Abstract
Rationale
18-25-year-olds show the highest rates of alcohol use disorders (AUD) and heavy drinking, which may have critical neurocognitive implications. Regions subserving memory may be particularly susceptible to alcohol-related impairments.
Objective
We used blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to examine the neural correlates of visual encoding and recognition among heavy drinking college students. We predicted that heavy drinkers would show worse memory performance and increased frontal/parietal activation and decreased hippocampal response during encoding.
Methods
Participants were 23 heavy drinkers and 33 demographically matched light drinkers, ages 18-20, characterized using quantity/frequency of drinking and AUD diagnosis. Participants performed a figural encoding and recognition task during fMRI. BOLD response during encoding was modeled based on whether each stimulus was subsequently recognized or forgotten (i.e., correct vs. incorrect encoding).
Results
There were no group differences in behavioral performance. Compared to light drinkers, heavy drinkers showed: 1) greater BOLD response during correct encoding in right hippocampus/medial temporal, right dorsolateral prefrontal, left inferior frontal, and bilateral posterior parietal cortices; 2) less left inferior frontal activation and greater bilateral precuneus deactivation during incorrect encoding; and 3) less bilateral insula response during correct recognition (clusters >10,233ul, p<.05 whole-brain).
Conclusions
This is the first investigation of the neural substrates of figural memory among heavy drinking older adolescents. Heavy drinkers demonstrated compensatory hyperactivation of memory-related areas during correct encoding, greater deactivation of default mode regions during incorrect encoding, and reduced recognition-related response. Results could suggest use of different encoding and recognition strategies among heavy drinkers.
Keywords: Alcohol, Adolescent, Young Adult, fMRI, Learning, Memory, Cognition
Alcohol consumption often escalates during the college years, and the highest rates of alcohol use disorders (AUD) are reported among 18-25-year-olds (SAMHSA 2011). However, little is known about the neurobiological sequelae of heavy drinking during late adolescence and emerging adulthood. Heavy alcohol use during this period may have implications for neuromaturation (Jacobus and Tapert 2013; Silveri 2012; Tapert and Schweinsburg 2005) as well as academic achievement (Hanson et al. 2011). In particular, memory processing is imperative to college education, and may be susceptible to the deleterious effects of alcohol (Jacobus and Tapert 2013). Therefore, it is of great importance to characterize the potential influence of heavy drinking on neural pathways involved in memory functioning among college students.
Neuropsychological studies have consistently demonstrated deficient learning and memory associated with heavy alcohol use in adults (for review, see Grant 1987) and adolescents (Brown et al. 2000; Jacobus and Tapert 2013; Parada et al. 2011; Squeglia et al. 2009b). Notably, frontal and temporal lobe structures that subserve memory (Budson 2009; Squire and Schacter 2002) continue to develop into the 20s (Gogtay et al. 2004) and may be uniquely vulnerable to the neurotoxic effects of alcohol (Crews and Boettiger 2009; Jacobus and Tapert 2013). Adolescents with AUD demonstrate smaller volumes of the hippocampus (De Bellis et al. 2000; Nagel et al. 2005) and prefrontal cortex (Medina et al. 2008; Thomasius et al. 2003). More recently, we reported that binge drinking in adolescence was associated with increased prefrontal and parietal blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) response but decreased hippocampal BOLD response during verbal learning, reflecting over-engagement of task-related frontoparietal systems in order to compensate for deficient medial temporal involvement and maintain task performance (Schweinsburg et al. 2010).
Although the neural substrates of verbal learning impairments have been explored in adolescent drinkers, visuospatial memory may be differentially impacted (Hanson et al. 2011). For instance, heavy alcohol use in adolescence is associated with compromised delayed recall of complex figures (e.g., Rey-Osterrieth Complex Figure test), but normal verbal learning and delayed recall (Brown et al. 2000; Squeglia et al. 2009b). Moreover, despite poorer delayed figure recall among adolescent drinkers, initial visuospatial learning and immediate recall may remain unimpaired (Brown et al. 2000; Hanson et al. 2011; Squeglia et al. 2009b), which could indicate a disparity in visuospatial acquisition and recognition processes. To our knowledge, the possible neurobiological abnormalities of complex figure encoding and recognition have not yet been explored among heavy drinking adolescents. Therefore, the current study was designed to characterize fMRI response during figural encoding and recognition in college drinkers.
There are numerous methods of ascertaining learning and memory using fMRI. One powerful approach is the subsequent memory paradigm (Kim 2011b), wherein subsequent performance on a memory task is used to code encoding trials as either subsequently remembered (correctly encoded) or forgotten (incorrectly encoded). Accordingly, fMRI response can be modeled separately for correct vs. incorrect encoding, usefully distinguishing brain systems involved in successful learning from neural activity thought to interfere with successful learning (Kim 2011b). Typically, correct encoding activates left inferior frontal cortex, fusiform, hippocampus, premotor cortex, and posterior parietal cortices (Kim 2011b). In contrast, incorrect encoding is associated with response in the default mode network (DMN), including medial prefrontal cortex, posterior cingulate, precuneus, and temporo-parietal junction (Kim 2011b). DMN response supports ongoing internally-oriented functions typically repressed during cognitively demanding tasks (Buckner et al. 2008; Raichle et al. 2001); activation of this network during incorrect encoding may reflect a failure to suppress these ongoing processes and redirect resources to the task (Daselaar et al. 2009; Kim et al. 2010).
Recent work has also characterized distributed networks associated with successful recognition of previously encountered stimuli (hits). During correct recognition, DMN response may reflect mental re-experiencing of old stimuli, prefrontal and posterior parietal regions may support cognitive control, and caudate may mediate positive reinforcement from correct responses (Kim 2011a). Several other regions have been less consistently identified, but may also be involved in retrieval functions (Spaniol et al. 2009). Some work differentiates between the recognition requirements of various paradigms, which may tap into different implicit and explicit processes involved in recognition (Spaniol et al. 2009).
In the current study, we examined the neural correlates of visual encoding and recognition associated with heavy drinking in college students using BOLD fMRI. Participants performed an established figural memory task that ascertains BOLD response during nonverbal visual encoding and subsequent “old/new” recognition (Beason-Held et al. 2005; Jamadar et al. 2013). Based on the literature on the subsequent memory paradigm (Kim 2011a; b) and our previous work examining fMRI response during verbal encoding (Schweinsburg et al. 2010), we predicted that 1) during correct encoding, heavy drinking college students would exhibit increased response compared to light drinkers in task-relevant regions including bilateral prefrontal cortex, posterior parietal cortex, and hippocampus, indicating compensatory neural recruitment to maintain task performance; 2) during incorrect encoding, heavy drinkers would show less DMN response, reflecting reduced suppression of irrelevant information and shifting of attention toward the task; and 3) during correct recognition, heavy drinkers would show reduced response in regions typically involved in correct recognition, suggesting diminished retrieval processing.
Method
Participants
Participants were 56 18- to 20-year-old college students who were recruited as part of an ongoing study of alcohol and neurocognitive function of 2100 first-year college students, the Brain and Alcohol Research in College Students (BARCS) study (Dager et al. 2013). A representative subset of individuals from the larger BARCS study participated in neuroimaging completed the figural memory task during scanning. Participants provided written informed consent, approved by the institutional review boards at Yale University, Hartford Hospital, Trinity College and Central Connecticut State University. Exclusion criteria included current DSM-IV-TR anxiety and mood disorders, current or past psychotic disorders or substance use disorder (other than AUD in the heavy drinking group), positive urine toxicology screen at the time of scanning, history of seizures, head injury with loss of consciousness >10 minutes, left handedness, poor performance (<50% accuracy) or unavailable behavioral data on the figural memory task, excessive motion during scanning, and MRI contraindications (see Electronic Supplement for additional details). Eligible participants were divided into heavy drinkers and light drinkers based on a combination of AUD diagnosis and quantity/frequency of current alcohol consumption (Dager et al. 2013). Light drinkers 1) did not meet current or past criteria for AUD, and 2) drank <50% of the weeks during the preceding six months. Heavy drinkers either 1) met criteria for current AUD, or 2) drank ≥50% of the weeks in the preceding six months and typically binge drank (≥4 drinks/occasion for females or ≥5 drinks/occasion for males (e.g., Courtney and Polich 2009; Schweinsburg et al. 2010)). The final sample consisted of 33 light drinkers and 23 demographically similar heavy drinkers (see Table 1).
Table 1. Participant Demographic and Substance Use Characteristics.
| Heavy Drinkers (n = 23) M (SD) or % [range] | Light Drinkers (n = 33) M (SD) or % [range] | p value | |
|---|---|---|---|
| Age (range 18 – 20) | 18.9 (0.63) | 18.7 (0.42) | 0.072 |
| Female | 52.2% | 60.6% | 0.530 |
| Caucasian | 74.3% | 60.0% | 0.918 |
| Family history negative for alcoholism | 56.5% | 72.7% | 0.208 |
| Past mood or anxiety disorder | 17.4% | 6.1% | 0.177 |
| Spielberger State-Trait Anxiety Inventory T-scorea,b | 48.7 (11.1) | 49.1 (12.5) | 0.91 |
| [30 – 68] | [30 – 76] | ||
| Beck Depression Inventory total scorea | 3.3 (4.9) | 2.6 (3.6) | 0.61 |
| [0 – 19] | [0 – 12] | ||
| Lifetime drinks | 152.0 (216.3) | 24.2 (26.8) | 0.01 |
| [10 – 1000] | [0 – 100] | ||
| # weeks drinking, past 6 months | 13.0 (7.4) | 2.3 (2.6) | < 0.001 |
| [2 – 26] | [1 – 10] | ||
| Drinking days/week, past 6 months | 3.5 (1.7) | 1.1 (1.4) | < 0.001 |
| [1 – 7] | [2 – 5] | ||
| Drinks/day, past 6 months | 6.7 (3.2) | 2.1 (2.6) | < 0.001 |
| [2 – 15] | [0 – 10] | ||
| Drinks/week, past 6 months | 22.3 (15.2) | 4.1 (5.0) | < 0.001 |
| [5 – 72] | [0 – 14] | ||
| Current alcohol dependence | 39.1% | 0.0% | < 0.001 |
| Current alcohol abuse | 91.3% | 0.0% | < 0.001 |
Data available for 16 heavy drinkers and 29 light drinkers
Normed for college students
Measures
Drinking history was obtained using the alcohol use module of the SCID (First et al. 2002), which ascertains AUD symptoms, usage patterns (e.g., quantity and frequency, lifetime drinks) and alcohol-related consequences (e.g., number of black-outs and pass-outs). Current and past DSM-IV-TR diagnoses for psychotic, anxiety, mood, and substance use disorders were ascertained using the MINI (Sheehan et al. 1998). Current use of other drugs was characterized through monthly online surveys as part of the ongoing BARCS study. Family history of alcohol use disorders was assessed with the Family History Assessment Module (FHAM) (Rice et al. 1995). The Fagerstrom Test of Nicotine Dependence (Heatherton et al. 1991) obtained information on cigarette smoking. The Spielberger State-Trait Anxiety Inventory (Form Y trait scale) (Spielberger et al. 1983) and Beck Depression Inventory (Beck 1978) were collected with monthly online surveys as part of the larger ongoing study in order to characterize anxiety and depressive symptoms. To best estimate anxiety and depression symptoms present at the time of scanning, the current study examined scores from the 30-day period that included the scan date. At the time of scanning, participants were free of alcohol and illicit substances as verified by breathalyzer and urine toxicology, and females provided negative pregnancy screens.
Figural Memory Task
The figural memory task (Beason-Held et al. 2005) is a visual encoding and recognition task designed to minimize verbal encoding of picture stimuli. The task stimuli (20 targets and 20 distractors) consisted of black line drawings presented against a white background. Participants performed an encoding phase and a recognition phase during fMRI scanning. The encoding phase presented 20 target stimuli (duration 3 sec, interstimulus (ISI) interval 4 sec), which participants were instructed to silently memorize. Participants pressed a fiber optic response box button following each stimulus presentation to confirm that they saw the stimulus. The recognition phase followed the encoding phase after a 5-minute delay (with no other cognitive task presented during the delay). During the recognition phase, 20 target and 20 distractor stimuli were presented in a fixed pseudo-random order, each for 3 sec with an ISI of 4 sec. Participants pressed a button with their right index (“yes”) and middle (“no”) fingers to indicate whether they had previously seen each stimulus, and accuracy was emphasized over speed.
Image Acquisition
Imaging data were collected on a Siemens 3T Allegra high performance head-dedicated system. Structural imaging was acquired with a sagittal T1 MPRAGE protocol using the following parameters: TR = 2500 ms, TE=2.74 ms, flip angle = 8°, FOV=176 × 256 mm, matrix = 256 × 208, voxel size = 1 mm3, 176 slices, total scan time =7:20. Functional images were collected in the axial plane using a T2*-weighted echoplanar image (EPI) gradient-echo pulse sequence covering the whole brain: TR = 1860 ms, TE = 27 ms, flip angle 70°, FOV = 240 mm, matrix=64 × 64, in-plane resolution=3.44 mm × 3.44 mm, slice thickness = 3 mm, gap = 1 mm, 36 slices, total scan time = 12:33.
Data Analyses
Stimuli from the recognition phase were classified as hits, misses, correct rejections, and false alarms. We used a signal detection approach (Macmillan and Creelman 2005) to examine accuracy. The discriminability index, d′, represents the ability to discriminate targets from distractors and was calculated as z(hit rate) – z(false alarm rate), with a standard correction for false alarm rates of zero. We compared d′ between groups with an independent samples t-test. Reaction times during the recognition phase were compared between groups using repeated measures ANOVA with two within-subjects factors (target vs. distractor and response “yes” vs. “no”) and one between-groups factor (drinking group).
Functional images from the figural memory task were preprocessed and modeled in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The first six volumes were discarded to allow for T1 saturation effects. Images were realigned, spatially normalized to Montreal Neurological Institute (MNI) standardized space, resampled to 3×3×3 mm voxels, and smoothed with a 9 mm full-width, half-maximum Gaussian filter. Datasets were inspected for motion, and those with >3mm displacement or >3 degrees rotation were not included in the current study (n = 20 not reported on here).
BOLD response was modeled as in our previous work (Jamadar et al. 2013), based on behavioral performance, while covarying for the degree of motion and linear baseline trends. Trials from the encoding phase were modeled as correctly encoded if they were subsequently identified as targets during the recognition phase. Targets that were subsequently designated as distractors in the recognition phase were categorized as incorrectly encoded. Events in the recognition phase were coded as hits, misses, false alarms, and correct rejections. BOLD response for each event was modeled using a canonical hemodynamic response function fitted to the onset of the event. The duration of each event was determined by reaction time. BOLD response contrast was determined for correctly encoded vs. incorrectly encoded, hits vs. implicit baseline, misses vs. implicit baseline, and correct rejections vs. implicit baseline. Although false alarm events were modeled at the individual level, we did not examine group differences in BOLD response to false alarms because there were too few such responses to model at the group level (Jamadar et al. 2013).
Group level analyses of BOLD response contrasts were conducted using Analysis for Functional NeuroImages (AFNI) (Cox 1996). First, images were transformed to Talairach space (Talairach and Tournoux 1988) and AFNI format files using the AFNI program 3dWarp. Then, independent samples t-tests characterized BOLD response differences between drinking groups for each contrast. We performed a whole-brain correction for multiple comparisons using a combination of cluster volume and individual voxel threshold (e.g., Forman et al. 1995) as determined through a Monte Carlo simulation (Ward 2000). Clusters were considered significant if they comprised ≥379 contiguous activated voxels (voxel p < .05, cluster volume 10,233 μl), yielding a whole-brain α = .05. To determine the nature of group differences, post-hoc analyses (p < .05, uncorrected) were performed on average activation from 8mm spheres centered at each peak voxel within group difference cluster.
Hippocampal ROI
Given the importance of the hippocampus in encoding, we also analyzed BOLD response within left and right hippocampal regions of interest (ROI) defined a priori using the ROI feature of AFNI (Schweinsburg et al. 2010; Schweinsburg et al. 2011). BOLD response during correct and incorrect encoding was averaged across the hippocampal ROIs for each subject, and then examined between groups using independent samples t-tests.
Exploratory Analyses
We conducted several exploratory supplementary analyses to determine the relationships between alcohol use characteristics, family history of alcoholism, behavioral performance, and BOLD response (see Electronic Supplement for detailed methods).
Results
Demographic Results
Heavy and light drinking groups were similar on age, sex, race, family history of AUD, and personal history of psychiatric disorders (see Table 1). Online survey data were incomplete, such that mood and substance use data for the month of the scan were unavailable for 11 participants. One light drinker and five heavy drinkers reported some (<10 times) marijuana use in the month of the scan, yet all provided negative urine toxicology screens for cannabinoids. One heavy drinker reported limited smoking fewer than 10 cigarettes per day and scored 2 out of 10 points on the Fagerstrom Test of Nicotine Dependence, indicating non-dependent use; all other participants were nonsmokers. All but three participants were free from psychoactive medication use: one light drinker and one heavy drinker reported using antidepressants, and one heavy drinker reported a past diagnosis of ADHD and current use of Adderall, yet provided a negative urine toxicology screen at the time of scanning. All other participants denied past diagnoses of ADHD. On each of the alcohol use indices in Table 1, heavy drinkers demonstrated significantly greater alcohol involvement than light drinkers (see Table 1, all p values < 0.01).
Behavioral Results
All participants correctly identified at least 50% of targets. There were no group differences in d′ [t(54) = 0.71, p = .48; see Table 2 for behavioral results]. There were no main effects on reaction time related to drinking group [F(1, 54) = 1.10, p = .30], stimulus type [target vs. distractor; F(1, 54) = 2.84, p = .10] or response type [yes vs. no; F(1, 54) = 2.70, p = .11]. There was a stimulus type × response type interaction for reaction time, such that participants reacted more slowly when responding “no” to targets (i.e., misses) than to other stimulus and response types [F(1, 54) = 21.87, p < .001]. There were no reaction time effects related to a stimulus type × response type × drinking group interaction, stimulus type × group interaction, or response type × group interaction (all ps > .3).
Table 2.
Figural Memory Task Performance.
| Heavy Drinkers (n = 23) M (SD) | Light Drinkers (n = 33) M (SD) | |
|---|---|---|
| Number of Responses (max=20) | ||
| Hits | 14.09 (0.46) | 14.18 (0.39) |
| Misses | 5.35 (0.46) | 5.64 (0.38) |
| False Alarms | 3.17 (0.40) | 2.88 (0.34) |
| Correct Rejections | 16.39 (0.44) | 16.70 (0.37) |
| d′ | 1.64 (0.69) | 1.76 (0.54) |
| Reaction Time (seconds) | ||
| Hits | 1.40 (0.49) | 1.44 (0.41) |
| Misses | 1.67 (0.82) | 1.74 (0.68) |
| False Alarms | 1.45 (0.13) | 1.63 (0.11) |
| Correct Rejections | 1.41 (0.53) | 1.43 (0.45) |
fMRI Results
BOLD response during encoding
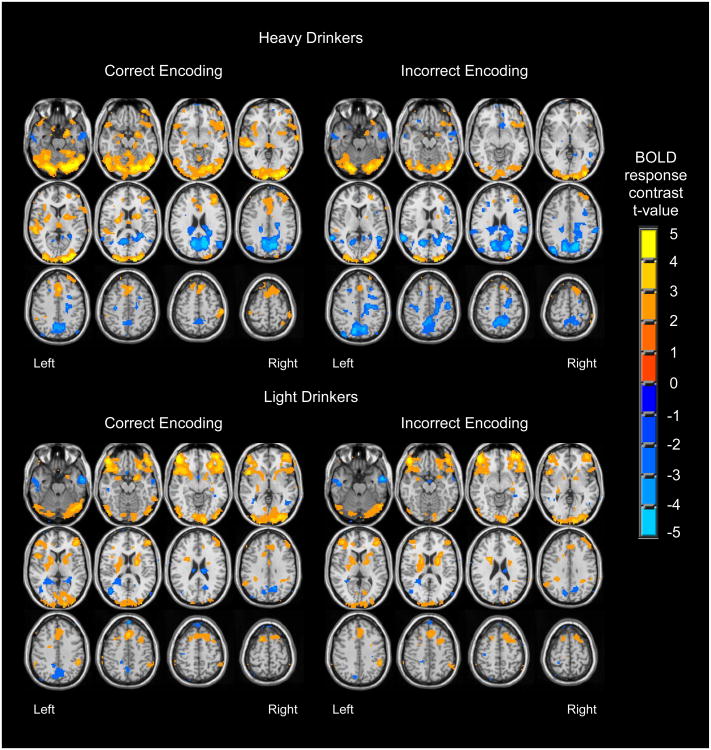
As in our previous work (Jamadar et al. 2013), participants (n = 56) showed activation during correct encoding in bilateral medial temporal cortices, medial frontal cortices and cingulate, anterior insula and inferior frontal gyri, inferior parietal lobules, and occipital cortices, and deactivation in precuneus and posterior cingulate (Figure 1). During incorrect encoding, response patterns were similar but diminished, with notable DMN deactivation.
Figure 1.
Regions showing significant BOLD response during correct encoding and incorrect encoding, among heavy drinkers (n = 23) and light drinkers (n = 33) (p < .05 uncorrected).
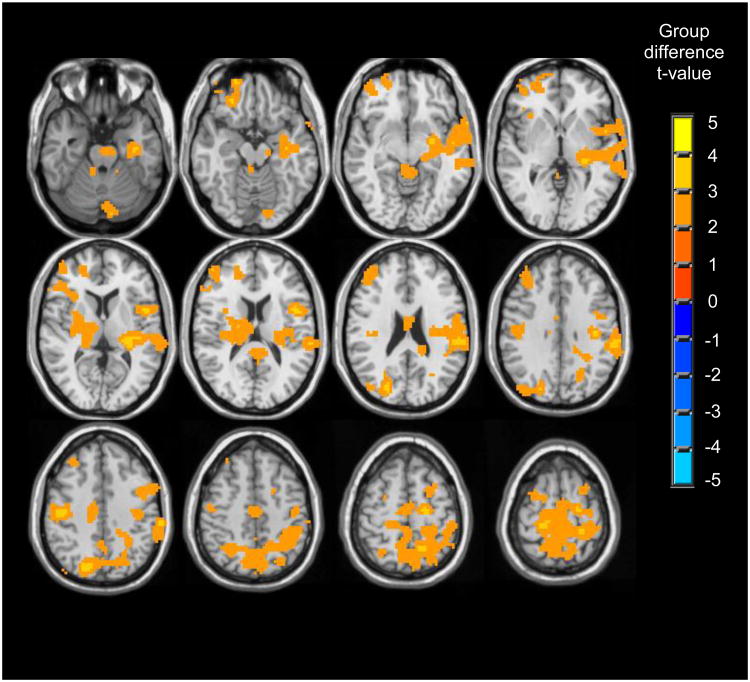
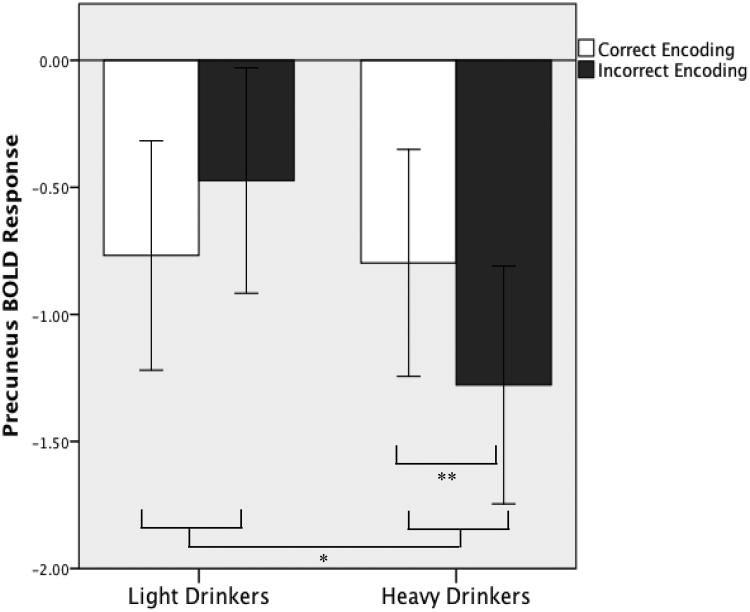
Whole-brain t-tests revealed group differences in BOLD response to correct vs. incorrect encoding primarily in three widespread clusters (clusters > 10,233 μl, p < .05) that each spanned several regions (see Table 3 and Figure 2). Follow-up single sample t-tests (p < .05, uncorrected) revealed the nature of group differences (see Figure 1 and Table 3). In right temporal cortex, right hippocampus and parahippocampus, and lateral portions of posterior parietal cortex, both heavy drinkers and light drinkers showed greater activation during correct encoding compared to incorrect encoding; however, this difference was more pronounced for heavy drinkers. In left ventral middle/inferior frontal cortex, light drinkers showed substantial activation during both correct and incorrect encoding, yet heavy drinkers did not demonstrate significant BOLD response during correct or incorrect encoding. In only one region, precuneus, group differences were accounted for by deactivation differences. Here, light and heavy drinkers showed deactivation during both correct and incorrect encoding, although heavy drinkers showed enhanced deactivation during incorrect encoding (Figure 3).
Table 3.
Regions showing significant group differences for correct vs. incorrect encoding (clusters > 10,233 μl, p < .05 whole brain corrected). In all regions, heavy drinkers showed greater BOLD response to correctly vs. incorrectly encoded stimuli relative to light drinkers.
| Anatomic Region (BA) | MNI Coordinates | Activation Mean (SD) | Between-Groups Cohen's d Effect Size | |
|---|---|---|---|---|
|
| ||||
| x, y, z | Heavy Drinkers | Light Drinkers | ||
| Cluster 1 | ||||
| Right hippocampus/parahippocampal gyrus (36) | 30, -14, -26 | 0.27 (0.49)* | -0.01 (0.33) | 0.70 |
| Right middle frontal gyrus (9) | 55, 7, 40 | 0.37 (0.61)* | 0.02 (0.41) | 0.70 |
| Right superior temporal gyrus, insula (22, 13, 44) | 45, -15, -8 | 0.46 (0.66)* | 0.08 (0.43) | 0.71 |
| Right superior temporal gyrus, inferior parietal lobule (40, 41, 42) | 55, -28, 12 | 0.55 (1.14)* | 0.24 (0.46) | 0.65 |
| Right superior temporal gyrus (21, 22) | 61, 6, -3 | 0.58 (0.82)* | 0.10 (0.50) | 0.74 |
| Bilateral cerebellum | 0, -40, -6 | 0.65 (1.58) | -0.34 (1.22) | 0.72 |
|
| ||||
| Cluster 2 | ||||
| Left precuneus, superior parietal lobule (7) | -3, -79, 42 | 0.48 (1.36) | -0.29 (0.83)* | 0.72 |
| Left cuneus, middle occipital gyrus (19, 31) | -21, -75, 22 | 0.32 (0.50)* | -0.01 (0.30) | 0.85 |
| Bilateral paracentral lobule, cingulate (31) | 0, -21, 48 | 0.40 (0.79)* | -0.02 (0.50) | 0.67 |
| Right precuneus, superior/inferior parietal lobules (7, 40) | 45, -55, 53 | 0.41 (0.78)* | 0.05 (0.54) | 0.56 |
| Bilateral medial frontal gyrus (6) | 3, -13, 74 | 0.66 (0.85)* | 0.09 (0.48) | 0.88 |
| Left thalamus, putamen | -3, -16, 15 | 0.34 (0.94) | -0.23 (1.03) | 0.57 |
|
| ||||
| Cluster 3 | ||||
| Left superior/middle frontal gyrus (9, 10, 46) | -21, 65, 10 | 0.31 (0.78) | -0.06 (0.49) | 0.59 |
| Left inferior frontal gyrus, insula (11, 13, 45) | -35, 21, 8 | 0.27 (0.41)* | 0.01 (0.44) | 0.60 |
Note: MNI coordinates refer to peak voxels within cluster; Group activation and between groups Cohen's d refer to 8mm spheres centered on peak voxels;
single sample t-test shows that BOLD response to correct vs. incorrect encoding significantly different from 0 (p < .05); positive mean indicates greater response during correct vs. incorrect encoding, negative mean indicates greater response during incorrect vs. correct encoding.
Figure 2.
Regions showing group differences in BOLD response to correct encoding vs. incorrect encoding (clusters > 10,233 μl, p < .05 whole brain corrected).
Figure 3.
Average precuneus BOLD contrasts during correct and incorrect encoding, where heavy drinkers showed enhanced deactivation during incorrect encoding. BOLD response is averaged from an 8 mm sphere around the peak MNI coordinate, -3, -79, 42. Error bars represent +/- 1 standard error.
* Significant group by encoding condition interaction, p < .05
** Significant difference between encoding conditions, p < .05
BOLD response during recognition
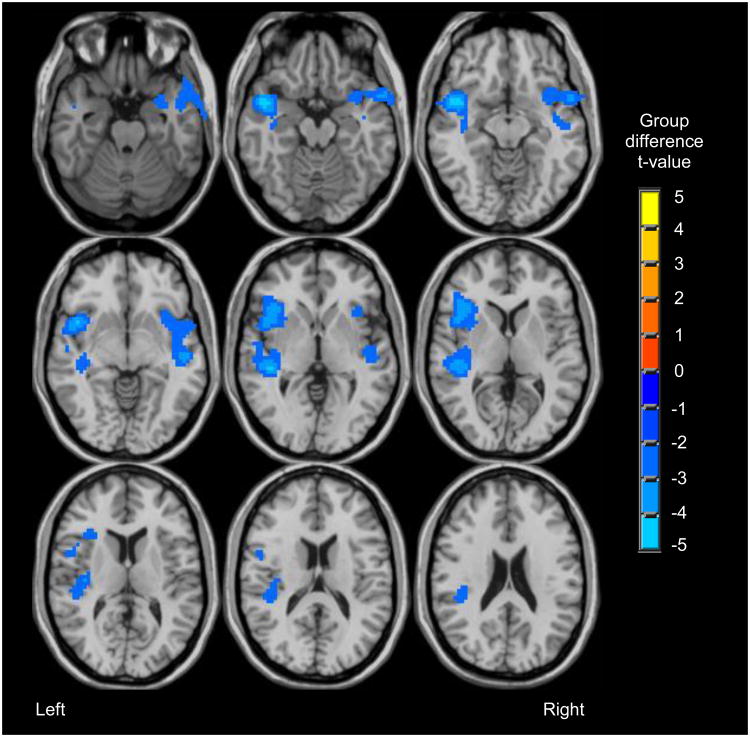
Consistent with our previous work using this task (Jamadar et al. 2013), participants showed BOLD response during recognition trials for hits vs. baseline, correct rejections vs. baseline, and missed targets vs. baseline in bilateral occipital, subcortical, inferior frontal, anterior cingulate, and posterior parietal regions. Compared to light drinkers, heavy drinkers showed less BOLD response during correct recognition trials (“hits”) in two clusters in bilateral insula and superior temporal gyri (clusters > 10,233 μl, p < .05; see Table 4 and Figure 4). There were no group differences in BOLD response to correct rejections, or missed targets.
Table 4.
Regions showing significant group differences for correct recognition (clusters > 10,233 μl, p < .05). In all regions, heavy drinkers showed less response during correctly recognized stimuli compared to light drinkers.
| Anatomic Region (BA) | MNI Coordinates | Activation Mean (SD) | Between-Groups Cohen's d Effect Size | |
|---|---|---|---|---|
|
|
||||
| x, y, z | Heavy Drinkers | Light Drinkers | ||
| Cluster 1 | ||||
| Left superior temporal gyrus (38, 41) | -45, 7, -9 | -1.13 (1.43)* | 0.67 (1.56)* | 1.19 |
| Left insula, inferior frontal gyrus (13, 47) | -45, 12, 4 | 0.41 (0.96) | 1.40 (1.25)* | 0.87 |
|
| ||||
| Cluster 2 | ||||
| Right superior temporal gyrus, insula (38, 22, 13) | 54, 7, -11 | -0.88 (1.16)* | 0.18 (1.35) | 0.84 |
Note: MNI coordinates refer to peak voxels within cluster; Group activation and between groups Cohen's d refer to 8mm spheres centered on peak voxels;
single sample t-test shows that BOLD response to correct vs. incorrect encoding significantly different from 0 (p < .05); positive mean indicates activation during correct recognition, negative mean indicates deactivation correct recognition.
Figure 4.
Regions showing group differences (heavy drinkers < light drinkers) in BOLD response during correct recognition (clusters > 10,233 μl, p < .05).
Hippocampal ROI
In parallel with the whole-brain analyses, the hippocampal ROI analyses revealed that heavy drinkers demonstrated greater BOLD response than light drinkers during correct vs. incorrect encoding in right hippocampus/parahippocampus [t(54) = 2.18, p = .034] but not the left [t(54) = 0.64, p = .17].
Discussion
This is the first study to examine fMRI response during visual encoding and recognition among heavy drinking older adolescents. Although we observed no group effects on task accuracy or reaction times, heavy drinkers showed three main BOLD response differences compared to light drinkers: 1) greater activation during encoding of subsequently remembered stimuli in widespread frontal, posterior parietal, and medial temporal systems; 2) less left inferior frontal activation and greater precuneus deactivation during incorrect encoding; and 3) decreased response during correct recognition in bilateral inferior frontal and insular cortices.
Correct Encoding
As predicted, while encoding subsequently remembered stimuli, heavy drinkers over-engaged prefrontal and parietal structures typically involved in successful encoding (Kim 2011b; Spaniol et al. 2009). Similarly, our previous work has characterized increased dorsal prefrontal response during verbal learning in binge drinking adolescents (Schweinsburg et al. 2010; Schweinsburg et al. 2011), as well as lateral posterior parietal hyperactivation among adolescent heavy drinkers both during spatial working memory (Schweinsburg et al. 2008; Tapert et al. 2004) and verbal encoding (Schweinsburg et al. 2010; Schweinsburg et al. 2011). Dorsal frontal regions may subserve working memory organization during encoding (Blumenfeld and Ranganath 2007; Spaniol et al. 2009), whereas posterior parietal response may support attention during correct encoding, particularly during visual learning tasks (Kim 2011b; Spaniol et al. 2009). In the context of intact performance, over-recruitment of task-related frontoparietal regions by heavy drinkers may represent an attempt to compensate for inefficient processing or greater difficulty with task demands (e.g., Gould et al. 2003; Schweinsburg et al. 2010).
Also consistent with previous neuroimaging work (Jamadar et al. 2013; Kim 2011b), both groups showed hippocampal and medial temporal activation during encoding of successfully remembered stimuli. In the right hippocampus, this effect was amplified among heavy drinkers. Although we did not test specifically for laterality effects, this finding is compatible with studies suggesting greater right medial temporal lobe involvement during encoding of abstract visual stimuli (Banks et al. 2012; Golby et al. 2001). Enhanced hippocampal response has been observed with increasing encoding demands, and may reflect more effortful processing among heavy drinkers (Leshikar et al. 2010; Ulrich et al. 2010). Importantly, the hippocampus may be particularly susceptible to alcohol-related neurotoxicity (Crews and Boettiger 2009), and has shown volumetric reductions among adolescent heavy drinkers (Medina et al. 2007; Nagel et al. 2005). In contrast to the current results, our previous work showed lack of significant hippocampal activation and somewhat poorer performance in adolescent binge drinkers during verbal learning (Schweinsburg et al. 2010). These somewhat conflicting results may be related to differences in task design and image analyses, as degree of hippocampal response may depend on task stimuli, encoding paradigm, and analytic approach (Kim 2011b). Our previous study examined verbal paired associates learning rather than complex nonverbal item learning, and did not distinguish neural response to correctly vs. incorrectly encoded items, which may contribute to differing hippocampal results compared to the current study (Kim 2011b).
Incorrect Encoding
During incorrectly encoded trials, heavy drinkers demonstrated attenuated left inferior frontal response. This region has been repeatedly identified in memory paradigms, and may facilitate conceptual processing of stimulus content during successful pictorial encoding (Kim 2011b; Spaniol et al. 2009). Moreover, inefficient interactions between left frontal and medial temporal cortices may be a crucial component of unsuccessful encoding (Buckner et al. 1999; Fernandez and Tendolkar 2001; Kim 2011b).
In addition, heavy drinkers exhibited greater deactivation during incorrect encoding in the precuneus, which is a well-established component of the DMN (Raichle et al. 2001) involved in internally-oriented processing (Buckner et al. 2008). DMN suppression during a cognitively demanding task likely represents shifting of attention to task-relevant regions, and is reflected as deactivation during task “on” periods (Whitfield-Gabrieli and Ford 2012). DMN deactivation during memory paradigms is associated with successful encoding, whereas DMN activation (i.e., failure to suppress DMN activity) is associated with encoding failure, reflecting that resources are not appropriately allocated to encoding functions (Daselaar et al. 2009; Kim et al. 2010). This “typical” pattern of diminished DMN suppression during incorrect encoding was observed among light drinkers in the current study. In contrast, heavy drinkers demonstrated more DMN suppression during incorrect encoding. DMN suppression (i.e., degree of task-related deactivation) increases with greater task difficulty, as more neural effort is focused toward task performance (Whitfield-Gabrieli and Ford 2012). Thus, greater deactivation among heavy drinkers in the current study could reflect more difficulty encoding stimuli that are later forgotten. Moreover, recent work has hypothesized that better task performance is associated with concurrent DMN suppression and task-positive network activation (Kelly et al. 2008; Prado and Weissman 2011; Sutherland et al. 2012). This model proposes that optimal performance is achieved when greater DMN suppression is accompanied by greater task-positive response, and argues that coherence both between and within these networks reflects efficient processing (Kelly et al. 2008). During incorrect encoding, heavy drinkers in the current study demonstrated enhanced DMN suppression without concomitant increases in task-related regions, which could suggest aberrant shifting between DMN and task-positive networks during trials that were unsuccessfully encoded. Accordingly, functional connectivity analyses have suggested that poor coupling between these systems may mediate cognitive decrements observed in addiction (Sutherland et al. 2012). Others have observed that adults with alcohol dependence show disrupted DMN functional connectivity during both rest and working memory (Chanraud et al. 2011), and greater alcohol dependence severity was linked to weaker frontostriatal functional connectivity during inhibitory processing (Courtney et al. 2013). During a response inhibition task, recovering alcoholics demonstrated less connectivity between posterior cingulate and mid-cingulate but greater connectivity between mid-cingulate and striatum (Schulte et al. 2012). Together, these studies support the hypothesis of aberrant between- and within-network function among heavy drinkers performing executive tasks. Future functional connectivity analyses may better address these relationships in the context of learning and memory.
Recognition
Our previous investigations of verbal learning in adolescent heavy drinkers ascertained fMRI response only during encoding (Schweinsburg et al. 2010; Schweinsburg et al. 2011); therefore, the neurobiological underpinnings of retrieval have not yet been examined in heavy drinkers. Heavy drinkers in the current study exhibited attenuated insula response to correctly recognized stimuli. Prior studies using this task have observed recognition-related insula response (Beason-Held et al. 2005; Jamadar et al. 2013), and meta-analyses reveal that insula activation is associated with successful recognition and retrieval in a variety of memory paradigms (Kim 2011a; Spaniol et al. 2009). The insula is involved in attention, cognitive control, and performance monitoring (Nelson et al. 2010), which may contribute to recognition success. In addition, insular function has been indicated in intolerance of uncertainty, as well as anticipation of negative outcomes, during cognitive tasks (Samanez-Larkin et al. 2008; Simmons et al. 2008). Blunted recognition-related insula response among heavy drinkers could reflect reduced arousal or distress while making recognition judgments. It is also possible that heavy drinkers approach the task in a different manner. Neuropsychological studies of heavy drinking adolescents have observed poorer complex figure retention, despite intact learning and immediate recall (Brown et al. 2000; Hanson et al. 2011), which could implicate altered consolidation or retrieval functions. In our exploratory analyses (see Electronic Supplement), more severe lifetime alcohol involvement (greater number of drinks, pass-outs, and blackouts) was also associated with slower reaction times among heavy drinkers, which may reflect less efficient processing or lower confidence in recognition judgments (Wixted 2009). Thus, during retrieval, heavy drinkers may utilize a different, slower approach that ultimately results in similar accuracy. This finding parallels work examining the speed/accuracy tradeoff in adults with alcoholism, which generally suggests less efficient processing associated with alcoholism (Glenn and Parsons 1991; 1992). In particular, when task instructions emphasize accuracy over speed (as in the current study), individuals with alcoholism demonstrate slower reaction times but intact accuracy compared to controls (Glenn and Parsons 1991). The current study provides initial insight into the neural mechanisms underlying these processes.
Limitations and Future Directions
The current study gains strength and novelty from the large, well-characterized imaging sample, separate modeling of correct and incorrect encoding, and assessment of fMRI response both during encoding and recognition phases. We excluded participants with current psychiatric disorders and history of other substance use disorders in order to differentiate unique alcohol-related effects, but this may limit our ability to generalize results. There are a number of limitations that should be addressed in future studies. Our anxiety and mood assessments may not have fully captured symptoms that were present at the time of scanning. We excluded for past substance use disorders, but did not collect detailed information on lifetime use of other drugs. We also did not ascertain current ADHD or prior disruptive behavior disorder symptomatology. We conducted exploratory analyses (see Electronic Supplement) indicating little influence of alcoholism family history on BOLD response patterns; however, our sample was relatively small for characterizing such effects. Future investigations should further explore these possible moderators (Jacobus and Tapert 2013; Silveri 2012; Tapert and Schweinsburg 2005).
As with other cross-sectional studies, it is unclear whether the observed BOLD response differences predate the onset of drinking, or represent cumulative effects of alcohol exposure. In particular, some differences in visuospatial working memory may exist before heavy drinking is initiated (Spadoni et al. 2008; Squeglia et al. 2012). However, additional work suggests that visuospatial decrements are exacerbated by escalating drinking (Squeglia et al. 2009a; Squeglia et al. 2012) and that hippocampal abnormalities emerge with the initiation of heavy drinking in adolescence (Hanson et al. 2010; Silveri 2012). Thus, work to date implicates both pre-existing abnormalities as well as deleterious effects of alcohol exposure on visuospatial memory processing in adolescents (Jacobus and Tapert 2013; Silveri 2012). Longitudinal studies could characterize BOLD response trajectories throughout drinking initiation and escalation, and explore the hypothesis that the BOLD effects we observed presage the subsequent development of performance decrements in heavy drinkers.
We also did not observe group differences in behavioral performance, which could make the implications of fMRI differences unclear. However, absence of differences in behavioral performance highlights neural strategies that are used to maintain performance, and also ensures that fMRI differences are not ascribable to differential achievement, motivation, or other factors that may lead to performance differences. Future studies utilizing tasks with a wider range of difficulty levels will characterize differences in coupling between performance and BOLD response between groups. In addition, it is possible that performance decrements would be observed with longer histories of heavy drinking (Tapert et al. 2001), and may be uncovered in subsequent longitudinal analyses. Finally, functional connectivity analyses will better elucidate the relationships between various task networks, such as the potentially opposing processes of DMN and task-positive systems that differ between heavy and light drinkers.
Conclusions
In this first study of brain response to nonverbal learning and recognition in heavy drinking college students, heavy drinkers showed an overall exaggerated response pattern during both correct and incorrect encoding, characterized by hyperactivity of task-relevant frontal, parietal, and medial temporal regions, and greater DMN deactivation. During recognition, heavy drinkers demonstrated reduced insula activation. Together, these findings could indicate more effortful encoding, and utilization of alternate encoding and retrieval strategies, compared to light drinkers. Future work is needed to determine the behavioral implications of these neural differences.
Supplementary Material
Acknowledgments
This research was made possible by grant support from the National Institute on Alcohol Abuse and Alcoholism (AA016599 and AA019036-01, Pearlson). The authors thank Gregory Book, Broderick Sawyer, Samantha Leen, Meredith Ginley, Krishna Pancholi, Balaji Narayanan, and Laura Mickes. Portions of this work were presented at the Society of Biological Psychiatry 67th Annual Scientific Convention, May 3-5, 2012, Philadelphia PA.
References
- Banks SJ, Jones-Gotman M, Ladowski D, Sziklas V. Sex differences in the medial temporal lobe during encoding and recognition of pseudowords and abstract designs. Neuroimage. 2012;59:1888–95. doi: 10.1016/j.neuroimage.2011.08.087. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Golski S, Kraut MA, Esposito G, Resnick SM. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiol Aging. 2005;26:237–50. doi: 10.1016/j.neurobiolaging.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp; San Antonio, TX: 1978. [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–91. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–71. [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–4. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–9. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–81. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2013;18:593–604. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–56. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante J, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcoholism: Clinical and Experimental Research. 2013;37:E161–71. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: human declarative memory formation at the system level. Brain Res Bull. 2001;55:1–9. doi: 10.1016/s0361-9230(01)00494-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Research Version, Non-patient Edition (SCID-I/NP, 11/2002 revision) Biometrics Research Department, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. Effects of alcoholism and instructional conditions on speed/accuracy tradeoffs. Alcohol Clin Exp Res. 1991;15:612–9. doi: 10.1111/j.1530-0277.1991.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. Neuropsychological efficiency measures in male and female alcoholics. J Stud Alcohol. 1992;53:546–52. doi: 10.15288/jsa.1992.53.546. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. Epub 2004 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–54. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, ffytche DH, Howard RJ. fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage. 2003;20:1006–19. doi: 10.1016/S1053-8119(03)00365-3. [DOI] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. Journal of Consulting and Clinical Psychology. 1987;55:310–24. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav. 2011;25:127–42. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am J Drug Alcohol Abuse. 2010;36:161–7. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol. 2013;9:703–21. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Assaf M, Jagannathan K, Anderson K, Pearlson GD. Figural memory performance and fMRI activity across the adult lifespan. Neurobiol Aging. 2013;34:110–127. doi: 10.1016/j.neurobiolaging.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: An Activation Likelihood Estimation Meta-Analysis. Hum Brain Mapp. 2011a doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011b;54:2446–61. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: roles of the task-positive and task-negative networks. Neuroimage. 2010;49:1045–54. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Gutchess AH, Hebrank AC, Sutton BP, Park DC. The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex. 2010;46:507–21. doi: 10.1016/j.cortex.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user's guide. 2nd. Erlbaum; Mahwah, NJ: 2005. [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–80. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Caamano-Isorna F, Mota N, Crego A, Holguin SR, Cadaveira F. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res. 2011;35:1475–84. doi: 10.1111/j.1530-0277.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- Prado J, Weissman DH. Heightened interactions between a key default-mode region and a key task-positive region are linked to suboptimal current performance but to enhanced future performance. Neuroimage. 2011;56:2276–82. doi: 10.1016/j.neuroimage.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–3. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings NHSDA Series H-41. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A. Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biol Psychiatry. 2012;71:269–78. doi: 10.1016/j.biopsych.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–7. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–73. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harvard review of psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–7. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–45. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009a;40:31–8. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. Journal of studies on alcohol and drugs. 2012;73:749–60. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009b;23:715–22. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Schacter DL. The neuropsychology of memory. 3. Guilford; New York: 2002. [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–95. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain Three-dimensional proportional system: An approach to cerebral imaging. Thieme; New York: 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–45. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD. The human adolescent brain and alcohol use disorders. Recent Dev Alcohol. 2005;17:177–97. doi: 10.1007/0-306-48626-1_9. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown GG, Brown SA, Frank LR, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology (Berl) 2003;167:85–96. doi: 10.1007/s00213-002-1383-9. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Jonas C, Gron G. Functional compensation of increasing memory encoding demands in the hippocampus. Neuroreport. 2010;21:59–63. doi: 10.1097/WNR.0b013e3283340d36. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2000. [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Remember/Know judgments in cognitive neuroscience: An illustration of the underrepresented point of view. Learn Mem. 2009;16:406–12. doi: 10.1101/lm.1312809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.