Abstract
Frontal-dependent task performance is typically modulated by dopamine (DA) according to an inverted-U pattern, whereby intermediate levels of DA signaling optimizes performance. Numerous studies implicate trait differences in DA signaling based on differences in the catechol-O-methyltransferase (COMT) gene in executive function task performance. However, little work has investigated genetic variations in DA signaling downstream from COMT. One candidate is the dopamine- and cAMP-regulated phosphoprotein of molecular weight 32 kDa (DARPP-32), which mediates signaling through the DA D1-type receptor, the dominant DA receptor in the frontal cortex. Using an n-back task, we used signal detection theory to measure performance in a healthy adult population (n=97) genotyped for single nucleotide polymorphisms in the COMT (rs4680) and DARPP-32 (rs907094) genes. Correct target detection (hits), and false alarms were used to calculate d' measures for each working memory load (0-, 2-, and 3-back). At the highest load (3-back) only, we observed a significant COMT×DARPP-32 interaction, such that the DARPP-32 T/T genotype enhanced target detection in COMTValVal individuals, but impaired target detection in COMTMet carriers. These findings suggest that enhanced dopaminergic signaling via the DARPP-32 T allele aids target detection in individuals with presumed low frontal DA (COMTValVal) but impairs target detection in those with putatively higher frontal DA levels (COMTMet carriers). Moreover, these data support an inverted-U model with intermediate levels of DA signaling optimizing performance on tasks requiring maintenance of mental representations in working memory.
Keywords: COMT, DARPP-32, DRD1, executive function, n-back
INTRODUCTION
A variety of evidence indicates that dopamine (DA) plays a critical neuromodulatory role in the functioning of the prefrontal cortex (PFC) (Goldman-Rakic, 1996; Seamans & Yang, 2004). Pharmacological investigation of the primate PFC suggests a critical role for D1-type DA receptor (DRD1) signaling in working memory (WM) tasks (Sawaguchi & Goldman-Rakic, 1991). More recent data from electrophysiological studies of PFC neurons support the notion that DA enhances the signal-to-noise ratio in active PFC networks to enhance signal processing (Kroener, Chandler, Phillips, & Seamans, 2009). More is not always better, however. Converging evidence from animal models supports an “inverted-U” model of PFC DA, whereby an intermediate level of DRD1 stimulation results in optimal WM performance (Goldman-Rakic, Muly, & Williams, 2000; Williams & Castner, 2006), including performance on a delayed response task in rhesus monkeys (Cai & Arnsten, 1997), a delayed version of the radial maze task in rats (Floresco & Phillips, 2001), and a spatial delayed alternation task in mice (Lidow, Koh, & Arnsten, 2003).
While the tools for investigating the contribution of frontal DA to WM performance in humans are more limited, available evidence supports the view that WM may be similarly regulated in the human PFC. For example, the Val158Met polymorphism (rs4680) in the gene encoding the catechol-O-methyltransferase (COMT) enzyme, which regulates PFC DA levels (Gogos et al., 1998; Kaenmaki et al., 2010; Karoum, Chrapusta, & Egan, 1994; Tunbridge, Bannerman, Sharp, & Harrison, 2004; Yavich, Forsberg, Karayiorgou, Gogos, & Mannisto, 2007), has been associated with individual differences in WM performance. Work using the Wisconsin Card Sorting Task (WCST) (Egan et al., 2001; Malhotra et al., 2002), which engages a variety of executive processes, including WM, have found that individuals with the COMT Met/Met genotype (and thus higher putative tonic PFC DA) perform better than do Val/Val individuals. Likewise, COMT Met/Met individuals were found to perform better in the 1- and 2-back conditions of a verbal n-back WM task relative to Val/Val individuals, but COMT genotype did not predict performance on a continuous performance task that required no updating (Goldberg et al., 2003). The presumed greater persistence of PFC DA in COMTMet allele carriers is proposed to enable more sustained PFC activity and increased stability of PFC WM representations (Durstewitz & Seamans, 2008; Durstewitz, Vittoz, Floresco, & Seamans, 2010), processes common to the WCST and n-back tasks. However, other investigators have failed to replicate an association between COMT genotype and WM performance in humans (Bruder et al., 2005; Dennis et al., 2010). A recent meta-analysis of the role of COMT in cognition suggested that the effect of this polymorphism is minor and that Val homozygotes show higher n-back accuracy (Barnett, Scoriels, & Munafo, 2008). These seemingly contradictory findings may be reconciled if one considers that performance on these tasks engages multiple cognitive processes, each of which may require a distinct optimal level, or be entirely independent, of DA signaling in the PFC (Bilder, Volavka, Lachman, & Grace, 2004; Cools & D'Esposito, 2011). Furthermore, growing evidence points to the fact that WM function also depends on DA signaling in the striatum (Cools, Gibbs, Miyakawa, Jagust, & D'Esposito, 2008; Landau, Lal, O'Neil, Baker, & Jagust, 2009; Rieckmann, Karlsson, Fischer, & Backman, 2011; Stelzel, Basten, Montag, Reuter, & Fiebach, 2010; van Schouwenburg, Aarts, & Cools, 2010), where DA is primarily regulated by the DA transporter (DAT), rather than COMT. Thus, considering how COMT may interact with other factors that impact DA signaling may prove useful in understanding the role of frontal DA in WM and other cognitive processes.
As COMT regulates the presynaptic concentration of DA in the PFC, we chose to investigate the interaction between COMT genotype and a downstream (postsynaptic) regulator of DA signaling in the PFC. As DRD1 receptors are relatively more abundant in the rodent (Gaspar, Bloch, & Le Moine, 1995) and primate PFC than are D2-type DA receptors (Goldman-Rakic, Lidow, Smiley, & Williams, 1992; Lidow, Goldman-Rakic, Gallager, & Rakic, 1991; Sesack, Snyder, & Lewis, 1995), and PFC DRD1 activity is critically involved in WM function (Sawaguchi & Goldman-Rakic, 1991), we chose to investigate a component of the DRD1 signaling cascade with a previously characterized functional polymorphism. Specifically, we investigated a functional single nucleotide polymorphism (SNP; rs907094 A→G/T→C) in the PPP1R1B gene, which encodes the dopamine- and cAMP-regulated phosphoprotein of molecular weight 32 kDa (DARPP-32; (Svenningsson et al., 2004), a downstream mediator of DRD1 stimulation (Nishi, Snyder, & Greengard, 1997; Walaas & Greengard, 1984) thought to play an important role in DA signaling (Greengard, Allen, & Nairn, 1999). DRD1 activation phosphorylates DARPP-32, which ultimately regulates a number of downstream proteins important in neural excitability, including ligand- and voltage-gated ion channels, the sodium/potassium pump, and transcription factors (Greengard et al., 1999). In rodents, DARPP-32 phosphorylation levels in the PFC predict performance in PFC-dependent tasks (Hotte et al., 2006; Kolata et al., 2010; Seamans, Floresco, & Phillips, 1998), and DARPP-32 knockout mice demonstrate behavioral deficits suggesting frontal impairment (Heyser, Fienberg, Greengard, & Gold, 2000). While extensive data from animal models support a role of DARPP-32 in frontal DA-dependent cognitive performance, few studies have investigated DARPP-32 in human cognition. One study found that a DARPP-32 haplotype that includes the rs907094 “A” (or “T”) allele is associated with enhanced 3-back performance in an n-back task, increased PPP1R1B mRNA expression in the PFC (specifically the middle frontal gyrus, MFG) of post-mortem human brain, and greater structural and functional connectivity between striatum and lateral PFC during n-back performance (Meyer-Lindenberg et al., 2007).
While DARPP-32’s modulation of DRD1 signaling clearly suggests a role for DARPP-32 in regulating DA signaling in the PFC, most work on DARPP-32 to date has focused on its role in modulating striatal function. For example, human studies of the rs907094 SNP have associated it with variations in reinforcement learning (Frank, Doll, Oas-Terpstra, & Moreno, 2009; Frank, Moustafa, Haughey, Curran, & Hutchison, 2007), where it appears to contribute to subtle discrimination abilities (Frank & Fossella, 2011). These findings have been interpreted in terms of DARPP-32 effects in the striatum, where it also modulates DRD2 signaling, albeit with opposing downstream effects: inhibition of cell excitability (Greengard et al., 1999). As a means of specifically isolating cognitive processes in which strong evidence implicates frontal DA and the DRD1 (Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007), we selected a WM task for which load increases preferentially engage the MFG, an effect that is modulated by putative frontal DA signaling, as indexed by COMT genotype and enzyme activity (Jacobs & D'Esposito, 2011). Distractor-resistant maintenance is thought to rely more specifically on frontal DA, whereas striatal DA appears to primarily subserve successful updating (van Schouwenburg et al., 2010). As load increases boost maintenance demands without markedly changing updating demands, observed effects of DARPP-32 on load-dependent performance would likely reflect mainly maintenance processes, which are largely mediated at the level of PFC.
Based on evidence that DARPP-32 potentiates DRD1 signaling, and that the rs907094 T/T genotype is associated with enhanced DARPP-32 expression in the PFC and enhanced 3-back performance, we hypothesized that this postsynaptic modulator of PFC DA signaling would interact with a presynaptic regulator of PFC DA signaling, the COMT Val158Met SNP, to influence WM performance in humans. Specifically, we expected to see greatest interactive effects of COMT and DARPP-32 genotype on correct target detection under high load in an n-back task. We expected this interaction to conform to an inverted-U model of PFC DA signaling, whereby too much or too little DA would impair performance (Cools & D'Esposito, 2011; Goldman-Rakic et al., 2000). To test this hypothesis, we tested healthy adult participants’ performance in an n-back WM task (Gray, Chabris, & Braver, 2003; Jacobs & D'Esposito, 2011), used signal detection analysis (Macmillan & Creelman, 2005) to investigate target detection in the presence of competing stimuli, and genotyped participants for the Val158Met COMT and DARPP-32 polymorphisms. We then tested for interacting effects of COMT and DARPP-32 genotype on d' measures of target detection calculated for each WM load.
As DARPP-32 is also known to modulate DA signaling in the striatum (Frank et al., 2007; Meyer-Lindenberg et al., 2007; Ouimet, Miller, Hemmings, Walaas, & Greengard, 1984), we also tested whether replacing DARPP-32 genotype in our statistical models with a genetic marker of striatal DA tone could produce similar results. To do so, we genotyped participants for a variable number tandem repeat (VNTR) polymorphism in the 3′ untranslated region of the DAT gene (SLC6A3) (Vandenbergh et al., 1992). This choice was based on evidence that DAT is the primary mechanism of DA clearance in the striatum (Giros, Jaber, Jones, Wightman, & Caron, 1996; Jones, Gainetdinov, Wightman, & Caron, 1998; Lewis et al., 2001; Sesack, Hawrylak, Matus, Guido, & Levey, 1998), and that variations in the number of repeats in this VNTR affect DAT availability in the striatum, with the most support for increased DAT availability in 9 repeat (9R) carriers (Shumay, Chen, Fowler, & Volkow, 2011; van de Giessen et al., 2009), but cf. (Heinz et al., 2000; VanNess, Owens, & Kilts, 2005).
METHODS
Participants
Participants (n=97) were recruited from the University of North Carolina, Chapel Hill (UNC) and surrounding community. All subjects were healthy individuals 22–40 years old with no history of neurological or psychiatric disorders, and no current use of psychoactive medications or other psychoactive substances aside from moderate caffeine, nicotine or alcohol. Participants were native English speakers, had at least a high-school education, and reported lifetime alcohol consumption of ≥1 drink. We excluded people under 22 based on the finding that age modulates COMT genotype effects on another aspect of cognition (Smith & Boettiger, 2012). This age cutoff is also supported by data showing that brain maturation asymptotes at ∼22 years of age (Dosenbach et al., 2010). We collected information regarding participants’ personal and parental occupation and education via questionnaire to quantify socioeconomic status (SES; Hollingshead, 1975). Participants gave written informed consent, as approved by the UNC Office of Human Research Ethics. Subjects received monetary compensation for participating.
Genotyping
COMT and DARPP-32 Single Nucleotide Polymorphisms
DNA samples were extracted from saliva samples using Oragene DNA Collection Kits (DNAGenotek, Kanata, Ontario, Canada). DNA samples from each participant were genotyped for the Val158Met COMT (rs4680) and DARPP-32 (rs907094) polymorphisms using TaqMan technology (Applied Biosystems, Foster City, CA) as described previously (Boettiger et al., 2007; Smith & Boettiger, 2012). Genotyping was performed by the Duke Center for Human Genetics. Both genotypes were in Hardy-Weinberg equilibrium (COMT: χ2=1.23, df=2, p=0.54; DARPP-32: χ2=0.785, df=2, p=0.675). The COMT genotype distribution was 29:43:25 (Val/Val:Val/Met:Met/Met). The DARPP-32 genotype distribution was 9:35:53 (C/C:C/T:T/T). The C/C and C/T genotypes were combined as C-carriers for all analyses to achieve comparable group sizes. COMT distributions did not differ between DARPP-32 groups, χ2=1.38, df=2, p=0.5.
DAT 3′ UTR VNTR Polymorphism
DNA samples were also genotyped for the DAT 3′ UTR VNTR using a modification of a previously described protocol (Anchordoquy, McGeary, Liu, Krauter, & Smolen, 2003). The PCR primer sequences were 5’-TGT GGT GTA GGG AAC GGC CTG AG-3’ and 5’-CTT CCT GGA GGT CAC GGC TCA AGG-3’.
PCR was carried out in a 20 µl final reaction volume with 1.0 U AmpliTaq Gold® polymerase (Life Technologies, Foster City, CA), 1x PCR buffer II solution (Life Technologies, Foster City, CA), 10% dimethyl sulfoxide (DMSO; Hybra-Max® grade; Sigma, St. Louis, MO)], 180 µM dNTP, 20 ng of DNA, with 7’-deaza-2’-deoxyGTP (Roche Applied Science, Indianapolis, IN) substituted for one half of the dGTP, and 480 nM for forward and reverse DAT primers (Life Technologies, Foster City, CA). Cycling conditions were as follows: 95°C for 10 min, with a series of touch down reactions for 2 cycles at 95°C for 30 s, 65°C for 30 s, 72°C for 1 min, 2 cycles each with a 65°C, 63°C, 61°C, 59°C, and 57°C annealing, respectively, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min. Final extension was at 72°C for 30 min.
After DAT VNTR amplification, all DNA samples were read for base pair (bp) length by the Genome Analysis facility at UNC using an ABI 3730xl DNA Analyzer (ABI, Foster City, CA) to determine the number of 40 bp repeats in the 3′ UTR region of the DAT gene. Data was visualized using PeakScanner software (ABI, Foster City, CA), and base pair calls were made by two independent observers. Known DAT VNTR control samples were provided by Andrew Smolen (University of Colorado, Boulder) and ABI (CEPH 1347–02) for quality assurance of PCR reaction and VNTR readout. We ascertained DAT genotypes for 96 subjects. The distribution of DAT genotypes was 54:29:11:2 (10/10:9/10:9/9:8/10), which is consistent with published data (Kang, Palmatier, & Kidd, 1999; Mitchell et al., 2000; Vandenbergh et al., 1992). Participants with rare alleles were excluded from our analyses (n=2).
n-back Task
Our behavioral paradigm was based on a previously described task (Gray et al., 2003; Jacobs & D'Esposito, 2011). Briefly, the task included 16 blocks of differing WM load (eight 0-back blocks, four 2-back blocks, and four 3-back blocks) ordered in one of two pseudorandom sequences which were assigned alternatively to each participant (i.e., odd subject numbers received one block sequence, while even subject numbers received the same trials presented in the other block sequence). Subjects were instructed on the condition (0-, 2-, or 3-back) at the start of each block. A block consisted of 20 serially presented consonant letter stimuli (duration: 1 sec), with each letter followed by a 1 sec delay. 0-back blocks consisted of 20% targets and 80% non-lures, while 2- and 3-back blocks consisted of 20% targets, 15% lures, and 65% non-lures. For 0-back trials, the target letter was defined as the letter “X” while in 2- and 3-back trials, target letters were those that matched the letter seen 2 or 3 previously in the stream, respectively. Subjects were asked to press one button for targets and another button for non-targets. Lures were letters seen previously in the stream but not at the target position; these were always ±1 position from the target position. Accuracy and reaction time measures were collected for each trial.
Data Analysis
Discriminating targets from non-targets
To quantify participants’ ability to correctly detect targets from the stimulus stream, we calculated a target discriminability index. The index, d-prime (d′), is the most commonly employed index derived from signal detection theory (Green & Swets, 1966). Proportion of hits (correct target identification) and false alarms (responding to non-target non-lures and/or lures as targets) were calculated for 0-, 2- and 3-back conditions and used to calculate a d' measure of target detection for each WM load. We calculated d′ for each participant at each load as follows:
d′= Z transform (p(Hits)) – Z transform (p(False Alarms))
Where p(X)=proportion of X instances (Hits or False Alarms) across all trials of a common load (0-, 2-, or 3-back). Hits reflected correct identification of a target as a target. The maximum Hit probability was set at 31/32 (0.969, Z score of 1.863) for 0-back trials and 15/16 (0.938, Z score of 1.534) for 2- and 3-back trials to allow for Z-transform computation. Incorrect identification of any non-target (non-lure or lure) as a target was classified as a False Alarm. The minimum False Alarm probability was set at 1/128 (0.0078, Z-score of −2.418) for 0-back trials and 1/64 (0.0156, Z-score of −2.154) for 2- and 3-back trials. Our Z transform reflects the Z-score transform of the proportion of responses based on a normal distribution of response probabilities mean centered at a probability of 0.5 (Z-score=0).
Discriminating targets from lures
For the 2- and 3-back conditions, a separate d′ measure was calculated based on target hits versus lure false alarms as follows:
d′= Z transform (p(Hits)) – Z transform (p(Lure False Alarms))
Where Lure False Alarms reflect incorrect identification of lures as targets. The maximum Hit probability was set at 15/16 (0.938, Z-score of 1.534) for 2- and 3-back trials, as for the d′ calculation above. The minimum Lure False Alarm probability was set at 1/12 (0.0833, Z-score of −1.383) for 2- and 3-back trials.
d′ slope
The use of different WM load levels allowed us to calculate the d′ slope (the difference in d′ between the 2- and 3-back conditions divided by the load difference). This measure quantifies the effect of increasing WM load on performance per unit of memoranda.
Statistical analyses
To test the significance of across group comparisons, we used 3 (COMT group: Met/Met, ValMet, Val/Val individuals) × 2 (DARPP-32 group: C carriers, T/T individuals) ANOVA for continuous measures and χ2 tests for categorical measures. Repeated measures ANOVA with genotype group (3×2) as between subjects factors and WM load as a within subjects factor (3 levels) were performed on accuracy and reaction time data. Analyses including DAT data were identical, with the substitution of DAT genotype (9R carrier, 10/10 individuals), for DARPP-32 genotype. All ANOVA included participant sex and ethnicity as covariates (see Demographic data in Results (below) for justification). When necessary, a Greenhouse-Geisser non-sphericity correction was applied. Post-hoc comparisons were performed where indicated using ANOVA and two-tailed t-tests. All statistical procedures were carried out in SPSS (SPSS Inc., Chicago, IL). Values reported as mean ± SEM, unless otherwise stated.
RESULTS
Demographic data
Demographic factors, including years of education, SES (Hollingshead, 1975), and age, did not significantly vary as a function of either COMT or DARPP-32 genotype in our sample (Table 1). Furthermore, gender ratios were well balanced across groups. While a greater proportion of DARPP-32 T/T individuals were white, this was not unexpected, given that allelic distribution of this polymorphism is known to vary as a function of ethnicity, with T being the major allele in Caucasian and Japanese populations, and C being the major allele in an African population (Yoruba, Nigeria) (Frazer, Ballinger, Cox, Hinds, & et al., 2007). To control for this potential confound of ethnic distribution differences across our genotype groups, ethnicity was included as a covariate in our analyses. As indicated in the results, we also repeated our ANOVA and post-hoc testing within the white participants (n=71) to verify that any significant DARPP-32 × COMT interaction effects in the full sample were not driven by differences in ethnic distribution between genotype groups. We also included sex as a covariate in our analyses, based on data showing that sex moderates COMT activity in post-mortem human PFC (Chen et al., 2004).
Table 1. Demographic data by genotype groups.
Values are reported as mean ± standard deviation. Reported p-values reflect COMT × DARPP-32 interaction effects on the variable of interest following 3 (COMT) × 2 (DARPP-32) ANOVAs. Exact p-values reported unless p < 0.001.
| COMT Val/Val D-32 C (n = 15) |
COMT Val/Met D-32 C (n = 20) |
COMT Met/Met D-32 C (n = 9) |
COMT Val/Val D-32 T/T (n=14) |
COMT Val/Met D-32 T/T (n=23) |
COMT Met/Met D-32 T/T (n =16) |
F(2, 91) (p) |
|
|---|---|---|---|---|---|---|---|
| Age (yrs) | 25.5 ± 4.2 | 26.7 ± 5.6 | 25.9 ± 4.2 | 24.8 ± 4.0 | 24.8 ± 4.7 | 25.1 ± 4.6 | 0.18 (0.84) |
| Edu (yrs) | 16.6 ± 1.5 | 16.4 ± 1.4 | 17.0 ± 2.4 | 16.8 ± 2.3 | 16.7 ± 1.6 | 16.1 ± 0.8 | 0.96 (0.39) |
| Subject SES | 44.1 ± 5.3 | 46.1 ± 6.0 | 48.3 ± 4.0 | 46.1 ± 6.2 | 47.2 ± 6.3 | 46.1 ± 5.8 | 0.89 (0.42) |
| Sex (% female) |
53.3 | 50.0 | 77.8 | 35.7 | 60.9 | 68.8 | (0.34) † |
| Ethnicity (% white) |
40 | 55.0 | 66.7 | 92.9 | 91.3 | 87.5 | (0.001) † |
p-value represents results of χ2 test. D-32 C, carrier of DARPP-32 C allele; D-32 T/T, DARPP-32 T/T individuals; SES, socioeconomic status; Edu, education.
Overall n-back performance declines as a function of load
The load manipulation produced significant differences in both Target accuracy (F(2,178)=6.97, p=0.001), and reaction time (RT) to correctly identify Targets (F(2,178)=23.21, p<0.001). Load effects on accuracy reflected near significant decreases in Target accuracy between 0- and 2-back trials (F(1,89)=3.69, p=0.058) and between 2- and 3-back trials (F(1,89)=3.57, p=0.062). Load effects on RT for correct Target identification reflect significant slowing from 0- and 2-back trials (F(1,89)=20.7, p<0.001) and from 2- to 3-back trials (F(1,89)=5.00, p=0.03). We observed no significant main or interacting effects of COMT or DARPP-32 genotype on Target accuracy or RT (max. F=2.68, min. p=0.07, main effect of COMT on target accuracy; Table 2).
Table 2. n-back task performance for target stimuli by genotype groups.
Values are reported as mean ± standard error of the mean. Reported p-values reflect COMT × DARPP-32 interaction effects in a 3 (COMT genotype group) × 2 (DARPP-32 genotype group) ANOVA with white ethnicity and sex included as covariates. Conventions as for Table 1.
| COMT Val/Val D-32 C (n = 15) |
COMT Val/Met D-32 C (n = 20) |
COMT Met/Met D-32 C (n = 9) |
COMT Val/Val D-32 T/T (n=14) |
COMT Val/Met D-32T/T (n=23) |
COMT Met/Met D-32 T/T (n =16) |
|
|---|---|---|---|---|---|---|
| Target Accuracy (%) |
||||||
| 0-back | 92.9 ± 2.0 | 90.7 ± 1.6 | 85.2 ± 2.4 | 92.9 ± 2.0 | 89.6 ± 1.5 | 92.9 ± 1.8 |
| 2-back | 88.8 ± 4.2 | 86.0 ± 3.5 | 70.9 ± 5.2 | 82.4 ± 4.3 | 81.4 ± 3.3 | 77.2 ± 3.9 |
| 3-back | 66.0 ± 4.1 | 69.0 ± 3.5 | 62.5 ± 5.1 | 70.2 ± 4.1 | 61.7 ± 3.2 | 66.5 ± 3.8 |
| Target Correct RT (ms) |
||||||
| 0-back | 481.1 ± 18.1 | 497.5 ± 15.2 | 507.8 ± 22.4 | 516.1 ± 18.3 | 494.8 ± 14.2 | 537.5 ± 16.9 |
| 2-back | 690.1 ± 32.1 | 685.0 ± 27.0 | 724.4 ± 39.6 | 710.9 ± 32.4 | 699.1 ± 25.2 | 707.6 ± 29.9 |
| 3-back | 729.0 ± 33.6 | 758.5 ± 28.3 | 724.2± 41.5 | 771.8 ± 33.9 | 726.2 ± 26.4 | 793.3 ± 31.4 |
RT, reaction time.
Target versus non-target discrimination decreases with increasing load
Beyond simple performance estimates, we wished to determine how well subjects were able to discriminate targets imbedded within a stream of non-targets. To do so, we used signal detection theory to calculate d-prime (d´) as a discriminability index (see Methods). We performed this analysis first including all trial types (Table 3). A repeated measures ANOVA (load × COMT × DARPP-32) taking d′ as the dependent measure found a significant main effect of WM load on d′ (F(2,178)=22.58, p<0.001). This reflected significant decreases in d´ from both the 0- (3.76±0.04) to 2-back condition (2.57±0.08; F(1,89)=13.15, p<0.001), and the 2- to 3-back condition (1.86±0.06; F(1,89)=8.35, p=0.005). In contrast, we observed no significant main effect of COMT (F(2,89)=2.8, p=0.066) or DARPP-32 (F(1,89)=0.59, p=0.45), nor any significant COMT*DARPP-32 interaction (F(2,89)=2.45, p=0.093) on basic target detection.
Table 3. Basic target discrimination across WM loads by genotype groups.
Target discrimination quantified as a d′ measure of correct target hits versus non-target false alarms (see Methods). Values are reported as mean ± standard error. Other conventions as for Tables 1 and 2. Significant main effect of WM load: F(2,178)=22.58, p<0.001; no significant main or interacting effects of COMT or DARPP-32 genotype on basic target detection (max F=1.69, min p=0.19).
| COMT Val/Val D-32 C (n = 15) |
COMT Val/Met D-32 C (n = 20) |
COMT Met/Met D-32 C (n = 9) |
COMT Val/Val D-32 T/T (n=14) |
COMT Val/Met D-32T/T (n=23) |
COMT Met/Met D-32 T/T (n =16) |
|
|---|---|---|---|---|---|---|
| d' | ||||||
| 0-back | 3.87 ± 0.11 | 3.76 ± 0.09 | 3.47 ± 0.14 | 3.93 ± 0.11 | 3.70 ± 0.09 | 3.86 ± 0.10 |
| 2-back | 2.76 ± 0.21 | 2.85 ± 0.17 | 2.03 ± 0.25 | 2.58 ± 0.20 | 2.70 ± 0.16 | 2.50 ± 0.19 |
| 3-back | 1.86 ± 0.15 | 1.95 ± 0.12 | 1.70 ± 0.18 | 2.09 ± 0.15 | 1.70 ± 0.12 | 1.85 ± 0.14 |
Discrimination of targets from lures
As previous work with this particular n-back paradigm found an inverted-U shaped relationship between DA signaling and both WM performance and neural activity when considering lure trials (Jacobs & D'Esposito, 2011), we investigated the relationship between COMT and DARPP-32 genotypes on discrimination between target and lures for 2- and 3-back trials (target/lure d′; see Table S1 for Lure RT and accuracy data by genotype group). A 3(COMT) × 2(DARPP-32) × 2(WM load) mixed repeated measures ANOVA with target/lure d′ as the dependent measure found a significant effect of WM load on d′ (F(1,89)=8.98, p=0.004), with increasing WM maintenance demands from the 2- to 3-back condition decreasing d′ from 1.65±0.09 to 0.93±0.07, respectively. We did not observe significant main effects of either COMT (F(2,89)=1.41, p=0.25) or DARPP-32 genotype (F(1,89)=0.028, p=0.87) on overall target/lure discrimination. Critically, we did find a significant COMT × DARPP-32 × load interaction effect on target versus lure discrimination (F(2,89)=4.61, p=0.012). Given our somewhat ethnically mixed sample (see Table 1) and reported ethnic differences in COMTval158met allele frequencies (e.g. (McLeod, Fang, Luo, Scott, & Evans, 1994; McLeod et al., 1998), it is important to note that the COMT genotype × DARPP-32 genotype × WM load interaction remained nearly significant when our analyses were restricted to the white participants, the largest ethnic group included (F(2,64)=3.09, p=0.052), despite the substantial loss of statistical power. We also found a significant COMT × load effect (F(2,89)=6.03, p=0.003), but no DARPP-32 × load effect (F(1,89)=0.36, p=0.55) on target/lure d′.
We next performed post-hoc ANOVA to investigate the COMT × DARPP-32 effect on target/lure discrimination separately for the 2- and 3-back conditions. While we observed a main effect of COMT for target/lure discrimination in 2-back trials (F(2,89)=3.97, p=0.022), no main effect of DARPP-32 or interaction effects were observed (max F=1.37, min p=0.26). In contrast, for 3-back trials, we found a significant COMT×DARPP-32 interaction (F(2, 89)=4.37, p=0.016, Figure 1), with no main effect of either COMT (F(2, 89)=0.45, p=0.64) or DARPP-32 (F(1, 89)=0.03, p=0.86). This COMT × DARPP-32 interaction effect on 3 back target/lure d′ also remained when only white participants were considered (F(2, 64)=4.05, p=0.022). This interaction primarily reflects impaired target/lure discrimination in COMTVal/Met individuals with the DARPP-32 T/T genotype (F(1,39)=6.23, p=0.017; Figure 1). In contrast, the DARPP-32 T/T genotype was associated with enhanced target/lure discrimination in COMTVal/Val individuals, although the latter effect failed to reach statistical significance (F(1, 25)=2.14, p=0.156; Figure 1). No significant effects of DARPP-32 genotype on 3-back target/lure discrimination were observed among COMTMet/Met individuals (F(1, 21)=0.058, p=0.81). When restricting our post-hoc analyses to white participants, the DARPP-32 T/T genotype remained associated with impaired target/lure discrimination in COMTVal/Met individuals (F(1,29)=4.65, p=0.04), and we observed a trend toward enhanced target/lure discrimination in COMTVal/Val individuals (F(1,16)=3.64, p=0.074); again, no effect of DARPP-32 was observed among COMTMet/Met individuals (F(1,17)=0.06, p=0.81).
Figure 1.
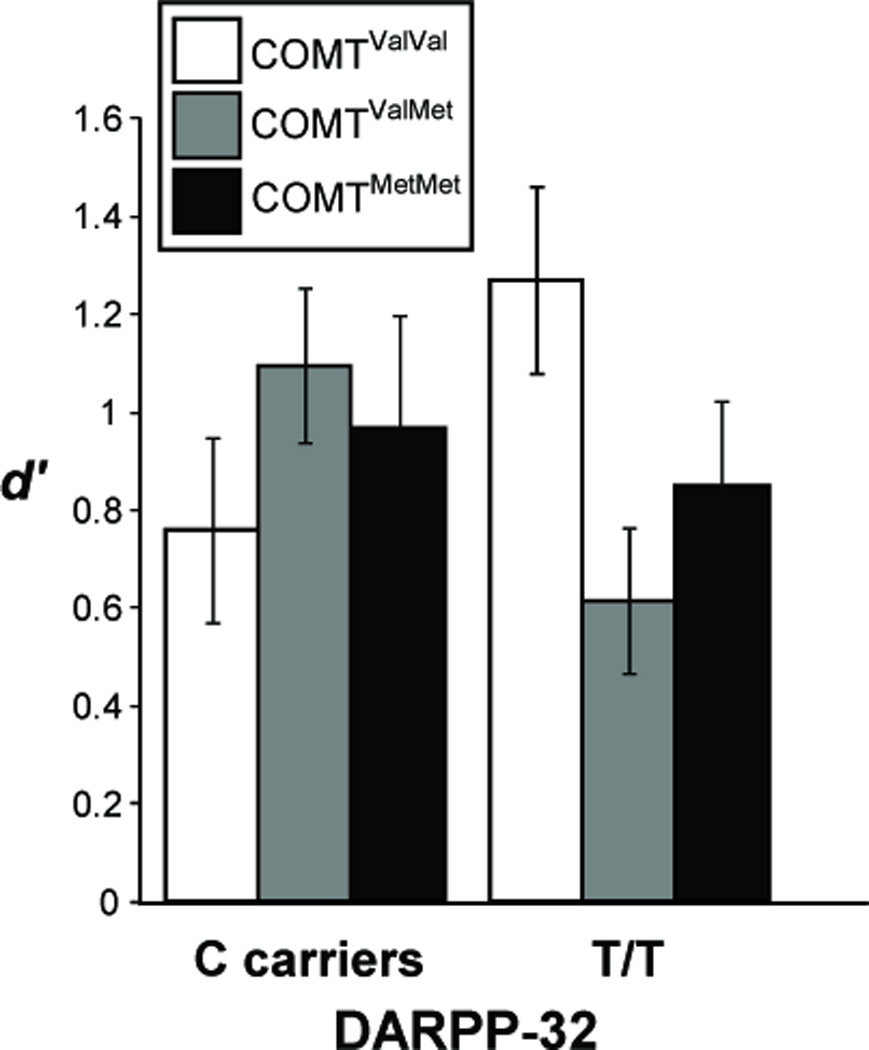
COMT and DARPP-32 genotype interaction effects on working memory performance. Graphs depict target discrimination (d′ for targets versus lures; mean ± SEM). Under high working memory load (3-back trials), COMT and DARPP-32 genotype significantly interact to affect d′, according to an inverted-U relationship (F(2, 89)=4.37, p=0.016). A post-hoc ANOVA found a significant effect of DARPP-32 genotype on 3-back target/lure detection in COMTValMet individuals, F(1,39)=6.23, p=0.017.
We directly compared the effect of DARPP-32 genotype in the COMTVal/Met group relative to the COMTVal/Val and COMTMet/Met groups. The COMTVal/Met group differed significantly from the COMTVal/Val group on 3-back target/lure discrimination (F(1,66)=8.31, p=0.005), but did not differ from the COMTMet/Met group (F(1,62)=1.2, p=0.28).
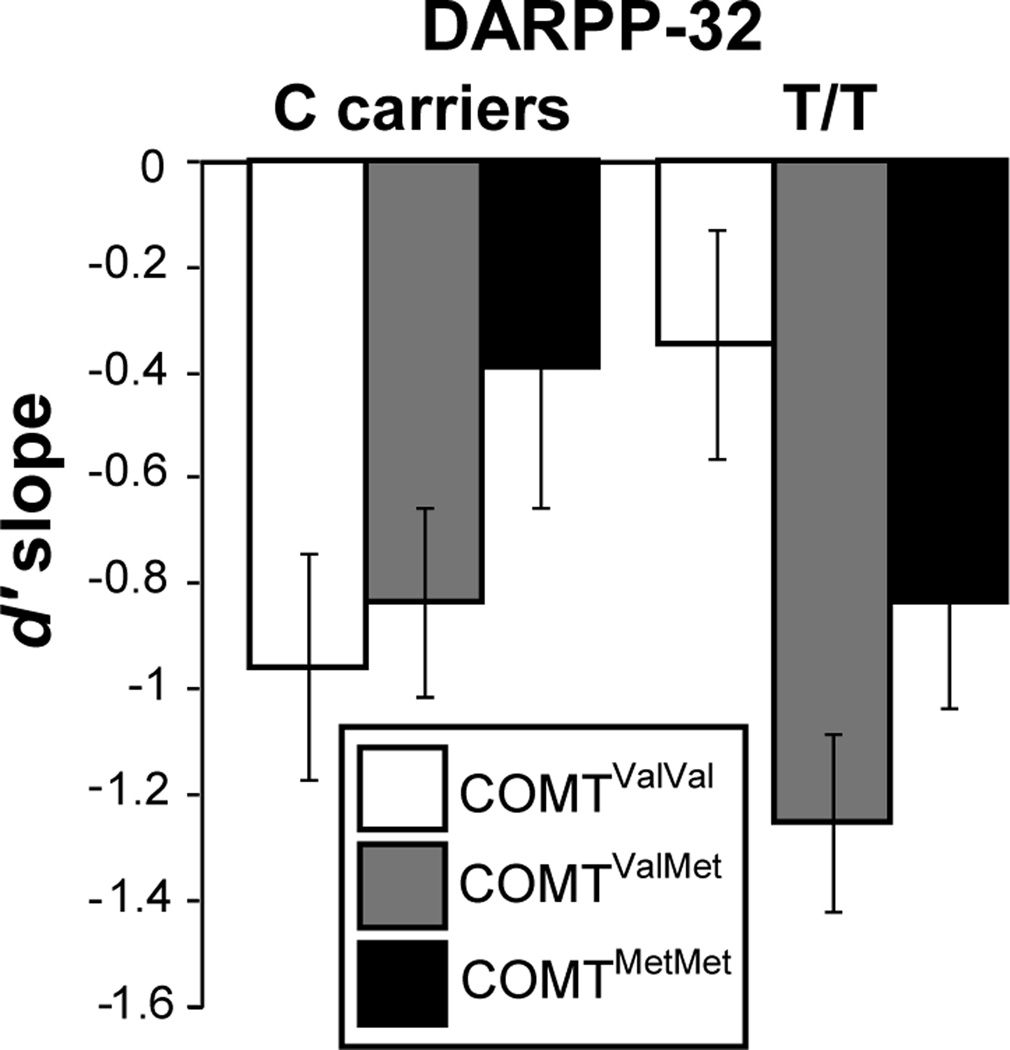
To test for interacting effects of DARPP-32 and COMT genotypes on target/lure discrimination with increasing load, controlling for baseline WM performance, we next evaluated the d′ slope for target/lure discrimination. In addition to normalizing high WM load (3-back) performance by baseline WM (2-back) performance, the d’ slope quantifies the change in target detection per unit of memorandum in WM, and can be more readily compared across studies with differing load levels. More negative slopes indicate poorer target discrimination as a function of WM load. A 3 × 2 ANOVA (COMT × DARPP-32) found a significant COMT × DARPP-32 interaction (F(2,89)=4.26, p=0.017; Figure 2). This COMT × DARPP-32 interaction effect on d’ slope was also present when considering only white participants (F(2,64)=3.18, p=0.048). Although post-hoc ANOVA within each COMT group did not reveal statistically significant effects of DARPP-32 genotype, we observed opposing directions for the effects of a putative increase in DRD1 signaling (DARPP-32 T/T) in those with putative low tonic PFC DA (COMTValVal), and COMTMet allele carriers. We observed a tendency toward less decrement in target discrimination with increasing load, i.e. a less negative d′ slope in the COMTValVal group (t(27)=1.45, p=0.16). In contrast, we observed greater decrement in target discrimination with increasing WM load in those with one (t(41)= −2.39, p=0.022) or two (t(23)=-1.48, p=0.15) COMTMet alleles, and when considering COMTMet carriers as a combined group (t(66)=-2.53, p=0.014). In considering only white participants, the DARPP-32 T/T genotype was associated with significantly less decrement in WM performance with increasing load (less negative d′ slope) in COMTValVal individuals (F(1,16)=4.13, p=0.02) and non-significant increases in the d′ slope in those with one (F(1,29)=1.76, p=0.20) or two (F(1,17)=0.64, p=0.44) COMTMet alleles. Finally, we directly compared the effect of DARPP-32 genotype on d′ slope in COMT heterozygotes, and each homozygous group. The effect of DARPP-32 T/T genotype in COMTVal/Met group differed significantly from that in the COMTVal/Val group (F(1,66)=8.17, p=0.006), but did not differ from the COMTMet/Met group (F(1,62)=0.04, p=0.85).
Figure 2.
Interacting effects of COMT and DARPP-32 genotype on target discrimination (target/lure d′; mean ± SEM) as a function of working memory load, as indexed by d′ slope (F(2, 89)=4.26, p=0.017). The magnitude of d′ slope follows an inverted-U pattern, with putatively intermediate levels of frontal dopamine D1 receptor signaling associated with better performance.
COMT×DARPP-32 interaction effect: frontal DA or fronto-striatal DA?
The fact that DARPP-32 boosts DRD1 signaling strongly implicates DARPP-32 in modulating frontal DA-dependent behaviors. Indeed the work of Hotte et al., 2006, Kolata et al., 2010, and Meyer-Lindenberg et al., 2007, all suggest that DARPP-32 modulates frontal function. However, DARPP-32 is most highly expressed in the striatum (Ouimet et al., 1984; Walaas & Greengard, 1984), and the majority of work on DARPP-32 in humans to date has focused on its role in modulating striatal function (Frank et al., 2009; Frank & Fossella, 2011; Frank et al., 2007). Furthermore, striatal DA also plays a role in WM function, where it is thought to primarily subserve successful updating, whereas distractor-resistant maintenance is thought to rely more specifically on frontal DA (see van Schouwenburg et al., 2010). Thus, the interacting effects of DARPP-32 and COMT may not simply reflect effects on frontal DA signaling, but rather interactions between frontal and striatal DA. If the DARPP-32 effect is largely striatal, one would expect an alternative marker of striatal DA to effectively replace it in our statistical models.
To test this possibility, we assayed DAT VNTR genotypes in these participants, and repeated our ANOVA for both target/lure discrimination (d′) and d′ slope, replacing our DARPP-32 genotype factor with DAT genotype: DAT 10/10 repeat individuals (n=54) versus DAT 9 repeat carriers (9R; n=40), with the 9R genotype most frequently associated with enhanced DAT availability and, thus, reduced striatal DA signaling (Costa, Riedel, Muller, Moller, & Ettinger, 2011; Jacobsen et al., 2000; Shumay et al., 2011; van de Giessen et al., 2009; van Dyck et al., 2005). Considering target/lure d′ as our dependent measure, in contrast to our significant load×COMT×DARPP-32 interaction, we observed no significant interaction between WM load, COMT, and DAT (F(2,86)=1.85, p=0.16). Likewise, when taking target/lure d′ slope as our dependent measure, we observed no significant COMT×DAT interaction (F(2,86)=1.72, p=0.19). Although not definitive, these findings fail to support the idea that our observed WM load×COMT×DARPP-32 interaction primarily reflects an interaction between frontal and striatal DA tone.
Theoretical model
Together, our findings are consistent with an inverted-U model of DRD1 signaling, possibly at the level of the PFC, on WM task performance under high load (Figure 3), which is expected based on findings in the animal literature (Vijayraghavan et al., 2007). In brief, based on individual putative tonic PFC DA level (COMT Val158Met genotype), greater DRD1 signaling (DARPP-32 T/T genotype) may facilitate, impair, or have no effect on high load WM performance by shifting where an individual falls on the inverted-U curve (see model, Figure 3).
Figure 3.
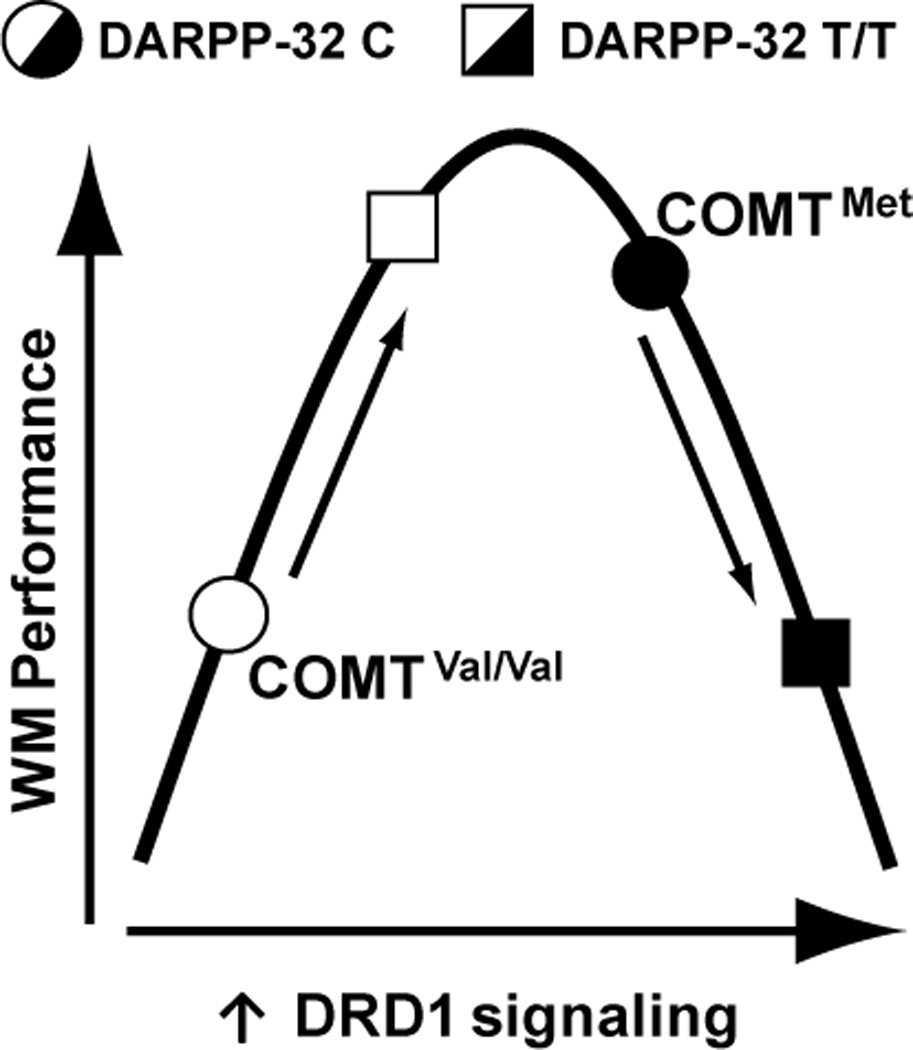
Model depicting an inverted-U relationship of dopamine D1 receptor (DRD1) signaling, which accounts for interacting effects of COMT and DARPP-32 genetic polymorphisms on working memory (WM) performance. Intermediate levels of DRD1 signaling, which we propose are acting at the level of the PFC, are hypothesized to enable optimal discrimination of target stimuli from interfering stimuli under high WM load.
DISCUSSION
The present findings support an inverted-U model for the role of DA signaling in PFC-dependent WM task performance, in that putatively enhanced DRD1 signaling (DARPP-32 T/T genotype) had differing effects based on putative tonic PFC DA levels (COMT genotype). We found that putatively enhanced DRD1 signaling via the DARPP-32 T allele tends to aid target detection in individuals with presumed low frontal DA tone (COMTValVal) but tends to impair target detection in those with putatively higher frontal DA levels (COMTMet carriers). These findings suggest that DRD1 signaling, perhaps largely in the PFC, is critical for optimal discrimination of targets from lures when maintenance demands on WM are high. The interpretation that DRD1 signaling is especially important in maintaining target representation in WM amid interfering stimuli is consistent with the demonstrated role of PFC DRD1 signaling in top-down control of visual attention (Noudoost & Moore, 2011).
Prefrontal DRD1 signaling and WM – pharmacology studies
Electrophysiological work in non-human primates long ago identified neurons near the principal sulcus (dorsolateral PFC) that sustain firing activity over a delay interval and encode target location information in oculomotor delayed-response (ODR) tasks (Funahashi, Bruce, & Goldman-Rakic, 1989). Pharmacological studies of these neurons subsequently demonstrated that sustained firing during a delay is critically sensitive to DRD1 manipulations (Sawaguchi, 2001). For example, DRD1 antagonists prevent maintenance of task-relevant information during the delay period in ODR tasks (Sawaguchi & Goldman-Rakic, 1991, 1994). Furthermore, investigations of both DRD1 agonists and antagonists demonstrated that WM performance is sensitive to DRD1 stimulation according to an inverted-U function, such that either insufficient or excessive DRD1 stimulation degrades performance and that pharmacological effects depend on tonic catecholamine levels (Arnsten, Cai, Murphy, & Goldman-Rakic, 1994; Cai & Arnsten, 1997). Although DRD1-specific agents are not currently approved for use in human subjects, available human studies support this inverted-U model for the role of DRD1 in WM processes as DA stimulation enhances WM performance in those with poor baseline WM (Costa, Peppe, Dell'Agnello, Caltagirone, & Carlesimo, 2009; Mehta et al., 2000) and hinders performance in those with high baseline WM capacity (Mattay et al., 2000). Thus, our present findings support the conclusion drawn from pharmacological studies that DRD1 signaling in the PFC modulates WM performance according to an inverted-U function.
Prefrontal DA and WM – genetic studies
Another approach to understanding the role of neuromodulators in WM is by evaluating the effect of functional polymorphisms in genes that regulate signaling in one or more neuromodulatory systems. A rather extensively studied example is the COMT Val158Met polymorphism, which is thought to roughly index tonic PFC DA, a notion supported by recent PET data (Wu et al., 2012). Accounting for tonic PFC DA levels via COMT genotype can help explain the inverted-U effect of pharmacological stimulation of DA signaling on WM performance. For example, the monoamine agonist amphetamine impairs WM in individuals with high tonic PFC DA (COMTMetMet), but enhances WM in individuals with low tonic frontal DA (Mattay et al., 2003). Furthermore, inhibiting COMT improves WM performance in COMTValVal individuals but worsens performance in COMTMetMet individuals (Farrell, Tunbridge, Braeutigam, & Harrison, 2012). Elevating catecholamine signaling in the PFC by inducing stress (Arnsten, 2009) impairs WM performance in COMTMetMet relative to COMTValVal individuals (Buckert, Kudielka, Reuter, & Fiebach, 2012). Of particular note, Buckert et al. (2012) tested participants in a similar age range to that tested here and used a similar n-back task and d′ index of WM performance. Thus, converging evidence indicates that amplifying DA signaling in the PFC interacts with COMT Val158Met genotype according to a unified inverted-U model of PFC DA effects on WM performance.
PFC DRD1 signaling and maintaining WM representations amid interference
Our findings support the role of putative PFC DRD1 signaling in WM performance following the inverted-U model under specific task demands. In the particular n-back task employed here, we found that COMT and DARPP-32 genotype interacted to affect target discrimination only when maintenance demands are high (3-back trials). Importantly, this effect was most pronounced when maintenance of a target representation amid interfering stimuli was considered (i.e. d′ for correct target detection versus lure false alarms) as opposed to maintenance of a target amongst any non-targets, including low interfering non-lures. Thus, the present data suggests that intermediate frontal DRD1 signaling is required for optimal performance specifically in tasks with high maintenance demands under considerable interference. Such a finding is consistent with the proposition that COMT’s effect on cognition is most apparent in cognitive tasks which engage the frontal cortical neurobiology tuned by DA (Goldman, Weinberger, Malhotra, & Goldberg, 2009).
Neurocomputational models of PFC function suggest that DRD1 activation enhances persistent high activity (or “up”) states thought to underlie the maintenance of information in WM, while depressing spontaneous background activity (Durstewitz & Seamans, 2002). Thus, DRD1 signaling is thought to enhance the “signal” of a WM representation relative to background noise. Importantly, modeling suggests that DRD1-mediated changes in NMDA and GABAA currents make persistent activity in PFC networks less susceptible to interruption by interfering stimuli and enable WM representations robust to interference (Durstewitz, Seamans, & Sejnowski, 2000). Thus, our finding that genetic variations which affect DA tone in the PFC presynaptically and DRD1 signaling postsynaptically predict individual differences in the capacity to detect targets under conditions requiring a high WM load within the context of significant interfering stimuli support existing models of PFC DA-dependent WM function.
Study Limitations
While the present data are consistent with, and significantly extend, the existing literature regarding DA regulation of WM, we acknowledge the limitations of the present study. First, our sample size is relatively small and was thus not adequately powered to detect effects of other polymorphisms that impact DA signaling on WM performance. While we have assumed a role for the DRD1 modulator DARPP-32 in PFC function, based on the predominance of DRD1 in the PFC (Goldman-Rakic et al., 1992; Lidow et al., 1991; Sesack et al., 1995) and the key role for the PFC in the maintenance aspect of WM, DARPP-32 has also been implicated in forms of reward learning that depend on striatal DA (Frank et al., 2009; Frank et al., 2007). However, our data lean toward the interpretation that the interacting effects of COMT and DARPP-32 on high load WM performance reflects PFC DRD1 signaling, as when we replaced DARPP-32 genotype in our models with a DAT VNTR genotype associated with variation in striatal DA tone, we found no significant interaction. Even considering these negative DAT results, however, we can’t rule out the possibility that our results here reflect an interaction between frontal and striatal DA, perhaps via striatal gating of goal-related information into WM at the level of the PFC (Frank, Loughry, & O'Reilly, 2001; Gruber, Dayan, Gutkin, & Solla, 2006; van Schouwenburg et al., 2010). Future PET studies quantifying striatal and extrastriatal DA signaling as a function of genotype coupled with measures of WM performance will allow for a more definitive examination of this issue. Finally, it is important to note that COMT and DARPP-32 modulate both DRD1- and DRD2-mediated signaling, albeit with different downstream effects in the case of DARPP-32 (Greengard et al., 1999). However, based on evidence demonstrating the critical role of DRD1 signaling localized to the PFC in WM maintenance (Goldman-Rakic et al., 2000; Sawaguchi & Goldman-Rakic, 1991; Vijayraghavan et al., 2007), it seems parsimonious to interpret our COMT × DARPP-32 interaction effect as reflecting DRD1-mediated WM maintenance processes in the PFC. Moreover, we observed this effect on 3-back but not 2-back trials, which differ only in the number of items that must be maintained in memory to correctly identify targets. Nonetheless, further dissection of the roles of DRD1 and DRD2 signaling in these WM processes is warranted.
Dopaminergic functioning varies with age
With regard to the role of COMT in cognition, not all of the published literature agrees. The present data argues that DARPP-32 variation could underlie some differences within COMT genotype. However, developmental changes in frontal DA neurotransmission (Tunbridge et al., 2007; Wahlstrom, Collins, White, & Luciana, 2010) likely interacts with genetic effects on cognition. Such effects may contribute to conflicting results from studies investigating the role of COMT in executive function. For instance, the participants in (Mattay et al., 2003) were in their mid 30’s, while participants in a recent study that failed to replicate Mattay et al.’s (2003) effects had a mean age of 23 (Wardle, Hart, Palmer, & de Wit, 2013). While the current study focused on participants aged 22–40 to limit age-related variability in frontal DA signaling, whether similar genotype effects on WM are present among the frequent subjects of cognitive studies, emerging adults, remains an open question.
Conclusion
Our results are consistent with existing computational models for the role of PFC DRD1 signaling in WM processes (Durstewitz & Seamans, 2002). Specifically, we have shown that putative DRD1 signaling (indexed by COMT and DARPP-32 genotype) predicts the stability of target representation in the face of interfering stimuli according to an inverted-U function (Cools & D'Esposito, 2011). Furthermore, our findings support the conclusion that inverted-U-shaped DA actions on human WM and cognitive control may be particularly strong under conditions of high cognitive load.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. Bardowell, V. Benson, A. Desai, J. Drost-Lopez, E. Jacobs, K. Kelm, C. Lang, M. Le, E. Steel, and R. Kaplan for valuable technical assistance. This work was supported by Award Numbers KL2RR025746, UL1RR025747 (now UL1TR000083), and P60AA011605 (CAB) and by T32DA007244, F31AA020132 (CTS).
REFERENCES
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behav Genet. 2003;33(1):73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116(2):143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Buckert M, Kudielka BM, Reuter M, Fiebach CJ. The COMT Val158Met polymorphism modulates working memory performance under acute stress. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283(1):183–189. [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28(5):1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Peppe A, Dell'Agnello G, Caltagirone C, Carlesimo GA. Dopamine and cognitive functioning in de novo subjects with Parkinson's disease: effects of pramipexole and pergolide on working memory. Neuropsychologia. 2009;47(5):1374–1381. doi: 10.1016/j.neuropsychologia.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Costa A, Riedel M, Muller U, Moller HJ, Ettinger U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse. 2011;65(10):998–1005. doi: 10.1002/syn.20927. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters-Metenier S, Cirulli ET, Kragel J, et al. COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cereb Cortex. 2010;20(3):672–683. doi: 10.1093/cercor/bhp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15(4–6):561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64(9):739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83(3):1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66(3):438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115(4):934–939. [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12(8):1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Fossella JA. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology. 2011;36(1):133–152. doi: 10.1038/npp.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci U S A. 2007;104(41):16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, et a. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7(5):1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. The anatomy of dopamine in monkey and human prefrontal cortex. J Neural Transm Suppl. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, Williams GV., 3rd D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Goldberg TE. The role of COMT Val158Met in cognition. Biol Psychiatry. 2009;65(1):e1–e2. doi: 10.1016/j.biopsych.2008.07.032. author reply e3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Green D, Swets J. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23(3):435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci. 2006;20(2):153–166. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Fienberg AA, Greengard P, Gold LH. DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res. 2000;867(1–2):122–130. doi: 10.1016/s0006-8993(00)02272-1. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Hollingshead's Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Hotte M, Thuault S, Lachaise F, Dineley KT, Hemmings HC, Nairn AC, et al. D1 receptor modulation of memory retrieval performance is associated with changes in pCREB and pDARPP-32 in rat prefrontal cortex. Behav Brain Res. 2006;171(1):127–133. doi: 10.1016/j.bbr.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D'Esposito M. Estrogen Shapes Dopamine-Dependent Cognitive Processes: Implications for Women's Health. J Neurosci. 2011;31(14):5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, et al. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157(10):1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114(6):1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3'-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46(2):151–160. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kolata S, Light K, Wass CD, Colas-Zelin D, Roy D, Matzel LD. A dopaminergic gene cluster in the prefrontal cortex predicts performance indicative of general intelligence in genetically heterogeneous mice. PLoS One. 2010;5(11):e14036. doi: 10.1371/journal.pone.0014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4(8):e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40(3):657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47(2):101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- Macmillan N, Creelman C. Signal Detection Theory: A User's Guide. 2 ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12(3):268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod HL, Fang L, Luo X, Scott EP, Evans WE. Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans. J Pharmacol Exp Ther. 1994;270(1):26–29. [PubMed] [Google Scholar]
- McLeod HL, Syvanen AC, Githang'a J, Indalo A, Ismail D, Dewar K, et al. Ethnic differences in catechol O-methyltransferase pharmacogenetics: frequency of the codon 108/158 low activity allele is lower in Kenyan than Caucasian or South-west Asian individuals. Pharmacogenetics. 1998;8(3):195–199. [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Straub RE, Lipska BK, Verchinski BA, Goldberg T, Callicott JH, et al. Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117(3):672–682. doi: 10.1172/JCI30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, et al. Distribution of the 3' VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol. 2000;72(2):295–304. [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17(21):8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474(7351):372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4(1):111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Karlsson S, Fischer H, Backman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J Neurosci. 2011;31(40):14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res. 2001;41(2):115–128. doi: 10.1016/s0168-0102(01)00270-x. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71(2):515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363(2):264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and ancestry modulate brain's DAT availability in healthy humans. PLoS One. 2011;6(8):e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology (Berl) 2012;222(4):609–617. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50(1):45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46(5):745–751. [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des. 2010;16(18):2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984;4(1):84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Hart AB, Palmer AA, de Wit H. Does COMT genotype influence the effects of d-amphetamine on executive functioning? Genes Brain Behav. 2013;12(1):13–20. doi: 10.1111/gbb.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139(1):263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wu K, O'Keeffe D, Politis M, O'Keeffe GC, Robbins TW, Bose SK, et al. The catechol-O-methyltransferase Val(158)Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson's disease: a PET study. Brain. 2012;135(Pt 8):2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27(38):10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.