Abstract
Rationale
Abuse of synthetic cathinones, popularized as “bath salts,” has increased dramatically in the United States since their debut in 2010. Preclinical behavioral studies may clarify determinants of the abuse-related effects produced by these compounds.
Objectives
This study examined behavioral effects of (±)-methcathinone, (±)-3,4-methylenedioxypyrovalerone (MDPV), (±)-3,4-methylenedioxymethcathinone (methylone) and (±)-4-methylmethcathinone (mephedrone) in rats using intracranial self-stimulation (ICSS).
Methods
Male Sprague-Dawley rats (n=18) with electrodes targeting the medial forebrain bundle responded for multiple frequencies of brain stimulation and were tested in two phases. First, dose-effect curves for methcathinone (0.1–1.0 mg/kg), MDPV (0.32–3.2 mg/kg), methylone (1.0–10 mg/kg) and mephedrone (1.0–10 mg/kg) were determined. Second, time courses were determined for effects produced by the highest dose of each compound.
Results
Methcathinone produced dose- and time-dependent facilitation of ICSS. MDPV, methylone and mephedrone produced dose- and time-dependent increases in low rates of ICSS maintained by low brain stimulation frequencies, but also produced abuse-limiting depression of high ICSS rates maintained by high brain stimulation frequencies. Efficacies to facilitate ICSS were methcathinone ≥ MDPV ≥ methylone > mephedrone. Methcathinone was the most potent compound, and MDPV was the longest acting compound.
Conclusions
All compounds facilitated ICSS at some doses and pretreatment times, which is consistent with abuse liability for each of these compounds. However, efficacies of compounds to facilitate ICSS varied, with methcathinone displaying the highest efficacy and mephedrone the lowest efficacy to facilitate ICSS.
Keywords: methcathinone, methylenedioxypyrovalerone, methylone, mephedrone, intracranial self-stimulation, rat, drug abuse
INTRODUCTION
Cathinone and methcathinone are the β-ketone analogs of amphetamine and methamphetamine, respectively. Like their amphetamine analogs, cathinone and methcathinone function as monoamine releasers that selectively promote release of dopamine (DA) and norepinephrine (NE) over serotonin (5-HT) (Kalix and Glennon 1986; Wagner et al. 1982; Nielsen and Schechter 1985; Cozzi et al. 1999, 2013). Cathinone and methcathinone produce amphetamine-like stimulant effects (Glennon et al. 1986, 1987; Dal Cason et al. 1997) and are classified as Schedule I drugs by the Drug Enforcement Agency (DEA). Methylenedioxypyrovalerone (MDPV), methylenedioxymethcathinone (methylone) and 4-methylmethcathinone (mephedrone) are synthetic cathinone analogs that have recently emerged as designer drugs of abuse in Europe and the United States (Vardakou et al. 2011; Spiller et al. 2011, McElrath and O’Neill 2011) and have been marketed under deceptively benign names, including the term “bath salts.” Methylone and mephedrone display lower selectivity to release DA/NE versus 5-HT than methcathinone, and MDPV differs from methcathinone by functioning as a monoamine reuptake inhibitor rather than as a monoamine releaser (Cozzi et al. 1999; Baumann et al. 2012a, 2012b; Cameron et al. 2013a, 2013b). These three synthetic cathinone analogs were emergency classified as Schedule I drugs by the DEA in October 2011 due to a surge in popularity that was perceived as an imminent threat to public safety. In 2012, Schedule I classification of MDPV and mephedrone was made permanent by the Food and Drug Administration Safety and Innovation Act, and emergency scheduling of methylone was extended. These scheduling decisions have been founded not only on emerging clinical and law enforcement experiences with these drugs, but also on a growing body of preclinical data that addresses their abuse liability.
Intracranial self-stimulation (ICSS) is one family of experimental procedures that has been used to assess abuse liability of stimulants and other drugs (Kornetsky and Esposito 1979; Wise 1996; Bauer 2013). In ICSS, subjects are trained to lever press for pulses of brain stimulation delivered via microelectrodes implanted in brain regions like the medial forebrain bundle, and different frequencies or intensities of brain stimulation maintain different rates of lever-pressing. Many drugs of abuse increase (or “facilitate”) low rates of ICSS maintained by low frequencies or intensities of brain stimulation and, as a result, ICSS facilitation is often interpreted as an abuse-related drug effect. ICSS shows substantial congruence with other preclinical measures of abuse liability, such as drug self-administration and conditioned place preference (CPP) (Vlachou and Markou 2011) and has therefore gained increasing support as a tool for preclinical abuse liability assessment. An additional benefit of ICSS is its utility for discriminating both abuse-related and abuse-limiting drug effects in a single procedure. Specifically, monoamine releasers and reuptake inhibitors can simultaneously produce DA-mediated, abuse-related effects (facilitation of low ICSS rates) and 5-HT-mediated, abuse-limiting effects (depression of high rates of ICSS). For example, DA-selective monoamine releasers like amphetamine and methamphetamine produce exclusive facilitation of ICSS across a broad range of doses (Esposito et al. 1980; Bauer et al. 2013). Conversely, the 5-HT-selective releaser fenfluramine, which has little abuse liability, produces exclusive depression of ICSS (Olds and Yuwiler 1992; Bauer et al. 2013). Lastly, mixed-action DA/5-HT releasers like MDMA can produce simultaneous facilitation of low ICSS rates and depression of high ICSS rates (Bauer et al. 2013). These studies lend support to the proposition that ICSS is capable of discerning abuse-related and abuse-limiting drug effects, and that a drug’s selectivity to promote DA versus 5-HT release correlates with its profile of abuse-related facilitation and/or abuse-limiting depression of ICSS.
The goal of the present study was to compare the potency and time course of ICSS effects produced by methcathinone and the three recently scheduled "bath salts" cathinone analogs: MDPV, methylone and mephedrone. Recent studies have reported facilitation of ICSS by MDPV in rats (Watterson et al. 2012) and mephedrone in mice (Robinson et al. 2012); however, effects of methcathinone and methylone on ICSS have not yet been described, nor have ICSS effects of these structurally, pharmacologically and epidemiologically-related drugs been directly compared. Based on the in vitro selectivity of these compounds to promote release or block reuptake of DA versus 5-HT, we predicted that methcathinone and MDPV would display the greatest efficacy to produce abuse-related facilitation of ICSS, whereas methylone and mephedrone would produce mixed effects that would include both DA-mediated facilitation of low ICSS rates and 5-HT-mediated depression of higher ICSS rates.
MATERIALS AND METHODS
Subjects
Eighteen adult male Sprague-Dawley rats (Harlan, Frederick, MD, USA) weighing 314–387 g at the time of surgery were individually housed and maintained on a 12 h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with the National Institutes of Health guidelines for the care and use of animal subjects in research (National Academy of Sciences, 2011) and adhered to guidelines of the Committee for Research (National Research Council, 2003). All animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Intracranial self-stimulation (ICSS) Procedure
Surgery
Rats were anesthetized with isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) until unresponsive to toe-pinch prior to implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode of each bipolar electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, whereas the anode was 0.125 mm in diameter and uninsulated. The cathode was stereotaxically implanted into the left medial forebrain bundle (MFB) at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to midsagittal suture, 8.8 mm ventral to skull). Three screws were placed in the skull, and the anode was wrapped around one screw to serve as the ground. The skull screws and electrode were secured to the skull with dental acrylic. Ketoprofen (5mg/kg) was used for post-operative analgesia immediately and 24 h after surgery. Animals were allowed to recover for at least 7 days prior to commencing ICSS training.
Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic and metal test chambers (29.2×30.5×24.1 cm) equipped with a response lever (4.5 cm wide, 2.0 cm deep, 3 cm off the floor), three stimulation lights (red, yellow, and green, positioned 7.6 cm directly above the response lever), a 2 W house light and an ICSS stimulator (Med Associates, St. Albans, VT, USA). Electrodes were connected to the stimulator via a swivel commutator (Model SL2C, Plastics One, Roanoke, VA, USA). The stimulator was controlled by Med-PC IV computer software that also controlled programming parameters and data collection (Med Associates).
Training
Following initial shaping of lever press responding, rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using a behavioral procedure identical to that previously described (Bauer et al. 2013). During behavioral sessions, each lever press resulted in the delivery of a 0.5 s train of square wave cathodal pulses (0.1 ms pulse duration) and illumination of the stimulus lights over the lever. Stimulation intensity and frequency were set at 150 µA and 126 Hz, respectively, during initial 60 min training sessions. Stimulation intensity was then individually adjusted for each rat to the lowest value that sustained ICSS rates > 30 stimulations/min. This intensity (130–240 µA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of three sequential 10 min components. During each component, a descending series of 10 frequencies (158 to 56 Hz in 0.05 log increments) was presented, with each frequency available for a 1 min trial. Each frequency trial consisted of a 10 s time-out, during which five non-contingent “priming” stimulations were delivered at the frequency of stimulation that would be available during that trial, followed by a 50 s “response” period, during which responding produced electrical stimulation under a FR 1 schedule as described above. Training continued until rats reliably responded for only the first three to six frequency trials of each component over a period of at least three consecutive training days.
Testing
Studies with the racemates of methcathinone (0.1–1.0 mg/kg), MDPV (0.1–3.2 mg/kg), methylone (0.32–10 mg/kg) and mephedrone (1.0–10 mg/kg) were conducted in two phases. In the first phase, a 1.0 to 1.5 log unit range of doses was tested for each drug with the goal of testing a dose range from an ineffective dose to a high dose that maximally facilitated ICSS. Dose ranges were based on extant literature (Aarde et al. 2013;Baumann et al. 2012a; Cozzi et al. 2013; Hadlock et al. 2011; Shortall et al. 2012; Watterson et al. 2012) and our own empirical results. For these dose-effect studies, test sessions consisted of three sequential “baseline” components followed by a 30 min time-out period and then by three sequential “test” components. A single dose of test drug was administered intraperitoneally (i.p.) at the beginning of the time-out period. In the second phase, a time course was determined for effects produced by the highest dose of each compound. Time course test sessions consisted of three consecutive baseline components followed by immediate drug injection, and then by pairs of consecutive test components beginning after 10, 30, 100 and 300 min. In the case of MDPV, which had a longer duration of action, an additional pair of test components was initiated 24 h after drug injection. Test sessions were completed on Tuesdays and Fridays, and three-component training sessions were conducted on all other weekdays. The order of testing with vehicle and drug doses was varied across subjects using a Latin-square design. Methcathinone, MDPV and mephedrone were tested in separate groups of six rats each, and methylone was tested in five rats from the group that initially received methcathinone (the sixth rat lost its headcap and could no longer be tested). Methylone testing began one week after completion of methcathinone testing to minimize potential for carryover effects, and a vehicle test session was conducted in this interval to confirm stable responding.
Data Analysis
The primary dependent variable was reinforcement rate in stimulations per minute during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (%MCR), with MCR defined as the mean of the maximal rates observed during the second and third baseline components of any given session in any given rat. Thus, %MCR values for each trial were calculated as (reinforcement rate during a frequency trial)/(MCR)×100. For each test session, data from the second and third baseline components were averaged to yield a baseline frequency-rate curve, and data from test components were averaged to generate test frequency-rate curves. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analyses, results were compared by repeated measures two way ANOVA with ICSS frequency as one factor and either dose or time as the second factor. A significant ANOVA was followed by the Holm-Sidak post hoc test and the criterion for significance was set at p < 0.05.
Two other analytic strategies were also used to provide summary measures for stratification of drug efficacies to facilitate ICSS (Altarifi and Negus 2011; Bauer et al. 2013; Bauer et al. in press). The first approach calculated the total number of stimulations delivered per component across all 10 frequency-trials. Test data were normalized to individual baseline data using the equation % baseline total stimulations per component = (mean total stimulations per test component)/(mean total stimulations per baseline component) × 100. Data were then averaged across rats in each experimental condition. The second approach employed rate-dependency analysis to provide a measure of the degree of facilitation of low ICSS rates maintained by low brain stimulation frequencies. Specifically, baseline and test frequency-rate curves were used to generate rate-dependency plots where the x-axis was log baseline rate and the y-axis was log [(test rate/baseline rate)×100]. Each rate-dependency plot consisted of 10 points for baseline and test rates maintained by each brain stimulation frequency. These plots were then subjected to linear regression analysis to determine two parameters: (1) the slope (expressed as -slope such that increasingly steep slopes were increasingly positive numbers), and (2) Y-intercept (expressed as the intercept at x=1, where the baseline rate equaled 10% MCR and log baseline rate=1). Summary measures across conditions were considered to be significantly different if 95% confidence limits did not overlap between drugs. Note that ICSS thresholds were not used to compare drug effects for reasons discussed previously (Bauer et al. 2013).
Drugs
(±)-Methcathinone HCl and (±)-3,4-methylenedioxymethcathinone HCl (methylone) were prepared as previously reported (Glennon et al. 1987; Dal Cason et al. 1997). (±)-3,4-Methylenedioxypyrovalerone HCl (MDPV) and (±)-4-methylmethcathinone HCl (mephedrone) were available from a recent investigation (Cameron et al. 2013a). Compounds were prepared in sterile saline and delivered i.p.
RESULTS
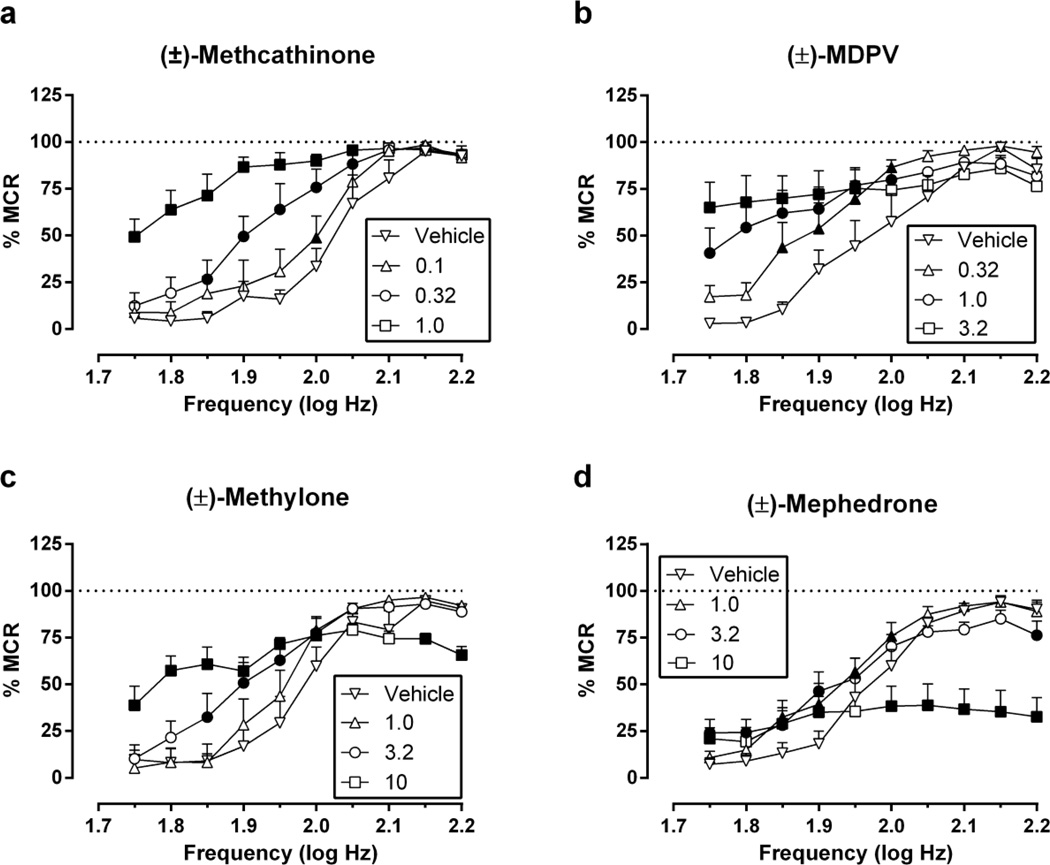
Electrical brain stimulation maintained a frequency-dependent increase in ICSS rates under baseline conditions (e.g. “vehicle” data in Figure 1). Across the 18 rats used in these studies, the average ± SEM baseline MCR was 64.4 ± 1.98 stimulations per trial, and the mean ± SEM number of total baseline stimulations was 330 ± 20.7 stimulations per component. Figure 1 shows dose-effect data for methcathinone (0.1–1.0 mg/kg), MDPV (0.32–3.2 mg/kg), methylone (1.0–10 mg/kg) and mephedrone (1.0–10 mg/kg). Two-way ANOVA indicated significant main effects of frequency and dose and significant frequency × dose interactions for all drugs, and interaction effects are reported below for each drug. Methcathinone [F(27,135)=6.43, p<0.0001] and MDPV [F(27,135)=5.11, p<0.0001] produced dose-dependent facilitation of low ICSS rates maintained by low brain stimulation frequencies with no evidence at these doses and pretreatment times of depression of high ICSS rates maintained by high brain stimulation frequencies. Methylone [F(36,144)=7.94, p<0.0001] also produced a dose-dependent facilitation of low ICSS rates maintained by low stimulation frequencies; however, the highest dose of 10 mg/kg methylone also significantly decreased high ICSS rates. Lastly, mephedrone [F(27,135)=13.9, p<0.0001] produced weak facilitation of low ICSS rates while dose-dependently depressing high ICSS rates maintained by high brain stimulation frequencies. Methcathinone was the most potent compound to alter ICSS (significant effects at doses ≥0.1 mg/kg), followed by MDPV (≥0.32 mg/kg), and methylone and mephedrone(≥1.0 mg/kg). Lower doses of MDPV (0.1 mg/kg) and methylone (0.32 mg/kg) were also tested but had no effect (data not shown).
Fig. 1.
Effects of (±)-methcathinone, (±)-MDPV, (±)-methylone and (±)-mephedrone on full ICSS frequency-rate curves. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: percent maximum control reinforcement rate (%MCR). Drug doses are indicated in legends in units of mg/kg. Filled points represent frequencies at which reinforcement rates were statistically different from vehicle rates as determined by two-way ANOVA followed by Holm-Sidak post hoc test, p<0.05. All data show mean ± SEM for five rats (methylone) or six rats (all other drugs).
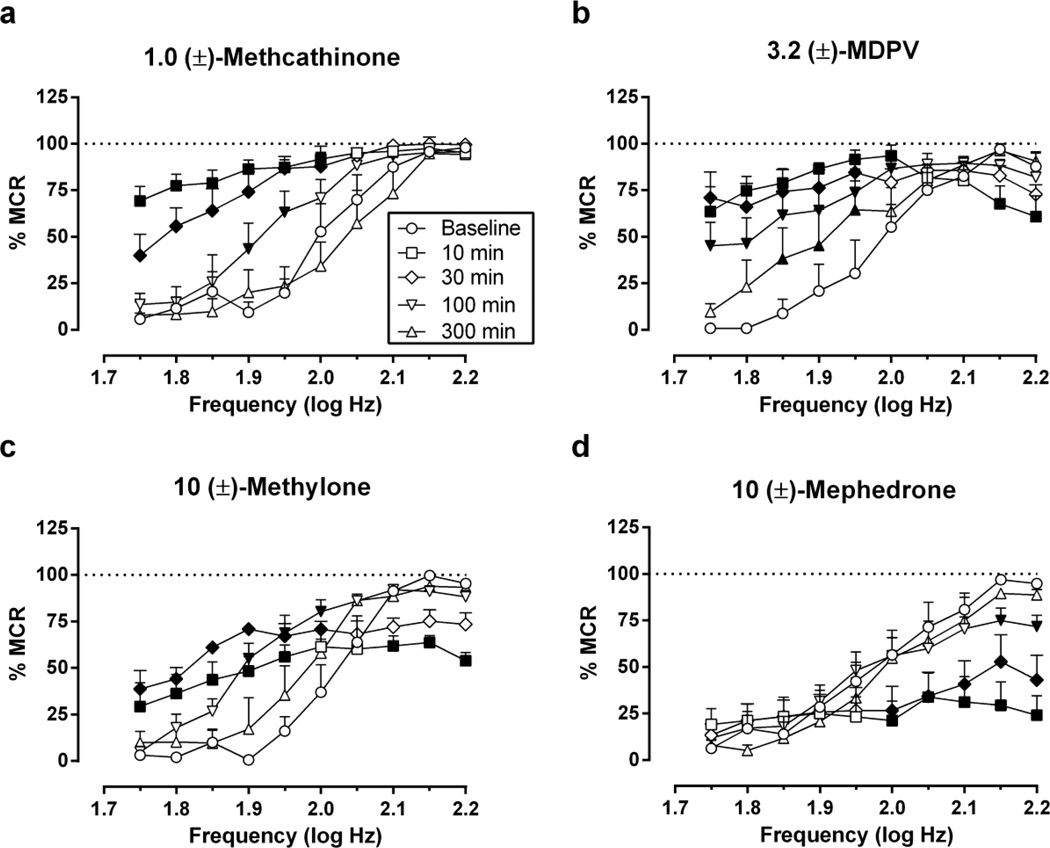
Figure 2 shows the time course of effects produced by the highest dose of each compound. Two-way ANOVA indicated significant main effects of frequency and time and significant frequency × time interactions for all drugs, and interaction effects are reported below for each drug. Methcathinone (1.0 mg/kg; [F(36,180)=5.23, p<0.001]) produced maximal facilitation of ICSS at the earliest time point (10 min), and significant ICSS facilitation was no longer apparent after 300 min. MDPV (3.2 mg/kg; [F(45,225)=5.52, p<0.0001]) produced maximal facilitation of ICSS maintained by low stimulation frequencies after 10 min, but it also depressed ICSS at the two highest stimulation frequencies at this same early time point. At later times, MDPV produced only ICSS facilitation, and this facilitation was still significant after 300 min (1.85–1.95 log Hz) and 24 hr (1.85 log Hz; data not shown). Methylone (10 mg/kg; [F(36,144)=8.93, p<0.0001]) yielded a mixed profile of effects with both rate-increasing and maximal rate-decreasing effects at 10 min. Rate-decreasing effects were no longer significant after 30 min, but significant rate-increasing effects persisted at 30 and 100 min. Mephedrone (10 mg/kg; [F(36,180)=6.26, p<0.0001]) produced only rate-decreasing effects in the time course study. ICSS depression peaked at 10 min and was no longer significant after 300 min.
Fig. 2.
Time courses of (±)-methcathinone, (±)-MDPV, (±)-methylone and (±)-mephedrone effects on full ICSS frequency-rate curves. Drug doses are expressed in mg/kg and are indicated in front of the drug name in the title of each panel. Other details as in Figure 1.
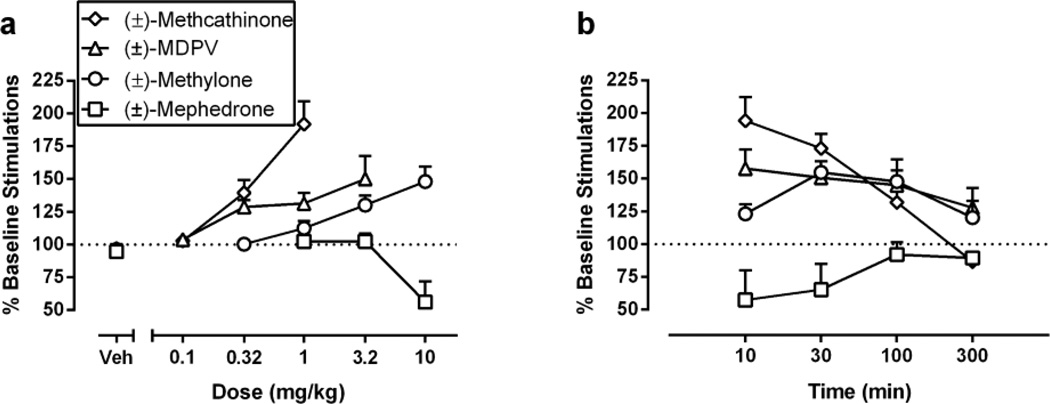
Figure 3 shows dose-dependence and time course of each compound expressed as total number of stimulations per component, a summary measure that integrates both rate-increasing and rate-decreasing drug effects on ICSS. All drugs produced dose-dependent changes in this metric (Figure 3A), and Table 1 compares peak effects of each drug. By this metric, the rank order of efficacy was methcathinone ≥ MDPV ≥ methylone ≥ mephedrone. All drugs had a rapid onset, and effects at 10 min provided further evidence for efficacy differences between methcathinone, MDPV and methylone. Durations of action were MDPV > methylone > methcathinone = mephedrone.
Fig. 3.
Summary of effects of (±)-methcathinone, (±)-MDPV, (±)-methylone and (±)-mephedrone on ICSS expressed as percent pre-drug baseline number of stimulations delivered across all frequencies of brain stimulation. Left panel (a) compares potencies and efficacies of drugs. Abscissa: drug dose in mg/kg. Ordinate: percent pre-drug baseline number of ICSS reinforcers. Right panel (b) compares time course profiles of drugs. Abscissa: pretreatment time in min. Ordinate: percent pre-drug baseline number of ICSS reinforcers.
Table 1.
Efficacy to facilitate ICSS as indicated by maximal drug effects on (a) % Baseline Number of Stimulations per Component, and (b) Y-intercept of rate-dependency plots. The dose producing the maximum effect on each measure is also indicated. Doses are expressed in mg/kg, and data for % Baseline Stimulations and Y-Intercepts are given as mean (95% CL). Values were considered to be significantly different if 95% confidence limits did not overlap.
| Drug | Dose | Maximum % Baseline Stimulations |
Dose | Maximum Y- Intercept |
|---|---|---|---|---|
| Methcathinone | 1.0 | 192 (147–237) | 1.0 | 0.86 (0.80–0.92) |
| MDPV | 3.2 | 150 (105–196) | 3.2 | 0.84 (0.82–0.86) |
| Methylone | 10 | 148 (117–180) | 10 | 0.78 (0.72–0.84) |
| Mephedrone | 3.2 | 102* (87–118) | 10 | 0.36* (0.30–0.43) |
Significantly different from methcathinone as indicated by non-overlapping 95% confidence limits.
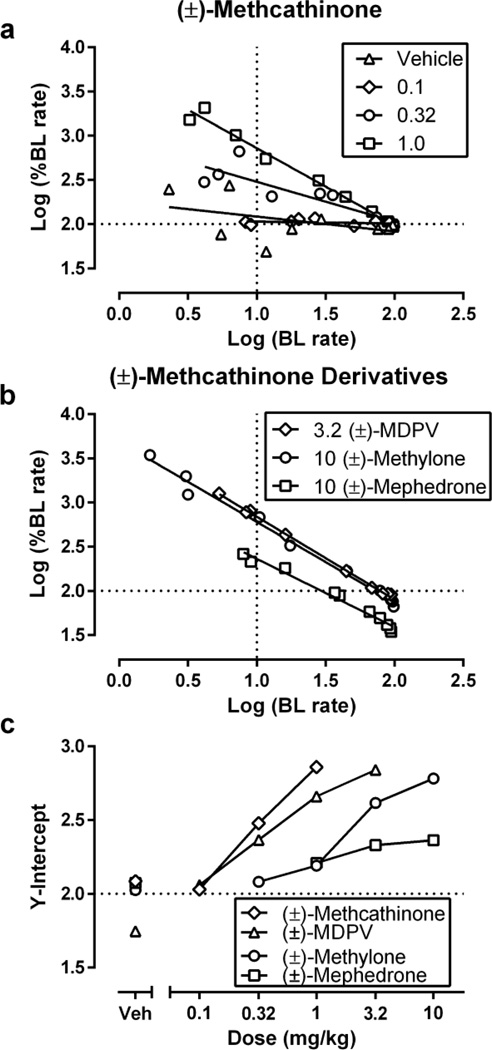
Figure 4 shows results of rate-dependency analysis to assess efficacy of ICSS facilitation. Figure 4A shows rate-dependency plots for each dose of methcathinone. Figure 4B shows ratedependency plots for doses of other drugs that produced peak Y-intercepts. Figure 4C shows dose-dependent effects of each drug on Y-intercept, and Table 1 compares peak increases in Y-intercept produced by each drug. The rank order of efficacy was methcathinone ≥ MDPV ≥ methylone > mephedrone.
Fig. 4.
Rate-dependency analysis of methcathinone and its derivatives effects on ICSS. (a, b) Abscissae: log baseline ICSS rate. Ordinates: log percent baseline ICSS rate. Horizontal line at Y=2.0 indicates no change from baseline rates. Vertical line at X=1.0 indicates the position of the Y-intercept values used in Panel c. (c) Abscissa: drug dose in mg/kg. Ordinate: Y-intercept from linear regression analysis of rate-dependency plots. All points show mean data for 5–6 rats.
DISCUSSION
This study compared effects produced by methcathinone and three recently scheduled "bath salts" cathinone analogs, MDPV, methylone and mephedrone, on ICSS in rats. There were three main findings. First, all four analogs facilitated ICSS at some dose. To the extent that facilitation of ICSS is suggestive of a drug’s abuse potential, these findings are consistent with abuse liability for all four compounds. Second, the compounds differed in their relative efficacy to facilitate ICSS, with a rank order of methcathinone ≥ MDPV ≥ methylone > mephedrone based on the maximal Y-intercept in rate-dependency analysis. Third, the compounds differed in their time courses. All compounds displayed a rapid onset of action, but the methylenedioxy compounds MDPV and methylone had longer durations of action.
Methcathinone: the β-ketone analog of methamphetamine
This is the first study to report methcathinone’s effects on ICSS, and its potency, efficacy and time course to facilitate ICSS directly parallel effects of its amphetamine counterpart, methamphetamine (Bauer et al. 2013). Previous studies have demonstrated that methcathinone functions as a monoamine releaser with approximately 200-fold selectivity for promoting in vitro release of DA versus 5-HT (Cozzi et al. 1999, 2013). Accordingly, the present data are consistent with previous evidence to suggest that the maximal degree of ICSS facilitation produced by monoamine releasers correlates with pharmacological selectivity to release DA versus 5-HT (Bauer et al. 2013). Additionally, the ICSS effects of methcathinone from the present study agree with other measures of psychostimulant effects and abuse liability. For example, methcathinone substituted for amphetamine in rats trained to discriminate amphetamine from saline (Glennon et al. 1986), and reciprocally, rats trained to discriminate methcathinone exhibited stimulus generalization to amphetamine, methamphetamine and cocaine (Young and Glennon 1998). Methcathinone also produced a dose-related increase in spontaneous locomotor activity in rats (Glennon et al. 1986) and maintained dose-dependent self-administration in baboons with rates comparable to those maintained by cocaine (Kaminski and Griffiths 1994). Taken together, results from the present study support previous data in suggesting that methcathinone is a DA-selective psychostimulant with significant potential for abuse, consistent with its Schedule I classification by the DEA.
Methylone and mephedrone: mixed-action monoamine releasers
In contrast to methcathinone, methylone and mephedrone function as monoamine releasers with little selectivity between their in vitro potencies to release DA and 5-HT (Cozzi et al. 1999; Cozzi et al. 2013; Baumann et al. 2012b; Rosenauer et al. 2013). We showed previously that selectivity of monoamine releasers to promote DA versus 5-HT release correlated with efficacy to facilitate ICSS (Bauer et al. 2013; Bauer et al. in press), and in agreement with this relationship, both methylone and mephedrone produced mixed effects that included abuse-related facilitation of low ICSS rates and abuse-limiting depression of high ICSS rates. This is the first study to evaluate methylone’s effects on ICSS; however, methylone is the β-ketone analog of MDMA, and methylone’s effects in the present study were similar to effects reported previously in this assay for MDMA (Bauer et al. 2013). The present results with mephedrone in rats agree with a previous study that reported facilitation of low ICSS rates and depression of high ICSS rates by mephedrone in mice (Robinson et al. 2012). Results of the present study also complement previous data on stimulant effects and abuse liability of methylone and mephedrone. For example, both drugs produce significant ambulatory hyperactivity in rodents (López-Arnau et al. 2012; Marusich et al. 2012; Shortall et al. 2012), and mephedrone has been shown to support intravenous self-administration in rats (Hadlock et al. 2011; Aarde et al. 2013; Motbey et al. 2013).
The present results add to this literature by suggesting that mephedrone has relatively low efficacy to facilitate ICSS. To the degree that methylone and mephedrone display similar in vitro selectivities to release DA versus 5-HT, the greater ICSS depressant effects of mephedrone suggest that factors other than 5-HT release may also contribute to abuse-limiting ICSS depressant effects. As one possibility, methylone and mephedrone also block DA and 5-HT reuptake, and one previous study suggests that mephedrone has lower selectivity than methylone to block reuptake of DA versus 5-HT (Rosenauer et al. 2013). A second possibility is that mephedrone may act directly on 5-HT receptors (López-Arnau et al. 2012; Simmler et al. 2013). Regardless of mechanism, the present results are consistent with the conclusion that mephedrone has lower abuse liability than methcathinone or the other recently scheduled “bath salts.” One implication of this finding is that mephedrone abuse might be expected to decline over time not only because of recently enacted legal constraints but also because of its pharmacological profile.
MDPV: the most common component of "bath salts."
MDPV, the primary constituent of "bath salts" in the United States prior to its emergency scheduling in October 2011 (Spiller et al. 2011; Kyle et al. 2011), is distinct among the synthetic cathinones because it functions as a reuptake inhibitor rather than as a monoamine releaser (Baumann et al. 2012b; Cameron et al. 2013b). Nonetheless, it displays high in vitro selectivity to block DA versus 5-HT reuptake (Baumann et al. 2012b) and, like cocaine and other dopamine reuptake inhibitors (Esposito et al. 1978; Rosenberg et al. 2013), MDPV produced robust facilitation of ICSS with an efficacy similar to that of methcathinone. This agrees with an earlier report that MDPV facilitated ICSS in rats responding under a discrete-trial current-threshold ICSS procedure (Watterson et al. 2012). Moreover, this evidence from our ICSS studies agrees with other evidence of psychostimulant and abuse-related effects of MDPV. For example, MDPV maintained intravenous self-administration in rats (Watterson et al. 2012) and produced both stimulant-like discriminative stimulus effects and locomotor-activating effects in mice (Marusich et al., 2012; Fantegrossi et al. 2013). Taken together, these data converge in suggesting that MDPV has high, stimulant-like abuse liability.
A distinguishing feature of the behavioral effects of MDPV in this study was its long duration of action. MDPV maintained significant facilitation of ICSS beyond 300 min (see Figure 2), with facilitation still apparent as long as 24 h after drug administration. While the present study provides the first in vivo data to suggest a long duration of action for MDPV, previous in vitro studies also support an extended time course profile for MDPV. For example, electrophysiological data show that MDPV blocked DAT longer and was more resistant to washout than cocaine (Cameron et al. 2013b). Consequently, the long duration of ICSS effects observed with MDPV may be explained by its high potency at and slow dissociation from DAT.
ICSS data analysis
One goal of the present study was to stratify the relative efficacies of methcathinone, MDPV, methylone and mephedrone to facilitate ICSS. This was accomplished using two approaches that have been described and validated previously with amphetamine and a series of 10 other monoamine releasers (Bauer et al. 2013; Bauer et al. in press). In those previous studies, both approaches yielded metrics of efficacy that correlated with in vitro selectivity of compounds to release DA versus 5-HT and in vivo efficacy to maintain self-administration in nonhuman primate assays of drug self-administration. Moreover, in the present study, both approaches yielded similar rank-ordering of efficacies to facilitate ICSS. These approaches differ from more conventional approaches to ICSS data analysis, which often focus on calculating “threshold” intensities or frequencies of brain stimulation to maintain ICSS (Miliaressis et al. 1986; Carlezon and Chartoff 2007). As discussed previously (Bauer et al. 2013), threshold measures have proven useful for dissociating hedonic from motor effects of experimental manipulations on brain reward substrates. However, the abuse liability of drugs likely reflects an integration of hedonic and motor effects, thus the approaches used here provide analytical strategies for quantifying that integration.
Despite the general agreement in results from ICSS and drug self-administration approaches to abuse liability assessment, results with mephedrone suggest a potential disconnect. Mephedrone produces a mixed profile of ICSS facilitation and depression in both rats and mice (present study; Robinson et al. 2012) that is generally associated with significant but relatively weak self-administration (Bauer et al. 2013). However, recent studies in rats have reported robust mephedrone self-administration (Hadlock et al. 2011; Aarde et al. 2013; Motbey et al. in press). Determinants of this apparent discrepancy and implications for human abuse liability will require further research.
Acknowledgements
This research was supported by National Institutes of Health grant R01 DA033930 and R01 DA026946
Footnotes
Disclosure
The authors declare no conflict of interest.
REFERENCES
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wisar rats. Addict Biol. 2013 doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Brit J Pharm. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: Implications for abuse liability assessment. Behav Pharmacol. 2013 doi: 10.1097/FBP.0b013e328363d1a4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012a;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2012b:1–11. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharm. 2013a doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013b;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE. Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. Eur J Pharm. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, Sitte HH, Baumann MH. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharm. 2013;699:180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several n-alkyl and methylenedioxy-substituted analogs. Pharm Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Esposito RU, Motola AH, Kornetsky C. Cocaine: acute effects on reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacol Biochem Behav. 1978;8:437–439. doi: 10.1016/0091-3057(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology. 1980;69:187–191. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharm Biochem Behav. 1986;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem Pharmacol. 1986;35:3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. J Miss State Med Assoc. 2011;52:375–377. [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath PB, O’Neill C. Experiences with mephedrone pre- and post-legislative controls: perceptions of safety and sources of supply. Int J Drug Policy. 2011;22:120–127. doi: 10.1016/j.drugpo.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: Neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013 doi: 10.1177/0269881113490325. in press. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Schechter MD. Behavioral and neurochemical effects of (-)- and ()- cathinone: dose-response and time-course. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:739–743. doi: 10.1016/0278-5846(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Olds ME, Yuwiler A. Effects of acute and chronic fenfluramine on self-stimulation and its facilitation by amphetamine. Eur J Pharmacol. 1992;216:363–372. doi: 10.1016/0014-2999(92)90432-4. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenauer R, Luf A, Holy M, Freissmuth M, Schmid R, Sitte HH. A combined approach using transporter-flux assays and mass spectrometry to examine psychostimulant street drugs of unknown content. ACS Chem Neurosci. 2013;4:182–190. doi: 10.1021/cn3001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;4:246–259. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M, Kornetsky C. Methamphetamine’s action on brain-stimulation reward threshold and stereotypy. Exp Clin Psychopharmacol. 1995;3:112–117. [Google Scholar]
- Shortall SE, Macerola AE, Swaby RTR, Jayson R, Korsah C, Pillidge KE, Wigmore PM, Ebling FJP, Green AR, Fone KCF, King MV. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.09.005. http://dx.doi.org/10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via internet and the example of mephedrone. Toxicol Letters. 2011;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal Models of Drug Addiction. Totowa, NJ: Humana Press; 2011. pp. 3–56. [Google Scholar]
- Wagner GC, Preston K, Ricaurte GA, Schuster CR, Seiden LS. Neurochemical similarities between d,l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982;9:279–284. doi: 10.1016/0376-8716(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(-)-methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology. 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]