Abstract
Mortality rates for maintenance hemodialysis patients are much higher than the general population and are even greater soon after starting dialysis. Here we analyzed mortality patterns in 86,886 patients in 11 countries focusing on the early dialysis period using data from the Dialysis Outcomes and Practice Patterns Study; a prospective cohort study of in-center hemodialysis. The primary outcome was all-cause mortality, using time-dependent Cox regression, stratified by study phase adjusted for age, sex, race, and diabetes. The main predictor was time since dialysis start as divided into early (up to 120 days), intermediate (121–365 days), and late (over 365 days) periods. Mortality rates (deaths/100 patient-years) were 26.7 (95% confidence intervals 25.6, 27.9), 16.9 (16.2, 17.6), and 13.7 (13.5, 14.0) in the early, intermediate, and late periods, respectively. In each country, mortality was higher in the early compared to the intermediate period with an adjusted range from 3.10 (2.22, 4.32) in Japan to 1.15 (0.87, 1.53) in the United Kingdom. Adjusted mortality rates were similar for intermediate and late periods. The ratio of elevated mortality rates in the early to the intermediate period increased with age. Within each period, mortality was higher in the United States than in most other countries. Thus, internationally, the early hemodialysis period is a high-risk time for all countries studied, with substantial differences in mortality between countries. Efforts to improve outcomes should focus on the transition period and first few months of dialysis.
Introduction
Annual mortality rates for patients on maintenance hemodialysis (HD) are several times higher than those of the general population (1). Compared to prevalent dialysis patients, patients new to dialysis (incident patients) experience an even higher mortality within the first few months after initiation of dialysis (1–3).
Many of the earlier studies which had assessed early mortality for incident dialysis patients reported an elevated mortality within the first 90 days after initiation of dialysis (4, 5, 6). However, the pattern of early mortality, and whether such elevation was limited to the first 90 days, was unclear. Subsequently, using the US cohort of the Dialysis Outcomes and Practice Patterns Study (DOPPS), Bradbury et al. showed that the elevated early mortality rate appeared to be maintained throughout the first 120 days following HD initiation, with mortality rates thereafter declining (2). Evidence from large national and regional renal registries suggested that the period of elevated mortality rate continues beyond the first 90 days (1, 3, 7), and the degree and duration of elevation were more pronounced among elderly patients (1, 3). However, studies of early dialysis mortality have focused primarily on a few countries, such as the US and UK. To our knowledge, mortality in the first few months after HD initiation has not been otherwise studied and compared across regions of the world.
The main purpose of this study was to evaluate mortality patterns over the course of HD treatment in 11 countries participating in the DOPPS, with particular focus on the elevated mortality rate soon after initiation of HD. We hypothesized that the elevated early mortality rate is universal in all DOPPS countries, but that the degree of elevation may differ by country. We also examined the degree of elevated early mortality by patient age, and we hypothesized that, across countries, older HD patients are less able to tolerate the initial impact of starting HD and thus have relatively higher early mortality.
Results
Study population
Among 86,886 participants, the median follow-up time was 1.2 years (range: 0 to 3.9 years), and 47,621 patients remained in the study to the end of follow-up. Of 22,172 patients that were censored, the causes of censoring included: switch to peritoneal dialysis (n=1,957), kidney transplant (n=3,594), recovery of kidney function (n=1,052), transfer out of a DOPPS dialysis facility (n=15,565), and other (n=4). There were 16,907 deaths (overall mortality rate=15.0 per 100 patient-years (95% confidence interval [CI]=14.7, 15.2). Of these, 1,939 deaths occurred in the early period; 2,299 in the intermediate period; and 12,669 in the late period. Mortality rates, in deaths per 100 patient-years, were 26.7 (95% CI=25.6, 27.9) in the early period, 16.9 (95% CI=16.2, 17.6) in the intermediate period, and 13.7 (95% CI=13.5, 14.0) in the late period.
Demographics
Patient demographics by country are shown in Table 1. The mean age overall was 62.9 years, ranging from 59.6 years in Australia/New Zealand (ANZ) to 66.8 years in Belgium. There were 58.9% male patients, ranging from 55.4% in the US to 63.9% in Sweden. In the US, 29.8% of patients were black, versus 0% in Japan and 2.7% in other countries. Diabetes was the cause of ESRD for 35.5% of patients, ranging from 19.1% in Italy to 49.1% in the US. As expected, higher mortality was observed in patients who were older (adjusted HR per 5 years=1.22, 95% CI=[1.21, 1.23], p<0.001), male (HR=1.05 95% CI = [1.02, 1.09], p=0.001), non-black (HR=1.26 95% CI = [1.14, 1.38], p<0.001), and for whom diabetes was the cause of ESRD (HR=1.27 95% CI = [1.23, 1.32], p<0.001). The interaction between the covariate black (v. non-black) and country was not significant (p=0.70).
Table 1.
Patient characteristics by country (DOPPS 2 and 3 combined)
| DOPPS country
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Australia-New Zealand | Belgium | Canada | France | Germany | Italy | Japan | Sweden | UK | US | |
| Number of patients | 5951 | 5544 | 10102 | 5956 | 5589 | 4288 | 16033 | 4600 | 5592 | 23231 |
| Age (mean) | 59.6 | 66.8 | 63.1 | 63.7 | 63.7 | 65.6 | 62.8 | 63.6 | 61.4 | 62.3 |
| <45 | 17.2 | 7.9 | 13.8 | 13.1 | 11.9 | 9.5 | 8.7 | 12.3 | 16.8 | 14.5 |
| 45–54 | 17.6 | 10.5 | 13.4 | 13.5 | 13.2 | 11.6 | 16.8 | 14.0 | 14.1 | 15.9 |
| 55–64 | 22.1 | 17.1 | 20.4 | 18.0 | 19.5 | 18.0 | 27.8 | 20.2 | 19.1 | 21.1 |
| 65–74 | 26.0 | 29.6 | 25.0 | 26.6 | 30.7 | 31.3 | 27.2 | 24.9 | 27.0 | 22.9 |
| ≥75 | 17.1 | 34.9 | 27.4 | 28.8 | 24.8 | 29.5 | 19.4 | 28.7 | 23.0 | 25.7 |
| Male (%) | 58.7 | 58.0 | 58.4 | 59.7 | 59.3 | 59.2 | 61.8 | 63.8 | 61.9 | 55.4 |
| Black (%) | 0.4 | 2.5 | 4.8 | 4.7 | 0.3 | 0.6 | 0.0 | 0.9 | 5.1 | 29.8 |
| Diabetes as cause of ESRD (%) | 33.2 | 28.6 | 40.1 | 22.8 | 31.9 | 19.1 | 32.6 | 28.9 | 23.1 | 49.1 |
Mortality at the early and late (vs. intermediate) period of dialysis
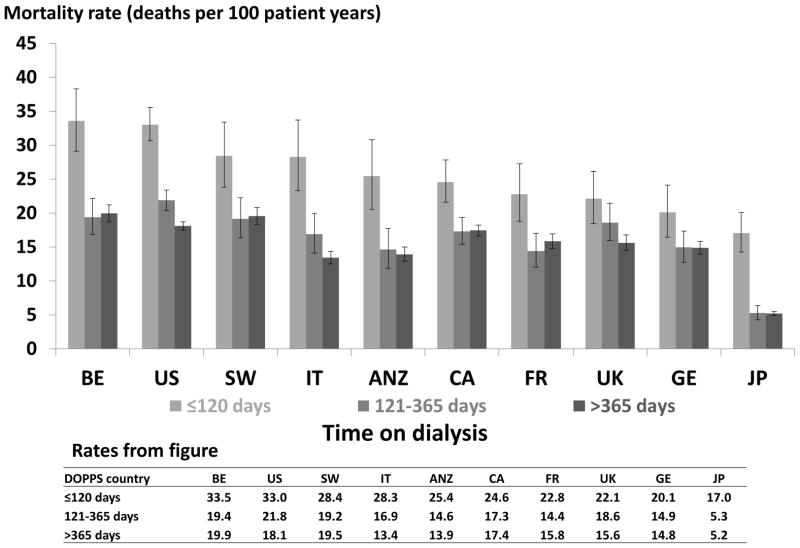
Crude mortality rates were highest in the early dialysis period (≤120 days) in all countries (Figure 1). Mortality rates in the intermediate period (121–365 days) were similar to those in the late (>365 days) period. A sensitivity analysis limited to patients incident to dialysis at DOPPS enrollment resulted in rates that followed a similar pattern, with higher rates in the early dialysis period in every country. Table 2 shows the adjusted hazard ratios for the early and late (vs. intermediate) period for each country. The adjusted hazard ratios for the early period vs. the intermediate were 3.1 in Japan; 1.6–1.8 in ANZ, Belgium, and Italy; 1.3–1.5 in Canada, France, Germany, Sweden, and the US; and 1.2 in the UK. The adjusted hazard ratios for the late period vs. the intermediate period were closer to 1, ranging from 0.93 (95% CI = 0.78, 1.12) in the UK to 1.25 (95% CI = 1.03, 1.52) in France.
Figure 1.
Mortality After the Start of Dialysis
Countries were ordered by mortality rate at ≤120 days.
ANZ=Australia and New Zealand; BE= Belgium; CA= Canada; FR= France; GE= Germany; IT: Italy; JPN= Japan; SW= Sweden; UK= United Kingdom; US= United States
Error bars correspond to 95% confidence intervals calculated using the Byer approximation
Table 2.
Adjusted all-cause mortality across time periods on hemodialysis within each country (DOPPS 2 and 3)*
| First 120 days (compared with 121 to 365 days) | After 365 days (compared with 121 to 365 days) | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Australia-New Zealand | 1.76 | (1.19, 2.60) | 1.01 | (0.81, 1.25) |
| Belgium | 1.69 | (1.32, 2.16) | 1.10 | (0.92, 1.31) |
| Canada | 1.42 | (1.15, 1.75) | 1.12 | (0.95, 1.31) |
| France | 1.49 | (1.12, 1.98) | 1.25 | (1.03, 1.52) |
| Germany | 1.34 | (1.03, 1.74) | 1.18 | (0.97, 1.42) |
| Italy | 1.62 | (1.19, 2.20) | 0.92 | (0.77, 1.09) |
| Japan | 3.10 | (2.22, 4.32) | 1.21 | (0.96, 1.52) |
| Sweden | 1.42 | (1.00, 2.01) | 1.13 | (0.95, 1.35) |
| UK | 1.15 | (0.87, 1.53) | 0.93 | (0.78, 1.12) |
| US | 1.44 | (1.28, 1.62) | 0.96 | (0.87, 1.05) |
Model adjusted for age, sex, race, and diabetes as cause of ESRD at enrollment, stratified by phase and accounted for facility clustering
The percentage of patients who switched modality was 4% or less in all countries except Australia/New Zealand (8%), and most of these patients switched before 120 days. If these patients were excluded from the analysis, the adjusted hazard ratios comparing the early period to the intermediate period were substantially similar to the results presented in table 2.
Mortality in other countries compared to the US
In each dialysis period, the adjusted hazard ratio, comparing each country with the US, was less than 1, with Japan displaying the lowest mortality rates in all periods (Figure 2). During the early period, the adjusted hazard ratios ranged from 0.50 (95% CI =0.36–0.69) in Japan to 0.93 (95% CI =0.74–1.15) in Belgium; during the intermediate period, those estimates ranged from 0.23 (95% CI =0.18–0.30) in Japan to 0.91 (95% CI =0.70–1.17) in the UK; and during the late period, they ranged from 0.29 (95% CI =0.25–0.34) in Japan to 0.98 (95% CI =0.86–1.13) in Sweden.
Figure 2.
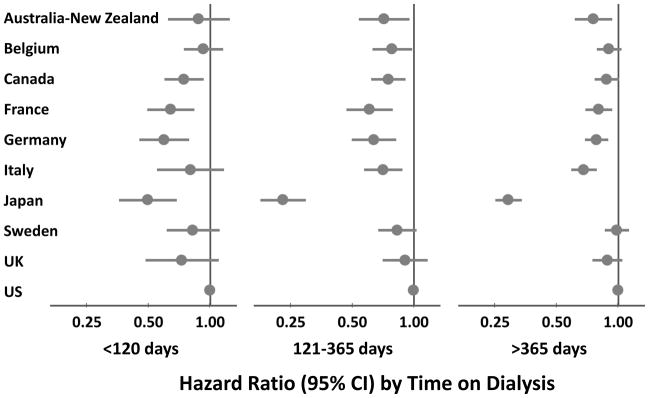
Mortality in Each DOPPS Country vs. the US by Time on HD
Model adjusted for age, sex, race and diabetes as cause of ESRD, stratified by study phase (N=86,886 HD patients from DOPPS census [2002–2008])
Early mortality by age, sex, and cause of ESRD (diabetes v. other)
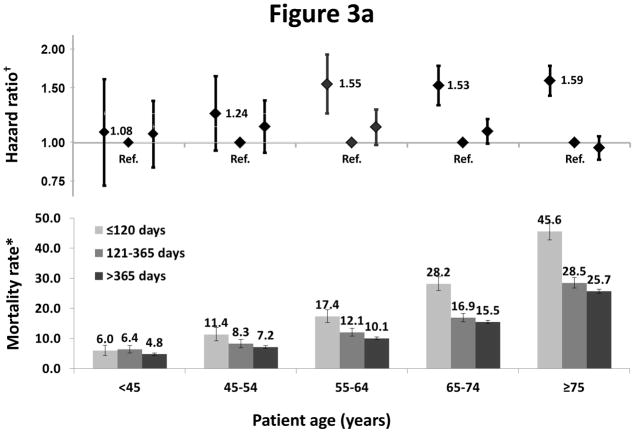
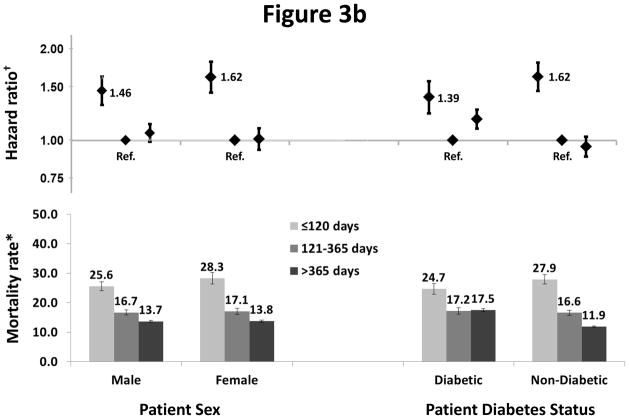
We observed interactions between dialysis period (< 120, 121–365, and >365 days) and age (p=0.002) (Figure 3a), and sex (p=0.02) and primary cause of ESRD (p<0.001) (Figure 3b), on the risk of death. A greater elevation in risk in the first 120 days was seen in older compared to younger patients (HR=1.59 vs. 1.08), females compared to males (HR=1.62 vs. 1.42), and those without versus with diabetes as a primary cause of ESRD (HR=1.62 vs. 1.39). In the subsequent periods, similar patterns were not observed. Crude mortality rates were consistently higher at ≤ 120 days compared to >120 days in each country by age stratum (not shown).
Figure 3.
Figure 3a: Association of mortality with age and vintage
†One model was fitted to each patient subgroup based on age at study enrollment (<45, 45–55, 55–64, 65–74, ≥75 years)
Models were adjusted for age, sex, race, and diabetes as cause of ESRD, stratified by countries and study phase, and accounted for facility clustering.
*Mortality rate: unadjusted number of deaths per 100 patient-years. Error bars correspond to 95% confidence intervals calculated using the Byer approximation.
Figure 3b: Association of mortality with sex, diabetes status, and vintage
†One model was fitted to each patient subgroup based on sex or diabetes status at study enrollment
Models were adjusted for age, sex, race, and diabetes as cause of ESRD, stratified by countries and study phase, and accounted for facility clustering.
*Mortality rate: unadjusted number of deaths per 100 patient-years. Error bars correspond to 95% confidence intervals calculated using the Byer approximation.
Monthly (30-day) mortality rates in first year of dialysis
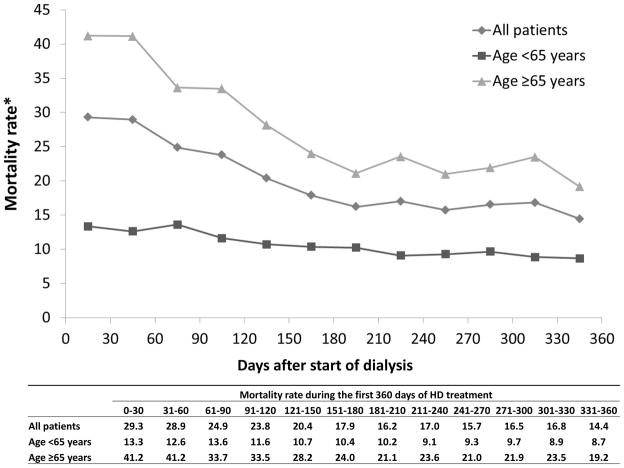
We estimated crude mortality rates by month, overall and by age (<65 vs. ≥65) (Figure 4). Overall, the mortality rate was highest in the first 2 months of dialysis, then decreased steadily to 180 days, and leveled off during the rest of the year. This temporal trend differed appreciably by patient age. The decline in mortality during the first 180 days was substantially greater among patients 65 years and older (53%) than in younger patients (about 35%).
Figure 4.
Overall mortality rate by age at study enrollment
*Mortality rate: number of deaths per 100 patient-years
Withdrawal from Dialysis
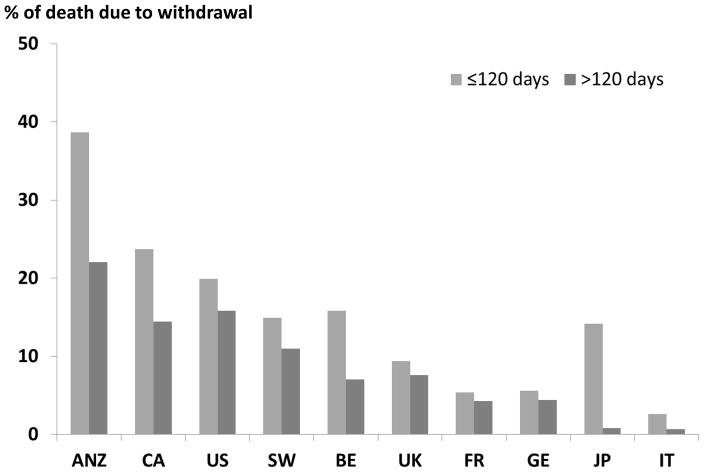
Among patients who died, 14% were coded as withdrawing from dialysis as their stated reason for leaving the dialysis facility. Among these patients, 74% had reported death dates and, of these, 82% had a death date at least four days after their date of last dialysis. Death was considered due to withdrawal in these patients. Among these patients, over half died by 9 days after their last dialysis and 74% died by 14 days. Figure 5 shows the percentage of deaths due to withdrawal, by country and dialysis period. The percentage of deaths due to dialysis withdrawal was higher in every country during the earlier period (<120 days) than in the later period, and ranged from 39% (<120 days) and 22% (120+ days) in Australia/New Zealand to 3% (<120 days) and 1% (120+ days) in Italy.
Figure 5.
Proportion of Deaths due to Withdrawal from Dialysis by Dialysis Period and Country
Countries were ordered by percent of deaths due to withdrawal from dialysis.
Discussion
In the international DOPPS cohort, we found consistently higher mortality rates in the first 120 days after starting HD, but the rate during this early period differed among countries, varying nearly two-fold between Japan at the low end and Belgium and the US at the high end. The magnitude of excess mortality in the first 120 days compared to later periods was substantial in all DOPPS countries except the UK, and the degree of elevated early mortality was more pronounced among patients at least 45 years of age. In our analysis of monthly mortality rates, we found that mortality was highest in the first 2 months of dialysis; then it decreased steadily and leveled off after 180 days.
Though mortality was higher in the early dialysis period in all DOPPS countries, differences between countries were dramatic with the adjusted hazard ratio for the early (vs. intermediate) period ranging from 3.10 in Japan to 1.15 in the UK. These differences may have a variety of complex determinants, such as: (1) patient characteristics and medical care before starting dialysis, as well as attitudes towards acceptance of dialysis, (2) dialysis-related care and adherence to care, and (3) withdrawal from dialysis. These are discussed further below.
First, characteristics of patients starting dialysis may differ between countries because of differences in the epidemiology of chronic kidney disease (CKD), access to care and quality of medical care for CKD patients, and the acceptance for and timing of initiation of dialysis. Processes surrounding “acceptance” for dialysis are complex, as these reflect a combination of patient preferences, provider preferences, and contextual effects reflecting cultural and societal differences. In Japan, our understanding is that the markedly elevated HR for early vs. later mortality is driven by the standard that dialysis facilities initiate dialysis treatment on all patients with terminal kidney failure, regardless of health condition (8). Thus, patients with poor short-term prognosis typically start dialysis and may die shortly thereafter. By contrast, other countries may be more likely to forgo dialysis in such patients, based on the judgment that its use would be futile or detrimental to patient well-being. For example, the lower hazard ratio, comparing the early vs. intermediate period, in the UK may be due, at least in part, to the decision not to dialyze some patients with short life expectancy. In keeping with this practice approach, in the UK a pathway for end-of-life care without dialysis, termed ‘conservative kidney management,’ has recently been developed for selected patients with advanced kidney disease (9).
Second, differences in dialysis-related medical care influence early dialysis mortality. Interventions identified as important include timely access to pre-ESRD nephrology care, ESRD education and support services, treatment of biochemical abnormalities in advanced CKD, and timely placement of a permanent vascular access. The crude early dialysis mortality rate in Japan is lower than in other countries, a finding likely attributable in part to high quality care including national screening programs for kidney disease, greater focus on management of patients with CKD, readily accessible nephrologist care, and excellent preparation for dialysis (8, 10, 11), as well as exceptionally high early fistula use (12).
DOPPS has previously demonstrated wide variation in catheter use among incident patients in its 12 countries, with prevalence (in the DOPPS study sample) ranging from 26% in Japan and 23% in Germany to 73% in Belgium and 70% in Canada (12). Interestingly, early mortality rates presented now (from DOPPS census data) were lowest in the two countries with lowest catheter use (Japan, Germany) and highest in 3 of the 4 counties with the highest catheter use (Belgium, United States, Sweden). A separate DOPPS analysis reported that the percent of patients receiving pre-ESRD nephrology care was lowest in Belgium and the United States, the two countries with the highest early mortality in the current study (20). Studies elsewhere have highlighted catheter use and late or absent pre-ESRD nephrology care, as well as malnutrition as captured by hypoalbuminemia, as major ‘reversible’ determinants of early dialysis mortality (2, 13–16). Though one study noted that survival among older patients starting dialysis in the US has not materially improved despite earlier pre-ESRD nephrology consultation, DOPPS and others have consistently found pre-ESRD nephrology care to be associated with improved early dialysis mortality and hospitalization rates (2, 17–21). Greater awareness of high first-year mortality in general has stimulated focus on interventions, before and after dialysis initiation, to address this problem. In the US, an example of this is Performance Excellence and Accountability in Kidney Care (PEAK), a voluntary quality improvement campaign to reduce mortality among first-year dialysis patients (22). To translate this goal into improved outcomes, interventions have been launched, such as a systematic case management program in the first three months of dialysis which has been largely successful (23–24). Additional efforts centering on eliminating barriers in access to pre-ESRD nephrology care, improving coordination of care, or reducing early catheter use are needed.
Third, withdrawal from dialysis is more common in the early dialysis period (Figure 5). Early withdrawal will contribute to elevated early mortality to the extent that patients withdrawing early would otherwise have survived to a later dialysis period. Our clinical intuition is that many, though not all, patients who were coded as withdrawing from dialysis would have died relatively soon if not withdrawn from dialysis. In our study, the percentage of deaths due to dialysis withdrawal ranged dramatically, from the highest in Australia/New Zealand to the lowest in Italy. Our finding of that a high proportion of death is due to withdrawal in Canada is corroborated by a recent study of Canadian registry data (25). It is tempting to speculate that the variation in withdrawal across countries is due to differences in cultural attitudes toward acceptance for dialysis of frail patients (e.g., initiating a “trial of dialysis”), as well as cultural attitudes about the appropriateness of withdrawal of a treatment needed to sustain life (26–28). Additional investigations to confirm and understand these differences that we report by country are needed.
This study also helps to clarify the duration of elevated early mortality after initiation of dialysis. Although the first 90-day period has been used as a convention, studies and registries have reported declining rates of death over an extended period within the first year of dialysis treatment (1, 2, 3, 7). In this study, we presented monthly mortality rates over the first year of dialysis treatment, and showed that the crude mortality rate continued to decrease over the first 180 days of dialysis treatment, particularly among HD patients ≥65 years (Figure 4). Since both the first 90 and 120 days contained the period of most elevated mortality after initiation of dialysis, characterizing early mortality by using the first 120-day period, as chosen in the earlier DOPPS paper by Bradbury et al. (2), may be a prudent approach in future studies.
For many years, mortality on dialysis has been higher in the US than in many other countries (1, 29, 30). In this study, we extend this finding by demonstrating that mortality is higher in the US than most DOPPS countries in the early dialysis period, as well as at later dialysis vintages. Early mortality is also especially high in Belgium. While this is in part explained by age (Belgium has the highest mean age among DOPPS countries), mortality adjusted for age remains high in Belgium as in the US. Though not evaluated in this analysis (because it was limited to DOPPS census data), the high early use of catheters in these countries likely contributes to this finding, as a previous DOPPS analysis demonstrated that facility catheter use explains much of the difference in mortality between the US and Europe (31). In the United States, the reduction in catheter use among prevalent hemodialysis patients since the launch of the Fistula First Initiative is encouraging (32). However, achieving substantial reductions in catheter use among incident patients is a vital next step. Efforts including improving access to pre-ESRD nephrology care and coordination of care are underway, and additional focus is warranted (22–24).
A unique advantage of this analysis is that mortality rates can be compared directly across DOPPS countries because of the uniform, representative sampling methods and data collection methods. Consistent with previous DOPPS findings (30, 33), we showed large variation across countries in annual mortality rates among prevalent HD patients, with the highest mortality rate in Belgium, Sweden, and the US (approximately 20 deaths per 100 patient-years), an intermediate rate in ANZ and European countries (between 14 and 17), and the lowest rate in Japan (approximately 6). In each country, a representative random sample of dialysis facilities is invited to enter the DOPPS. As in most studies, sites agreeing to participate may have somewhat higher performance on average than other facilities. Despite this possibility, the annual mortality rates reported by DOPPS in many of the study’s countries have been similar to, or only slightly lower, those reported by national registries (33–35). The annual mortality rate among Japanese DOPPS patients was lower than reported by the Japanese Society of Dialysis Therapy (9.6% in 2009) (36). In Japan, DOPPS applies proportional sampling of the 9 or 10 different types of facilities defined by the JSDT, but may not capture all deaths, possibly due to difficulties in obtaining complete follow-up for patients who are hospitalized for illness and subsequently transferred to specialized care settings. If the hypothesized missing deaths in Japan were distributed across the time periods in a way that differed greatly from the way deaths are distributed in the reported data, then the conclusions with respect to Japan may not hold.
Our results can be considered generalizable across participating countries because the DOPPS enrolls representative national samples of HD facilities (33, 35). This analysis relied on the DOPPS census (the entire population of patients in DOPPS facilities) because it had three times as many patients as in the DOPPS study sample and because it did not require patients to give informed consent, which tends to bias selection toward healthier patients. On the other hand, DOPPS census data are limited to the inclusion of few patient variables: age, sex, race, and cause of ESRD, as well as date of death and facility departure events. Although these variables are the ones typically used for making adjustments in national registries (with DOPPS census data having advantages over international registry comparisons that include standard data collection methods, standard covariate data, and reliable capture of incident patients) (1, 3), they comprise many fewer variables than the detailed patient-level data in the DOPPS study sample. For example, the DOPPS census does not contain detailed information on the cause of death, comorbidities, laboratory test results, and treatment data. Previous analyses with detailed data from the DOPPS study sample have shown that there are substantial differences in patient case-mix across countries (30), and that adjustment for these differences and for vascular access practices markedly attenuate the crude survival differences across countries (31). To date, an analysis focused on explaining mortality differences across countries in incident dialysis patients has not been feasible because the DOPPS study sample has had a relatively small number of incident dialysis patients in certain countries. By sampling a high proportion of incident dialysis patients, the current phase of DOPPS (DOPPS 5) will make this analysis feasible. Additionally, studies that focus on case-mix and practice patterns among advanced CKD patients and the dialysis transition period are warranted to gain understanding of their influence on early dialysis mortality and to identify practices to improve outcomes.
Mortality rates in the first few months following dialysis initiation were substantively higher in all countries participating in DOPPS, and this elevation was most pronounced among older patients. Furthermore, overall differences in mortality on dialysis between countries are strongly influenced by mortality in the early dialysis period. To the best of our knowledge, this study is the first to compare mortality rates soon after starting dialysis in a large, multi-national cohort of dialysis patients. These results better characterize this high-risk period for dialysis patients internationally, providing an important framework for future investigations to explore differences in practice and outcomes, to identify optimal approaches to extend survival, and to target early mortality as a means to lessen disparities in dialysis survival internationally. We anticipate that the burden of excess early mortality may be lessened by improving access to high-quality medical care for CKD patients, improving coordination of care, optimizing approaches for acceptance and preparation for dialysis, reducing early catheter use, and delivering effective dialysis-related care.
Methods
Data Source
The DOPPS is a prospective cohort study of in-center hemodialysis practice patterns and outcomes. Twelve countries, including Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, the United Kingdom (UK) and the United States (US), participated in the DOPPS phase 2 (2002–2004) and phase 3 (2005–2008). In each study phase, a random, stratified sampling method was used to select a generally representative sample of dialysis facilities for each country (37, 38).
The analyses for this paper used the DOPPS census database. This population consisted of all hemodialysis patients treated at each participating facility, including patients being treated at the start of each DOPPS phase and patients who started treatment during follow-up. Study coordinators were directed to enroll only chronic hemodialysis patients (less than 1% of the patients appeared to have reversible kidney injury, as they left the study due to recovery of kidney function). In the DOPPS census database (phase 2 and 3), we identified 100,411 HD patients at least 18 years old at census enrollment. 5,493 patients from Spain were excluded due to unreported mortality in the first 30 days of dialysis treatment in a substantial proportion of dialysis facilities. After also excluding 6,254 patients with missing dates of study enrollment and 1,778 patients with missing dates of dialysis initiation, 86,886 census patients (87% of the total) were included in this analysis.
Covariates and Outcome Data
Baseline patient information on age, sex, race, diabetes as cause of ESRD (Yes/No), and the date of first chronic dialysis were collected for all census patients. Outcome data including date of death or censoring were obtained for all patients during follow-up. Reasons for censoring included switch to peritoneal dialysis, kidney transplant, transfer out of a DOPPS facility, recovery of kidney function, and the end of follow-up. Patient status (dead/alive/unknown) was requested for up to 60 days after departure from the DOPPS facility. Ninety percent of deaths occurred during study follow-up or within 11 days of departure. For patients who had not died, follow-up was censored at 7 days after departure from the facility. All reported deaths were included as outcome events in these analyses.
Withdrawal was coded if it was the reported reason the patient left the dialysis facility. Death was considered due to withdrawal if the date of death was 4 or more days after withdrawal. If the patient withdrew and no date of death was reported, a date of death was imputed at 8 days (the median reported time to death after withdrawal).
Statistical Analysis
The primary outcome was all-cause mortality. Crude mortality rates (MRs) were estimated as the number of deaths per 100 patient-years at risk. 95% confidence intervals (CI) for rate estimates used Byer’s method (39). The main predictor was time since the start of dialysis, categorized as early (≤120 days after start of dialysis), intermediate (121–365 days), and late (>365 days), with the cut-point of 120 days based initially on previous DOPPS analyses (2). Follow-up started for each patient upon their entry into the DOPPS census.
Cox regression was used to compare mortality rates between dialysis periods (< 120 days, 121–365 days, and >365 days since start of dialysis) for each country, treating the intermediate period as the referent, and to compare mortality rates among countries within each dialysis period (US as referent). Both objectives were accomplished in the same model by using indicator variables for DOPPS countries, time-dependent indicators for dialysis periods (i.e., time-dependent vintage effect updated for each patient throughout his/her time-at-risk), and product (interaction) terms for country and dialysis period. The model was stratified by study phase, and adjusted for age, sex, race, and diabetes as the cause of ESRD.
Note that there were two different measures of time in the Cox model – time since the patient joined the census, and time since dialysis start as a time-dependent covariate. Time since joining the census was the time axis in the Cox model, while time since dialysis start was the predictor.
To evaluate mortality patterns by available patient characteristics, crude mortality rates for the early, intermediate, and late periods were estimated by age at census enrollment (<45, 45–54, 55–64, 65–74, ≥75 years), sex, and primary cause of ESRD (diabetes v. other). Adjusted hazard ratios and 95% confidence intervals for the early and late (vs. intermediate) periods were obtained from separate Cox models fitted to each age, sex, and cause of ESRD group. In each model, the primary predictor was dialysis period, modeled as time-dependent indicators. Age at census enrollment was included as a continuous covariate in each separate age group model to adjust for possible age confounding within the group. Models were stratified by study phase and adjusted for country, age, sex, race, and diabetes as the cause of ESRD. Cox models were used to test the overall interaction between dialysis period and age (as a categorical variable), sex, or cause of ESRD category using product terms. A likelihood ratio test compared models with and without all product terms for age and the <120 day dialysis period.
To more clearly identify patterns of changing mortality during the first year of dialysis, we estimated crude mortality rates by month, overall and dichotomized according to age <65 and ≥65 years. To avoid unstable estimates due to sparse data, monthly mortality rates were not estimated within countries or in a greater number of age categories.
The proportional hazard assumption for Cox models was checked graphically and using the stratification method. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
Acknowledgments
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbott (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, helped edit this manuscript.
Footnotes
DISCLOSURES
Drs. Bradbury and Ng work in the Center for Observational Research at Amgen, Inc.
Bruce Robinson, Jinyao Zhang, Keith McCullough, Francesca Tentori, and Ronald Pisoni are employees of Arbor Research Collaborative for Health. The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbott (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
Francesca Tentori is supported by award number 1K01DK087762-01A1 from the National Institute of Diabetes And Digestive And Kidney Diseases.
Hal Morgenstern has no conflicts of interest.
Brenda Gillespie has no conflicts of interest.
Raymond Hakim has no conflicts of interest.
Hugh Rayner has no conflicts of interest.
Joan Fort has no conflicts of interest.
Tadao Akizawa has received speaker’s fees and research grants from Kyowa Hakko Kirin.
References
- 1.U S Renal Data System, USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. [Google Scholar]
- 2.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 3.Caskey F, Dawnay A, Farrington K, Feest T, Fogarty D, Inward C, Tomson CRV. Report Nephron Clinical Practice. UK Renal Registry 2010; 13th Annual Report of the Renal Association; Bristol, UK: UK Renal Registry; 2011. 2010. [Google Scholar]
- 4.Khan IH, Catto GR, Edward N, et al. Death during the first 90 days of dialysis: a case control study. Am J Kidney Dis. 1995 Feb;25(2):276–80. doi: 10.1016/0272-6386(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Garcia G, Deddens JA, D’Achiardi-Rey R, et al. Results of Treatment in Patients with End-Stage Renal Disease: A Multivariate Analysis of Risk Factors and Survival in 341 Successive Patients. Am J Kidney Dis. 1985 Jan;1(5):10–18. doi: 10.1016/s0272-6386(85)80129-3. [DOI] [PubMed] [Google Scholar]
- 6.Wright LF. Survival in patients with end-stage renal disease. Am J Kidney Dis. 1991 Jan;17(1):25–8. doi: 10.1016/s0272-6386(12)80245-9. [DOI] [PubMed] [Google Scholar]
- 7.Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996 Oct;7(10):2169–75. doi: 10.1681/ASN.V7102169. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata K, Nakai S, Masakane I, et al. Ideal Timing and Predialysis Nephrology Care Duration for Dialysis Initiation: From Analysis of Japanese Dialysis Initiation Survey. Ther Apher and Dial. 2012;16(1):54–62. doi: 10.1111/j.1744-9987.2011.01005.x. [DOI] [PubMed] [Google Scholar]
- 9.NHS. Kidney Care, National End of Life Care Programme. End of Life Care in Advanced Kidney Disease: A Framework for Implementation. 2009 Jun; [Google Scholar]
- 10.Yamagata K, Iseki K, Nitta K, et al. Chronic kidney disease perspectives in Japan and the importance of urinalysis screening. Clin Exp Nephrol. 2008;12:1–8. doi: 10.1007/s10157-007-0010-9. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y, Ito S, Uemura O, et al. CKD Clinical Practice Guidebook. The essence of treatment for CKD patients. Clin Exp Nephrol. 2009 Jun;13(3):191–248. doi: 10.1007/s10157-009-0163-9. [DOI] [PubMed] [Google Scholar]
- 12.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradbury BD, Chen F, Furniss A, et al. Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis. 2009;53(5):804–814. doi: 10.1053/j.ajkd.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Astor BC, Eustace JA, Powe NR, et al. Type of vascular access and survival among incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16:1449–1455. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 15.Xue JL, Dahl D, Ebben JP, et al. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Chan KE, Maddux FW, Tolkoff-Rubin N, et al. Early Outcomes among Those Initiating Chronic Dialysis in the United States. Clin J Am Soc Nephrol. 2011 Nov;6(11):2642–9. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golper TA. Predialysis nephrology care improves dialysis outcomes: now what? Or chapter two. Clin J Am Soc Nephrol. 2007 Jan;2(1):143–5. doi: 10.2215/CJN.03711106. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmayer WC, Owen WF, Jr, Levin R, et al. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol. 2003 Feb;14(2):486–92. doi: 10.1097/01.asn.0000046047.66958.c3. [DOI] [PubMed] [Google Scholar]
- 19.Winkelmayer WC, Liu J, Chertow GM, et al. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171(15):1371–1378. doi: 10.1001/archinternmed.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa T, Bragg-Gresham JL, Yamazaki S, et al. Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol. 2009;4:595–602. doi: 10.2215/CJN.03540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kausz AT, Obrador GT, Arora P, et al. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000;11:2351–2357. doi: 10.1681/ASN.V11122351. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed: March 28, 2012];Performance Excellence and Accountability in Kidney Care website. http://www.kidneycarequality.com/AboutPeak.htm.
- 23.Wingard RL, Pupim LB, Krishnan M, et al. Early intervention improves mortality and hospitalization rates in incident hemodialysis patients: RightStart Program. Clin J Am Soc Nephrol. 2007;2:1170–1175. doi: 10.2215/CJN.04261206. [DOI] [PubMed] [Google Scholar]
- 24.Wingard RL, Chan KE, Lazarus JM, et al. The “right” of passage: surviving the first year on dialysis. Clin J Am Soc Nephrol. 2009;4:S114–120. doi: 10.2215/CJN.04360709. [DOI] [PubMed] [Google Scholar]
- 25.Ellwood AD, Jassal SV, Suri RS, et al. Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol. 2013;8:265–270. doi: 10.2215/CJN.01000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambie M, Rayner HC, Bragg-Gresham JL, et al. Starting and withdrawing haemodialysis — associations between nephrologists’ opinions, patient characteristics, and practice patterns (data from the Dialysis Outcomes and Practice Patterns Study) Nephrol Dial Transplant. 2006;21:2814–2820. doi: 10.1093/ndt/gfl339. [DOI] [PubMed] [Google Scholar]
- 27.Fissell RB, Bragg-Gresham JL, Lopes AA, et al. Factors associated with “Do not resuscitate” orders and rates of withdrawal from hemodialysis in the international DOPPS. Kidney Int. 2005;68(3):1282–1288. doi: 10.1111/j.1523-1755.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 28.Renal Physicians Association. Shared Decision Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2. Rockville, MD: 2010. [Accessed March 28, 2012]. Available at: http://www.renalmd.org. [Google Scholar]
- 29.Held PJ, Brunner F, Odaka M, et al. Five-year survival for end stage renal disease patients in the US, Europe, and Japan 1982–87. Am J Kidney Dis. 1990;15:451–457. doi: 10.1016/s0272-6386(12)70363-3. [DOI] [PubMed] [Google Scholar]
- 30.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States in the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14(12):3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 31.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis. 2009;53(3):475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 32. [Accessed August 31, 2012];Fistula First Initiative website. http://www.fistulafirst.org/
- 33.Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalisation in haemodialysis patients in five European countries: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19(1):108–120. doi: 10.1093/ndt/gfg483. [DOI] [PubMed] [Google Scholar]
- 34.Rayner HC, Greenwood R, MacTier R, et al. Estimated life expectancy of UK HD patients if clinical practice guidelines are met. Br J Renal Med. 2007;12(3):11–14. [Google Scholar]
- 35.Robinson B, Fuller D, Zinsser D, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: Rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis. 2011;57(6):822–831. doi: 10.1053/j.ajkd.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakai S, Iseki K, Itami N, et al. Overview of Regular Dialysis Treatment in Japan (as of 31 December 2009) Ther Apher Dial. 2012;16(1):11–53. doi: 10.1111/j.1744-9987.2011.01050.x. [DOI] [PubMed] [Google Scholar]
- 37.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int. 2000;57 (suppl 74):S-74–S-81. [Google Scholar]
- 38.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study: Design, data elements, and methodology. Am J Kidney Dis. 2004;44 (Suppl 2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Rothman Kenneth. Epidemiology: An Introduction. Oxford University Press; 2002. p. 134. [Google Scholar]