Abstract
Objective
To describe clinical, cognitive, and personality characteristics at baseline assessment of 249 participants 19 to 60 years of age in a multinational longitudinal study (DIAN) of autosomal dominant Alzheimer disease (ADAD).
Method
Participants (74% cognitively normal) were from ADAD families with mutations in one of three genes (APP, PSEN1, or PSEN2). Mixed model analyses including family as a random variable and controlling for years from expected time of symptomatic onset of ADAD based on parental age at onset compared three groups (cognitively normal mutation noncarriers, cognitively normal mutation carriers, very mildly impaired mutation carriers).
Results
Global cognitive deficits similar to those observed in late-life sporadic Alzheimer disease (AD) existed in very mild ADAD compared with cognitively normal carriers and noncarriers on all but two measures (Digit Span Backward, Letter Fluency for FAS) of episodic memory, semantic memory, working memory, attention, and speeded visuospatial abilities. Demented individuals were less extraverted, open, and conscientious than cognitively normal participants on the International Personality Item Pool. Differences in the relation between three measures (Logical Memory, Digit Symbol, attention switching) and time to expected age at symptomatic onset indicate that cognitive deficits on some measures can be detected in mutation carriers prior to symptomatic AD and hence should be useful markers in subsequent longitudinal follow-up.
Conclusions
Overall cognitive and personality deficits in very mild ADAD are similar to those seen in sporadic AD. Cognitive deficits also occur in asymptomatic mutation carriers who are closer to the expected time of dementia onset.
Keywords: Alzheimer disease, early onset, preclinical, cognition, personality
The Dominantly Inherited Alzheimer Network (DIAN) is a multinational collaborative prospective study of individuals at risk for autosomal dominant Alzheimer disease (ADAD) who have mutations in one of three genes (APP, PSEN1, or PSEN2). The structure, implementation, and underlying principles of DIAN and a description of some of the biological characteristics (e.g., markers obtained from cerebrospinal fluid and neuroimaging) of the initial cohort have been described recently (Bateman et al., 2012; Morris et al., 2012). Although one goal of this effort is the longitudinal description of the preclinical phase of the disease, the ultimate goal is to find ways to prevent symptomatic expression.
Because of the rarity of ADAD (< 1% of all cases of AD) descriptions of its earliest effects on cognition are limited. Given geographic limitations, most reports describe a single family (e.g., Mondadori et al., 2006), although in some instances the extended families are quite large (Acosta-Baena et al., 2011). Some research centers (e.g., Fox et al., 1998; Ringman et al., 2005) have collected data from multiple pedigrees, but their sample sizes are still relatively modest. A major goal of the DIAN cohort is to pool these research efforts to form a substantially larger sample. This will also pool the variability in the expression of the disease that appears to occur across the variety of specific mutations that lead to ADAD. Hence, in order to fully appreciate the influence of mutation status, it is necessary to include family in mixed model analyses.
Based on the limited information available (e.g., Lopera et al., 1997), ADAD appears to have deficits similar to those seen in far more common sporadic late-onset AD (LOAD). Specifically, it often begins with the hallmark deficit in episodic verbal memory as well as difficulties in some aspects of executive function and visuospatial abilities. Some studies have shown that language is particularly sensitive to asymptomatic AD (e.g., Jacobs et al., 1995; Jones, Laukka, & Backman, 2006; Snowden et al., 1996) as deficits may be detected prior to the diagnosis of symptomatic AD (Acosta-Baena, 2011; Fox et al., 1997). Hence, one purpose of this report was to describe the initial DIAN cohort’s clinical and psychological characteristics. This description can then be used to plan clinical trials for asymptomatic mutation carriers with the goal of preventing the cognitive consequences of the disease.
The goals of the present report were fourfold: First, we examined the extent to which the cognitive differences that have been observed in a wide range of tasks used in studies of sporadic AD also appear in this sample of DIAN participants with specific mutations. We expected global differences between the healthy control individuals and those with very mild symptomatic AD on a wide variety of cognitive measures, such as those that have already been reported for these tasks in LOAD (for a review see Salmon & Bondi, 2009). Of course, if the profile of the cognitive differences is dissimilar (e.g., cognitive differences within only selected domains), then one may be concerned about the generalizability of studying the DIAN sample in understanding the much more prevalent LOAD. Second, we examined the extent to which there is a difference between people without the mutation and carriers who at the time of testing did not produce sufficient cognitive deficit to be diagnosed with even very mild symptomatic dementia. This provides information regarding the sensitivity of the cognitive measures in the preclinical phase. Third, given that AD is a progressive neurodegenerative disease, we expected cognitive performance to vary as a function of how close a participant was to the expected age at onset of symptomatic AD based on parental history, especially in the cognitively normal mutation carriers, but not in the participants who were mutation noncarriers. This would provide information from this cross-sectional study about what might be expected as the longitudinal study unfolds. Finally, we examined differences in personality in this sample. There is accumulating evidence that at least some aspects of personality appear to change in sporadic AD (Balsis, Carpenter, Storandt, 2005; Duchek, Balota, Storandt, & Larson, 2007; Wilson et al., 2003; Wilson, Schneider, Arnold, Bienias, & Bennett, 2007), and we asked whether similar differences be detected in ADAD.
Method
Participants
The data examined in this report were from the initial assessment of the 249 DIAN participants aged 19 to 64 years with known mutation status who were enrolled from January 26, 2009 through June 30, 2012. Participants with known mutation status were recruited from 197 families at 6 sites in the United States, 1 in the United Kingdom, and 3 in Australia (Morris et al., 2012). Demographic characteristics including education and ethnicity were obtained by self-report. Table 1 shows the sample characteristics by Clinical Dementia Rating (CDR; Morris, 1993) and mutation status (carrier, noncarrier). Only mutation carriers were included in the groups with CDR status greater than 0 given that the focus of this project is on ADAD rather than dementia due to other causes.
Table 1.
Demographic and clinical characteristics at entry
| Characteristic | CDR 0 | CDR 0.5 | CDR 1 | CDR 2 or 3 | |
|---|---|---|---|---|---|
| Noncarrier | Carrier | ||||
| N | 96 | 89 | 40 | 18 | 6 |
| Age (yrs) | 40.4 (9.6) | 34.6 (8.7) | 44.1 (10.3) | 45.8 (8.1) | 54.3 (8.3) |
| Education (yrs) | 14.7 (2.6) | 14.4 (2.5) | 14.2 (2.5) | 12.9 (2.6) | 13.0 (2.4) |
| Time to onset (yrs) | − 6.6 (11.9) | −12.7 (8.1) | −1.9 (7.7) | 3.9 (5.6) | 1.5 (10.6) |
| Women (%) | 59 | 60 | 60 | 56 | 50 |
| APOE 1 E4 (%) | 28 | 22 | 26 | 6 | 17 |
| APOE 2 E4 (%) | 0 | 2 | 5 | 6 | 17 |
| CDR Sum of boxes | 0.0 (0.1) | 0.0 (0.1) | 1.5 (1.0) | 5.8 (1.0) | 13.5 (3.4) |
| MMSE | 29.1 (1.3) | 29.1 (1.3) | 26.8 (3.0) | 17.9 (4.9) | 9.5 (6.4) |
| FAQ | 0.1 (0.3) | 0.2 (0.8) | 2.5 (3.2) | 16.6 (6.6) | 26.8 (4.5) |
| Hachinski | 0.2 (0.7) | 0.1 (0.4) | 0.7 (1.4) | 0.5 (0.7) | 0.8 (1.0) |
| GDS | 1.2 (1.5) | 1.4 (1.8) | 3.6 (3.3) | 4.4 (3.0) | 2.0 (2.2) |
Entries are means with SDs in parentheses unless otherwise specified. Time to onset is time to expected symptomatic onset; MMSE = MiniMental Status Exam; FAQ = Functional Activities Questionnaire; GDS = Geriatric Depression Scale.
Clinical Evaluation
Experienced clinicians assessed each participant for the presence and severity of dementia based on semi-structured interviews with the research participant and a knowledgeable collateral source (usually a spouse or an adult child) followed by a neurologic examination of the participant. The assessment protocol assesses cognitive problems that represent a decline from a former level of function for that individual and interfere to at least some degree with the individual’s ability to carry out accustomed activities. For example, the informant’s report of recent onset of repetition of questions or statements or misplacing items without independent retrieval could represent memory deficit, unusual difficulty with dates or in finding familiar places could represent disorientation, and unaccustomed problems with calculations (such as in household finances) or in judgment (such as purchasing unneeded items) could indicate executive dysfunction. Diminished ability to carry out activities of daily living at the individual’s usual level was sought (among other examples) in operating household appliances, driving a motor vehicle, maintaining hobbies, and keeping appointments. Behavioral and personality change also was ascertained. The assessment protocol (Morris et al., 2006) also included demographic information, health history, an aphasia battery, medication history, and several commonly used scales: Functional Assessment Questionnaire (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982); Geriatric Depression Scale (Sheikh & Yesavage, 1986); MiniMental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975); Rosen modification of the Hachinski Ischemic Scale (Rosen, Terry, Fuld, Katzman, & Peck, 1980). The clinician synthesized all information to determine whether dementia was present or absent based on the principle of intraindividual cognitive decline relative to previously attained function (McKhann et al., 2011).
Importantly, without reference to the participant’s performance on the psychometric battery, the clinician’s judgment was operationalized with the Clinical Dementia Rating (CDR; Morris, 1993), where CDR 0 corresponds to cognitive normality and CDR 0.5, 1, 2, and 3 represent very mild, mild, moderate, and severe dementia. An etiologic diagnosis of dementia (i.e., CDR ≥ 0.5) was made by the clinician in accordance with standard criteria (Morris et al., 2006). The present study focuses on the discrimination between the earliest CDR .5 stage and CDR 0 (indicating no impairment), which as noted is primarily based on the informant’s report of cognitive change in the ability to carry out everyday activities together with observations from the examination of the individual. Previous studies have documented the diagnostic acumen of clinicians using this informant-based method in reliably discriminating nondemented persons from individuals at the earliest symptomatic stage of a dementing illness (Morris et al., 1991; Carr, et al., 2000; Galvin et al., 2005). The validated clinical criteria for AD used in this study (Morris et al., 1988) are confirmed by the neuropathologic presence of AD in 93% of cases, including those identified in the CDR 0.5 stage (Berg et al. 1988). The CDR ratings for the six domains assessed (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) can be summed for a CDR sum of boxes score providing a finer gradation of impairment, and are presented below in Table 1.
Genotyping for apolipoprotein E (APOE) and for pathogenic mutations in the APP, PSEN1, and PSEN2 genes was performed on DNA extracted from peripheral blood samples using methods described previously (Talbot et al., 1994). Clinicians were unaware of APOE genotype results, and genotype data were not used diagnostically (Morris et al., 2012).
Psychometric Assessment
A 2-hour battery was administered independently of the clinical assessment. The Uniform Data Set Neuropsychological Test Battery included the following standard tests: Wechsler Memory Scale-Revised (WMS-R; Wechsler, 1987) Logical Memory (Story A only, immediate and delayed recall) and Digit Span; Category Fluency (animals, vegetables; Goodglass & Kaplan, 1983); Trailmaking A and B (Armitage, 1945); Digit Symbol from the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981); the Boston Naming Test (30 odd items; Goodglass & Kaplan, 1983). Letter fluency for F, A, and S and immediate and delayed recall of a single presentation of a 16-item word list were also obtained. After completing these tests, participants completed a computerized battery of seven cognitive tests of attention and executive functioning, language, and memory as well as the 120-item International Personality Item Pool (IPIP; Goldberg et al., 2006). The collateral source also completed the IPIP with respect to the participant.
Detailed descriptions of the tasks in the computerized cognitive battery can be found at www.dian-info.org/computerbattery. The Simon (1969) task and the Consonant Vowel Odd Even Switching task (Rogers & Monsell, 1995) assessed attentional selection and control; scores were percentage correct (Duchek et al., 2009). Working memory was measured with Reading Span (Daneman & Carpenter, 1980) and Computation Span (Conway et al., 2005); these two measures were summed to form a composite Working Memory score (total correct trials) for data analysis (Tse, Balota, Yap, Duchek, & McCabe, 2010). A Semantic Categorization task (Smith, Shoben, & Rips, 1974) was developed that assessed retrieval of information from semantic memory under high attentional demands; the score was percentage correct (Aschenbrenner et al., 2012). Paper Folding (Salthouse, Mitchell, Skovronek, & Babcock, 1989) measured visuospatial ability; the score was total correct. Finally, a measure of associative episodic memory was administered; the Pair-Binding task (Naveh-Benjamin, 2000) produced recall scores for number of correctly identified pairs of three types: (a) intact pairs from the list as originally learned, (b) pairs in which responses from learned pairs were rearranged so that they were associated with a different stimulus word (repaired list) and (c) new pairs.
In addition to the total accuracy scores, percentage correct subscores were also computed for three of the computerized tasks (see Tse et al., 2010, for more details about the Simon and Switching tasks). Briefly, for the Simon task subscores were for congruent trials (arrow pointed the same direction as side of screen on which it appeared) and incongruent trials (arrow pointed opposite direction compared with side of screen). For the Switching task the subscores were for the trials during which the same response was used throughout the block (pure block) versus for trials on which the participant was required to switch between two responses across trials (switch block). The two subscores on the Semantic Categorization task were for trials on which the stimuli were exemplars of the category (BIRD-robin) or were not exemplars but associates (e.g., BIRD-butterfly).
Procedure for Standardization and Quality Control
After data for the demographic, clinical, and psychological measures were collected by site personnel, they were subjected to a quality control process and certified as available for analysis by the DIAN Clinical Core. New testers download the extensive administration and scoring manual accessible on the DIAN web page and view the training DVD of the administration of the Uniform Data Set instruments. Prior to beginning their work, they review all assessment and scoring materials by phone with the Clinical Core’s quality control supervisor for the psychometric battery (a licensed psychologist with a faculty appointment in the Psychology Department at Washington University). An audio recording of every tenth protocol is sent to the Clinical Core for review. Testers are also supervised on site by the site neuropsychologist, who reviews the scoring of each protocol (and initials it) before it is scanned into the central data base. Each scanned protocol is then reviewed by the Clinical Core quality control supervisor; any administration or scoring issues are resolved prior to releasing the data for use by investigators. The computerized battery is uploaded directly to the data base and is scored electronically.
Data Analyses
Because participants were recruited from multiple, distinct families, it is necessary to account for the variability in disease expression across different mutations. We conducted a mixed model regression analysis (MIXED; SAS 9.1, Cary, NC) with participants nested within family to account for the correlation between members of the same family. Cognitive performance at the individual level was then added to the model using fixed effects of time to expected onset, group and the interaction between time to expected onset and group. The proportional reduction in total variance after including these effects was calculated as a measure of effect size. We conducted pairwise Student’s t-tests to determine whether cognitive performance differed significantly between specific groups. To assess the significance of the fixed effects, F tests were conducted with degrees of freedom estimated using the Satterthwaite method. This method has been shown to maintain Type I error rates at nominal levels (Manor & Zucker, 2004). We report Cohen’s D as a measure of effect size of each of these comparisons.
Three groups were used in the comparison of the cognitive data: CDR 0 noncarriers, CDR 0 mutation carriers, and CDR 0.5 mutation carriers. A fourth group (CDR 1.0) is also presented but because of the small size of this sample, and the present interest is in early stage AD, we only present the descriptive data for this group. Demographic data are shown for a fifth group (CDR 2 and 3) that was omitted from all scientific analyses because of its small size (n = 6) and failure to perform many or all tests.
Descriptive and clinical data were analyzed using analysis of variance and chi-square analyses. As noted above, because descriptive analyses revealed that CDR 0 mutation carriers were approximately 6 years younger than CDR 0 noncarriers at the time of the entry visit, expected time to onset based on parental age of symptomatic onset was included as a covariate in all the mixed model analyses along with its interaction with group. Inclusion of the interaction term allowed testing of the parallelism assumption required in analysis of covariance. In addition, the potential for nonparallel regression lines between the covariate and the various dependent variables was of primary interest in terms of the third goal of the study.
Results
Table 1 displays demographic and clinical characteristics of the sample. The CDR sum of boxes scores, the FAQ, and the modified Hachinski scores increased (ps < .001) and the MMSE decreased (p < .0001) with increasing global CDR. Depression scores were higher in the CDR 0.5s and 1 groups than in the other groups (p < .0001). As noted, the CDR 0 mutation carriers were younger (34.6 years) than the CDR 0 noncarriers (40.4 years), and the CDR 0.5 mutation carriers (44.1) years (p < .001), and therefore the CDR 0 noncarriers (−6.6 years) were, on average, closer to parental age of onset compared with the CDR 0 mutation carriers (−12.7 years; p < .001). This difference occurred for two reasons: (a) only 3% of the CDR 0 mutation carriers were older than 50, compared with 17% of the noncarriers, because many older mutation carriers were no longer CDR 0, and (b) 26% of the CDR 0 mutation carriers were under age 30 compared with only 12% of CDR 0 noncarriers. As expected, the CDR 0.5 mutation carriers were closest to the expected age of symptomatic onset (−1.9 years). Including expected time to onset in the data analyses also functionally controls for age.
We now turn to the four major questions motivating this project. First, are there widespread cognitive differences between CDR 0s and very mild dementia (CDR 0.5s) as has been reported in previous studies with these tasks in LOAD? Table 2 provides means and standard deviations for the cognitive measures from the battery of tests for participants with CDR 0, 0.5, and 1 at entry; the 6 CDR 2s and 3s could not perform most of the tasks. As noted, although performance for the CDR 1 group is presented in the Tables, the analyses focus on the more important comparison of the two CDR 0 and CDR .5 groups. The results of the mixed model analyses along with Cohen’s D for each of the three group comparisons for each of dependent measures are displayed in Table 3. As shown, the mixed model analyses yielded significant main effects of group for all measures with the exception of Digit Span Backwards and Letter Fluency. Post hoc comparisons indicated that, with a few exceptions, the CDR 0.5 group performed more poorly than either of the CDR 0 groups. Although the main effects of group were significant, as shown in Table 3, the post hoc pairwise comparisons were not for the intact and repaired lists from Pair-Binding. In general, these results clearly indicate that the cognitive differences between CDR 0 individuals, regardless of whether they were mutation carriers or not, and 0.5 individuals were widespread across tasks that have been shown to be sensitive to very mild LOAD.
Table 2.
Mean (and SD) performance on cognitive measures at entry
| Measure | CDR 0 | CDR 0.5 | CDR 1 | |
|---|---|---|---|---|
| Noncarrier | Carrier | |||
| Letter fluency | 41.4 (10.8) | 43.1 (12.4) | 35.6 (12.9) | 26.5 (10.0) |
| Word list (immediate) | 5.9 (2.0) | 6.1 (2.3) | 3.9 (1.5) | 1.6 (1.2) |
| Word list (delayed) | 3.2 (2.0) | 3.2 (2.1) | 0.7 (1.1) | 0.1 (0.2) |
| Logical Memory (immed.) | 14.6 (3.8) | 14.2 (4.5) | 9.0 (4.6) | 3.7 (3.8) |
| Logical Memory (delayed) | 13.5 (4.1) | 12.8 (4.7) | 6.9 (5.4) | 1.9 (3.2) |
| Digit Span Forward | 6.9 (1.1) | 6.9 (1.0) | 6.2 (1.2) | 5.1 (1.2) |
| Digit Span Backward | 5.1 (1.2) | 5.1 (1.3) | 4.5 (1.3) | 3.2 (1.0) |
| Animal Naming | 22.4 (5.1) | 23.2 (6.6) | 17.5 (5.1) | 12.6 (5.0) |
| Vegetable Naming | 15.8 (3.3) | 14.9 (3.9) | 11.9 (3.6) | 6.9 (2.4) |
| Trailmaking A | 21.9 (6.2) | 23.0 (6.0) | 36.7 (29.5) | 86.4 (43.7) |
| Trailmaking B | 55.2 (18.4) | 57.2 (23.3) | 106.6 (80.3) | 235.8 (99.3) |
| Digit Symbol | 62.0 (11.0) | 62.1 (12.1) | 44.6 (16.0) | 16.9 (14.9) |
| Boston Naming Test | 27.7 (1.6) | 26.9 (2.9) | 25.5 (3.6) | 22.0 (6.1) |
| Simon | 98.4 (1.6) | 98.0 (2.9) | 94.0 (9.8) | 78.2 (25.5) |
| Switch | 98.6 (2.4) | 97.7 (4.7) | 89.5 (15.2) | 61.0(23.9) |
| Working Memory | 19.1 (7.7) | 17.1 (8.7) | 11.6 (7.5) | 3.1 (1.2) |
| Categorization | 92.0 (5.5) | 89.0 (8.8) | 83.2 (11.8) | 59.4 (23.7) |
| Paper Folding | 7.4 (2.7) | 6.9 (3.0) | 4.3 (2.0) | 3.1 (2.4) |
| Pair Binding - Intact | 8.8 (2.5) | 9.2 (2.2) | 7.7 (2.9) | 4.1 (2.4) |
| Mixed | 7.6 (3.0) | 7.5 (3.3) | 5.3 (2.8) | 6.3 (3.2) |
| New | 10.7 (2.4) | 10.7 (1.7) | 8.2 (3.6) | 6.6 (3.3) |
Table 3.
Test statistics for expected age of onset, group and the interaction for each outcome measure.
| (Degrees of Freedom) and F valuea | Cohen’s Dc | ||||||
|---|---|---|---|---|---|---|---|
| Measure | EAO | Group | E* G | R2b | C vs. NC | NC vs. 0 .5s | C vs. 0.5s |
| Letter Fluency | (1,214) 0.5 | (2,212) 2.7^ | (2,210) 0.0 | .03 | .19 | .28 | .43* |
| Word List Immed. | (1,215) 8.6** | (2,211) 10.8** | (2,209) 0.6 | .16 | .08 | .65** | .57** |
| Word List Delayed | (1,214) 16.0** | (2,212) 19.3** | (2,210) 0.5 | .27 | .22 | .98** | .78** |
| Logical Mem Immed | (1,214) 13.3** | (2,213) 24.1** | (2,211) 3.8* | .22 | .23 | .78** | .57** |
| Logical Mem Delay | (1,213) 21.5** | (2,213) 30.0** | (2,213) 6.7** | .27 | .33* | .80** | .50** |
| Digit Span Forward | (1,215) 0.6 | (2,210) 5.8** | (2,208) 0.1 | .06 | .01 | .50** | .49* |
| Digit Span Back | (1,215) 4.3* | (2,212) 2.2 | (2,210) 0.6 | .04 | .03 | .19 | .17 |
| Animal Naming | (1,217) 9.9** | (2,206) 8.5** | (2,204) 0.9 | .11 | .06 | .51** | .55** |
| Vegetable Naming | (1,212) 0.3 | (2,212) 15.6** | (2,211) 1.2 | .14 | .17 | .69** | .53** |
| Trailmaking A | (1,216) 6.7* | (2,216) 17.6** | (2,216) 2.8^ | .15 | .10 | .60** | .50* |
| Trailmaking B | (1,208) 9.0** | (2,206) 22.2** | (2,202) 2.7^ | .24 | .11 | .81** | .69** |
| Digit Symbol | (1,215) 31.6** | (2,211) 25.7** | (2,208) 4.9** | .29 | .18 | .78** | .61** |
| Boston Naming Test | (1,215) 5.2* | (2,196) 6.5** | (2,197) 0.3 | .10 | .18 | .71** | .55** |
| Simon | (1,205) 8.1** | (2,205) 19.2** | (2,205) 7.2** | .14 | .09 | .47* | .38^ |
| Switch | (1,189) 7.7** | (2,185) 22.3** | (2,182) 2.6^ | .18 | .21 | .86** | .67** |
| Working Memory | (1,197) 5.6* | (2,196) 8.4** | (2,197) 0.7 | .10 | .28^ | .57** | .32 |
| Categorization | (1,199) 3.3^ | (2,179) 14.9** | (2,184) 2.8^ | .12 | .31* | .66** | .39^ |
| Paper Folding | (1,200) 2.6 | (2,194) 7.4** | (2,194) 0.2 | .16 | .19 | .82** | .65** |
| Pair Binding | |||||||
| Intact | (1,203) 0.3 | (2,201) 3.9* | (2,202) 1.1 | .03 | .16 | .24 | .37^ |
| Mixed | (1,203) 5.2* | (2,200) 6.6** | (2,200) 2.0 | .08 | .09 | .36^ | .28 |
| New | (1,204) 2.5 | (2,203) 14.1** | (2,203) 1.6 | .12 | .03 | .66** | .62** |
indicates p < .1
indicates p < .05
indicates p < .01
F-test and approximate degrees of freedom estimated via the Satterthwaite method
Proportion of variance explained by the 3 predictor variables
Cohen’s D for each pairwise comparison of the group main effect
NC = CDR 0 noncarrier
C = CDR carrier
0.5s = CDR 0.5 carrier
The second question addressed was whether there were any differences between CDR 0 mutation carriers and noncarriers. As shown in Table 3, the analyses indicated better performance by the noncarriers than the carriers only on Logical Memory delayed recall and Semantic Categorization accuracy performance.
The third question addressed the influence of the expected time to onset on the dependent variables. If it differed across groups one would expect different regression functions for the correlation between expected time to onset and the dependent variable (i.e., a lack of parallelism). There were significant interactions in the analyses for Logical Memory immediate and delayed recall, the Digit Symbol task, and the accuracy estimates from the Simon attention task. These significant interactions indicate that at least two of the regression lines relating time to expected onset to performance on the task are significantly different from each other. For the Simon task, the interaction regression coefficients for the two CDR 0 groups were not significantly different from 0 (ps > .05), but the coefficient for the CDR 0.5 group was (ps < .05).
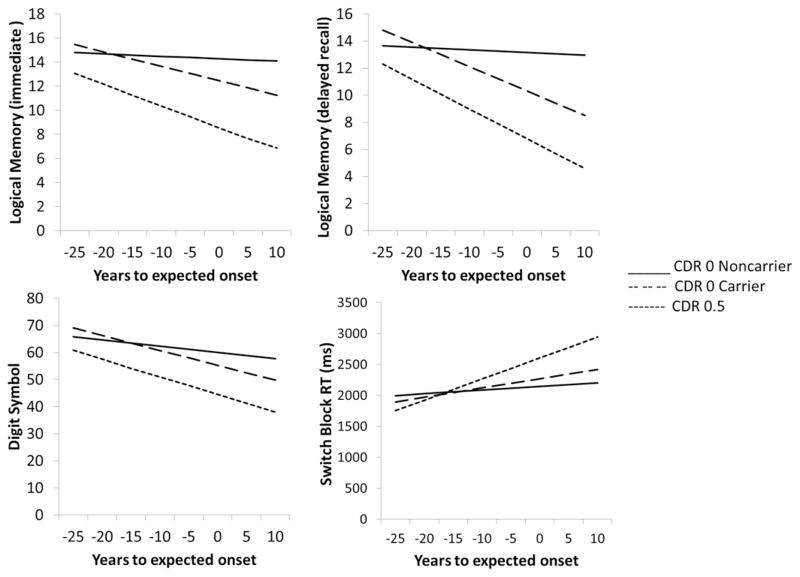
Importantly, time to expected onset was more strongly correlated with scores on the two Logical Memory measures and Digit Symbol not only in the CDR 0.5 group but also in the CDR 0 mutation carriers than it was in CDR 0 noncarriers. As shown in Figure 1 the regression lines as a function of time from age at baseline assessment to expected age of onset were quite flat for CDR 0 noncarriers (B weights = −.02 in both cases) and not significantly different from 0 for the two Logical Memory measures (immediate and delayed recall). There was, however, a significant downward slope to the regression lines for both the CDR 0 mutation carriers and CDR 0.5 individuals. The B weights for the CDR 0 mutation carriers were −.12 (p = .03) for Logical Memory immediate and −.18 (p = .003) for delayed recall; for the CDR 0.5 group the weights were −.26 (p = .004) for immediate and −.35 (p = .0003) for delayed recall. There was a significant downward slope in Digit Symbol performance in the CDR 0 noncarriers (B weight = −.21, p < .05) that is consistent with the well-known age effect on this measure (e.g., Storandt, 1976). The slope of the regression line was even steeper for the CDR 0 mutation carriers (−.55, p < .005) and the CDR 0.5s (−.98, p < .0001), indicating effects of both age and disease.
Figure 1.
Relations between baseline assessment and time to expected age of symptomatic onset vary between CDR 0 mutation carriers and noncarriers for four cognitive measures.
Means (and SDs) of the accuracy subscores from three of the computerized tasks are shown for each group in the upper portion of Table 4, with the corresponding reaction time data displayed in the bottom half of the table. The results from mixed model analyses, along with Cohen’s D for these measures are displayed in Table 5. First, consider the accuracy data. There were main effects of group on all accuracy measures with the exception of the relatively easy exemplar items on the categorization task. In addition, there were reliable interactions between group and time to expected onset in the more difficult conditions in the Simon task (incongruent trials), Switch task (Switch trials), and the Semantic Categorization task (associate trials). These interactions reflected greater slopes for the CDR .5s, than for CDR 0 carriers or the CDR 0 noncarriers, which did not differ from zero. Also, it is important to note that for the more difficult associate condition for the categorization task, there was a reliable difference between the CDR 0 carriers and noncarriers.
Table 4.
Mean (SD) accuracy and RT of subscores for three of the computerized tasks
| Measure | CDR 0 | CDR 0.5 | CDR 1 | |
|---|---|---|---|---|
| Noncarrier | Carrier | |||
| Accuracy | ||||
| Simon | ||||
| Congruent | 99.5 (1.6) | 99.0 (2.6) | 96.9 (7.0) | 82.0 (26.6) |
| Incongruent | 96.7 (3.3) | 95.8 (5.9) | 89.7 (14.4) | 74.0 (25.5) |
| Switch | ||||
| Pure | 98.8 (2.0) | 98.1 (4.2) | 91.6 (15.9) | 62.1 (26.8) |
| Switch | 98.2 (3.9) | 97.2 (6.6) | 85.8 (19.8) | 59.4 (23.9) |
| Categorization | ||||
| Exemplars | 91.7 (4.8) | 90.2 (5.7) | 89.4 (8.0) | 82.8 (6.8) |
| Associates | 92.3 (9.0) | 87.7 (15.9) | 77.0 (19.3) | 54.3 (16.5) |
| Reaction time | ||||
| Simon | ||||
| Congruent | 573 (111) | 565 (122) | 725 (268) | 1209 (498) |
| Incongruent | 636 (113) | 637 (148) | 831 (289) | 1300 (499) |
| Switch | ||||
| Pure | 933 (208) | 914 (282) | 1271 (573) | 1933 (874) |
| Switch | 2117 (363) | 2066 (438) | 2528 (687) | 2449 (1100) |
| Categorization | ||||
| Exemplars | 1084 (245) | 1116 (329) | 1200 (276) | 2404 (1027) |
| Associates | 1218 (373) | 1269 (453) | 1592 (521) | 3586 (1983) |
Table 5.
Test statistics for expected age of onset, group and the interaction for the subscores on three of the computerized tasks.
| (Degrees of Freedom) and F valuea | Cohen’s Dc | ||||||
|---|---|---|---|---|---|---|---|
| Measure | EAO | Group | E* G | R2b | C vs. NC | NC vs. 0 .5s | C vs. 0.5s |
| Accuracy | |||||||
| Simon | |||||||
| Incongruent | (1,204) 13.1** | (2,201) 21.3** | (2,202) 11.4** | .15 | .11 | .37^ | .27 |
| Congruent | (1,204) 3.3^ | (2,201) 9.8** | (2,203) 2.6^ | .07 | .14 | .41* | .28 |
| Switch | |||||||
| Switch | (1,199) 9.3** | (2,156) 23.8** | (2,165) 4.9** | .22 | .15 | .67** | .55** |
| Pure | (1,196) 4.2* | (2,196) 12.8** | (2,197) 1.3 | .11 | .16 | .68** | .53* |
| Categorization | |||||||
| Associate | (1,197) 3.5^ | (2,191) 15.9** | (2,193) 3.1* | .12 | .33* | .72** | .42* |
| Exemplar | (1,199) 0.1 | (2,163) 1.0 | (2,172) 1.2 | .00 | .12 | .19 | .09 |
| Reaction Time | |||||||
| Simon | |||||||
| Incongruent | (1,206) 10.4** | (2,184) 10.6** | (2,190) 0.0 | .19 | .17 | .80** | .65** |
| Congruent | (1,206) 6.2* | (2,182) 7.1** | (2,188) 0.3 | .14 | .12 | .77** | .65** |
| Switch | |||||||
| Switch | (1,190) 16.8** | (2,189) 10.4** | (2,187) 3.2* | .15 | .10 | .42* | .33 |
| Pure | (1,194) 17.3** | (2,189) 13.7** | (2,188) 2.2 | .17 | .14 | .63** | .52* |
| Categorization | |||||||
| Associate | (1,191) 4.4* | (2,186) 6.8** | (2,188) 0.2 | .07 | .21 | .65** | .45* |
| Exemplar | (1,192) 1.9 | (2,188) 1.7 | (2,190) 0.2 | .00 | .17 | .25 | .10 |
indicates p < .1
indicates p < .05
indicates p < .01
F-test and approximate degrees of freedom estimated via the Satterthwaite method
Proportion of variance explained by the 3 predictor variables
Cohen’s D for each pairwise comparison of the group main effect
NC = CDR 0 noncarrier
C = CDR carrier
0.5s = CDR 0.5 carrier
Turning to the response latency data from subscores of the computerized tasks, the results of the mixed model analyses on RTs revealed significant group differences on all RT measures except the exemplars from the Semantic Categorization task. As shown in Table 5, post hoc comparisons following significant group effects indicated that the two CDR 0 groups did not differ from each other, and both of these groups were faster than the CDR 0.5 group, with only one exception. The CDR 0 carriers did not differ from the CDR 0.5 group on RTs from the switch block from the Switching task.
The analyses of the RTs also yielded a significant interaction between group and time to expected onset only on the more difficult switch block from the Switching task (p = .04). As shown in Figure 1, this interaction reflected a pattern that was similar to that reported for Logical Memory and Digit Symbol. Time to expected symptomatic onset was strongly correlated with the RTs in the block of trials in which participants had to switch back and forth between responding to letters versus numbers not only in the CDR 0.5s (B = 34, p = .003) but also in the CDR 0 mutation carriers (B = 15, p = .01). This did not occur in the CDR 0 noncarriers (B = 6, p = .15).
The fourth and final question addressed in this project was whether there were differences in personality as a function of group. The means and standard deviations of scores on the five personality factors from the IPIP supplied by the participants and by the informants (about the participants) are shown in Table 6. In addition, we display at the bottom of Table 6, the correlations between the informant and participant reports. As shown, most correlations are of moderate size between the informant and the participant, with agreeableness showing the smallest correlations. One must be cautious not to over interpret these correlations, especially for the CDR 1.0 group which only included 18 participants.1
Table 6.
Mean (SD) performance on International Personality Item Pool at entry
| CDR 0 | CDR 0.5 | CDR 1 | ||
|---|---|---|---|---|
| Noncarrier | Carrier | |||
| Participant | ||||
| Neuroticism | 59.2 (16.2) | 60.0 (12.7) | 65.7 (16.2) | 66.3 (18.2) |
| Extraversion | 86.2 (13.0) | 85.8 (11.1) | 78.8 (13.4) | 78.8 (13.6) |
| Openness | 78.3 (12.0) | 79.8 (11.8) | 75.3 (12.3) | 72.7 (8.5) |
| Agreeableness | 97.0 (9.6) | 97.1 (9.6) | 96.8 (10.2) | 95.1 (12.3) |
| Conscientiousness | 98.1 (11.6) | 97.2 (12.0) | 88.7 (13.4) | 86.1 (15.5) |
| Informant | ||||
| Neuroticism | 56.8 (16.1) | 60.6 (15.6) | 64.7 (16.2) | 76.8 (12.6) |
| Extraversion | 87.3 (11.6) | 85.0 (12.8) | 75.0 (14.9) | 63.8 (13.6) |
| Openness | 76.4 (9.8) | 77.9 (10.0) | 69.5 (12.8) | 62.2 (6.9) |
| Agreeableness | 97.8 (10.1) | 97.8 (9.7) | 94.7 (11.9) | 91.0 (14.0) |
| Conscientiousness | 100.0 (13.8) | 96.4 (11.9) | 87.4 (17.3) | 71.9 (16.8) |
| Participant-Informant Correlation | ||||
| Neuroticism | .462** | .402** | .602** | .534* |
| Extraversion | .635** | .353** | .731** | .686** |
| Openness | .505** | .622** | .758** | .625* |
| Agreeableness | .294** | .223* | .266 | .441 |
| Conscientiousness | .538** | .381** | .551** | .330 |
p < .05,
p < .01
The data in the upper portion of Table 6 provide the personality estimates from the participants’ reports, with the results from the mixed model analyses and Cohen Ds for the individual comparisons in Table 7. As shown, there were significant group main effects in the participants’ self-reports for both extraversion and conscientiousness. On post hoc comparisons the cognitively normal mutation carriers and noncarriers did not differ from each other, but the CDR .5s were less conscientious than either of the CDR 0 group. Also, as shown in Table 7, the noncarriers were also less neurotic and more extroverted than the CDR .5 group. There was no evidence of a group by time to expected age of onset interaction for any of the participants’ self reported personality measures.
Table 7.
Test statistics for expected age of onset, group and the interaction for each measure of the International Personality Item Pool for the Participant and Informant Reports
| (Degrees of Freedom) and F valuea | Cohen’s Dc | ||||||
|---|---|---|---|---|---|---|---|
| Measure | EAO | Group | E* G | R2b | C vs NC | NC vs 0.5s | C vs 0.5s |
| Participant | |||||||
| Neuroticism | (1,216) 0.4 | (2,216) 2.9^ | (2,216) 0.3 | .01 | .05 | .40* | .35^ |
| Extraversion | (1,212) 0.1 | (2,213) 7.4** | (2,212) 2.4^ | .06 | .08 | .42* | .34^ |
| Openness | (1,213) 3.1^ | (2,212) 1.6 | (2,214) 2.6^ | .02 | .01 | .11 | .10 |
| Agreeableness | (1,215) 5.3* | (2,214) 0.0 | (2,215) 0.7 | .01 | .06 | .18 | .23 |
| Conscientiousnes | (1,211) 1.2 | (2,213) 7.8** | (2,212) 1.1 | .07 | .10 | .79** | .68** |
| Informant | |||||||
| Neuroticism | (1,203) 0.1 | (2,203) 3.0^ | (2,203) 0.1 | .01 | .23 | .43* | .26 |
| Extraversion | (1,203) 0.0 | (2,203) 14.3** | (2,203) 0.8 | .10 | .12 | .67** | .55** |
| Openness | (1,201) 0.1 | (2,201) 6.1** | (2,202) 1.4 | .07 | .08 | .48* | .54** |
| Agreeableness | (1,202) 0.7 | (2,200) 2.0 | (2,198) 1.2 | .01 | .00 | .25 | .25 |
| Conscientiousnes | (1,201) 2.2 | (2,200) 11.6** | (2,197) 0.1 | .08 | .25 | .80** | .58** |
indicates p < .1
indicates p < .05
indicates p < .01
F-statistic and denominator degrees of freedom estimated via the Satterthwaite method
Proportion of variance explained by the 3 predictor variables
Cohen’s D for each pairwise comparison of the group main effect
NC = CDR 0 noncarriers
C = CDR 0 carriers
0.5s = CDR 0.5 carrier
The data displayed in the middle third of Table 6 are for the informant reports, with the results from the mixed level analyses and Cohen Ds for the individual comparisons in Table 7. As shown, the analyses revealed significant group differences in the informants’ reports for extraversion, openness, and conscientiousness. Post hoc comparisons revealed that the informants viewed the CDR .5 group as significantly less extraverted, open, and conscientious than both of the CDR 0 groups. Although the means were in the predicted direction for neuroticism, the only significant difference was between the CDR 0 noncarriers and the CDR .5 group. Again, none of the interactions between group and time from onset were significant.
Discussion
There were four goals in the present study of the DIAN cohort. The first goal was to determine if the overall pattern observed in these individuals is similar to the pattern observed in LOAD. The results are very clear. There were differences across the wide spectrum of tasks in this younger DIAN cohort that are consistent with the pattern observed in LOAD. These results extend the conclusions of Lopera et al. (1997) to a larger cohort and to less severely impaired individuals. In their study the demented group had a mean MSSE value of 14 as opposed to 27 in the present study. These results are also consistent with a more recent paper by Acosta-Baena et al. (2011) on a larger cohort of 449 PSEN1 carriers in showing relatively widespread impairments across multiple domains in very early stage symptomatic AD. There may be tasks that show more or less sensitivity, but the present results clearly suggest a wide spectrum of deficits in the very mildly symptomatic individuals in the DIAN cohort.
The second goal was to determine if any of the present tasks were particularly sensitive to mutation presence in asymptomatic mutation carriers. The literature on asymptomatic cognitive deficits in ADAD is somewhat mixed. Specifically, some studies suggest that there may be specific deficits in verbal memory and performance IQ (Fox, Warrington, Seiffer, Agnew, & Rossor, 1998), whereas other studies have shown localized differences in lexical-semantic tasks (e.g., famous face naming, Arango-Lasprilla, Cuetos, Valencia, Uribe, & Lopera, 2007). Ringman et al. (2005) provided evidence that mutation carriers had lower visuospatial and executive function working memory performance but did not find evidence of differences in verbal memory or language composite scores. Although there were tendencies for the carriers to perform worse than the noncarriers in the present sample, only Logical Memory delayed recall and the semantic categorization task produced reliable differences. At first glance, the present results might suggest that episodic memory performance and language may be especially sensitive. It is unlikely, however, that any of the tasks in the present study or in other cognitive batteries are process pure (Jacoby, 1991). For example, the present semantic verification task was developed to measure attentional control in semantic memory retrieval (Aschenbrenner et al., 2012), and it is well established that there is a strong contribution of attention to episodic memory performance as reflected in tasks such as the Logical Memory task (e.g., Balota, Burgess, Cortese, & Adams, 2002). Finally, in making comparisons across studies one must be certain there are no impaired individuals in asymptomatic groups. In the present study, the CDR 0 individuals were highly functional as reflected by the MMSE mean scores of 29.1 for both mutation carriers and noncarriers and by the well-established CDR measure. Ongoing longitudinal work will be especially important in tracking the cognitive changes in these asymptomatic individuals.
The third goal of the present study was to examine the relationship between how far the person was from the expected age of symptomatic onset and cognitive decline. One might expect increased cognitive deficits in the mutation carriers as one first approaches and then passes the expected age of onset. In most measures, there was no evidence of a reliable interaction between expected time of onset and group. However, there was evidence found in two measures that were localized for the very mild CDR 0.5 individuals. Specifically, accuracy estimates from the more difficult conditions of the Simon task (incongruent condition) and the Semantic Categorization task (associate condition) were lower in the CDR 0.5 group as the time from expected age of symptomatic onset increased. This relation did not reliably occur on these specific tests in the two CDR 0 groups. This is noteworthy given the very early stages of dementia in the CDR 0.5 individuals.
In addition to this pattern in the very mildly demented individuals, there was also evidence that even in the CDR 0 individuals, there were three tasks that produced reliable effects of time from expected age of symptomatic onset in the mutation carriers but not in the noncarriers. This effect was seen in both scores from Logical Memory, in Digit Symbol, and in the RT subscore from the switch blocks on the Switching task. Thus, even though the CDR 0 mutation carriers were very high functioning, there were preclinical indications of deficits in the domain of episodic memory (Logical Memory), speeded interference-based performance (Digit Symbol), and executive control (Switching). Although these effects were limited to only a few tasks in the present study, these results suggest that sensitive measures across multiple cognitive domains are sensitive to mutation status.
Finally, because one may be concerned about self-insight regarding personality, we were especially interested in the results from the informant sources, although these indeed were correlated with self-reports. It is clear that the informants see those who are cognitively normal as more extraverted, open, and conscientious than those who are demented. The participant reports yielded the same pattern for conscientiousness, whereas, for extraversion, the difference was only reliable between the CDR 0 noncarriers and the CDR .5 participants. There was a similar pattern in terms of neuroticism, but here both participants and the informants reported that the CDR 0 mutation carriers were similar to the CDR 0.5 group. There were no reports of differences between the two CDR 0 groups. Thus, although the differences found between the CDR 0 and the CDR .5 groups seen in the DIAN cohort are consistent with a degraded personality profile occurring with DAT, there was no evidence of any differences in personality presymptomatically in the CDR 0 carriers vs CDR 0 noncarriers.
In sum, the primary purpose of the analyses reported here was to provide a baseline description of the clinical and cognitive status of a valuable multinational sample of primarily cognitively normal people from families with one of three autosomal dominant mutations that causes early-onset AD. As expected, those who were not demented performed better on clinical and cognitive measures than those who were, even those only in the very mild symptomatic stage of the disease. Those who were demented in the DIAN cohort had a widespread pattern of cognitive deficits identical to the pattern typically observed in LOAD. In addition, cross-sectional results from the cognitive measures indicated that as the study progresses longitudinal changes in not only episodic memory but also in speeded visuospatial abilities and attention may also be detected prior to dementia onset, indicating a preclinical cognitive signal in CDR 0 mutation carriers. Performance by cognitively normal mutation carriers who were closer to their expected age of onset of dementia was poorer on both immediate and delayed recall of Logical Memory, Digit Symbol, and the switch block of trials from a test of attention. These results are similar to those reported for LOAD (Johnson, Storandt, Morris & Galvin, 2009). The present results also indicate that there are widespread differences in personality during the earliest detectable stage of the disease.
Acknowledgments
This study was supported by grant U01AG032438 from the National Institute on Aging, the generous support of F. Simmons and O. Mohan, and a private nonprofit foundation. This work involved an international consortium, which included contributions by the following Site/Core leaders: Randy Bateman, Tammie Benzinger, Nigel Cairns, Anne Fagan, Bernardino Ghetti, Alison Goate, Daniel Marcus, Ralph Martins, Colin Masters Richard Mayeux, Eric McDade, John Morris, John Ringman, Martin Rossor, Stephen Salloway, Peter Schofield, Reisa Sperling, and Chengjie Xiong. The authors thank the DIAN participants and their families for their dedication and altruism and the research and support staff at each of the DIAN sites for their contributions to the study.
Footnotes
A Fisher Z test was run on all the pairwise comparisons and only 3 correlations were significantly different. The carriers had a lower correlation on extraversion compared to both the noncarriers and the CDR 0.5s The noncarriers has a lower correlation on openness than the .5s
References
- Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, Saldarriaga A, Lopera F. Predementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: A retrospective cohort study. Lancet Neurology. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- Arango-Lasprilla JC, Cuetos F, Valencia C, Uribe C, Lopera F. Cognivite changes in the precelinica phase of familial Alzheimer’s disease. Journal of Clinical And Experimental Neuropsychology. 2007;29:892–900. doi: 10.1080/13803390601174151. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs. 1945;60(1, Whole No. 177):1–48. [Google Scholar]
- Aschenbrenner AJ, Balota DA, Duchek J, Tse CS, Fagan A, Holtzman D, Ratcliff R. Healthy aging and early stage AD: Examination of CSF biomarkers and RT distributions in a category verification task. Poster session presented at the Cognitive Aging Conference; Atlanta, GA. 2012. Apr, [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, Adams DR. The word-frequency mirror effect in young, old, and early-stage Alzheimer’s disease: Evidence for two processes in episodic recognition. Journal of Memory & Language. 2002;46:199–226. [Google Scholar]
- Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. Journal of Gerontology: Psychological Sciences. 2005;60B:98–101. doi: 10.1093/geronb/60.2.p98. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England Journal Of Medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, Miller JP, Storandt M, Duchek J, Morris JC, Rubin EH, Burke WJ, Coben LA. Mild senile dementia of the Alzheimer type: 2. Longitudinal assessment. Annals of Neurology. 1988;23:477–484. doi: 10.1002/ana.410230509. [DOI] [PubMed] [Google Scholar]
- Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724–1727. doi: 10.1212/WNL.55.11.1724. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilheim O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Duchek JM, Balota DA, Storandt M, Larsen R. The power of personality in discriminating between healthy aging and early stage Alzheimer’s disease. Journal of Gerontology: Psychological Sciences. 2007;62B:353–361. doi: 10.1093/geronb/62.6.p353. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse CS, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23:746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rosor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer’s disease: A longitudinal prospective study. Brain. 1998;121:1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Morris JC. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, Gough HC. The International Personality Item Pool and the future of public-domain personality measures. Journal of Research in Personality. 2006;40:84–96. [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination Booklet. III. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer’s disease. Neurology. 1995;45:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer’s disease. Archives of Neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Laukka EJ, Bäckman L. Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex. 2006;42:347–355. doi: 10.1016/s0010-9452(08)70361-7. [DOI] [PubMed] [Google Scholar]
- Lopera F, Ardilla A, Martinez A, Madrigal L, Arango-Viana JC, Lemere CA, Kosik KS. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. Journal of the American Medical Association. 1997;277:793–799. [PubMed] [Google Scholar]
- Manor O, Zucker DM. Small sample inference for the fixed effects in the mixed linear model. Computational Statistics & Data Analysis. 2004;46:801–817. [Google Scholar]
- McKann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori CR, Buchmann A, Mustovic H, Schmidt CF, Boesiger P, Nitsch RM, Henke K. Enhanced brain activity may precede the diagnosis of Alzheimer’s disease by 30 years. Brain. 2006;129(11):2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Annals of Neurology. 1988;24(1):17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Storandt M, Rubin EH, Price JL, Grant EA, Berg L. Very mild Alzheimer’s disease Informant–based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41(4):469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Aisen PS, Bateman RJ, Benzinger TLS, Cairns NJ, Fagan AM, Bhetti B, Goate AM, Holtzman DM, Klunk WE, McDade E, Marcus DS, Martins RN, Masters CL, Mayeux R, Oliver A, Quaid K, Ringman JM, Rossor MN, Salloway S, Schofield PR, Selsor NJ, Sperling RA, Weiner MW, Xiong C, Moulder KL, Buckles VD for the Dominantly Inherited Alzheimer Network. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clinical Investigation. 2012;2:975–984. doi: 10.4155/cli.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities of older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Diaz-Olavarrieta C, Rodriguez Y, Chavez M, Fairbanks L, Varpetian A, Maldonado HC, Macias-Islas MA, Murrell J, Ghetti B, Kawas C. Neuropsychological function in nondemented carriers of presenilin-1 mutations. Neurology. 2005;65:552–558. doi: 10.1212/01.wnl.0000172919.50001.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Annals of Neurology. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Mitchell DR, Skovronek E, Babcock RL. Effects of adult age and working memory on reasoning and spatial abilities. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology. 1986:165–173. [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. Journal of Experimental Psychology. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Smith EE, Shoben EJ, Rips LJ. Structure and process in semantic memory: A featural model for semantic decisions. Psychological Review. 1974;1:214–241. [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. Journal of the American Medical Association. 1996;275:528–532. [PubMed] [Google Scholar]
- Storandt M. Speed and coding effects in relation to age and ability level. Developmental Psychology. 1976;12:177–178. [Google Scholar]
- Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet. 1994;343:1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- Tse CS, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual: Wechsler Adult Intelligence Scale -Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Manual: Wechsler Memory Scale-Revised. San Antonio, Texas: Psychological Corporation; 1987. [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of ‘Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of General Psychiatry. 2007;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]