SUMMARY
Anterior cingulate cortex (ACC) is known to be involved in functions such as emotion, pain, and cognitive control. While studies in humans and non-human mammals have advanced our understanding of ACC function, the subjective correlates of ACC activity have remained largely unexplored. In the current study, we show that electrical charge delivery in the anterior mid-cingulate cortex (aMCC) elicits autonomic changes and the expectation of an imminent challenge coupled with a determined attitude to overcome it. Seed-based, resting-state connectivity analysis revealed that the site of stimulation in both patients was at the core of a large-scale distributed network linking aMCC to the frontoinsular and frontopolar as well as some subcortical regions. This report provides compelling, first-person accounts of electrical stimulation of this brain network and suggests its possible involvement in psychopathological conditions that are characterized by a reduced capacity to endure psychological or physical distress.
INTRODUCTION
The greatest of life’s challenges leave us mired in “a sea of troubles,” battered by the “slings and arrows of outrageous fortune.”(Shakespeare, 1603). It is at such moments that an individual’s will to persevere is put to the test. While some are able to marshal the necessary physical and psychological resources in the face of challenges, others have a pathologically lowered motivation and mental strength for enduring physical or psychological pain. Understanding the structure and physiology of the brain networks mediating attributes, such as the resolve to overcome upcoming challenges, will create new diagnostic and therapeutic frontiers for disorders such as depression and chronic pain that are characterized, in part, by reduced motivation, endurance, and perseverance.
Two lines of evidence suggest that the anterior cingulate cortex (ACC) and a set of connected regions might be the key network in this context. First, studies in humans and non-human mammals suggest that the ACC (including its mid-cingulate region) is essential for initiating changes in behavior, making associations between reward and action, determining the action necessary to obtain a goal, and synthesizing information about reinforcers ranging from pain and threatening conspecifics to aversive cues and negative feedback (Carter et al., 1999; Devinsky et al., 1995; Hayden et al., 2009; Rushworth et al., 2011; Shackman et al., 2011; Shenhav et al., 2013; Vogt and Sikes, 2000). Second, the ACC is anatomically well-situated for such functions. For instance, anatomical tracing studies in non-human primates, as well as tractography and functional connectivity studies in humans, have suggested strong anatomical and functional connectivity between the ACC and brain structures known to be important for pain, pleasure, emotion, and decision making (for original references see (Seeley et al., 2007; Van Hoesen et al., 1996; Vogt et al., 2004).
While lesion studies in humans have shown that the ACC is important for decision-making and emotional processing, the anatomical imprecision of this approach can be problematic. It is often unclear the extent to which the cognitive and behavioral deficits in these patients are due to the compromise of the ACC itself rather than the adjacent cortical gray and white matter tissue frequently included in the lesion. In addition, subjective correlates of ACC activity have been methodologically difficult to assess given its hidden position deep within the mesial surface of the brain.
In the current multimodal study, we provide detailed, first-person accounts of neuromodulation in the anterior mid-cingulate cortex (aMCC) and its associated functional network using a combination of electrical brain stimulation (EBS) and pre-operative resting-state functional magnetic resonance imaging (fMRI) in two epilepsy patients implanted with intracranial electrodes. In both patients, we demonstrate a remarkably stereotyped set of autonomic, cognitive, and emotional changes and establish a common functional connectivity map linking the aMCC stimulation site to a distributed network of regions, often referred to as the “emotional salience” or “cingulo-opercular” network (Seeley et al., 2007).
RESULTS
Two patients with refractory epilepsy were implanted with intracranial depth electrodes to localize the source of seizure activity. We localized the anatomical position of intracranial electrodes in each subject’s native neuroanatomical space. As part of their routine clinical diagnosis, a volley of electrical charge was delivered in a select number of electrode contacts. These electrodes were clinically selected to probe the target region’s function and its potential involvement in the patients’ seizures. EEG and clinical signs in both patients suggested medial temporal lobe epilepsy. To rule out the involvement of other limbic areas in the patients’ seizures, a mild electrical current was delivered in extra-temporal sites within the orbitofrontal, cingulate, and retrosplenial regions, and the patient was instructed to report if the electrical stimulation caused their typical seizure auras.
Each stimulation trial was defined as the time when the patient thought his brain was being stimulated. These trials were defined as “real” if electrical charge was delivered and “sham” if the patient thought he was being stimulated, but no electrical charge was delivered. During some of the sham trials, we specifically tested the placebo effect by counting “1, 2, 3” before clicking the stimulator button while the electrical current was set at 0 mA.
While electrical charge delivered in the medial temporal lobe structures caused electrographical seizures and typical clinical auras, EBS in non-temporal target sites was void of any EEG after-discharges or seizures and the patients’ perceptual changes were distinct from their typical clinical auras.
In both patients, electrical stimulation in the anterior mid-cingulate cortex (aMCC) (Figure 1), caused a strikingly similar and consistent set of perceptual and behavioral changes of a physical and psychological nature (Table 1). These changes were absent during sham trials and when electrical current was delivered below a certain threshold (i.e., subthreshold: 6 mA in P1 and 4 mA in P2). Both patients reported autonomic symptoms including “shakiness” or “hot flashes” in the upper chest and neck region. Heart rate seemed to increase in both cases. Trends of heart rate changes in the three conditions of sham, sub-threshold EBS, and real EBS trials were (in mean beats per minute ± standard deviation) 77±6, 86±9, 92±7 in P1 and 81±8, 90±5, 94±7 in P2. Moreover, both patients recounted a sense of “challenge” or “worry” (also known as foreboding) but remained motivated and aware that they would overcome the challenge. Patient 1 used an interesting analogy to explain his feeling. He reported that he felt as if he were driving a car into a storm. In his words, “like you’re headed towards a storm that’s on the other side, maybe a couple of miles away, and you’ve got to get across the hill and all of a sudden you’re sitting there going how am I going to get over that”. With repeated stimulation, he reported “Let’s say … if you knew you were driving your car and it was … one of the tires was half flat and you’re only halfway there and you have no other way to turn around and go back, you have to keep going forward”. When cued whether the feeling was negative or positive, he reported “it was more of a positive thing like … push harder, push harder, push harder to try and get through this…” (Movie S1). Patient 2 recounted feeling worried and anxious about something negative that was going to happen, but simultaneously knowing that he had to fight to make it through and not give up. Both patients described the same physical or psychological phenomena during each of the six repetitions of real trials. By contrast, no perceptual changes were reported during any repetitions of the sham trials in which all parameters of the EBS procedure were the same except the electrical current, which was set to 0 mA. Stimulation of subgenual or retrosplenial cingulate regions or the adjacent electrodes in the white matter did not elicit any perceptual or behavioral responses.
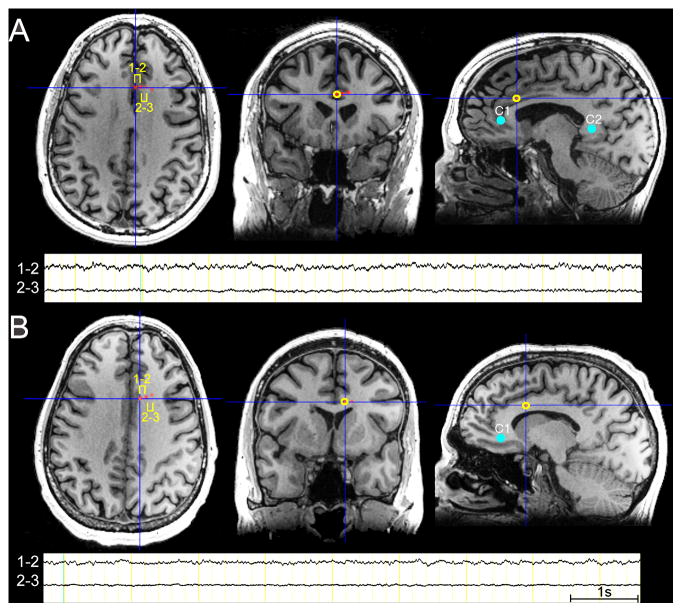
Figure 1. Anatomical Location of the Intracranial Electrodes and Electroencephalographic Activity.
In P1 (A) and P2 (B) electrical stimulation was bipolar between contacts 1 and 2. As noted in the magnetic resonance images (MRIs) and the electroencephalographic (EEG) traces, contacts 1 and 2 in P1 and contact 1 in P2 were clearly in the gray matter. Because the EBS was performed in a bipolar manner (i.e., between electrode contacts 1 and 2), we attribute the results of the EBS to the engagement of the aMCC gray matter and its white matter connections with other brain regions. Note the decrement of EEG activity between sites 2–3 compared to the activity recorded between sites 1 and 2. The MNI coordinates of the EBS targets were as follows: P1 (electrode 1=; 2.00, 26.00, 26.00; electrode 2=8.00, 26.00, 26.00) and P2 (electrode 1=10.00, 18.00, 26.00; electrode 2=16.00, 20.00, 26.00). Note that the right side of the image corresponds to the right side of the brain. One second (1s) EEG time scale shown. Sites of stimulation in the subgenual and retrosplenial regions are shown as controls sites 1 or 2 (C1 and C2 – blue filled circles). Besides these cingulate control regions, sites immediately adjacent to the aMCC were also stimulated as control sites (see Table 1). Stimulation of these control sites did not elicit similar cognitive and emotional effects as the stimulation of the aMCC sites.
Table 1.
Details of Electrical Stimulation Procedure and Subjective Reports
| P1 | ||||
|---|---|---|---|---|
| EBS Target | Current (mA) | Prompting Question | Patient Response | Stimulation Duration (s) |
| Electrodes 1&2 | 2 | Any difference? | “No” | 1.8 |
| 4 | No change? | “No, probably the next one I would start feeling it, it’s starting to get kind of disillusional. It’s starting to get to the point where I’m starting to have a hard time making a decision about what answer I want to say, or how to pursue it or what. It’s not clear.” | 2.2 | |
| 6 | Let me know what you feel. | “Yeah, my upper respiratory … started, kind of … my chest and respiratory system started getting shaky, like it was wanting to … go push itself out the door.” | 2.4 | |
| Any change in your emotion and mood? | “Not in my emotion, but my mood, I started getting this feeling like … I was driving into a storm. That’s the kind of feeling I got. Like, almost like you’re headed towards a storm that’s on the other side, maybe a couple of miles away, and you’ve got to get across the hill and all of a sudden you’re sitting there going how am I going to get over that, through that? And that’s the way my brain started functioning.” | |||
| 8 | Weaker or stronger? Longer or shorter? | “That’s just about the same way as last time. My chest never sits there pounding like it’s, you know, like you’re a football player getting ready to go out and make his first touchdown for the season or something; it’s not that type of thing. It’s more like this thing of trying to figure out your way out of, how you’re going to get through something. It’s not a matter of how you’re going to production-wise do something … Let’s say … if you knew you were driving your car and it was … one of the tires was half flat and you’re only halfway there and you have no other way to turn around and go back, you have to keep going forward … That type of a, you know, feeling you have. You’re like, you’re like (pats chest) am I gonna, am I gonna to get through this? Am I gonna get through this?” | 3.2 | |
| Was it negative or positive? | “I’d say it is a question, not a … not a worry like a negative like I’m not …’cause there’re not too many things I don’t … it was more of a positive thing like … push harder, push harder, push harder to try and get through this and that’s when my heart started … my, my … I don’t know if you were reading my pulse or anything, or heart rate. Was it starting to go up at that time or …?” | |||
| Was it stronger of the same as the time before? | “The same.” | |||
| SHAM | “nothing, absolutely nothing” | |||
| SHAM | “my ears.. Well this ear [right] starting to get a lot of pressure and sensitivity built up in it. Thinks it’s the one that got banged up real good when they were starting to put screws in it.” | |||
| Electrodes 2&3 | 2 | anything? | “nothing” | 2.4 |
| 4 | “no” | 2.6 | ||
| 6 | “no” | 2 | ||
| 8 | “no” | 2.6 | ||
| Electrodes 9&10 | 2 | anything? | “no” | 1.1 |
| 4 | “no” | 2.6 | ||
| 6 | “no” | 2.4 | ||
| 8 | “no” | 2.5 | ||
| P2 | ||||
|---|---|---|---|---|
| EBS Target | Current (mA) | Prompting Question | Patient Response | |
| Electrodes 1&2 | SHAM | Any change? | “No.” | |
| SHAM | One more time. | “No.” | ||
| 1 | How about now? | “No.” | 2.1 | |
| 2 | What about now? | “Yeah, that felt weird. Umm … I felt like a clicking here and then like a twitch starting in my shoulder.” | 2.3 | |
| A twitch in your shoulder? | “Yeah … and then my ear at the same time.” | |||
| 3 | Now? | “No. Maybe … little bit of irritable. No physical, just emotional. I just, I get impatient sometimes. I know it’s just an attitude.” | 2.3 | |
| Did I make you impatient? | “I think it’s-it’s the overall. I don’t believe you did anything to me.” | |||
| I’m going to do a couple of times with electricity and without, and we’ll see if you can guess. I’m going to do it four times … two of them with, two of them without. See if that emotional change happens. Do you want to close your eyes? | “Open will be fine.” | |||
| 5 | “A little hot flash. “ | 2.9 | ||
| Where was it? | “Just … in my chest and a little in my face.” | |||
| Emotional change? | “No, not really.” | |||
| SHAM | How about now? Hot flash? | “No. No.” | ||
| SHAM | How about now? | “No. Nothing.” | ||
| 5 | Now? | “No … just a little bit as the last one, same thing. Just, just like a hot flash and I started to get a little irritable.” | 2.5 | |
| What does irritability mean to you? | “Impatient. Yeah … you get short. Short-fused, you know, irritable. Yeah …” | |||
| Alright. Let’s do it this way now: Once with, once without. You were pretty good at guessing by the way. | “I’m trying not to guess, like you said.” | |||
| No, I know. But you did a good job. | ||||
| SHAM | Now? | “No.” | ||
| 5 | Now? | “Yes. Same thing, I get this hot flash. (motions to neck) Starts in my neck and you know … then I try to fight it to calm down.” | 2.2 | |
| Is that the hot flash that makes you irritated or is it your emotions? | “Yeah … it’s the emotion of I know something is going happen that I can’t control. Then I get irritated and then from there it’s all…” | |||
| Do you think you became anxious or worried or super excited? | “Worried. Worried that something bad is going to happen.” | |||
| Something bad is going to happen? | “You know … physically. Not that I’m going to do something bad but something, physically, I’m not going to be able to control.” | |||
| Sure. But how was your stamina? Doomed to fail or you knew you were going to make it? | “No, I knew I had to fight to make it.” | |||
| Tell us more. | “If I don’t fight I give up. I can’t give up. If I give up, then … then, you know …” | |||
| I’m going to do it once now. You can pretty much guess when I’m stimulating, because when I don’t stimulate you don’t feel anything. I’m going to stimulate and I want you to pay attention to whether you feel that you are going to fail or that you should try harder. One is optimistic. There is trouble but you know you are going to make it, and the other is pessimistic, the world is black and you are doomed. Pay attention to that attitude part. | ||||
| 4 | Now? | “Yeah … I don’t feel like there’s nothing I can do about it. I feel like …’cause I have to fight it, you know? I have to make it through.” | 3.0 | |
| Can you give us some examples of how this could happen in your daily life? Let’s say you are driving … | (laughs) “I don’t get to drive.” | |||
| I know, but let’s say you were driving when you were 30 … what should happen on the road that would give you this feeling? | “You mean what would happen when I would start to feel like that before? Something like that would only be triggered by a major accident, you know, cause anything small in life you have to be able to handle. Cause there are so many millions of small things that happen to you daily that, you have to handle them, you have to deal with them. It’s the major things that if you give up on, you’re in trouble. You can’t give up.” | |||
| Can you tell us more about your feeling of not giving up? | “I feel like if I give up, then I’ve let everybody else down.” | |||
| But, right now, do you think I made you stronger or weaker? | “I felt the flash and as we talked through it … it made me stronger. | |||
| Electrodes 2&3 | SHAM | Tell me if the same thing or a different thing happens? Any change? | “No.” | |
| SHAM | Now? | “No.” | ||
| SHAM | Now? | “No.” | ||
| Are you okay? | “Yeah, just trying to relax, yeah.” | |||
| SHAM | Now? | “No.” | ||
| 4 | Now? | “Yeah, a little bit. I was, no not really hardly … same thing, anticipation … I really didn’t feel anything.” | 2.9 | |
| Anticipation of what? | “About what’s going to get stimulated? You know, what’s going to happen? How’s it going to feel?” | |||
| 4 | Now I’m going to do the same thing. Don’t anticipate any change, don’t guess that I’m going to make any change. Pay attention to your emotions. Describe if anything changed. | No. | 2.6 | |
| Electrodes 2&4 | 4 | Now? | No, not really, no. | 2.3 |
| Are you sure? | Yeah. | |||
| SHAM | Now? | No, nothing. | ||
| 6 | Now? | No, it’s just, same. Frustrated a little bit. You know … | 2 | |
Knowing that each cortical site has a specific set of anatomical connections linking it to a broader network of cortical as well as subcortical regions (Parvizi, 2009), the electrical current applied to the aMCC site should not only affect the local activity of the aMCC neuronal populations but also modulate activity in the network of regions connected to it. Reasoning from this perspective, we hypothesized that the complex perceptual and behavioral changes provoked by aMCC stimulation were attributable to functional changes in a large-scale distributed network, which we sought to identify with a resting-state fMRI analysis.
To test our hypothesis, we used seed-based, resting-state connectivity and mapped the resting-state correlations of a single seed region (the anatomical site between electrode contacts 1 and 2) with every other voxel in the brain. As seen in Figure 2, seeding the site of EBS in both patients generated a well-characterized network of regions variably referred to as the emotional salience or the cingulo-opercular network (Dosenbach et al., 2007; Seeley et al., 2007).
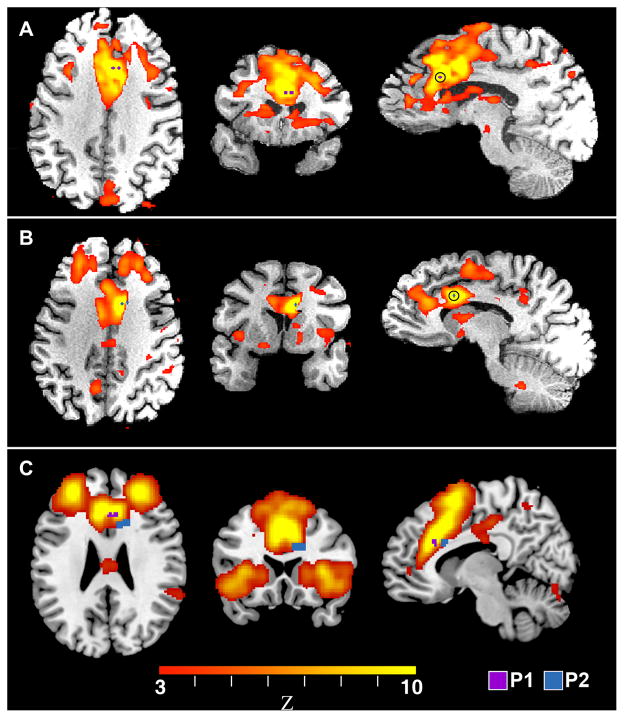
Figure 2. Functional Connectivity Analyses of the Stimulation Sites Identify the EBS Target Sites as Part of the Emotional Salience Network.
Panels A and B show the functional connectivity network derived by seeding a small region-of-interest (ROI) centered between electrodes 1 and 2 in patients P1 and P2, respectively. The ROI is indicated in blue for P1 and purple for P2. These results are presented in standard MNI space with the right side of the image corresponding to the right side of the brain. A goodness-of-fit analysis, comparing the networks in A and B to a set of 14 previously characterized networks, found that each patient’s network best matched the “emotional salience” network. Panel C shows the location of the electrodes in P1 and P2 overlaid onto the standard emotional salience network derived from a group of normal human subjects (Shirer et al., 2012b).
DISCUSSION
Our findings provide a striking subjective account of the feelings associated with the stimulation of the aMCC. Both subjects reported a stereotyped set of changes i.e., increased heart rate, induced physical sensation in the chest or the neck, anticipation of challenge coupled with strong motivation to overcome it (Movie S1). For the sake of simplicity, we refer to this stereotyped set of complex autonomic, emotional, and cognitive experience as the “will to persevere” but we are mindful that the experiential phenomena caused by the aMCC stimulation may be more complex than we have been able to decipher.
Our findings are consistent with the current knowledge of the aMCC function and dysfunction. For instance, neuroimaging studies in human subjects have shown aMCC activation, accompanied by autonomic changes, during the subjective experience of intense affective states such as the anticipation and/or perception of aversive stimuli (References in (Shackman et al., 2011)). Intriguingly, rodents with cingulate lesions involving the homologue of aMCC show reduced ability to endure hardship when attempting to reach a rewarding target. These rodents gave up more easily during a task in which they had to climb over a barrier before reaching a food pellet (Rudebeck et al., 2006).
Our findings are also in line with previous preliminary EBS observations. While electrical stimulation has been reported for several brain regions (Selimbeyoglu and Parvizi, 2010), EBS studies of the ACC are exceedingly uncommon because this area is rarely implanted for clinical reasons. However, Talairach and colleagues (Talairach et al., 1973) conducted a pioneering EBS investigation of the anterior cingulate and reported that stimulation of the anterior to the mid-portion of the cingulate gyrus elicited rubbing of the upper part of the chest and lower part of the neck. They further noted that these responses “were sometimes accompanied by a change in mood or level of consciousness or by autonomic phenomena which included mydriasis, rubefaction of the face, increase in heart rate and increase in respiratory frequency”. Despite the interesting responses observed, the subjective reports of the patients were not detailed and the precise locations of the stimulation sites, within the large mantle of the cingulate gyrus, were not reported.
Taking advantage of imaging technologies, we were able to localize the stimulation site in both of our subjects to the aMCC. However, we are mindful that in EBS, the delivered electrical charge does not remain stationary, but rather travels along the hardwired neuroanatomical pathways connected with the site of stimulation (Borchers et al., 2012; Selimbeyoglu and Parvizi, 2010). Thus, it is reasonable to assume that the perceptual and autonomic changes caused by EBS are due to changes of activity not only in the aMCC but also in the network of brain regions connected with it – including cortical and subcortical nodes of the network.
As an indirect measure of the putative brain network affected by the electrical charge delivery, we used resting-state fMRI data, and localized the target EBS site in both participants as belonging to a network often referred to as the “emotional salience network”. The nomenclature applied to this network is imperfect and varies across different labs. “Salience” here refers not to perceptual salience but rather to “emotional salience”, reflecting the tendency for regions in this network to respond to environmental stimuli of significant valence and to mediate appropriate autonomic changes that will facilitate the proper response (Seeley et al., 2007). While the nomenclature for this resting state network varies across imaging laboratories, the anatomy and functional attributes of this network are quite consistent. The aMCC and the bilateral frontoinsular cortices are the most reliably identified regions and form the core of this network. The bilateral frontal polar regions and temporoparietal junctions are usually identified as well, with more variable demonstration of connected regions in the amygdala, hypothalamus, and brainstem (Seeley et al., 2007). These regions are typically activated in concert with the sympathetic nervous system, by a variety of emotionally, cognitively, or physically salient stimuli (Critchley et al., 2000). Subsequent work has shown that this network is targeted fairly specifically by frontotemporal dementia, a disorder characterized, in part, by apathy and reduced motivation (Seeley et al., 2009; Zhou et al., 2010). It should be noted that anatomical tracing studies in primates have also confirmed hardwired connections between the ACC and some of the sites identified in our resting state analysis (Pandya et al., 1981; Van Hoesen et al., 1993; Vogt and Pandya, 1987). Therefore, the notion that EBS led to changes in this network is based not only on our own functional imaging findings, but also on the classical anatomical tracing data obtained in non-human primates.
Our findings are of clinical relevance given the recent focus on cingulate dysfunction in neuropsychiatric disorders. To take one example, our findings are helpful in interpreting variable therapeutic responses to cingulate surgeries in conditions such as obsessive-compulsive disorder (OCD)(Richter et al., 2004; Sheth et al., 2013). While many patients show measurable therapeutic responses, many others have no response and/or require repeated surgeries. Though there are other possible explanations, it is clear that adjacent regions of the anterior cingulate have distinct functional roles and these variable response profiles might relate to relatively coarse and anatomical targeting. The findings reported here suggest that individualized functional assays for segregating the large mantle of the cingulate gyrus should ultimately allow for safer and more effective therapeutic targeting.
In closing, identifying the subjective correlates of the aMCC stimulation in humans, and the putative brain network that was affected, will have ramifications for future research. If, indeed, “the will to persevere” is mediated by a specific brain region and its neuroanatomical network, it follows that differences in structure and function of this network are associated with innate differences in our abilities to cope with physical or psychological distress. This raises a number of critical questions relating to individual differences in the aMCC function and its associated network: how early can these differences be identified? Are they genetically determined? And to what degree can they be modified by behavioral therapy, medication, or, as suggested here, electrical stimulation?
EXPERIMENTAL PROCEDURES
Intracranial Implantation
In both patients, several depth probes were implanted with lateral-medial trajectory to reach deep brain structures in the medial temporal lobe, ventromedial prefrontal, anterior cingulate and retrosplenial regions. Each probe consisted of 8–10 electrode contacts interspaced 5mm apart. Each electrode contact has a shape of a cylinder with 2.4mm length, 1.3mm diameter, and total surface area of approximately 12mm2.
Electrical Brain Stimulation
Electrical stimulation was delivered in bipolar square waves between two adjacent electrode contacts 1 and 2. Stimulation occurred at 2–8 mA for real trials and 0 mA for control sham trials using a 200μs pulse width at a frequency of 50 Hz. The EEG was simultaneously monitored for after-discharges and seizures and any sessions leading to epileptic activity were excluded. Patients were asked to describe any perceptual or physical changes they experienced during or after each stimulation trial. Patients’ reports were captured on the video and transcribed ad verbatim.
Heart Rate Analysis
Heart rate was monitored simultaneously with the EEG recordings. Trends in heart rate changes were calculated on trial-by-trial basis. After each real or sham trial, heart rate was assessed in a 5-second window. Since EBS and the clinical setting may cause some changes in heart rate throughout the whole procedure, we only compared the median heart rate values for the first 5 seconds after real and sham trials (P1: n=4 real and 2 sham; P2: 4 real and 5 sham). Some of the real EBS trials that were delivered with smaller currents did not cause any subjective changes. We labeled these as sub-threshold EBS trials.
Anatomical MRI acquisition and electrode localization
High-resolution anatomical MRIs were acquired on a GE 3 Tesla scanner at the Center for Cognitive and Neurobiological Imaging, Stanford University. The whole brain scans were attained in 0.9 mm slices axially using a T1-weighted SPGR sequence. Data were resampled to 1 mm voxels. Post-implant CT images were aligned to pre-operative T1 MRIs using Statistical Parametric Mapping V8 software (http://ACC.fil.ion.ucl.ac.uk/spm) in order to correct for surgical brain shift and visualize electrode probe locations. In both patients the electrical stimulation was bipolar between electrode contacts 1 and 2. The MRIs and the electroencephalographic (EEG) traces obtained from these electrodes indicated that contacts 1 and 2 in P1 and contact 1 in P2 were clearly in the gray matter whereas the more proximal contacts were in the white matter.
Resting-state fMRI acquisition
Whole brain pre-operative resting-state fMRI scans were acquired in 30 slices with 4.0 mm isotropic voxels. A 32-channel surface coil (Nova Medical Inc.) was used in the GE EPI sequence (FOV= 100 mm, TR = 2000 ms, TE= 30 ms, flip angle= 77 degrees and bandwidth =127.68 kHz.) Patients were instructed to close their eyes and relax while allowing their minds to wander for the duration of the scan.
Structural MRI Data Analysis
Anatomical data were analyzed with FSL(Smith et al., 2004). First, anatomical images were brain extracted using the Brain Extraction Tool(Smith, 2002). Next, tissue-type segmentation was performed using FAST4(Zhang et al., 2001). The resultant gray matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001), followed by nonlinear registration using FNIRT (Andersson, 2007a; Andersson, 2007b) (FMRIB technical reports TR07JA1 and TR07JA2; available at ACC.fmrib.ox.ac.uk/analysis/techrep), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999).
Resting-State FMRI Data Processing
Data were processed and analyzed using the FMRIB Software Library (FSL: version 4.1): motion correction(Jenkinson et al., 2002); removal of non-brain structures via BET brain extraction of the functional data(Smith, 2002); spatial smoothing with a 6 mm FWHM Gaussian; and high-pass temporal filtering to remove low-frequency signal ( <0.008 Hz) from the data. We aligned the functional data to standard space using a two-level registration using affine linear registration(Jenkinson et al., 2002). The participant’s functional data is first registered to the participant’s high-resolution T1-weighted image. The high-resolution T1-weighted image is then registered to the MNI152 standard space (average T1 brain image constructed from 152 normal subjects at Montreal Neurological Institute (MNI)), and the transformation matrix calculated to register the high-resolution T1-weighted image to standard space is then applied to the T1-aligned functional data. We then regressed the following noise signals from the fMRI data: white matter (time series extracted from a 3-mm radius spherical ROI centered in the WM of the MNI152 standard space atlas, coordinates: 26, −12, 34), cerebral spinal fluid (time series extracted from a 3-mm radius spherical ROI centered in the CSF of the MNI152 standard space atlas, coordinates: 18, −34, 18), head motion time series (measured across six dimensions: lateral, vertical, and horizontal translation, and yaw, pitch, and roll rotation), global signal (time series extracted from a binarized whole brain mask covering the brain matter voxels of the MNI152 standard space atlas), and voxels outside the brain (time series extracted from a binarized mask of all voxels not included in the whole brain mask).
Determining Functional Connectivity
For each participant, a 3-mm spherical ROI was centered between the two electrodes whose stimulation elicited a positive response (P1 coordinates: 12, 18, 26; P2 coordinates: 2, 26, 26). Because of their proximity to the grey-white matter junction, each ROI was masked with the corresponding participant’s standard-space grey matter segmentation. Therefore, any voxels of the 3-mm spherical ROIs that covered the participants’ white matter were excluded. The resulting ROIs were used as subject-specific seeds in separate whole-brain functional connectivity (FC) analyses of the resting-state scans. For each participant, the time series of the ROI was calculated from the resting-state scan by averaging the time series of all voxels within the ROI. The resulting time series was then entered as a covariate of interest in a whole-brain linear regression analysis using the FSL software library. A whole-brain mask was used, so that voxels outside the brain were excluded from this analysis. Contrast images corresponding to this regressor were determined individually for each subject and thresholded at p < 0.001 uncorrected (Fig. 2a and Fig. 2b), producing network connectivity maps for each subject-specific ROIs.
Network identification
The subject-specific network connectivity maps were identified as being the “emotional salience” network using a standard goodness-of-fit analysis (Greicius et al., 2004), in which the networks generated by the FC analysis were compared with an atlas of 14 functional networks (Shirer et al., 2012a). In each case, the algorithm selected the salience network (Fig. 2c) as the best fit (highest spatial correlation).
Supplementary Material
MOVIE S1: Both participants (P1 and P2) gave their consent to release their video files to reviewers of the manuscript at the time of submission, and P1 gave his additional consent to publish his video. Details of P1 and P2’s subjective reports during the EBS procedure are shown in Table 1. Sham trials refer to the trials when the electrical current was set at zero, but the patient thought he was being stimulated.
Highlights.
Electrical stimulation of the aMCC region was performed in 2 subjects.
A stereotyped set of cognitive and autonomic changes was elicited in both subjects.
This included feeling of foreboding combined with strong motivation to persevere.
Stimulation sites were in a network linking aMCC to frontopolar/insular regions.
Acknowledgments
We thank the patients for their involvement in the study ; Stanford Epilepsy Monitoring Unit staff for help with electrophysiological recordings; and Dr. Abbas Milani for his insightful comments about the ramifications of our findings. This research was funded by Stanford NeuroVentures Program, R01 NS078396-01 to JP, and R01 NS073498 to MDG.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JL, Jenkinson M, Smith S. FMRIB technical report TR07JA2. 2007a. Non-linear registration, aka Spatial normalisation. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. FMRIB technical report TR07A1. 2007b. Non-linear optimisation. [Google Scholar]
- Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nature reviews Neuroscience. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural Activity Relating to Generation and Representation of Galvanic Skin Conductance Responses: A Functional Magnetic Resonance Imaging Study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Fictive reward signals in the anterior cingulate cortex. Science. 2009;324:948–950. doi: 10.1126/science.1168488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the Rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Parvizi J. Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn Sci. 2009;13:354–359. doi: 10.1016/j.tics.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Richter EO, Davis KD, Hamani C, Hutchison WD, Dostrovsky JO, Lozano AM. Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery. 2004;54:622–628. doi: 10.1227/01.neu.0000108644.42992.95. discussion 628–630. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in human neuroscience. 2010;4:46. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare W. Prince of Denmark. New York: Penguin Group; 1603. The tragedy of Hamlet. [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR, Eskandar EN, Dougherty DD. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg. 2013;118:491–497. doi: 10.3171/2012.11.JNS12389. [DOI] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012b;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G, Rusu M. The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol. 1973;34:45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Semendeferi K, Fogel BS, Schiffer RB, Rao SM. Functional neuroanatomy of the limbic system and prefrontal cortex. Neuropsychiatry. 1996:113–143. [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA, Gabriel M. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkhauser; 1993. Connections of the Monkey Cingulate Cortex; pp. 249–284. [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Sikes RW. The medial pain system, cingulate cortex, and parallel processing of nociceptive information [Review] [70 refs] Prog Brain Res. 2000;122:223–235. doi: 10.1016/s0079-6123(08)62141-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Farber NB, Paxinos G. The rat nervous system. New York: Elsevier (USA); 2004. Cingulate Cortex and Disease Models; pp. 705–727. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain : a journal of neurology. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MOVIE S1: Both participants (P1 and P2) gave their consent to release their video files to reviewers of the manuscript at the time of submission, and P1 gave his additional consent to publish his video. Details of P1 and P2’s subjective reports during the EBS procedure are shown in Table 1. Sham trials refer to the trials when the electrical current was set at zero, but the patient thought he was being stimulated.