Abstract
Microtechnology offers great prospects for cellular research by enabling controlled experimental conditions that cannot be achieved by traditional methods. This study demonstrates the use of a microfluidic platform for long-term cultivation (3 weeks) of human mesenchymal stem-like cells (MSCs), a cell population of high interest for tissue engineering. The typical high motility of the MSCs required a strategy for preventing cells from inhabiting the feeding channels and thus interfere with a steady perfusion of medium to the cell cultivation chamber. Hence, a straightforward and long-term patterning method was developed and implemented for reliable cell positioning within the device. This method was based on the modification of a polystyrene substrate into cell supportive and non-supportive regions by the use of selective oxygen plasma treatment and the triblock copolymer Pluronic. Also, a novel and size-effective “flip-chip” set-up for operating the devices was invented. Successful and reproducible adipogenic and osteogenic differentiation of MSCs in the device was demonstrated, verifying that an adequate long-term microfluidic cultivation environment was obtained. Strengths of the experimental protocol include ease of fabrication and maintenance (gravity driven), good cell performance (viability/differentiation), as well as the possibility of exposing the culture to heterogeneous laminar flow for experimental purposes.
Keywords: Microfluidics, SU-8, PDMS, Polystyrene, Pluronic, Cell patterning, Gravitational flow, Flip-Chip, Differentiation, Mesenchymal stem cells, Adipocytes, Osteoblasts
Introduction
The bone marrow (BM) mesenchymal stem cell (MSC) population has a remarkably broad biological potential being an essential support for hematopoiesis (blood cell formation) and founder cells of connective tissues such as bone, cartilage and fat. 1, 2 Hence, methods for manipulating these cells in controlled manners can contribute significantly to progress within stem cell research and tissue engineering. Microfabrication enables production of versatile tools that allow for experimental culture conditions not achievable by traditional methods. For instance, cells can be precisely positioned 3-6 and signals can be delivered locally by the use of laminar flow in microchannels. 7-9 Also, microfluidic cell cultures can be exposed to gradients of stimulatory agents, e.g cytokines and morphogens. 10 These means enable the controlled creation of heterogeneous cellular in vitro environments mimicking some of the complexity found in vivo. As the role of the microenvironment is paramount for the realisation of a cell’s potential, these strategies can conceivably be used to better embrace the potency of cultivated stem cells. While it is hard to imagine that the homogeneous environment offered by conventional cultivation can be used for directing stem cells to produce functional tissue units, the methods for precisely organising cells and signals in position and time bring great prospects for regenerative medicine.
The nature and abilities of stem cells are typically elucidated by differentiation studies, in which days or weeks are required before specialised offspring of the stem cells can be detected. Consequently, stem cell culture systems must be long-term supportive and continuously provide for proper physiological conditions, such as correct pH, osmotic pressure, gas and nutrient concentration. Tourovskaia et al. pioneered differentiation-on-a-chip by developing a microfluidic platform for differentiation of skeletal muscle cells, 7, 11 Recently, microfluidic systems have been utilised for differentiation of MSCs into hepatocytes, 12 adipocytes 13-15 and osteoblasts. 14-16 However, there is still limited documentation of efficient on-chip differentiation of MSCs.
In contrast to the microfluidic devices previously used for MSC differentiation, the device of Tourovskaia et al. is equipped with channels for creating heterogeneous laminar flows, thereby allowing for unique manipulation of the culture, both with respect to cellular composition and chemical environment. According to the laminar flow patterns, cells can be locally seeded or removed by trypsination, and various agents can be locally delivered (e.g. growth factors, genetic vectors, hypoxic medium etc.). These directions may advance stem cell research and be critical for the preparation of stem cell niches in vitro. Further, the device is rather simple to reproduce, making it available for cell biologists. This study was initiated to evaluate the ability of this system to maintain MSCs in the long-term perspective necessary for cellular differentiation. The need for restricting the cell localization within the device in order to preserve proper flow was acknowledged, and two experimental modifications to the original system were developed: 1) a simpler patterning method using a polystyrene (PS) substrate, eliminating the need for harmful chemicals and 2) a novel “flip-chip” set-up for operating the device, making fluid connectors superfluous. Extensive differentiation of MSCs into adipocytes and osteoblasts was achieved, and verified the capability of the modified device to accommodate healthy MSCs during the incubation period of 3 weeks. As MSCs can serve as supporting cells for hematopoietic stem cells (HSCs), a future application of this device includes MSC/HSC co-cultures and the investigation of using heterogeneous laminar flow in order to build artificial BM HSC niches.
Experimental
Cell culture
The cell line used for this study was iMSC#3, a human telomerised BM-derived mesenchymal cell line developed by Ola Myklebost and co-workers (unpublished). The cells display an MSC-like morphology and have maintained their differentiation potential. They are non-tumorigenic, have a normal karyotype and a doubling time around 60h. Cells were maintained in T25 cell culture flasks using α-modified essential medium, α-MEM (E15-832, PAA Laboratories, Austria), supplemented with 10% fetal bovine serum, FBS (FBS-1, Saveen Werner AB, Sweden) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin, P11-010, PAA laboratories). Cell stocks were subcultured every 4-5 days by trypsination (trypsin/EDTA, 0.05%/0.02%, L11-004, PAA laboratories). Adipogenic differentiation was induced by adding the following reagents to the cell medium: 1 μM dexamethasone (Fortecortin inject, Merck KGaA, Germany), 50 μM indomethacin (I7378, Sigma-Aldrich) and 0.5 μM 3-isobutyl-1-methylxantine (I7018, Sigma-Aldrich). Osteogenic differentiation was induced by the use of Complete MesenCult®Ostegenic Medium, containing 3.5 mM β-glycerophosphate, 10 nM dexamethasone and 50 μg/ml ascorbic acid (StemCell Technologies Inc.), as specified by the manufacturer. Cells in both T25 flasks and microfluidic devices were cultivated at 37°C in a humidified atmosphere with 5% CO2.
Cell patterning
Proof-of-principle experiments for the patterning procedure (Fig. 1, left panel) were carried out using conventional cultures. Heterogeneous PS substrates were made by exposing partially masked PS petri dishes (bacteriological grade) to an oxygen plasma using a March Plasmod apparatus (March Plasma Systems, Concord, CA) at ~30 Pa oxygen pressure and 300 W power for time intervals ranging from 5 to 60 sec. This treatment caused exposed areas of the dish to turn more hydrophilic (PSox), while masked areas remained hydrophobic (PS). Pattern-defining masks were made of silicone rubber; poly-dimethylsiloxane (PDMS, Sylgard 184 Silicone Elastomer, Dow Corning, US), which adheres spontaneously and reversibly onto most dry surfaces, including PS. Test masks were made from PDMS slabs by manually cutting, while masks of precise dimensions were produced by replica molding (see next section), using masters of either SU-8 or etched silicon. After substrate demasking, three blocking agents for preventing cell growth on unmodified PS areas were tested under sterile conditions. Respective substrate areas on the same petri dish were covered with one the following solutions: 0.5% polyethylene glycol, PEG (P2263, Sigma-Aldrich) in methanol, 2% Pluronic F108 or 2% Pluronic F127 (kindly donated by W.R.Glomm, NTNU, Norway), both in Dulbecco’s phosphate-buffered saline, PBS (H15-001, PAA laboratories). PBS was used as a negative control. The agents were kept from mixing by borders of soft paraffin (Kløver® Vaseline, Lilleborg AS, Norway). Samples were incubated at room temperature for 2 h, washed extensively with PBS, seeded with iMSC#3 (~20 000 cells/cm2) in maintenance medium and evaluated after short-term cultivation (Fig. 2a-d).
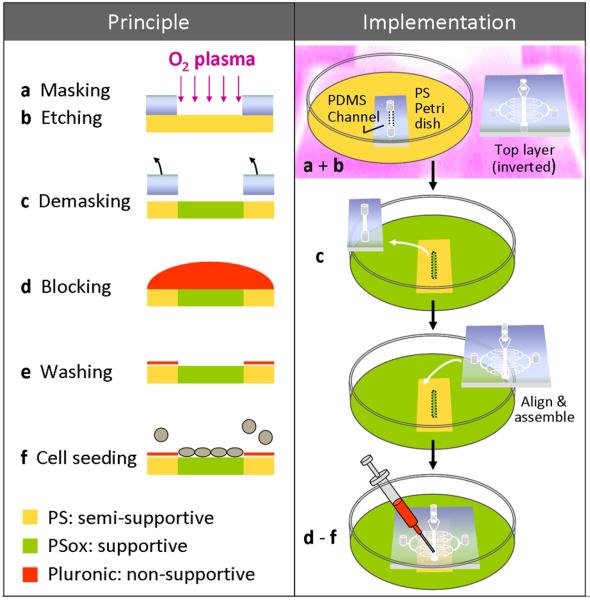
Fig. 1. Patterning of PS substrates.
Schematic outline of the patterning method used for confining MSC growth on a PS substrate; principle shown at left and implementation in a microfluidic device shown at right. PDMS masks were used for selectively exposing the dedicated cultivation areas of PS substrates (bacteriological grade) to oxygen plasma. This treatment resulted in a heterogeneous substrate, in which the oxidized PS (PSox) areas accommodated cell growth (a-c). After demasking, Pluronic 127 was used to block cell adhesion to the non-cultivation areas, binding to unmodified PS only (d-f). During the initial testing of blocking candidates, the steps of blocking, washing and cell seeding were performed by covering the substrate with the respective solutions. When preparing PDMS/PS devices, these steps were performed on-chip - after the device top layer had been bonded to the substrate in accordance with the footprint of the mask (right panel). Inclusion of the device top layer in the oxygen plasma treatment provided a hydrophilic and sterile PDMS surface (right panel, a).
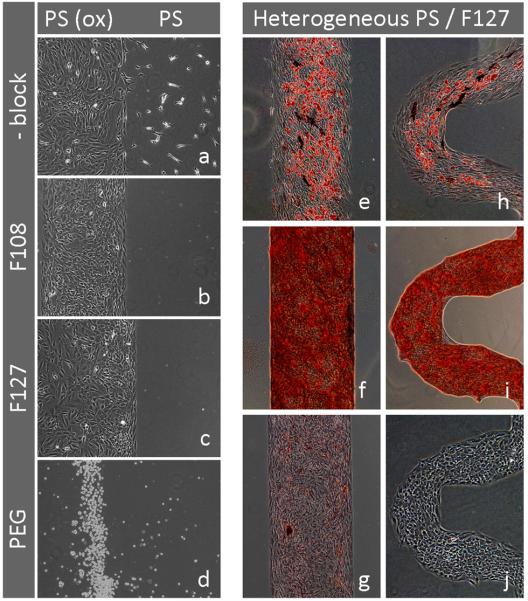
Fig. 2. Pluronic treatment of heterogeneous PS substrates (PS/PSox) provides stable MSC patterning and is compatible with adipogenic and osteogenic differentiation.
Images showing iMSC#3 cultivated conventionally in serum-containing medium (10% FCS) on modified PS substrates.
Left panel:Distribution of iMSC#3 on PS versus PSox areas without blocking (PBS control, a) and after blocking with 2% of the Pluronics F108 (b), F127 (c) or 0.5 % PEG (d). Images were acquired on day 2 (a-c), or on day 1 (d) after seeding.
Right panel:Long term patterning of iMSC#3 on Pluronic F127-treated heterogeneous PS substrates. Adipogenic (e, h) and osteogenic (f, i) differentiation were induced in separate cultures and confirmed after 3 weeks by Oil Red-O and Alizarin Red S staining, respectively. The medium control was stained by both Oil Red-O and Alizarin Red S in sequence (g). Some Alkaline Phosphatase (ALP) activity was revealed in both adipogenic and osteogenic cultures by the use of a ALP substrate (cat. no. B5655, Sigma-Aldrich), resulting in dark blue staining (e, f, h). Cell density at the time when differentiation regimes were initiated is shown (j). Edged features of curved patterns are addressed to the pattern-defining PDMS mask, being a replica of a silicon pattern made by anisotropic etching. All images were acquired by phase contrast microscopy. Magnification objectives: 4× (j) and 10× (a-i).
Additional tests were performed to verify whether the patterning regime was compatible with long-term cultivation and differentiation of iMSC#3. Cells were seeded on patterned PS substrates (heterogeneous PS blocked with 1% Pluronic F127 for 5 min) and cultivated in adipogenic or osteogenic differentiation media for 3 weeks with medium changes every 3-4 days (Fig. 2e-i).
Device fabrication
SU-8 masters were produced and used as templates for molding of open microchannels in PDMS. Subsequent bonding of the channels onto substrates resulted in closed microchannel devices. An overview of the process is presented in Fig. 3a.
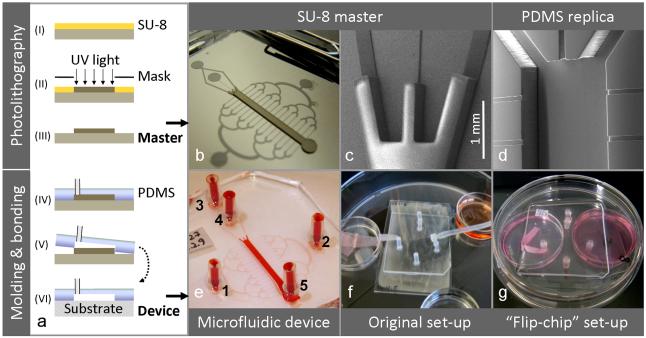
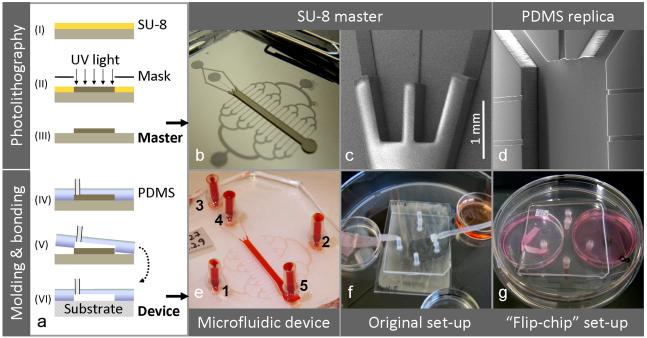
Fig. 3. Fabrication, device compositions and perfusion set-ups.
Schematic outline of the device fabrication process (a), involving photolithography for production of SU-8 master molds (step I-III) and PDMS replica molding and substrate bonding for realization of the microfluidic devices (step IV-VI). The overview image of an SU-8 master (b) illustrates the channel design: centrally positioned is the cell cultivation chamber and transversely arranged are the perfusion channels. The SEM images show a detail of the two-level SU-8 relief providing the two channel heights (c) and a PDMS replica resulting from the molding process (d). A test device filled with dye illustrates port annotations (e). Alternative device compositions and perfusion set-ups are shown: A PDMS/glass system run in a substrate-down orientation, as described originally (e), and a PDMS/PS system run in an inverted, substrate-up orientation (f), as developed in this study. Note: Schematic not to scale.
Fabrication of SU-8 master and PDMS microchannels
The two-layer relief of SU-8 on the master was obtained by two-layer photolithography, as previously reported. 7, 11 CoralDraw cdr-files specifying the two layers were kindly provided by A.Tourovskaia and A. Folch (University of Washington, Seattle, WA, US) and printed as transparency masks (5080 dpi) by Pageworks (Cambridge, MA, www.pageworks.com, US). The layers were made sequentially using two formulations of the negative photocurable resist SU-8 (MicroChem Corp, US). Layer 1 (thickness 22 μm, defining perfusion channels and ports): SU-8 50 was spincoated on a silicon wafer (ø10 cm, Ultrasil Corp, Hayward, CA) at a final speed of 5000 rpm, prebaked on a hotplate (50°C for 2 min, then ramped to 90°C and held for 4 min), UV-exposed (400 mJ/cm2) trough mask 1 (Karl Suss MJB55 mask aligner, Süss Microtech, Garching, Germany) and post-baked (50°C for 2 min, then ramped to 90°C and held for 5 min). Layer 2 (thickness ~ 300 μm, defining cultivation channel): SU-8 100 was spun on top of layer 1 at a final speed of 1060 rpm, prebaked (65°C for 28 min, then ramped to 95°C and held for 82 min), UV exposed (720 mJ/cm2) trough mask 2 after alignment, and post-baked (65°C for 1 min, then ramped to 95°C and held for 18 min). Alignment of the second mask was enabled by peripheral alignment marks in layer 1 that were selectively developed and protected against SU-8 100 coverage by a piece of tape. The two-layered SU-8 structure was released by immersion in SU-8 developer (MicroChem Corp, Newton, MA) for at least 20 min. To facilitate PDMS removal after replica molding, the master was coated with trichloro(1H, 1H, 2H, 2H-perfluorooctyl)-silane (448931, Sigma-Aldrich) in gas phase for 40 min using a vacuum desiccator. Silicon tubings (length 10 mm, ID 1mm) were glued to the master, serving as access ports of the final device. PDMS was mixed in a 1:10 ratio of curing agent and prepolymer, degassed in a desiccator under vacuum, poured onto the master (avoiding ports), re-degassed and cured in an oven (65°C, overnight). The PDMS microchannels (replica) were cut out from the master, and glue plugs in the integrated ports were pushed out using a syringe needle. The replica had a centrally positioned main channel for cell cultivation (height ~ 300 μm, width 1.5 mm, length 1.8 cm) and transversely arranged were a binary network of perfusion channels (height 22 μm, width of linear channels 50 or 100 μm). In order to test for a more elaborate fluid distribution, a variant with a doubled number of perfusion channels (=32) was produced by extending the binary tree of these channels with one level.
High resolution images of an SU-8 master and a PDMS replica were obtained by a Philips XL30 scanning electron microscope (SEM), Fig 3c, 3d. PDMS collects electronic charge during electron microscopy due to its low electrical conductivity. Thus, this material was protected from a build-up of charge by sputter-coating the replica with gold prior to imaging, using a Polaron SC500 apparatus (Quorum Tech., UK) at ~ 40 Pa, 20 mA for ~ 1 min with Argon as process gas.
Substrate bonding and conditioning
Microfluidic culture devices were made by bonding PDMS microchannels to a substrate of either glass or PS. The surfaces to be brought in conformal contact were pretreated with oxygen plasma (300 W, ~30 Pa, 20 sec), leading to covalent bonding when glass was used as substrate. Additionally, the oxygen plasma turned the surfaces both sterile and hydrophilic. These properties were maintained by handling the devices in sterile conditions in a laminar flow cabinet and filling the channels with PBS within 10 min. The liquid was introduced into the channels from a syringe with a needle (25G) via port 5, and vacuum was applied to ports 3 and 4, and then ports 1 and 2, see Fig. 3e for port annotations. Subsequent changes of reagents in the device were performed likewise. PDMS/glass devices were uniformly coated with 50 μg/ml fibronectin, FN (F-0895, Sigma-Aldrich), incubated overnight at 37°C and washed with PBS. PDMS/PS devices were made by implementing the method developed for cell patterning (Fig. 1), starting at the bonding step. A PDMS mask (~3 × 1.5 cm, height 1-2 mm) was designed to expose the future cultivation area of the substrate (inside the main channel) while protecting the adjacent area. It was placed centrally on a bacteriological grade PS petri dish (ø mm, Sarstedt AG & co, Germany) and reference marks were made on the reverse side. Both the masked substrate and the device top layer were treated simultaneously with oxygen plasma. After demasking, the device was assembled under a stereomicroscope, using the reference marks for aligning the main channel of the device top layer with the cultivation area of the substrate (PSox). As the PDMS/PS bonding was reversible, it was reinforced by hand-painting of partly cured PDMS around the perimeter of the top layer. The filled device was incubated in a cell incubator (37°C, overnight) to saturate the PDMS microchannels and complete curing of the PDMS used for reinforcement. Finally, cell adhesion to the hydrophobic substrate area outside the main channel region was blocked: A sterile solution of F127 (1% in PBS) was introduced to the channels for a few min at room temperature and removed by extensive washing with PBS.
Device operation
Cells were loaded into the main channel of the device via the lower port (5), Fig. 3e, using a sterile syringe with needle (25G) and suction from the upper port (3). iMSC#3 was seeded at 0.6 × 106 cells/ml for osteogenic cultures and 0.8 × 106 cells/ml for adipogenic cultures. During seeding, cell entrance into the perfusion channels was prohibited by keeping the fluid in the distal ports (1 and 2) at a higher level than in the remaining ports, causing a minute gravitational flow from the perfusion channels into the main channel. The cultures were incubated statically overnight to ensure cell attachment. Next day, perfusion of the main channel was established (Fig. S1b) by connecting port 1 to a filled medium reservoir (5 ml) and port 2 to an empty waste reservoir, thereby creating a hydrostatic pressure difference. Care was taken to avoid bubbles in the device, causing flow disruption. Petri dishes (ø35 mm) served as reservoirs. A wick was used to conduct fluid from the device to the waste reservoir. This was cut from a wiper (TX1109, ITW Texwipe, US), sprayed with 70% ethanol and inserted into the outlet (port 2). Medium was supplied via port 1 by two alternative set-ups, coinciding with the robustness of the PDMS/substrate bonding. Each PDMS/glass device (permanently bonded) was connected to the medium reservoir by a tube, as originally described 11 (Fig. 3f). Each PDMS/PS device, consisting of PDMS microchannels being reversibly bonded to a petri dish, was inverted (substrate facing upwards) and made resting on its lid, making the ports protrude into their respective reservoirs (Fig. 3g). This “flip chip” set-up permitted direct fluidic contact between the medium reservoir and the inlet. During microscopic observation, the device was flipped back in accordance with the working distance of the microscope. All devices were maintained every 1-3 days by removing medium expelled into the waste reservoir and refilling the medium reservoir with a corresponding volume. Upon termination of cultures, cells were analyzed in situ.
Assays
Cell in the microfluidic cultures were assayed by introducing reagents into the microchannels in a manner identical to the initial filling procedure. Cell viability was determined by the Live/Dead® Viability/Cytotoxity Kit for mammalian cells (L3224, Molecular Probes, Invitrogen, USA) and imaged by fluorescence microscopy, detecting green colour resulting from enzymatic conversion of calcein AM (non-fluorescent) to calcein (fluorescent) in living cells and red colour resulting from Ethidium homodimer-1 entrance through damaged membranes and binding to nucleic acids. Prior to further staining, cells were washed in PBS, fixed in 10% formalin (Merck) in PBS for 30 min at room temperature and washed in dH2O. Fat droplets in adipocytes were stained for 30 min by a Oil Red-O solution containing 2 parts dH2O and 3 parts Oil Red-O stock (0.5% Oil Red-O (O-0625. Sigma-Aldrich) in isopropanol). Calcium deposits of osteoblasts were stained for 30 min by a 40 mM Alizarin Red S solution (05600, Sigma-Aldrich) in dH2O at pH 4.1. Staining procedures were completed by washing with dH2O. Fluorescence, bright-field and phase-contrast images were acquired with an inverted microscope (Olympus IX51) equipped with a CCD camera (Olympus DP71) using the accompanying imaging software (Cell B Life Science Software).
Results and discussion
Device fabrication and characteristics
Microfluidic culture devices were made by bonding a top layer of PDMS microchannels to either a glass (unpatterned) or a PS substrate (patterned, Fig. 1). The top layers were fabricated by SU-8 photolithography and PDMS replica molding (Fig. 3a, step I-V). Using these standard processes, a two-layered relief pattern corresponding to the channel design was created (a SU-8 master, Fig. 3b, 3c), and replicated inversely in PDMS (Fig. 3d). The device contained a central channel designed for cell cultivation. Connected to this cell chamber were two sets of channels having a higher flow resistance. Perfusion channels for cell feeding were arranged transversely, and channels for delivery of heterogeneous laminar flows to the cultivation chamber were positioned longitudinally. The flow patterns during the two separate modes of operation (perfusion mode or heterogeneous flow mode) were visualised by the use of dyes (Fig. S1). The dimensions and design of the channels are discussed by the originators.7, 17
On-chip differentiation using homogeneous glass substrates
The microfluidic device used in this study was originally developed for differentiation of myoblasts into aligned myotubes, a process critically dependent on linear patterning of seeded cells. As cell orientation was not a subject of interest in the present study, initial experiments were performed with devices having uniform glass substrates that were coated inside channels with the extracellular matrix protein FN. In these PDMS/glass devices, the iMSC#3 cells spread out of the cell chamber and into the perfusion channels. Although some degree of differentiation occurred in these cultures (Fig. 4), aberrant cell morphology was observed and results were not consistent. Variations in the design of perfusion channels (width = 50 or 100 μm, number = 16 or 32) did not affect this outcome. The modest performance of the PDMS/glass devices was suspected to be caused by the cells inhabiting the perfusion channels. Evidently, they violated a steady perfusion of medium; thereby jeopardising proper nutrition of the culture. Also, cells in this region were experiencing high shear stress (due to a low channel height), a condition that might cause a release of mediators with undesired effects on the overall cell culture.
Fig. 4. Microfluidic differentiation on unpatterned glass substrates.
Bright field images showing cells inhabiting the feeding channels (arrowhead) as well as the cultivation area (center) and modest levels of iMSC#3 differentiation in devices having homogeneous, FN-coated glass substrates. The adipogenic culture was stained by Oil Red O after 3 weeks (a) and the osteogenic culture was stained by Alizarin Red S after 4 weeks (b). Flow direction is indicated by an arrow.
Cell patterning
In order to improve on-chip differentiation of MSCs, a straightforward patterning method for confining cells to the cultivation area of the device was developed.
Use of PS/PSox heterogeneous substrates
Preliminary experiments indicated a challenge in obtaining stable patterning of iMSC#3. Using micropatterned glass substrates, 18 the cells quickly invaded areas coated with bovine serum albumin (BSA) - a protein widely used for surface passivation (Fig. S2). However, it was found that iMSC#3 patterning could be achieved on PS dishes (bacteriological grade) that had been subjected to localised oxygen plasma treatment. PS does not normally support adherent cells as focal adhesions are scarcely formed on hydrophobic surfaces. 19 Hence, plasma oxidation is routinely used for modifying PS surfaces into the tissue culture grade quality suitable for adherent cell cultivation. This treatment leads to oxidation of the surface (Pox) and increased wettability, factors that influence the sort, amount and conformation of cell adhesion molecules to be adsorbed. 6, 20 Several doses of oxygen plasma (5, 10, 30 or 60 sec) were tested to have similar capability in transforming PS into a PSox state that permitted iMSC#3 adherence and growth (not shown). Localised plasma treatment was performed by the aid of reversibly bonded PDMS masks that protected the non-cultivation area from plasma modification (Fig. 1a-c).
Patterning enforcement using Pluronics
However, the outcome of iMSC#3 patterning on PS/PSox heterogeneous substrates was not consistent. Some brands of bacteriological grade PS acted semi-supportive towards iMSC#3, as demonstrated by scattered cells located outside the PSox growth areas (Fig. 2a). This tendency of iMSC#3 adhesion to untreated PS was successfully blocked by treatment with Pluronics (Fig. 2b, 2c), resulting in a sustained patterning for more than 3 weeks (Fig. 2e-i). Pluronics is a series of commercially available non-ionic surfactants. They are triblock copolymers, consisting of a central block of poly (propylene oxide) flanked by one block of poly (ethylene oxide) on each side, as described by the generic formula; (PEO)x-(PPO)y-(PEO)x. Given a hydrophobic surface immersed in an aqueous medium, the central PPO-block will adsorb spontaneously to the surface by hydrophobic interactions, while the hydrophilic tails (PEO-blocks) will extend into the medium and prevent protein adsorption and cell adhesion. 21, 22 Initial experiments were performed using 2% solutions of Pluronics in PBS for 2h (Fig. 2b, 2c) while further testing demonstrated effective blocking using a 1% solution for 2-3 min, a regime that was used for subsequent experiments (Fig. 2e-j, Figs. 5, 6). For sustaining long term patterning, F127 [(PEO)100-(PPO)70-(PEO)100] was slightly more effective than F108 [(PEO)129-(PPO)56-(PEO)129], as reported earlier. 23 Whereas the Pluronics tested did not affect cell growth on PSox areas, PEG treatment of heterogeneous PS substrates resulted in total lack of cell adherence, indicating ability for PEG to adsorb to and block both PSox and PS areas (Fig. 2d).
Fig. 5. Microfluidic adipogenesis on patterned PS substrates.
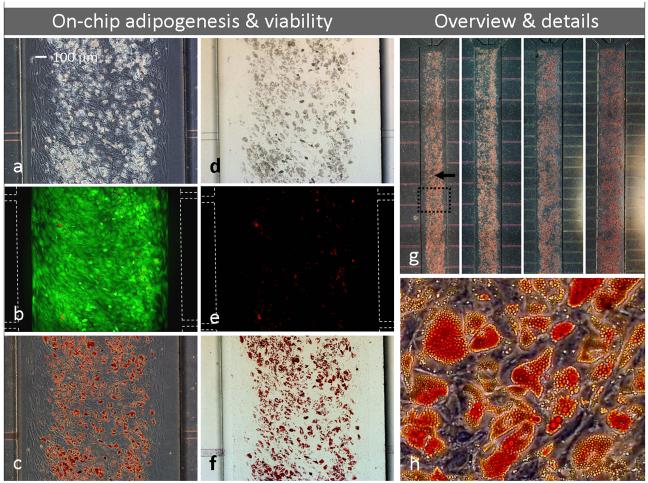
Adipocytes (containing lipid droplets) indicated by phase-contrast (a) and bright field (d) microscopy of iMSC#3 cultures after on-chip adipogenic stimulation for 3 weeks. Note that cells are stably patterned inside the main channel (patterned area being somewhat smaller than the channel itself) with no propagation into feeding channels. The culture was characterized by fluorescent microscopy, demonstrating good viability (b) and few dead cells (e). Oil Red-O staining confirmed extensive adipogenesis (c, f-h). Images (a-f) represent a section of one of 4 parallel microfluidic cultures (g, dotted frame). Details from the same culture are shown in (h). White dotted lines indicate channel wall position when not visible (b, e). Flow direction is indicated by an arrow. Magnification objectives: 4× (g), 10× (a-f) and 20× (h).
Fig. 6. Microfluidic osteogenesis on patterned PS substrates.
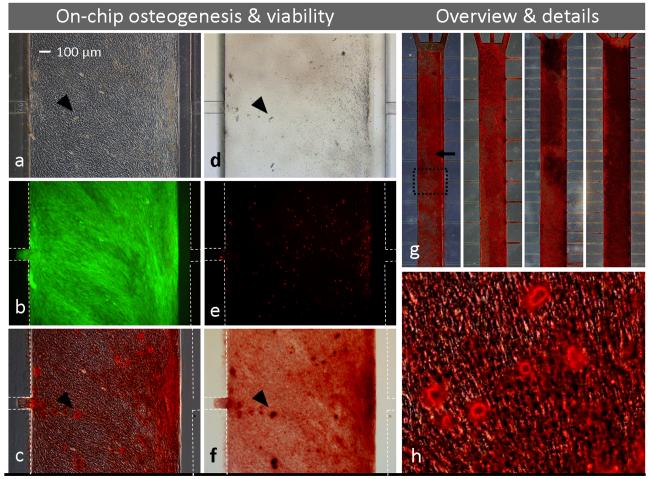
Osteoblasts (depositing Ca2+ nodules, arrowheads) indicated by phase-contrast (a) and bright field (d) microscopy of iMSC#3 cultures after on-chip osteogenic stimulation for 3 weeks. Note that cells are stably patterned (patterned area identical to the channel itself, but a bit misaligned) with no outward propagation. The culture was characterized by fluorescent microscopy, demonstrating good viability (b) and few dead cells (e). Alizarin Red S staining confirmed extensive osteogenesis (c, f-h). Images (a-f, h) represent a section of one of 4 parallel microfluidic cultures (g, dotted frame). White dotted lines indicate channel wall position when not visible (b, e) and when removed for better visualization of cells (c, f). Flow direction is indicated by an arrow. Magnification objectives: 4× (g), 10× (a-f) and 20× (h).
Evaluation and comparison with other methods
Patterning of iMSC#3 on Pluronic-treated PS/PSox heterogeneous substrates (referred to as “patterned PS substrates”) was found to be compatible with adipogenesis (Fig. 2e, 2h), as well as osteogenesis all the way to the stage of calcium deposition (Fig. 2f, 2i). Notably, this method permitted MSC cultures to stay patterned for a longer period (>3 weeks) than previously reported. By the use of a PDMS substrate, microcontact printing of FN-patterns and Pluronic blocking, Ruiz et al. achieved a sustained patterning for only 14 days and no calcium detection in osteogenic cultures was possible. 24
PS patterning by selective plasma oxidation treatment has previously been demonstrated in the literature. 6, 25 However, Rhee et al. 25 did not reinforce the patterning by the use of Pluronics, while Detrait et al. 6 used a much more labour intensive photolithographic technique for PS modification. The presented method has the following advantages: 1) long-term efficiency, 2) easy and rapid performance and 3) no involvement of harmful chemicals. On the other hand, PS has not been the traditional choice as a substrate material for PDMS microchannels, since bonding between these two materials is challenging. Means for obtaining permanent bonding 26-28 were not explored, as sufficient device integrity was obtained by 1) reinforcing the perimeter of the PDMS/PS interface by PDMS hand painting, and 2) operating the device inverted (see next section). In addition, the reversible bonding of PDMS to PS was exploited in the end-point evaluation of osteogenesis in PDMS/PS devices. Due to calcium deposition on the channel surface, the cells were best visualised after removal of the device top layer (see below).
“Flip-chip” set-up
The microfluidic cultures were maintained by medium perfusion, achieved by siphoning from a filled medium container into an empty waste container. 11 The use of gravity driven flow allows for several cultures to be run in parallel, irrespective of pump availability. Whereas PDMS/glass devices were run by the set-up described originally (Fig. 3f), a novel and simplified variant was invented and used for the PDMS/PS devices (Fig. 3g). The whole device, consisting of PDMS microchannels bonded to the bottom part of a petri dish was simply inverted and made to rest on its lid so that the ports were accommodated by their respective reservoirs. By this “flip-chip” set-up there was no need for connecting a tube between the medium reservoir and inlet, entailing the following benefits: 1) no risk of device delamination caused by mechanical stress on the port (no handling, no movable parts connected), 2) smaller dead volume, facilitating medium changes, and 3) reduced system size and complexity. Also, when facing the substrate upwards, any possible debris will separate from the cells by gravity.
Inverted cultures are of special relevance with respect to HSC research. Previously, HSC-MSC co-cultures have been observed in the inverted state by the use of sealed wells. 29 In this short term assay, the most immature hematopoietic cells stayed adherent to the MSC feeding layer, while those more differentiated dropped to the bottom of the chamber. Based on this finding, it is likely that long-term cultures of HSCs will benefit from a flipped set-up. In this state, the non-adherent hematopoietic progeny produced can gravitate away from their ancestors and be removed (and assayed) with minimal intrusion. Such a course of events will mimic normal physiology and relieve the cultures from medium exhaustion caused by an overload of highly proliferative cells.
On-chip differentiation using patterned PS substrates
Microfluidic differentiation of MSCs was tested in devices modified by the integration of a patterned PS substrate and perfused by the flip-chip set-up.
Cell localisation and flow rates
Upon introduction to a PDMS/PS device, iMSC#3 attached to the PSox area in the main channel and the culture was confluent on the next day, when a gravity driven perfusion with differentiation medium was initiated. The patterning strategy worked successfully in prohibiting cells from spreading into the perfusion channels (i.e. PS area blocked by Pluronic F127) and interfere with the delivery of medium to the cultivation chamber. Accordingly, higher flow rates were found in patterned versus unpatterned microfluidic cultures, providing for better nutrition of cells in the first group. Average flow rates were estimated from the volumes of medium expelled from the devices and equalled 311 μL/day in PDMS/PS devices (patterned and inverted, n=7), as opposed to 135 μL/day in corresponding PDMS/glass devices (unpatterned and run by traditional substrate-down orientation, n=7). The arithmetic means of the two groups were different by 130% (with p=0.006, Student’s unpaired t-test).
Cell performance
Whereas on-chip differentiation of iMSC#3 was unpredictable in devices having unpatterned glass substrates, osteogenesis and adipogenesis proceeded reproducibly and at high levels in 4 parallel microfluidic cultures on patterned PS (Figs. 5g, 6g). During the 3-weeks course of the experiments, cells differentiated at rates expected from traditional cultivation, as estimated by microscopic observations of lipid droplets in adipogenic cultures (Fig. 5a) and morphology of osteogenic cultures (Fig. 6a). At termination of the cultures, a fluorescent assay confirmed high cell viability (Fig. 5b, 6b) and few dead cells (Fig. 5e, 6e), visualised by green and red colour respectively. These results were similar to what was found for patterned cells grown in traditional petri dishes (not shown). Extensive cell differentiation in the PDMS/PS devices was confirmed by routine diagnostic methods; Oil Red-O staining of lipids accumulated in adipocytes (Fig. 5c, 5f-h) and Alizarin Red S staining of calcium deposited by osteoblasts (Fig. 6c, 6f-h).
Both microfluidic devices with 16 and 32 perfusion channels were capable of supporting adipogenic and osteogenic differentiation (Fig. 5g, 6g, double channel densities at right). However, calcium deposition in osteogenic cultures was more prominent in devices fed by 32 perfusion channels and increased towards the medium reservoir, indicating a correspondence between level of nutrient supply and osteogenesis and/or calcium production. Notably, the overall on-chip osteogenic activity was so pronounced that the cell morphology was disguised by extensive calcium staining at the PDMS surface of the cultivation chamber (facing down during cultivation and up during microscopic observation), Fig. 3g. Hence, all devices were disassembled before detailed imaging (Fig. 6c, 6f, 6h), an easy operation due to the reversible bonding of the PDMS channels to the PS substrates.
While osteogenic differentiation was not affected by the constraints of the pattern (Fig. 2f, 2i), adipogenic differentiation in both traditional and microfluidic cultures tended to be less prominent at the edges of the pattern (Fig. 2e, 2h, 5c). These observations are related, but not consistent with those recently presented by Ruiz et al., 24 who report that cytoskeletal tension can modulate MSC differentiation, mediating adipogenesis in central regions of patterns and osteogenesis in peripheral regions. However, this patterned segregation of lineages was reported to occur exclusively in cultures subjected to a mix of adipogenic and osteogenic media, a result that was not reproduced by using the iMSC#3 cell line in this study. Irrespective whether the differentiation agents were presented separately (Figs. 2, 5, 6) or in a mix (Fig. S3), osteogenesis proceeded throughout the pattern, while adipogenesis was reduced in peripheral areas. This discrepancy may be due to different cell sources and experimental parameters (differentiation formulation, substrate material, seeding density etc.).
The extended level of on-chip differentiation observed in patterned cultures (on PSox) versus unpatterned cultures (on glass) may not solely be ascribed to the elimination of clogging of the perfusion channels and elevated flow rates, but also to the chemical nature of the substrate. The biocompatibility of materials is crucial in microsystems, due to their high surface area to volume (SAV) ratios (cm2/mL). Glass, the original substrate for cell cultivation, is easily and permanently bonded to PDMS and is widely used as a substrate for cellular microsystems. However, in contrast to PS, glass has been found to have a strong inhibitory effect on HSCs at even lower SAV ratios than used here. 30 Even if a protein coat (e.g. FN) is used for masking, the use of glass substrates in microsystems might cause suboptimal performance of given cell types. Indeed, a cell adhesion study indicates that a conditioning film is depending on the underlying substrate properties rather than masking them. 20 In order to distinguish the impact of patterning from the substrate influence, on-chip MSC differentiation using patterned and unpatterned versions of glass and PS substrates should be compared.
PDMS is a popular choice for fabricating of microscale cell culture chambers. This silicon-based organic polymer is easily processed and has many physical attributes. It can be micromolded into fine structures (Fig. 3d), and adheres spontaneously to many materials (e.g. PS, Fig. 3g). It is also transparent (Fig. 3e-g), non-fluorescent (Fig. 5b, 5e, 6b, 6e) and gas permeable. However, although PDMS is commonly considered as a biocompatible material, concerns have been pointed towards its tendency to leach uncrosslinked oligomers 31 and to sequester small hydrophobic molecules from the culture media, 31, 32 properties that may cause culture artifacts and limit the use of PDMS in some contexts. Nevertheless, the substantial on-chip differentiation obtained here provides an example verifying that PDMS microchannel systems can be compatible with normal cell behaviour on a long term basis.
Comparison with other devices
On-chip differentiation of MSCs has previously been conducted for evaluating the potential of microfluidic devices as tools for MSC studies and with the general prospect of developing cost-effective and automated systems for cell cultivation.12-16 All previously reported microfluidic systems utilize glass substrates and have no means for preventing cells from spreading out of the cultivation area. The problem with obstruction of channels used for cell feeding may have been avoided in these devices due to the use of primary cells, inherent with lower proliferation rates than a cell line (as used here), combined with taller feeding channels (25-100 μm). Nevertheless, as previously argued, cells inhabiting feeding channels should presumably be avoided as their exposure to the elevated shear stress in these areas may cause undesired consequences for the overall cell culture. In comparison with earlier documentation of on-chip adipogenesis and ostogenesis, 13-16 the differentiated cells in the present study correlate better (judged by morphology and quantity) with those produced by conventional cultivation. These results invite to a more systematic approach for evaluating the contribution of the different interdependent system parameters to the overall outcome of a microfluidic culture; choice of substrate, channel dimensions, cell source, patterning, flow regime etc.
Conclusions
Microfluidic devices provide new avenues for experimental settings, and replica molding of PDMS using SU-8 masters allows for great design flexibility and rapid prototype production. Demonstrations of successful experimental use of such devices, as well as simplifications of the fabrication/operation processes, as shown here, may engage a broader scientific community in applying a microfluidic approach to their research.
A microfluidic device that maintains MSCs healthy during 3 weeks in culture is demonstrated in this study. Adaptive modifications to the original device included the use of a PS substrate and an easy and user-friendly patterning method to prevent cells from intrusion into feeding channels, causing flow obstruction. Also the experimental set-up was made more effective by arranging medium perfusion of the device in a novel “flip-chip” set-up. The device was proven able to provide for an adequate long-term environment for MSCs by efficiently and reproducibly accommodating the delicate developmental processes of adipogenesis and osteogenesis, respectively. Further studies are required to elucidate whether the improved performance of the modified device is due to unobstructed flow, better substrate suitability, the absence of influence from cells perceiving high shear stress, or a combination of these factors. The compatibility of the device with normal MSC behaviour encourages its further use in experimental research, including studies of interactions within the BM microenvironment. Equipped with channels for generating consistent medium heterogeneity, the device allows for localised application of factors governing stem cell renewal and differentiation, a strategy that should be investigated in order to fabricate artificial stem cell niches, both for studies of normal functions and for cancer stem cells.
Supplementary Material
Acknowledgements
This work was supported by the Royal Norwegian Ministry of Culture and Church Affairs. The authors wish to thank the following contributors: Dr. Anna Tourovskaia (VisionGate, Inc, Seattle, WA) for comments upon the manuscript, for information about the properties of Pluronic and for sharing masks and experience about the fabrication and operation of the original device, Dr. Albert Folch (University of Washington) and the Folch lab for hosting Tenstad’s 3 weeks training period, Dr. Dag Josefson (Rikshospitalet University Hospital, Oslo, Norway) for urging the need for an easy understandable presentation and for providing serum and osteogenic differentiation media, Dr. Wilhelm. R. Glomm (NTNU, Trondheim, Norway) for providing the Pluronics F108 and F127, Dr. Frank Karlsen and Norchip AS, Klokkarstua, Norway for financing the cell laboratory at Vestfold University College, Tormod Vinsand for acquisition of SEM pictures, and Erik Johannesen for comments upon the manuscript
References
- 1.Delorme B, Chateauvieux S, Charbord P. Regenerative Medicine. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Riminucci M, Gronthos S, Robey PG. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 4.Folch A, Jo B-H, Hurtado O, Beebe DJ, Toner M. Journal of Biomedical Materials Research. 2000;52:346–353. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal A, Macdonald A, Voldman J. Biomaterials. 2007;28:3208–3216. doi: 10.1016/j.biomaterials.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detrait E, Lhoest J-B, Knoops B, Bertrand P, van den Bosch de Aguilar P. Journal of Neuroscience Methods. 1998;84:193–204. doi: 10.1016/s0165-0270(98)00114-9. [DOI] [PubMed] [Google Scholar]
- 7.Tourovskaia A, Figueroa-Masot X, Folch A. Lab Chip. 2005;5:14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 8.Sawano A, Takayama S, Matsuda M, Miyawaki A. Developmental Cell. 2002;3:245–257. doi: 10.1016/s1534-5807(02)00224-1. [DOI] [PubMed] [Google Scholar]
- 9.Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Nature. 2001;411:1016–1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 10.Keenan TM, Folch A. Lab Chip. 2008;8:34–57. doi: 10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tourovskaia A, Figueroa-Masot X, Folch A. Nat. Protocols. 2006;1:1092–1104. doi: 10.1038/nprot.2006.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju X, Li D, Gao N, Shi Q, Hou H. Biotechnology Journal. 2008;3:383–391. doi: 10.1002/biot.200700152. [DOI] [PubMed] [Google Scholar]
- 13.Ni XF, Crozatier C, Sensebé L, Langonne A, Wang L, Fan Y, He PG, Chen Y. Microelectronic Engineering. 2008;85:1330–1333. [Google Scholar]
- 14.Gomez-Sjoberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR. Analytical Chemistry. 2007;79:8557–8563. doi: 10.1021/ac071311w. [DOI] [PubMed] [Google Scholar]
- 15.Wu H-W, Lin X-Z, Hwang S-M, Lee G-B. Biomedical Microdevices. 2009;11:869–881. doi: 10.1007/s10544-009-9304-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Sensébé L, Zhou YL, Lin CJ, Chen Y. Microelectronic Engineering. 2009;86:1459–1461. [Google Scholar]
- 17.Tourovskaia A, Kosar TF, Folch A. Biophys. J. 2006;90:2192–2198. doi: 10.1529/biophysj.105.074864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folch A, Toner M. Biotechnology Progress. 1998;14:388–392. doi: 10.1021/bp980037b. [DOI] [PubMed] [Google Scholar]
- 19.Keselowsky BG, Collard DM, García AJ. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 20.van Kooten TG, Spijker HT, Busscher HJ. Biomaterials. 2004;25:1735–1747. doi: 10.1016/j.biomaterials.2003.08.071. [DOI] [PubMed] [Google Scholar]
- 21.Li J-T, Caldwell KD. Colloids and Surfaces B: Biointerfaces. 1996;7:9–22. [Google Scholar]
- 22.Liu VA, Jastromb WE, Bhatia SN. Journal of Biomedical Materials Research. 2002;60:126–134. doi: 10.1002/jbm.10005. [DOI] [PubMed] [Google Scholar]
- 23.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS. Tissue Engineering. 2004;10:865–872. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz SA, Chen CS. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee SW, Taylor AM, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Lab Chip. 2005;5:102–107. doi: 10.1039/b403091e. [DOI] [PubMed] [Google Scholar]
- 26.Bubendorfer A, Liu X, Ellis AV. Smart Materials and Structures. 2007;16:367–371. [Google Scholar]
- 27.Kim L, Toh Y-C, Voldman J, Yu H. Lab Chip. 2007;7:681–694. doi: 10.1039/b704602b. [DOI] [PubMed] [Google Scholar]
- 28.Chung BG, Park JW, Hu JS, Huang C, Monuki ES, Jeon NL. BMC Biotechnology. 2007;7:60. doi: 10.1186/1472-6750-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner W, Wein F, Roderburg C, Saffrich R, Faber A, Krause U, Schubert M, Benes V, Eckstein V, Maul H, Ho AD. Experimental Hematology. 2007;35:314–325. doi: 10.1016/j.exphem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Noll T, Jelinek N, Schmidt S, Biselli M, Wandrey C. Adv Biochem Eng Biotechnol. 2002;74:111–128. doi: 10.1007/3-540-45736-4_6. [DOI] [PubMed] [Google Scholar]
- 31.Regehr K, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Lab Chip. 2009;9:2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Schwartz M, Ionescu-Zanetti C. J Biomol Screen. 2009;14:194–202. doi: 10.1177/1087057108327326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.