Abstract
Recently we have shown that docosahexaenoic acid complexed to albumin (DHA-Alb) is neuroprotective after experimental stroke in young rats. The purpose of this study was to determine whether treatment with DHA-Alb would be protective in aged rats after focal cerebral ischemia. Isoflurane/nitrous oxide-anesthetized normothermic (brain temperature 36–36.5°C) Sprague-Dawley aged rats (18-months old) received 2 h middle cerebral artery occlusion (MCAo) by poly-L-lysine-coated intraluminal suture. The neurological status was evaluated during occlusion (60 min) and on days 1, 2, 3 and 7 after MCAo; a grading scale of 0–12 was employed. DHA (5mg/kg), Alb (0.63g/kg), DHA-Alb (5mg/kg+0.63g/kg) or saline was administered i.v. 3 h after onset of stroke (n=8–10 per group). Ex vivo T2-weighted imaging (T2WI) of the brains was conducted on an 11.7T MRI on day 7 and 3D reconstructions were generated. Infarct volumes and number of GFAP (reactive astrocytes), ED-1 (activated microglia/microphages), NeuN (neurons)-positive cells and SMI-71 (positive vessels) were counted in the cortex and striatum at the level of the central lesion. Physiological variables were entirely comparable between groups. Animals treated with DHA-Alb showed significantly improved neurological scores compared to vehicle rats; 33% improvement on day 1; 39% on day 2; 41% on day 3; and 45% on day 7. Total and cortical lesion volumes computed from T2WI were significantly reduced by DHA-Alb treatment (62 and 69%, respectively). In addition, treatment with DHA-Alb reduced cortical and total brain infarction while promoting cell survival. We conclude that DHA-Alb therapy is highly neuroprotective in aged rats following focal cerebral ischemia and has potential for the effective treatment of ischemic stroke in aged individuals.
Introduction
Stroke is a major cause of death and disability in the elderly. However, experimental stroke research, including the evaluation of neuroprotective drugs, has almost universally relied on use of young animals despite the importance of aging on cerebrovascular disease in humans. This might reflect in part the considerable difficulties to establish a reproducible stroke model in aged animals. In addition, aging has been associated with a significant increase in cerebral infarct size and high mortality (Davis et al., 1995). The need to evaluate and develop an effective treatment for elderly stroke patients remains paramount. Thus animal studies which investigate the mechanisms and efficacy of novel treatments specifically in aged animal remain ideal.
High-dose human albumin (Alb) therapy is strongly neuroprotective in animal models of focal cerebral ischemia (Belayev et al., 2001), as well as in global cerebral ischemia (Belayev et al., 1999) and traumatic brain injury (Belayev et al., 1999). The neuroprotective efficacy of albumin is attributed to its multifunctional properties, which include antioxidant action, hemodilution and oncotic effects, binding of copper ions, fatty-acid transport, preservation of endothelial integrity, platelet anti-aggregatory effects and decreased red blood cell sedimentation under low-flow conditions (Belayev et al., 1997; Belayev et al., 1998; Belayev et al., 2002). Recently, Alb was studied in a phase III clinical trial for acute ischemic stroke (Ginsberg et al., 2011), but the trial was stopped, because it was associated with more adverse effects than saline control (administration of high-dose albumin in dose of 2 g/kg expanded intravascular volume, which leads to pulmonary edema in 13% of patients).
Recent studies have established that omega-3 fatty acids reduce inflammation and may help lower risk of chronic diseases such as heart disease, cancer, and arthritis. Docosahexaenoic acid (DHA; 22:6, n-3), a member of omega-3 fatty acid family, is highly concentrated in the brain and appears to be important for cognitive (brain memory and performance) and behavioral function. DHA therapy in low (3.5 mg/kg) and medium (5 mg/kg) doses improves neurological and histological outcome following focal cerebral ischemia in rats (Belayev et al., 2009). Recently, we complexed 25% human serum albumin to DHA and compared its neuroprotective efficacy with that of native albumin in young rats with 2 h of focal cerebral ischemia (Belayev et al., 2005). The DHA-Alb complex affords high-grade neurobehavioral neuroprotection in focal cerebral ischemia, equaling or exceeding that afforded by native albumin at considerably lower doses of Alb (0.63 g/kg). In addition, DHA-Alb leads to improved neurological outcomes and significant reductions of infarct volumes (especially in the salvageable penumbral region) even when treatment is initiated as late as 7 hours after onset of middle cerebral artery occlusion (MCAo) (Eady et al., 2012b). The purpose of this study was to determine whether treatment with DHA-Alb would similarly protect aged rats from focal cerebral ischemia.
Materials and methods
Animal preparation
All studies were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center, New Orleans. Twenty nine male Sprague-Dawley aged rats (18-months old, Harlan Laboratories, Inc, Indianapolis, IN) were fasted overnight but allowed free access to water. Atropine sulfate (0.5 mg/kg, i.p.) was injected 10 min before anesthesia. Anesthesia was induced with 3.5% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen. All rats were orally intubated and mechanically ventilated. During ventilation, the animals were paralyzed with pancuronium bromide (0.6 mg/kg, i.p.). Temperature probes were inserted into the rectum and the left temporalis muscles. Heating lamps were used to maintain rectal and temporalis temperatures at 36° to 37°C. Arterial blood gases, pH, hematocrit and plasma glucose were measured 15 min before, 15 min after stroke and 15 min after treatment (3 h 15 min after onset of MCAo). Body weight and rectal temperature were monitored daily during a one-week survival period.
Stroke model
Focal cerebral ischemia was induced by the intraluminal-filament method as we previously described (Belayev et al., 1996). The right middle cerebral artery (MCA) was temporarily occluded for 2 h by a filament coated with poly-L-lysine (Belayev et al., 1996). In brief, the right common carotid artery (CCA) was exposed through a midline neck incision and dissected free of the surrounding nerves. The occipital branches of the external carotid artery (ECA) were coagulated, and the pterygopalatine artery was ligated. A 4-cm length of 3-0 monofilament nylon suture was inserted via the proximal ECA into the internal carotid artery (ICA) and MCA. Prior to use, the tip of the suture was heat-blunted and a 20-mm distal segment of the suture was coated with poly-Llysine solution (0.1% [wt/vol]) and dried at 60°C for 1 h (Belayev et al., 1996). Following suture placement, the neck incision was closed, animals were allowed to awaken from anesthesia, and they were tested using a standardized neurobehavioral battery. After 2 h of MCAo, rats were reanesthetized with the same anesthetic combination. Temperature probes were reinserted, and intraluminal sutures were carefully removed. The neck incisions were closed with silk sutures and animals were allowed to survive with free access to food and water. Because of severe neurological deficit and high mortality after MCAo in the aged rats, we limited our study up to 7 days after stroke.
Neurobehavioral testing
Behavioral tests were performed by an observer blinded to the treatment groups at 60 min during MCAo and then on days 1, 2, 3 and 7 after MCAo. The battery consisted of two tests used previously to evaluate various aspects of neurologic function: (1) the postural reflex test, to examine upper body posture while the animal is suspended by the tail; and (2) the forelimb placing test, to examine sensorimotor integration in forelimb placing responses to visual, tactile and proprioceptive stimuli (Belayev et al., 1996). Neurological function was graded on a scale of 0–12 (normal score = 0, maximal score = 12), as previously described (Belayev et al., 1996). Only those animals with a high-grade neurological deficit (10 or greater) were used.
Treatment
Docosahexaenoic acid (DHA) in acid form (Cayman Chemical, Ann Arbor, MI) was physically complexed to human albumin (Alb) by incubating 20 ml of human serum albumin (25%; Baxter, Westlake Village, CA) with 5 mg DHA/g albumin (molar ratio = 0.2) as we previously described (Belayev et al., 2005). Animals were randomly assigned to four treatment groups: DHA (5mg/kg; n=6), Alb (0.63/kg; n=6), DHA-Alb (5mg/kg+0.63g/kg; n=8) or saline (n=9). Treatment was administered 3 h after onset of stroke into the femoral vein over the course of 3 min using an infusion pump.
Ex vivo MRI
High resolution ex vivo MRI was performed on 4% paraformaldehyde fixed brains on day 7 using an 11.7T Bruker Advance 8.9 cm horizontal bore instrument equipped with an 89 mm (ID) receiver coil (Bruker Biospin, Billerica, MA). MRI-derived lesion volumes were extracted from high resolution T2 weighted images (T2WI) and encompassed cortical and subcortical regions of the entire brain from which 3D reconstructions were generated to illustrate the extent of the lesions. Cheshire image processing software (Hayden Image Processing Group, Waltham, MA) and Amira (Mercury Computer Systems, Visage Imaging, Inc., San Diego, CA) were used for MRI analysis. We have previously reported no volumetric differences between in vivo and ex vivo MRI lesion assessment (Obenaus et al., 2011).
Histopathology and immunostaining
After completion of ex vivo MRI studies, brains were embedded in a gelatin matrix using MultiBrain™ Technology (NeuroScience Associates, Knoxville, TN). Coronal sections were stained with thionine (Nissl), digitized at nine standardized coronal levels and analyzed (MCID™ Core imaging software, Linton, Cambridge, UK) as we previously described (Belayev et al., 1996). An investigator blinded to the experimental groups then outlined the zones of infarction (which were clearly demarcated) as well as the left and right hemispheres of each section. Infarct volume was calculated as the integrated product of cross-sectional area and inter-section distance and corrected for brain swelling.
Immunohistochemical procedures were performed on the adjacent sections to identify specific vascular and neuronal elements in the infarct area. The following antibodies were used: glial fibrillary acid protein (GFAP, Santa Cruz, SDS Biosciences, Sweden) to label reactive astrocytes, Cd68/ED-1 (Serotec, Raleigh, NC) for activated microglia/microphages, Neuron-Specific Nuclear Protein (NeuN, Chemicon/Millipore, Billerica, MA) and rat blood-brain barrier (SMI-71, Sternberger Monoclonals, Inc., Baltimore, MD) as a vascular marker. Numbers of GFAP, ED-1, NeuN positive cells and SMI-71 immunopositive vessels were counted in the three cortical and one subcortical area (ipsi- and contralateral sides) at the level of the central lesion (bregma level −0.3 mm). Data were expressed as numbers of positive cells and vessels per high-power microscopic field (magnification ×40).
Statistical analysis
Data are presented as mean values ± SEM. Repeated measures analysis of variance (ANOVA) followed by Bonferroni procedures to correct for multiple comparisons were used for intergroup comparisons of behavioral scores over time and infarct areas across coronal levels. Two-tailed Student’s t tests were used for two-group comparisons. Differences at P<0.05 were considered statistically significant.
Results
Physiological variables
Rectal and cranial (temporal muscle) temperatures, arterial blood gases, plasma glucose and hematocrit showed no significant differences between groups (Table 1). There were no adverse side effects observed after treatment in all groups.
Table 1.
Physiological variables
| Saline (n=7) |
DHA (n=6) |
Albumin (n=5) |
DHA-Alb (n=8) |
|
|---|---|---|---|---|
| Before MCAo (15 min) | ||||
| Rectal temperature (°C) | 37.0 ± 0.1 | 36.8 ± 0.2 | 37.3 ± 0.3 | 37.0 ± 0.1 |
| Cranial temperature (°C) | 36.9 ± 0.1 | 36.6 ± 0.2 | 37.1 ± 0.2 | 37.0 ± 0.2 |
| pH | 7.44 ± 0.02 | 7.44 ± 0.01 | 7.46 ± 0.01 | 7.46 ± 0.01 |
| pO2, mm Hg | 100 ± 7 | 109 ± 9 | 113 ± 6 | 105 ± 5 |
| pCO2, mm Hg | 38 ± 0.8 | 39 ± 1.0 | 37 ± 0.4 | 39 ± 0.4 |
| Plasma glucose, mg/dL | 239 ± 17 | 246 ± 32 | 207 ± 9 | 208 ± 10 |
| Hematocrit | 46 ± 1 | 48 ± 1 | 49 ± 1 | 49 ± 0 |
| Body weight (g) | 578 ± 13 | 575 ± 9 | 502 ± 12 | 494 ± 3 |
| During MCAo (15 min) | ||||
| Rectal temperature (°C) | 37.6 ± 0.1 | 37.7 ± 0.2 | 37.5 ± 0.2 | 37.4 ± 0.1 |
| Cranial temperature (°C) | 37.2 ± 0.2 | 37.1 ± 0.1 | 36.8 ± 0.2 | 37.2 ± 0.1 |
| pH | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.46 ± 0.02 | 7.45 ± 0.0 |
| pO2, mm Hg | 90 ± 5 | 93 ± 2 | 104 ± 5 | 96 ± 7 |
| pCO2, mm Hg | 42 ± 1 | 40 ± 0 | 39 ± 1 | 39 ± 1 |
| Plasma glucose, mg/dL | 207 ± 9 | 231 ± 29 | 175 ± 11* | 191 ± 10 |
| Hematocrit | 46 ± 1 | 47 ± 1 | 49 ± 0 | 49 ± 1 |
| After treatment (15 min) | ||||
| Rectal temperature (°C) | 36.8 ± 0.3 | 36.4 ± 0.1 | 37.5 ± 0.2 | 37.4 ± 0.2 |
| Cranial temperature (°C) | 36.6 ± 0.1 | 36.9 ± 0.2 | 37.0 ± 0.2 | 37.0 ± 0.1* |
| Hematocrit | 44 ± 1.0 | 44 ± 0.9 | 37 ± 0.3 | 38 ± 0.5 |
| After treatment (24 h) | ||||
| Rectal temperature (°C) | 37.4 ± 0.4 | 37.4 ± 0.2 | 37.5 ± 0.4 | 37.4 ± 0.5 |
| Body weight (g) | 544 ± 11 | 553 ± 7 | 476 ± 9 | 471 ± 6 |
| After treatment (48 h) | ||||
| Rectal temperature (°C) | 37.4 ± 0.9 | 37.6 ± 0.3 | 37.1 ± 0.2 | 37.5 ± 0.3 |
| Body weight (g) | 510 ± 13 | 537 ± 6 | 467 ± 11 | 460 ± 8 |
| After treatment (72 h) | ||||
| Rectal temperature (°C) | 37.5 ± 0.5 | 37.6 ± 0.3 | 37.5 ± 0.2 | 37.8 ± 0.2 |
| Body weight (g) | 514 ± 13 | 528 ± 7 | 462 ± 12 | 452± 10 |
| After treatment (1 week) | ||||
| Rectal temperature (°C) | 37.3 ± 0.3 | 37.9 ± 0.2 | 37.4 ± 0.2 | 37.4 ± 0.2 |
| Body weight (g) | 510 ± 16 | 531 ± 7 | 462 ± 16 | 460 ± 12 |
Values are mean ± SEM
MCAo, middle cerebral artery occlusion
different from saline group (p<0.01, Student's t-test).
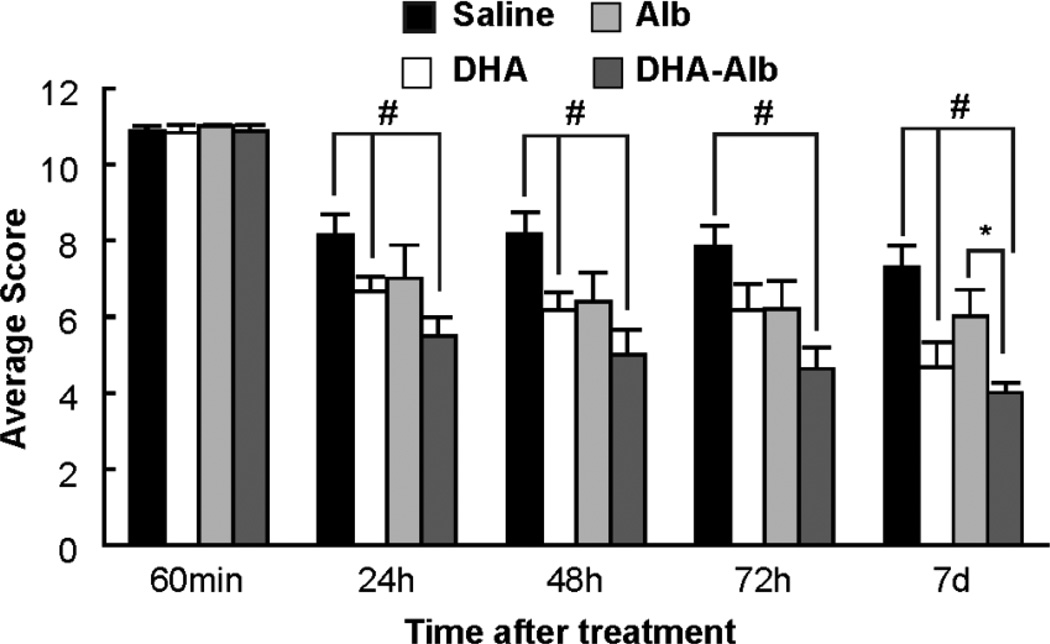
DHA-Alb improves behavioral function
Animals in all groups exhibited high-grade neurological deficits (score = 11) after 60 minutes of MCAo; thus, no animals required exclusion on the basis of an inadequate degree of cerebral ischemia (Fig. 1). Saline- and Alb-treated rats both developed severe neurological deficits. However, rats in the DHA and DHA-Alb groups showed improvements in neurological function compared to the saline group throughout the 7-day survival period (Fig. 1). DHA-Alb treatment significantly improved the neurological score compared to the Alb group on day 7 (Fig. 1).
Figure 1.
Total neurological score (normal score=0, maximal score=12) during MCAo (60 min) and on days 1, 2, 3 and 7 after treatment. All treatments were administered intravenously at 3 h after onset of stroke. DHA and DHA-Alb treatment significantly improved neurological score compared to the saline group during the 7-day survival period. In addition, DHA-Alb treatment significantly improved the neurological score compared to the corresponding Alb group on day 7. Values shown are means ± SEM. #P<0.05 versus saline group; *P<0.05 DHA-Alb versus Alb group (repeated measures ANOVA followed by Bonferroni tests).
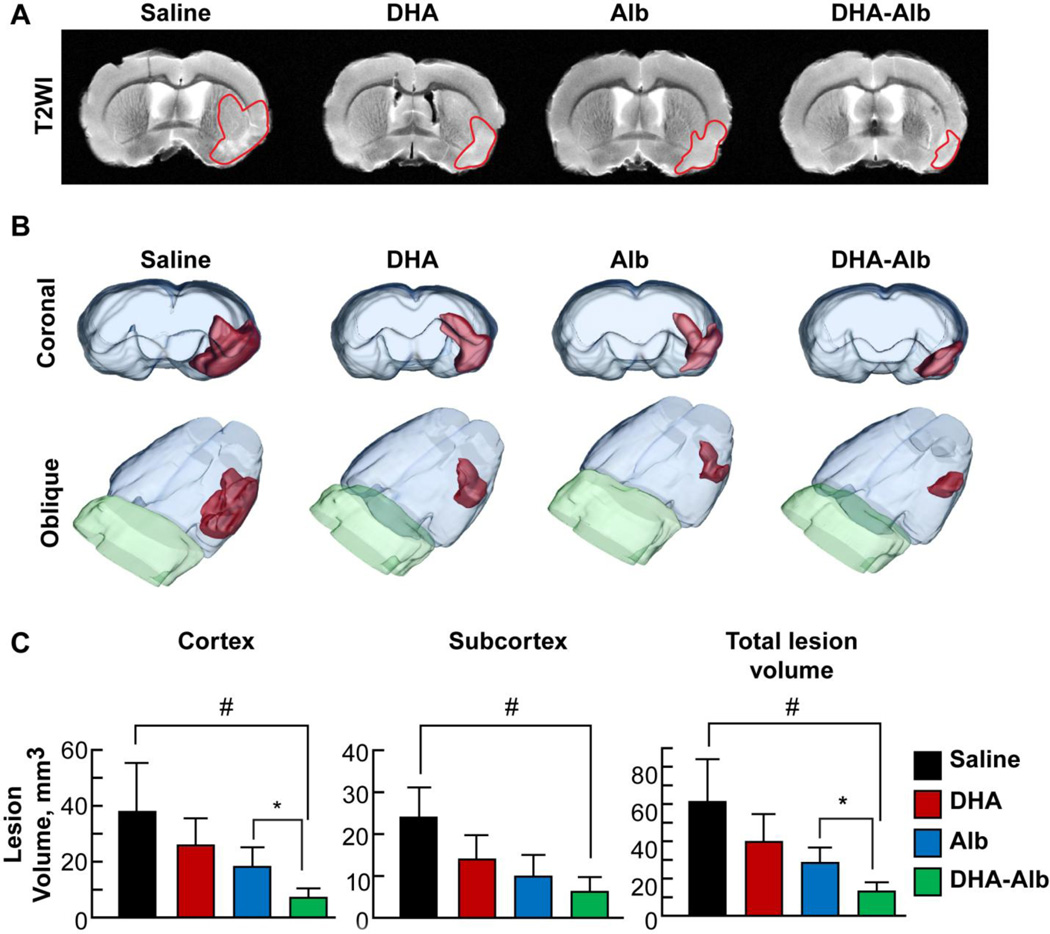
DHA-Alb reduces T2WI lesion volumes
T2 hyperintensities were observed in the cortex and striatum of saline-treated rats, consistent with ongoing edema formation (Fig. 2A). Conversely, lesion volume was reduced in the DHA and Alb groups and was markedly decreased in the DHA-Alb treatment group (Fig. 2A). Three-dimensional lesion volumes were computed from T2WI with saline-treated rats showing large cortical and subcortical lesion volumes (Fig. 2B). A reduction in lesion volume was observed in DHA and Alb-treated rats that was primarily localized to small cortical and subcortical areas. In contrast, DHA-Alb treatment further reduced lesion volumes (Fig. 2B). Lesion volumes computed from T2WI are presented in Figure 2C. Animals treated with DHA-Alb had dramatically reduced cortical (by 69%) and total lesion volumes (by 62%) as compared to the Albtreated group.
Figure 2. Ex vivo MRI at 7d after MCAo.
Panel A: Representative T2WI from saline, DHA-, Alb- and DHA-Alb-treated rats. T2 hyperintensities were observed in the cortex and striatum of saline-treated rats, consistent with ongoing edema formation. DHA- and Alb-treated animals had smaller lesion size, with only partial cortex and subcortical involvement. In contrast, DHA-Alb-treated rats had primarily only a small subcortical lesion. Panel B: 3D reconstructions of MRI-derived lesion volumes from high resolution T2 weighted images (T2WI). Saline-treated rats showed large cortical and subcortical lesion volumes. Rats treated with DHA or Alb show less lesion volume, mostly in the subcortical area. By contrast, lesion volume was dramatically reduced in rats treated with DHA-Alb and was mostly localized in the subcortex. Panel C: Cortical, subcortical and total lesion volumes were computed from T2WI. Only treatment with DHA-Alb significantly reduced cortical, subcortical and total lesion volumes compared to the saline-treated group. In addition, DHA-Alb therapy reduced cortical and total lesion volumes compared to the corresponding Alb group. DHA and Alb treatments alone also reduced lesion volumes, but were not significantly different from saline and from each other. Values shown are means ± SEM., #P<0.05 versus saline group; *P<0.05 DHA-Alb versus Alb group (repeated measures ANOVA followed by Bonferroni tests).
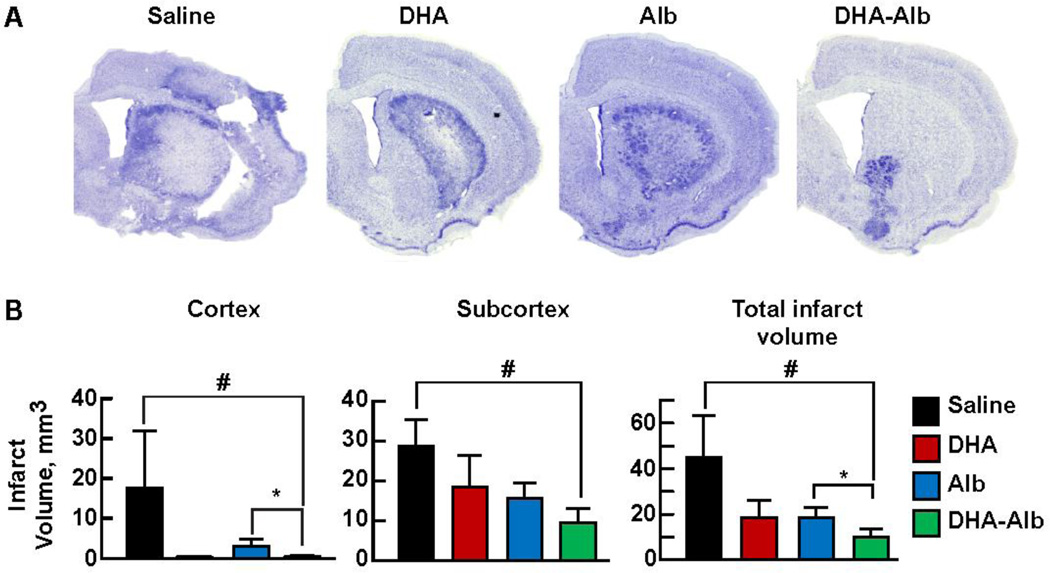
DHA-Alb decreases histological damage and promotes cell survival
The brains of saline-treated animals, studied after 7 days of survival, confirmed large zones of infarction involving both cortical and subcortical regions of the right hemisphere (Fig. 3A). These infarcted regions are characterized microscopically by destruction of neuronal, glial, and vascular elements. In contrast, DHA- and Alb-treated rats showed smaller infarct volumes that were mostly localized to the subcortical area. DHA-Alb treatment dramatically reduced lesion volume, which was limited to a small area of the subcortex (Fig. 3A). Treatment with DHA-Alb significantly reduced total infarct volume (by 50%) as compared to the corresponding Alb-group, and cortical infarct was almost eliminated by DHA-Alb therapy (Fig. 3B).
Figure 3. Histopathology at 7d after MCAo.
Panel A: Computer-generated MosaiX processed images (Zeiss Axio Imager.M1; AxioVision Release 4.6.3) of Nissl stained paraffin-embedded brain sections from rats treated with saline, DHA, Alb or DHA-Alb. Saline-treated rats showed large cortical and subcortical infarction. Rats treated with DHA or Alb show less extensive damage, mostly in the subcortical area. In contrast, DHA-Alb treated rat shows a very small infarction, involving only subcortical region. Panel B: Cortical, subcortical and total infarct volumes in four groups. Total infarct volume was corrected for brain swelling. DHA-Alb treatment reduced cortical and total infarct volumes compared to corresponding Alb group. DHA and Alb treatment alone also reduced infarct volumes, but were not significantly different from saline and from each other. Values shown are means ± SEM., *P<0.05 #P<0.05 versus saline group; *P<0.05 DHA-Alb versus Alb group (repeated measures ANOVA followed by Bonferroni tests).
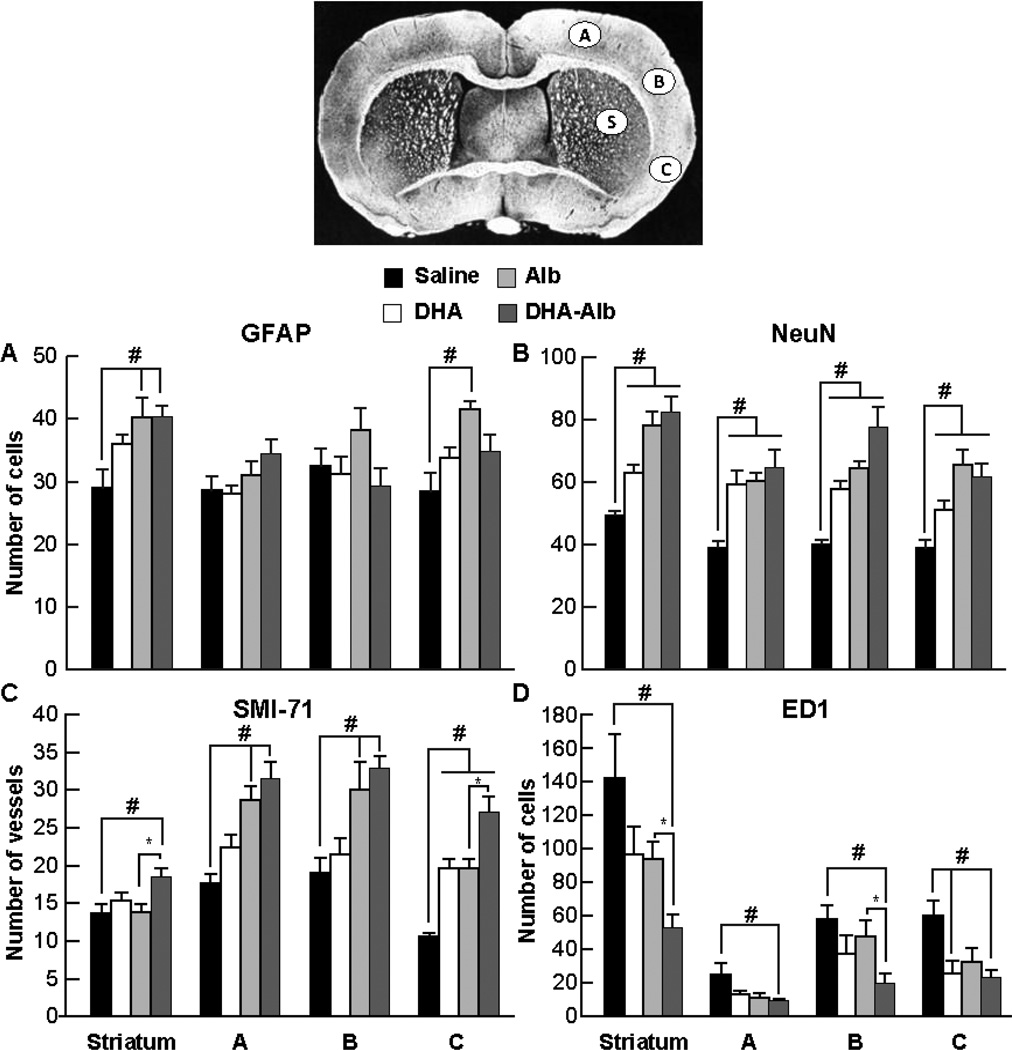
Cellular count for GFAP, NeuN, ED-1 positive cells and SMI-71 immunopositive vessels is presented in Figure 4. Saline-treated rats showed loss of neuronal, glial, vascular elements and massive ED-1 positive microglia/macrophage infiltration (Fig. 4A–D). In contrast, DHA-Alb-treated rats decreased ED-1 positive microglia/microphages and increased SMI-71 positive vessels compared to the Alb-treated group. Increased blood vessel density was observed in the cortical and subcortical areas of the ischemic hemisphere (Fig. 4C–D).
Figure 4.
Cell count for GFAP positive astrocytes, NeuN positive neurons, SMI-71 positive vessels and ED1 positive microglia cells on day 7 after 2h of MCAo. Coronal brain diagram showing locations of regions for cell count (bregma level - 0.3 mm) in cortex (A, B, and C) and striatum (S). Alb- and DHA-Alb-treated rats expressed increased numbers of GFAP compared to the saline-treated group (Panel A). All treatments (DHA, Alb, DHA-Alb) increased numbers of NeuN positive cells and SMI-71 immunopositive vessels compared to saline-treated rats (Panels B and C). In addition, DHA-Alb treatment increased SMI-71 positive vessels compared to the corresponding Alb group (Panel C). DHA, Alb, and DHA-Alb treatments decreased microglial invasion of the infarcted tissue while salinetreated rats showed significant microglial infiltration of the infarct regions (Panel D). In addition, DHA-Alb treatment decreased ED1 positive microglia compared to the corresponding Alb group (Panel D). Values shown are means ± SEM., *P<0.05 #P<0.05 versus saline group; *P<0.05 DHA-Alb versus Alb group (repeated measures ANOVA followed by Bonferroni tests).
Cell count for GFAP, NeuN, ED-1 positive cells and SMI-71 immunopositive vessels in the contralateral (non-ischemic) hemisphere of the brain was conducted as well. All treatment groups had a similar number of positive cells and vessels: the average number of GFAP positive astrocytes (striatum: 23–27; cortex: 16–25 cells), NeuN (striatum: 61–73; cortex: 51–73 cells) and SMI-71 vessels (striatum: 16–17; cortex: 17–30 vessels. There were no activated microglia cells present (striatum: 7–9; cortex: 4–8 cells).
Three animals died during the experiment: two rats in the saline group (on days 2 and 3) and one rat in the Alb group (on day 2).
Discussion
This study was prompted by our earlier findings demonstrating the neuroprotective efficacy of DHA-Alb therapy after focal cerebral ischemia in young rats (Belayev et al., 2005; Eady et al., 2012b). Previously, we have shown treatment with a moderate dose of DHA and low dose of Alb (5mg/kg+0.63g/kg) results in greatly improved behavioral outcomes, significantly reduced infarction volumes, and markedly diminished brain swelling after ischemia-reperfusion injury. This protection was clinically advantageous in that lower doses of albumin, when complexed with DHA, might reduce the likelihood of acute intravascular volume overload and congestive heart failure sometimes associated with albumin administration (Belayev et al., 2005). In subsequent studies, treatment with a moderate dose of DHA-Alb led to improved neurological outcomes and significant reductions of infarct volumes (especially in the salvageable penumbral region) even when treatment was initiated as late as 7 hours after onset of MCAo (Eady et al., 2012b). In this part of the study, we demonstrate that prompt DHA-Alb administration is efficacious in protecting the aged brain after focal cerebral ischemia, a more vulnerable target due to less protective reserves and increased cumulative damage as compared to the young brain (Davis et al., 1995). Our results clearly indicate that DHA-Alb therapy substantially improves neurological function, reduces T2WI lesion volumes, diminishes cortical and total brain infarcts, and promotes cell survival in the aged brain.
Docosahexaenoic acid is an essential omega-3 fatty acid that must be consumed from the diet. DHA can be found in fish (salmon, tuna, mackerel, herring, sardines, halibut), other seafood (algae, krill) and some nuts and plants. It is well established that DHA and other omega-3 fatty acids have an important role in heart health. The American Heart Association recommends consuming 400 to 500 mg/day of omega-3 fatty acids or eating fish, especially cold water fish, at least two times a week to reduce the risk of heart failure or myocardial infarctions (Harris et al., 2008). Omega-3 fatty acids also play a critical role in brain function, as well as normal growth and development (Bazan, 2007). We have discovered that DHA is a crucial component of the body's endogenous mechanism to protect the brain after injury. Cerebral ischemia-reperfusion results in the rapid accumulation of free fatty acids (FFA), including arachidonic acid (AA; 20:4 n-6) and DHA, which are released from membrane phospholipids. An early increase in unesterified (free) DHA during brain ischemia initiates a pathway for docosanoid biosynthesis, specifically neuroprotectin D1 (NPD1) (Bazan, 2003; Marcheselli et al., 2003). NPD1 functions to inhibit apoptosis and promotes brain cell survival (Bazan, 2005; Bazan, 2006). We have recently shown that NPD1 inhibits brain ischemia-reperfusion-mediated leukocyte infiltration and proinflammatory gene expression, promotes neurogenesis both in vitro and in vivo, and has been implicated in neuroprotection (Bazan, 2007; Marcheselli et al., 2003).
Preclinical studies in rodent models of ischemic stroke have established that that administration of Alb at high doses (25%, 2.5 g/kg, i.v.) decreases infarct volume, reduces brain swelling (Belayev et al., 1997, Belayev et al., 1998) and improves local cerebral perfusion in affected tissue (Huh et al., 1998). Among its actions in ischemia, albumin induces the systemic mobilization of omega-3 polyunsaturated fatty acids (PUFAs) and may help to replenish PUFAs lost from neural membranes (Rodriguez de Turco et al., 2002). We have shown when albumin is complexed with DHA, it is possible to achieve neuroprotection at lower albumin doses. This is desirable because high-dose albumin expands intravascular volume and may exacerbate congestive heart failure in certain patients.
The complex processes that occur after stroke require the targeting of multiple factors and cells, including glia, vascular, and inflammatory cells. Our results demonstrated that DHA-Alb treated brains showed attenuated cellular death of both astrocytes and neurons and fewer activated microglia, as compared to saline-treated brains within the infarcted region. One of the limitations of our study was to use a count of the remaining cells, which could be affected by proliferative/regenerative activity in the post-ischemic brain. We are planning to use a direct measurement of cell death using methods such as TUNEL staining in our future experiments.
In this study, not only did DHA-Alb significantly improve neurological outcomes, reduce infarct volumes, and promote cellular survival, but it had these effects in an aged rat model. This study reinforces the importance of using older animals to identify effective treatments for stroke victims. Nearly three-quarters of all strokes occur in people over the age of 65 (Feigin et al., 2003). Despite this, most preclinical studies utilize only young animals who tend to have a dramatic capacity to recover even from large strokes (Manwani et al., 2011). There are well-documented age-related changes in the central nervous systems of humans and laboratory animals including a relative deficiency in collateral circulation with age. The avoidance of aged animals in experimental studies is due in part to considerable difficulties to establish a reproducible stroke model, high mortality, and the high cost of these animals (Davis et al., 1995). It has been previously reported that in aged rats the passage of the filament through the ICA is difficult, which was attributed either to a narrower carotid channel or a more tortuous course of the ICA (Manwani et al., 2011). In an additional 50% of all rats, attempts resulted in a rupture of the intracranial arteries (most probably of the ICA) with subsequent subarachnoid hemorrhage. From our experience to date, the insertion of 3/0 monofilament sutures in aged rats was necessary for successful MCAo. The correct suture position was confirmed by feeling a certain resistance during filament forwarding or by advancing the suture a defined distance (23–24 mm) from the CCA bifurcation. We also used a Poly-L-lysine-coated suture in order to enhance its adhesion to the surrounding endothelium, which increased the reproducibility of the resulting infarction (Belayev et al., 1996). The severity of stroke injury was assessed by behavioral examination of each rat at 60 min after onset of MCAo. Rats that did not demonstrate high-grade contralateral deficit (score, 10–11) were excluded from further study.
As many therapeutic strategies to treat stroke have been developed in young animals, concerns exist regarding the translation of these therapies to aging animals, and more importantly, to patients at the highest risk for stroke, the elderly. It has been suggested that a lack of specific dietary nutrients, such as essential omega-3 fatty acids, may significantly contribute to cognitive decline and increased risk and severity of brain injury. In addition to decreased dietary intake, age-related defects in antioxidant systems result in an increase in lipid peroxidation that further reduces DHA levels (Uauy and Dangour, 2006). Considering aged individuals have reduced omega-3 fatty acid intake and enhanced lipid peroxidation, it follows that aging is associated with decreased levels of DHA in both rat and human brains (Uauy and Dangour, 2006). We have shown that aged rats have the capacity to synthesize NPD1, and that this synthesis is potentiated by DHA treatment (Eady et al., 2012a). Treatment with Alb appears to facilitate the delivery of DHA to injured brain tissue (Belayev et al., 2005, Eady et al., 2012b). Alb has several high-affinity fatty acid binding sites (Fujiwara and Amisaki, 2008; Simard et al., 2005). We and others have demonstrated that the DHA bond to albumin is readily provided to the brain (in vivo as in the present study). The results of this study indicate that after prompt treatment with a moderate dose of DHA complexed with low dose of Alb, the aged brain has the capacity to utilize DHA in a beneficial way, culminating in decreased infarct volume, increased cellular survival, and behavioral improvements after ischemia reperfusion injury.
In addition to histopathology, ex vivo MRI was performed in our study. The rationale for undertaking MRI is three-fold: 1) MRI can rapidly acquire and generate whole brain and lesion volumes (<30 min) which is considerably faster than traditional histological methods, 2) the MRI data are typically volumetric with no gaps in sampling, unlike histology where often tissue sections are skipped to facilitate analysis speed, and 3) MRI sequences used in the present study are also clinically relevant, allowing for translatability to future human studies.
In summary, DHA-Alb complex therapy given to aged animals after MCAo results in high-grade neuroprotection equaling or exceeding that afforded by native albumin, improves behavioral outcome even when treatment is delayed up to 3 h after stroke onset, and reduces subcortical and total infarct volumes. DHA-Alb complex, at relatively low albumin doses, is clinically advantageous in that it avoids the complications of acute intravascular volume overload and congestive heart failure sometimes induced in patients with compromised cardiovascular function. We conclude that DHA-Alb therapy is highly neuroprotective in aged rats following focal cerebral ischemia and has potential as an effective treatment for ischemic stroke in aged individuals.
Research Highlights.
We examine the effect of DHA-Alb treatment on MCAo in aged rats.
DHA-Alb therapy improves behavior during 7 days after stroke.
DHA-Alb treatment reduces T2WI lesion volumes.
DHA-Alb treatment reduces cortical and total infarct volumes.
Treatment with DHA-Alb may be beneficial for stroke patients.
ACKNOWLEDGEMENTS
This study was supported by R01 NS065786 (LB). The authors thank Neuroscience Associates, Inc., for histology service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Huh PW, Zhao W, Busto R, Ginsberg MD. Posttreatment with high-dose albumin reduces histopathological damage and improves neurological deficit following fluid percussion brain injury in rats. J. Neurotrauma. 1999;16:445–453. doi: 10.1089/neu.1999.16.445. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Clemens JA, Ginsberg MD. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J. Neurosurg. 1997;87:595–601. doi: 10.3171/jns.1997.87.4.0595. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, Bazan NG. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, Zhao W, Busto R, Ginsberg MD. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, Ginsberg MD. Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res. 1999;845:107–111. doi: 10.1016/s0006-8993(99)01952-6. [DOI] [PubMed] [Google Scholar]
- Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, Busto R, Ginsberg MD. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG. Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One. 2012a;7:e46151. doi: 10.1371/journal.pone.0046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady TN, Khoutorova L, Atkins KD, Bazan NG, Belayev L. Docosahexaenoic acid complexed to human albumin in experimental stroke: neuroprotective efficacy with a wide therapeutic window. Exp. Transl. Stroke Med. 2012b;4 doi: 10.1186/2040-7378-4-19. 19-7378-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara S-i, Amisaki T. Identification of high affinity fatty acid binding sites on human serum albumin by MM-PBSA method. Biophys. J. 2008;94:95–103. doi: 10.1529/biophysj.107.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Palesch YY, Martin RH, Hill MD, Moy CS, Waldman BD, Yeatts SD, Tamariz D, Ryckborst K ALIAS Investigators. The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011;42:119–127. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr. Atheroscler. Rep. 2008;10:503–509. doi: 10.1007/s11883-008-0078-z. [DOI] [PubMed] [Google Scholar]
- Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav. Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Dilmac N, Tone B, Tian HR, Hartman R, Digicaylioglu M, Snyder EY, Ashwal S. Long-term magnetic resonance imaging of stem cells in neonatal ischemic injury. Ann. Neurol. 2011;69:282–291. doi: 10.1002/ana.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD. Systemic fatty acid responses to transient focal cerebral ischemia: influence of neuroprotectant therapy with human albumin. J. Neurochem. 2002;83:515–524. doi: 10.1046/j.1471-4159.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- Simard JR, Zunszain PA, Ha C-E, Yang JS, Bhagavan NV, Petitpas I, Curry S, Hamilton JA. Locating high-affinity fatty acid-binding sites on albumin by x-ray crystallography and NMR spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17958–17963. doi: 10.1073/pnas.0506440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr. Rev. 2006;64:S24–S33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72–91. [DOI] [PubMed] [Google Scholar]