbB4 signaling pathway, which lead to identification of a modest signal for association of SNPs in NRG3 with prospective smoking cessation. Thus, our combination of functional and molecular analysis following chronic nicotine and withdrawal to inform candidate gene analyses in smokers may help identify novel molecular targets for development of new smoking cessation therapies. Furthermore, these types of cross-species analyses have the potential to accelerate target identification for smoking cessation therapies.
Supplementary Material
Acknowledgments
This research was funded by grants from the National Cancer Institute and National Institute on Drug Abuse, P50-CA143187, 1-F32-DA026236, K01-DA031747, 1-K99-DA032681, and U01-DA020830. We thank the Next Generation Sequencing Core at the University of Pennsylvania for sequencing support and technical assistance.
Footnotes
Conflict of Interest: The authors have no conflict of interest in relation to the work described.
References
- 1.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46(6):933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34(8):1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. Journal of neurochemistry. 2003;84(6):1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 5.Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. Journal of neurochemistry. 2001;77(3):943–952. doi: 10.1046/j.1471-4159.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 6.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 7.Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 8.Janes AC, Pizzagalli DA, Richardt S, de Frederick BB, Holmes AJ, Sousa J, et al. Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology. 2010;35(12):2339–2345. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology (Berl) 2010;210(4):577–583. doi: 10.1007/s00213-010-1862-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35(4):499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. The Journal of pharmacology and experimental therapeutics. 2012;343(2):342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 14.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36(1):30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 15.Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacology, biochemistry, and behavior. 1995;50(4):521–526. doi: 10.1016/0091-3057(94)00331-9. [DOI] [PubMed] [Google Scholar]
- 16.Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biological psychiatry. 2003;54(5):552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- 17.Bechtholt AJ, Hill TE, Lucki I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl) 2007;190(4):531–540. doi: 10.1007/s00213-006-0615-9. [DOI] [PubMed] [Google Scholar]
- 18.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neuroscience and biobehavioral reviews. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and Distinct Brain Regions Associated with the Anxiolytic Effects of Chlordiazepoxide and Chronic Fluoxetine. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- 20.Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, et al. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27(29):7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balu DT, Turner JR, Brookshire BR, Hill-Smith TE, Blendy JA, Lucki I. Brain monoamines and antidepressant-like responses in MRL/MpJ versus C57BL/6J mice. Neuropharmacology. 2013;67:503–510. doi: 10.1016/j.neuropharm.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11(5):529–538. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goeldner C, Spooren W, Wichmann J, Prinssen EP. Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64–6198 in rodent models of conflict anxiety and despair. Psychopharmacology (Berl) 2012;222(2):203–214. doi: 10.1007/s00213-012-2636-x. [DOI] [PubMed] [Google Scholar]
- 24.Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-a have differential effects on affective behavior. The Journal of pharmacology and experimental therapeutics. 2010;334(2):665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble-George JC, Conger JR, Hartley ND, Gupta P, Sumislawski JJ, Patel S. Dissociable effects of CB1 receptor blockade on anxiety-like and consummatory behaviors in the novelty-induced hypophagia test in mice. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolas L, Kolb Y, Prinssen E. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. European journal of pharmacology. 2006;547(1–3):106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13(1):41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuteja G, White P, Schug J, Kaestner KH. Extracting transcription factor targets from ChIP-Seq data. Nucleic Acids Res. 2009;37(17):e113. doi: 10.1093/nar/gkp536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Lay J, Tuteja G, White P, Dhir R, Ahima R, Kaestner KH. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 2009;10(1):55–62. doi: 10.1016/j.cmet.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2005;2(2):141–148. doi: 10.1016/j.cmet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JR, Larris B, Le PP, Peiris TH, Arsenlis A, Schug J, et al. Orthogonal analysis of C/EBPbeta targets in vivo during liver proliferation. Proc Natl Acad Sci U S A. 2004;101(35):12986–12991. doi: 10.1073/pnas.0402875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 2008;201(1):15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97(4):482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown R, Burgess E, Sales S, Whiteley J, Evans D, Miller I. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12 (2):101–112. [Google Scholar]
- 36.SRNT. Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 37.Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science (New York, NY. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 40.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995;12(5):921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- 41.Schaid DJ. Relative efficiency of ambiguous vs.directly measured haplotype frequencies. Genet Epidemiol. 2002;23(4):426–443. doi: 10.1002/gepi.10184. [DOI] [PubMed] [Google Scholar]
- 42.Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Intern Med. 2004;140(6):426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- 43.Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends in neurosciences. 2010;33(5):230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005;19(17):2078–2090. doi: 10.1101/gad.338505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. The Journal of pharmacology and experimental therapeutics. 1983;226(3):817–825. [PubMed] [Google Scholar]
- 46.Schwartz R, Kellar K. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science (New York, NY. 1983;220(4593):214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- 47.Houghtling RA, Davila-Garcia MI, Kellar KJ. Characterization of (+/−)(−)[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Molecular pharmacology. 1995;48(2):280–287. [PubMed] [Google Scholar]
- 48.Avalos M, Parker MJ, Maddox FN, Carroll FI, Luetje CW. Effects of pyridine ring substitutions on affinity, efficacy, and subtype selectivity of neuronal nicotinic receptor agonist epibatidine. The Journal of pharmacology and experimental therapeutics. 2002;302(3):1246–1252. doi: 10.1124/jpet.102.035899. [DOI] [PubMed] [Google Scholar]
- 49.Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. The Journal of pharmacology and experimental therapeutics. 2004;310(1):98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- 50.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 51.Turner JR, Wilkinson D, Poole RL, Gould TJ, Carlson GC, Blendy JA. Divergent Functional Effects of Sazetidine-A and Varenicline During Nicotine Withdrawal. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson SM, Brunzell DH. Low dose nicotine and antagonism of beta2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS One. 2012;7(11):e48665. doi: 10.1371/journal.pone.0048665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang YC, Chen JY, Chen ML, Chen CH, Lai IC, Chen TT, et al. Neuregulin 3 genetic variations and susceptibility to schizophrenia in a Chinese population. Biological psychiatry. 2008;64(12):1093–1096. doi: 10.1016/j.biopsych.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Chen PL, Avramopoulos D, Lasseter VK, McGrath JA, Fallin MD, Liang KY, et al. Fine mapping on chromosome 10q22-q23 implicates Neuregulin 3 in schizophrenia. Am J Hum Genet. 2009;84(1):21–34. doi: 10.1016/j.ajhg.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, et al. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci U S A. 2010;107(35):15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morar B, Dragovic M, Waters FA, Chandler D, Kalaydjieva L, Jablensky A. Neuregulin 3 (NRG3) as a susceptibility gene in a schizophrenia subtype with florid delusions and relatively spared cognition. Mol Psychiatry. 2011;16(8):860–866. doi: 10.1038/mp.2010.70. [DOI] [PubMed] [Google Scholar]
- 57.Sonuga-Barke EJ, Lasky-Su J, Neale BM, Oades R, Chen W, Franke B, et al. Does parental expressed emotion moderate genetic effects in ADHD? An exploration using a genome wide association scan. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1359–1368. doi: 10.1002/ajmg.b.30860. [DOI] [PubMed] [Google Scholar]
- 58.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, et al. Identification of heregulin, a specific activator of p185erbB2. Science (New York, NY. 1992;256(5060):1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 59.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69(3):559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 60.Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, et al. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, et al. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci U S A. 1997;94(18):9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. The Journal of comparative neurology. 2004;472(2):156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- 63.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54(4):583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prochaska JJ, Hall SM, Bero LA. Tobacco use among individuals with schizophrenia: what role has the tobacco industry played? Schizophr Bull. 2008;34 (3):555–567. doi: 10.1093/schbul/sbm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7(3):373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 66.Mathew SV, Law AJ, Lipska BK, Davila-Garcia MI, Zamora ED, Mitkus SN, et al. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16(23):2921–2932. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- 67.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21(15):5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang Q, Fischbach GD. An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J Neurosci. 2006;26(44):11295–11303. doi: 10.1523/JNEUROSCI.1794-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2008;28(37):9111–9116. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28(27):6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6(9):746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- 73.Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of Varenicline and Bupropion Sustained-Release Use Plus Intensive Smoking Cessation Counseling on Prolonged Abstinence From Smoking and on Depression, Negative Affect, and Other Symptoms of Nicotine Withdrawal. JAMA Psychiatry. 2013:1–12. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. J Stud Alcohol Drugs. 2013;74 (3):469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly MM, Grant C, Cooper S, Cooney JL. Anxiety and smoking cessation outcomes in alcohol-dependent smokers. Nicotine Tob Res. 2013;15(2):364–375. doi: 10.1093/ntr/nts132. [DOI] [PubMed] [Google Scholar]
- 76.Nakajima M, al’Absi M. Predictors of risk for smoking relapse in men and women: a prospective examination. Psychol Addict Behav. 2012;26(3):633–637. doi: 10.1037/a0027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alkam T, Kim HC, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, et al. Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav Brain Res. 2013;239:80–89. doi: 10.1016/j.bbr.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 78.Mannucci C, Navarra M, Calzavara E, Caputi AP, Calapai G. Serotonin involvement in Rhodiola rosea attenuation of nicotine withdrawal signs in rats. Phytomedicine. 2012;19(12):1117–1124. doi: 10.1016/j.phymed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Roni MA, Rahman S. Neuronal nicotinic receptor antagonist reduces anxiety-like behavior in mice. Neuroscience letters. 2011;504(3):237–241. doi: 10.1016/j.neulet.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 80.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dow HC, Kreibich AS, Kaercher KA, Sankoorikal GM, Pauley ED, Lohoff FW, et al. Genetic dissection of intermale aggressive behavior in BALB/cJ and A/J mice. Genes Brain Behav. 2011;10(1):57–68. doi: 10.1111/j.1601-183X.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology. 2006;31(11):2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- 83.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183(2):257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 84.CDC. Cigarette smoking among adults--United States, 2006. Morbid Mortal Wkly Rep. 2007;56(44):1157–1161. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.