Abstract
Cdc37 is a kinase-associated molecular chaperone whose function in concert with Hsp90 is essential for many signaling protein kinases. Here, we report that mammalian Cdc37 is a pivotal substrate of CK2 (casein kinase II). Purified Cdc37 was phosphorylated in vitro on a conserved serine residue, Ser13, by CK2. Moreover, Ser13 was the unique phosphorylation site of Cdc37 in vivo. Crucially, the CK2 phosphorylation of Cdc37 on Ser13 was essential for the optimal binding activity of Cdc37 toward various kinases examined, including Raf1, Akt, Aurora-B, Cdk4, Src, MOK, MAK, and MRK. In addition, nonphosphorylatable mutants of Cdc37 significantly suppressed the association of Hsp90 with protein kinases, while the Hsp90-binding activity of the mutants was unchanged. The treatment of cells with a specific CK2 inhibitor suppressed the phosphorylation of Cdc37 in vivo and reduced the levels of Cdc37 target kinases. These results unveil a regulatory mechanism of Cdc37, identify a novel molecular link between CK2 and many crucial protein kinases via Cdc37, and reveal the molecular basis for the ability of CK2 to regulate pleiotropic cellular functions.
Protein kinases play pivotal roles in controlling cellular functions by phosphorylating and regulating wide varieties of proteins. Protein kinases are often associated with their regulators and substrates but are also associated with a set of proteins called molecular chaperones. Molecular chaperones are a group of proteins that associate with cellular proteins and assist their proper folding and functions (41). Among many molecular chaperones, protein kinases are most frequently associated with the 90-kDa heat shock protein (Hsp90) and Cdc37. Hsp90 is one of the most abundant molecular chaperones that plays a vast array of roles in cellular function by associating with a wide variety of proteins (6, 8, 41, 57). On the other hand, Cdc37 has been widely accepted as an Hsp90 cochaperone that interacts both physically and genetically with signaling protein kinases (18, 27). In fact, many protein kinases, including Raf (15, 51); Src (38); IKK (7); Cdk4 (10, 22, 53); MOK, MAK, and MRK (32); Aurora-B (23); Cdc28 (12); and Akt (5), have been reported to interact with both Cdc37 and Hsp90 genetically or biochemically.
Cdc37 was originally identified as a candidate gene for cell division cycle mutants in Saccharomyces cerevisiae (43). Cdc37 is an essential protein for viability in yeast (14), in Drosophila melanogaster (9), and in Caenorhabditis elegans (19). Cdc37 was reported to function in tight collaboration with Hsp90 (9), and initially, Cdc37 was proposed to be a “kinase-targeting accessory factor” for Hsp90 (53). Later, Cdc37 was revealed to have molecular chaperone activity per se for certain substrates (20). Moreover, the kinase-interacting domain without the Hsp90-binding region of Cdc37 was shown to be nearly sufficient for its physiological function (24). In addition, it was recently suggested that there are certain Cdc37 targets other than kinases (13, 42, 55). Thus, Cdc37 should obviously perform more tasks than simply functioning as a stable bridge between kinases and Hsp90 (17, 27).
CK2 (casein kinase II) is a ubiquitously expressed and evolutionarily conserved pleiotropic protein kinase that is essential for viability. CK2 is constitutively active, and its activity is independent of either known second messengers or phosphorylation events (39). The levels of CK2 are elevated in a wide variety of tumors, and CK2 can be oncogenic when overexpressed in animals (46), suggesting its important role in cell growth and neoplasia (56). In addition, CK2 has been implicated to be essential for many biological processes such as cell cycle progression (40), proliferation (16), cell survival (2, 26), oncogenic processes (46, 56), and circadian rhythms (3, 25). Various kinds of CK2 substrates both in vitro and in vivo have been reported (29). Surprisingly, even long years after its discovery in the 1950s, molecular mechanisms by which CK2 regulates a vast array of cellular functions remain unknown.
Previously, a genetic observation of yeast suggested an intimate functional relationship between CK2 and Cdc37 (4). Moreover, Shao et al. recently suggested the importance of phosphorylation of Cdc37 for the binding to eIFα kinase in rabbit reticulocyte lysates (48). In this study, we found that Cdc37 is directly phosphorylated by CK2 on highly conserved Ser residue both in vitro and in vivo. The CK2-mediated phosphorylation was essential for the stable interaction of Cdc37 with a vast array of protein kinases and for the recruitment of Hsp90 to Cdc37-kinase complexes. Furthermore, the inhibition of CK2 in vivo diminished the phosphorylation of Cdc37 and thus induced the destabilization of Cdc37 target kinases. Altogether, we revealed functional regulation of Cdc37 by CK2-dependent phosphorylation, suggesting an intriguing possibility that CK2 may influence pleiotropic cellular functions by modulating numerous cellular Cdc37-dependent protein kinases via Cdc37 phosphorylation.
MATERIALS AND METHODS
Proteins and antibodies.
CK2 was purified from porcine testes (33). Anti-FLAG (clone M2), anti-hemagglutinin (HA) (clone 12CA5), and anti-Cdc37(C-19) antibodies were from Sigma, Roche Molecular Biochemicals, and Santa Cruz Biotechnology, respectively. Anti-Hsp90 antibody was described previously (21, 35). Horseradish peroxidase-conjugated secondary antibodies were from Amersham Pharmacia Biotech and Santa Cruz Biotechnology.
Construction of various Cdc37 mutants.
Rat Cdc37 was originally cloned by Ozaki et al. (37), but the reported nucleotide sequence contained apparent errors. We have recloned and resequenced rat Cdc37 cDNA from the same source, and the corrected sequence data have been submitted to the DDBJ, EMBL, and GenBank databases. Various mutants of Cdc37 were produced by in vitro mutagenesis as described below. C-terminal deletion mutants were made by introducing stop codons at indicated sites. For example, Cdc37(N093) encodes amino acids 1 to 93 of Cdc37. N-terminal deletion mutants were constructed by amplifying appropriate regions of Cdc37 by PCR introducing BglII sites at both ends of the fragments. Met (ATG) in the middle of the original Cdc37 sequence was used as a first Met codon for each of the N-terminal deletion mutants. For example, Cdc37(D105) encodes amino acids 106 to 379 (last) of Cdc37, lacking N-terminal amino acids 1 to 105, and the first Met of Cdc37(D105) corresponds to Met(106) of wild-type Cdc37 [Cdc37(WT)]. The whole coding regions were confirmed by direct sequencing for all of the mutants.
Site-directed mutagenesis.
Site-directed in vitro mutagenesis was performed essentially as described previously (30). The sense sequences of mutagenic primers used were 5′-GGAGGAGGAGCGCTAGCAGAGGCTAGG-3′ for Cdc37(N284), 5′-GACAGCCAGCTAGCTGGTTATCTGGTG-3′ for Cdc37(N189), 5′-GCAGCTGCGCTAGCAGGAGCGAAGCTG-3′ for Cdc37(N093), 5′-GATCACATCGAGGTCGCGGACGATGAGGAC-3′ for Cdc37(13SA), and 5′-GATCACATCGAGGTCGACGACGATGAGGAC-3′ for Cdc37(13SD). Sense primers for N-terminal deletion mutants were 5′-AGTCAGATCTATGCGCAAAAAGGAGAAGAACATG-3′ for Cdc37(D105), 5′-AGTCAGATCTATGGAGCAGGTGGCTCACCAGACC-3′ for Cdc37(D206), and 5′-AGTCAGATCTATGAAGGAGTACGAGGAGGAGGAG-3′ for Cdc37(D275), and the antisense primer with a stop codon and a BglII site was 5′-AGTCAGATCTTCACGCACTGATGTCTTTCTCGTT-3′.
For recombinant Cdc37 purification, BglII fragments that contain coding regions of Cdc37 were introduced into a BamHI site of pGEX-6P2 (Amersham Pharmacia) to construct glutathione S-transferase (GST)-Cdc37 fusion proteins. For the expression of Cdc37 in mammalian cells with an HA tag, the BglII fragments were introduced into a BglII site of pcDNA3-HA (30).
Expression plasmids for various kinases.
Plasmids encoding FLAG-tagged MOK, myc-tagged MRK, and MAK were described previously (32). Myc-tagged Aurora-B in SRα was from Toyoshima. An XbaI-BamHI fragment of the mouse Akt1 coding region was ligated into an XbaI-BamHI site of pFLAG-CMV2 (Sigma). An EcoRI fragment of the mouse Cdk4 coding region was ligated into an EcoRI site of pCMV-Tag2B (Stratagene). A BamHI fragment of human Raf1 was ligated into a BamHI site of pFLAG-CMV2. The coding region of chick v-Src was amplified by PCR with v-Src/pBabe-neo used as a template with primers containing a NotI site (sense, 5′-ATGCGCGGCCGCAATGGGGAGTAGCAAGAGCAAG-3′; antisense, 5′-TTTTGCGGCCGCACTACTCAGCGACCTCCAACAC-3′). The obtained NotI fragment was ligated into a NotI site of p3xFLAG-CMV7.1 (Sigma). The coding regions of all these kinases were directly sequenced and checked.
Expression and purification of various mutants of Cdc37 in Escherichia coli.
Expression in E. coli BL21-CodonPlus (DE3) RIL (Stratagene) and purification of various mutants of Cdc37 were performed essentially as described previously (32), with the following modifications. After reaching an A600 level of 0.7, 0.1 mM (final) isopropyl-1-thio-β-d-galactopyranoside was added, and the cultures were shaken for 4 h at 30°C. Final 1% bacterial protease inhibitor cocktail (Sigma) was included in B-PER (Pierce) extraction buffer. After glutathione-Sepharose purification and tag cleavage by protease digestion, eluted Cdc37 proteins were further purified by a ResourceQ ion exchange column (Amersham Pharmacia) with a linear gradient of 0 to 1 M NaCl in 50 mM Tris-Cl, 1 mM EDTA, and 1 mM dithiothreitol, pH 7.4.
In vitro phosphorylation of Cdc37.
Phosphorylation of Cdc37 with CK2 in vitro was performed by incubating purified Cdc37 with purified CK2 for 60 min at 30°C. The final composition of the phosphorylation mixture was 53 mM Tris-Cl, 13 mM HEPES, 0.17 mM EDTA, 0.8 mM dithiothreitol, 2% glycerol, 200 mM NaCl, 17 mM β-glycerophosphate, 1.7 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.67 mM sodium orthovanadate, 10 mM MgCl2, and 40 μM ATP including 0.1 MBq of [γ-32P]ATP, 8 μg of Cdc37, and 40 ng of CK2, pH 7.3.
Expression in mammalian cells and immunoprecipitation.
COS7 cells were cultured and transfected with mammalian expression vectors by electroporation (31), and cell extracts were prepared as described previously (30). Extracts with equal amounts of protein were incubated with appropriate antibodies for 12 h at 4°C with gentle rotation. A suspension of EZView protein G (Sigma) was added, and the mixtures were further rotated for 2 h at 4°C. The immunocomplexes were extensively washed and analyzed as described previously (32).
In vivo labeling of COS7 cells.
Forty-eight hours after transfection of COS7 cells, culture medium was replaced with phosphate-free Dulbecco's modified Eagle's medium containing 10% fetal calf serum and incubated for 4 h. The medium was again replaced with fresh phosphate-free medium, and 1.85 MBq of [33P]orthophosphate (Perkin-Elmer) per 100-mm dish was added. Cells were incubated at 37°C for 6 h with occasional swirling. 33P-labeled cell extracts were prepared as described above, followed by immunoprecipitation.
Inhibition of CK2 in vivo.
4,5,6,7-Tetrabromobenzotriazole (TBB), a specific inhibitor of CK2 (44, 45), was provided by F. Meggio and L. A. Pinna (University of Padua, Padua, Italy). COS7 cells were treated with indicated concentrations of TBB for 16 h. For the determination of the levels of MOK and Raf1, these kinases were expressed by transient transfection, and TBB treatment was started at 36 h after transfection. For the determination of in vivo phosphorylation of Cdc37, HA-Cdc37 was expressed by transient transfection, and TBB treatment was started at 36 h after transfection in phosphate-free medium. The cells were then labeled by adding 1.85 MBq of [33P]orthophosphate per 100-mm dish and incubated 4 h as described above. TBB was left included during the in vivo radiolabeling period.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 10 or 15% acrylamide gels. Western blotting was performed with horseradish peroxidase-conjugated secondary antibodies or peroxidase-conjugated primary anti-tag antibodies, and detection was performed by using the chemiluminescent system as described previously (30). For chemiluminescent detection of alkaline phosphatase-conjugated antibodies, CDP-Star was used according to the manufacturer's protocol (Roche Molecular Biochemicals).
Nucleotide sequence accession number.
Sequence data for rat Cdc37 cDNA have been submitted to the DDBJ, EMBL, and GenBank databases under accession number AB097113.
RESULTS
Phosphorylation of mammalian Cdc37 by CK2 uniquely on Ser13 in vitro.
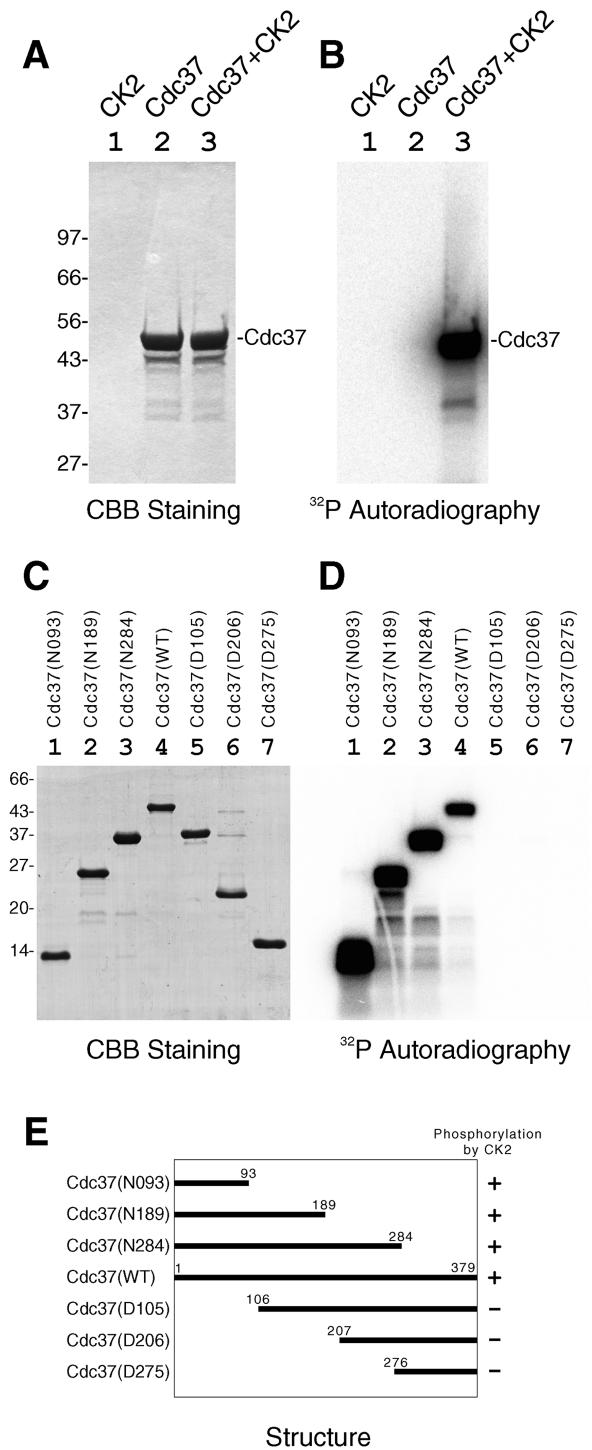
First, we have examined if purified mammalian Cdc37 is phosphorylated by CK2. Recombinant rat Cdc37 was heavily radiolabeled when incubated with [γ-32P]ATP and purified CK2 (Fig. 1A and B, lane 3), indicating that Cdc37 is phosphorylated by CK2 in vitro. Many molecular chaperones possess ATP-dependent autophosphorylation activity, but Cdc37 was not labeled when CK2 was omitted (Fig. 1A and B, lane 2), showing that the observed radiolabeling was produced by CK2-dependent phosphorylation but not by autophosphorylation of Cdc37.
FIG. 1.
Phosphorylation of Cdc37 by CK2 in vitro. Purified recombinant rat Cdc37 was incubated with or without purified CK2 in the presence of radiolabeled ATP. (A and B) The phosphorylation mixtures were analyzed by SDS-PAGE followed by CBB staining (A) or by autoradiography (B). Lane 1, CK2; lane 2, Cdc37; lane 3, CK2 and Cdc37. (C and D) Phosphorylation of various deletion mutants of Cdc37 by CK2 in vitro. Various deletion mutants of recombinant Cdc37 were incubated with CK2 in the presence of radiolabeled ATP in vitro. The phosphorylation mixtures were analyzed by SDS-PAGE followed by CBB staining (C) or autoradiography (D). Deletion mutants used were Cdc37(N093) (lane 1) Cdc37(N189) (lane 2), Cdc37(N284) (lane 3), Cdc37(WT) (lane 4), Cdc37(D105) (lane 5), Cdc37(D206) (lane 6), and Cdc37(D275) (lane 7). (E) The structures of Cdc37 deletion mutants and their phosphorylation by CK2 are schematically illustrated with numbers of amino acids. +, phosphorylated by CK2; −, not phosphorylated by CK2.
To determine the CK2-mediated phosphorylation site(s) within Cdc37, we made a series of N-terminal and C-terminal deletion mutants of Cdc37. All of the recombinant Cdc37 deletion mutants migrated at positions with expected molecular masses (Fig. 1C). The C-terminal deletion mutants Cdc37, Cdc37(N284), Cdc37(N189), and Cdc37(N093) were well phosphorylated by CK2, like Cdc37(WT) (Fig. 1D, lanes 1 to 4). On the other hand, the N-terminal deletion mutants Cdc37(D105), Cdc37(D206), and Cdc37(D275) were not phosphorylated by CK2 (Fig. 1D, lanes 5 to 7). The results, which were schematically summarized in Fig. 1E, clearly indicate that the phosphorylation site(s) of Cdc37 by CK2 locates in the N-terminal region of Cdc37, amino acids 1 to 93.
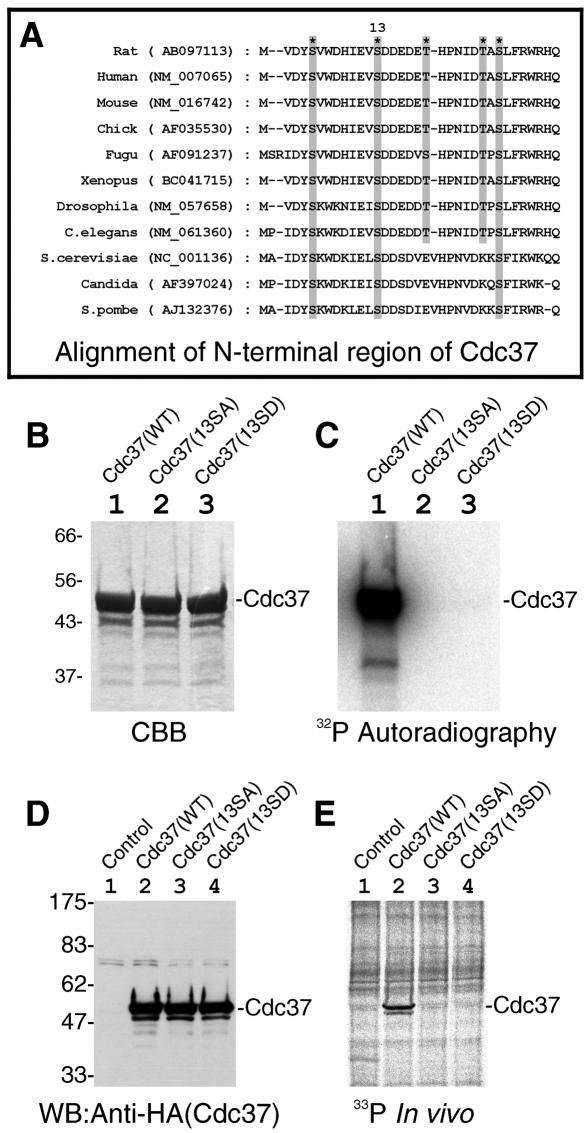
The N-terminal region (amino acids 1 to 93) of rat Cdc37 contains 3 serines and 2 threonines. All of them are clustered in the most N-terminal region of Cdc37 (amino acids 1 to 30), and this region shows the highest conservation between species (Fig. 2A). One of these serines, Ser13 of rat Cdc37 (Fig. 2A), is followed by a conserved stretch of acidic amino acids, making Ser13 a consensus sequence motif for CK2 phosphorylation. To show that Ser13 of Cdc37 is the CK2 phosphorylation site, we made two point mutants in which Ser13 was changed into Ala [Cdc37(13SA)] or Asp [Cdc37(13SD)]. As shown in Fig. 2C, CK2 phosphorylated Cdc37(WT) (lane 1) but not Cdc37(13SA) (lane 2) or Cdc37(13SD) (lane 3), indicating that Ser13 of Cdc37 is the sole phosphorylation site by CK2 in vitro.
FIG. 2.
Mutation in conserved Ser13 of Cdc37 abolished the phosphorylation of Cdc37 by CK2. (A) The alignment of amino acid sequences from N-terminal regions of Cdc37 for various species is shown. The positions of three serines and two threonines are shown by shaded boxes and asterisks. A CK2 target phosphorylation site is indicated as “13” according to the amino acid number of rat Cdc37. The names of the species are shown on the left with their GenBank accession numbers in parentheses. (B and C) Effect of Ser13 mutations of Cdc37 on the CK2-mediated phosphorylation in vitro. Recombinant Cdc37 and its Ser13 mutants were incubated with CK2, and the phosphorylation mixtures were analyzed by SDS-PAGE followed by CBB staining (B) or autoradiography (C). Lane 1, Cdc37(WT); lane 2, Cdc37(13SA); lane 3, Cdc37(13SD). (D and E) Mutations on Ser13 abolished the phosphorylation of Cdc37 in vivo. The equal expression of Cdc37 and its mutants in cells was shown by Western blotting of cell extracts with anti-HA antibody (D). The incorporation of phosphate in vivo into Cdc37 was visualized by 33P autoradiography after immunoprecipitation (E). Lane 1, control (nontransfected); lane 2, Cdc37(WT); lane 3, Cdc37(13SA); lane 4, Cdc37(13SD).
Ser13 is the unique phosphorylation site of Cdc37 in mammalian cells.
Next, we examined whether the same site is phosphorylated in vivo. Cdc37(WT), Cdc37(13SA), or Cdc37(13SD) with an HA tag was expressed in COS7 cells. The expression levels of both mutants were essentially the same as that of Cdc37(WT) as determined by Western blotting of cell extracts (Fig. 2D). Transfected cells were labeled with [33P]orthophosphate, and Cdc37 was immunoprecipitated with anti-HA antibody. The immunocomplexes were analyzed by SDS-PAGE and 33P autoradiography. As shown in Fig. 2E, Cdc37(WT) was indeed phosphorylated in vivo (lane 2). On the contrary, neither Cdc37(13SA) nor Cdc37(13SD) was phosphorylated at all (Fig. 2E, lanes 3 and 4). The results clearly indicate that Ser13 is the unique phosphorylation site of Cdc37 in mammalian cells and strongly suggest that Cdc37 is phosphorylated in vivo by CK2.
Protein kinase domain of MOK is essential for the stable association with Cdc37.
We then investigated the functional importance of the phosphorylation of Cdc37 by CK2. The principal physiological function of Cdc37 is its ability to interact with protein kinases. As a Cdc37 target kinase, we first chose MOK, a member of the MAP kinase superfamily (30). The association of MOK with Cdc37 and Hsp90 was reported previously (32). MOK consists of an N-terminal kinase domain and a C-terminal tail without a known functional motif. We have determined the region of MOK that is responsible for the stable association with Cdc37. A series of deletion mutants of MOK were expressed in COS7 cells and immunoprecipitated. Almost equal amounts of the wild type and deletion mutants of MOK, except MOK(N107), were expressed and immunoprecipitated as shown by Western blotting (Fig. 3A). The unstable characteristic of MOK(N107) was previously described (32). The binding of coimmunoprecipitated endogenous Cdc37 was revealed by Western blotting (Fig. 3B). Wild-type MOK (lane 2) and MOK(N285) (lane 5) were strongly associated with Cdc37, and MOK(N178) was associated with Cdc37 moderately (lane 4). None of the N-terminal deletion mutants, including MOK(D8-078), MOK(D8-195), or MOK(D8-309), was associated with Cdc37 (Fig. 3B, lanes 6 to 8). The results, which were schematically summarized in Fig. 3C, clearly indicate that the C-terminal tail region of MOK was dispensable for Cdc37 binding, whereas even the shortest deletion in the N-terminal protein kinase region abolished the Cdc37 association. In other words, the protein kinase domain of MOK is essential for the stable association with Cdc37.
FIG. 3.
Protein kinase domain of MOK was essential for the binding of Cdc37. Various MOK deletion mutants with a FLAG tag were expressed in COS7 cells and immunoprecipitated. (A) Amounts of MOK and its mutants in the immunoprecipitates were examined by Western blotting. Bands corresponding to deletion mutant proteins are indicated by asterisks [except MOK(N107) (see the text)]. Lane 1, control (not transfected); lane 2, MOK(WT); lane 3, MOK(N107); lane 4, MOK(N178); lane 5, MOK(N285); lane 6, MOK(D8-078); lane 7, MOK(D8-195); lane 8, MOK(D8-309). (B) Associations of endogenous Cdc37 with the same set of MOK deletion mutants as in panel A were examined by coimmunoprecipitation experiments and revealed by Western blotting with anti-Cdc37 antibody. Lane marks are the same as those for panel A. The positions of Cdc37, immunoglobulin heavy-chain (HC), immunoglobulin light-chain (LC), and molecular weight markers are indicated. (C) The relationship between structures and the Cdc37 binding abilities of MOK deletion mutants is schematically illustrated. ++, binds strongly; +, binds well; −, no binding.
Ser13 of Cdc37 is essential for the association of Cdc37 with MOK.
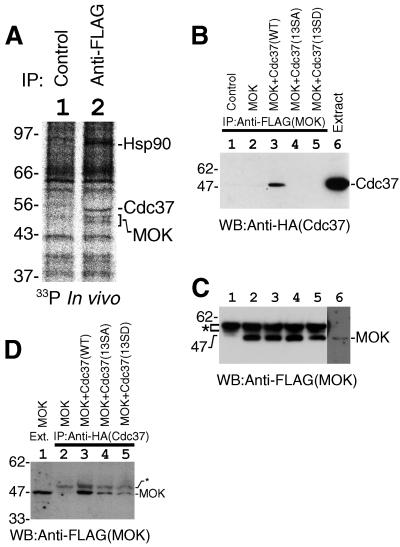
It is important to know whether endogenous Cdc37 is phosphorylated in vivo and whether the phosphorylation of Ser13 by CK2 regulates the protein kinase binding activity of Cdc37. Cells expressing FLAG-tagged MOK were labeled with [33P]orthophosphate. MOK was immunoprecipitated, and the phosphorylation of endogenous proteins associated with MOK was analyzed. The identification of all MOK-associated protein bands was precisely described previously (32). As shown in Fig. 4A, Hsp90 and Cdc37 were specifically immunoprecipitated with MOK, and all three proteins were phosphorylated in vivo (lane 2; compare with lane 1). This result clearly indicates that endogenous Cdc37 is a phosphoprotein in vivo, and the phosphorylated form of Cdc37 possesses the protein kinase binding activity. However, from this experiment, we couldn't determine whether the phosphorylation of Cdc37 on Ser13 is necessary for the protein kinase binding.
FIG. 4.
The CK2-phosphorylated form of Cdc37 was active in protein kinase binding. (A) Association of the endogenous phosphorylated form of Cdc37 and Hsp90 with MOK in vivo. FLAG-MOK and its associated proteins were isolated by immunoprecipitation from radiolabeled cells. Lane 1, control; lane 2, immunoprecipitation with anti-FLAG antibody. The positions of Hsp90, Cdc37, and MOK are shown. (B) Disruption of the CK2 phosphorylation site of Cdc37 abolished the association of Cdc37 with MOK. FLAG-MOK was expressed in COS7 cells and immunoprecipitated with anti-FLAG antibody. The association of Cdc37 was examined by Western blotting (WB) of the immunoprecipitates. Lane 1, control; lane 2, MOK only; lane 3, MOK plus Cdc37(WT); lane 4, MOK plus Cdc37(13SA); lane 5, MOK plus Cdc37(13SD). Cell extract was included in lane 6 to show the migration position of Cdc37. (C) The equality of immunoprecipitated MOK was confirmed by Western blotting of the immunoprecipitates with anti-FLAG antibody. Lane marks are the same as those for panel B. The position of cross-reacted immunoglobulin heavy-chain bands is shown by an asterisk. The position of MOK was revealed in lane 6 by longer exposure. (D) Ser13 of Cdc37 is important for the stable association with MOK. Cdc37 or its mutants were immunoprecipitated and the association of MOK was examined by Western blotting. In lane 1, extract of cells was included to show the migration position of MOK; lane 2, MOK only; lane 3, MOK plus Cdc37(WT); lane 4, MOK plus Cdc37(13SA); lane 5, MOK plus Cdc37(13SD). Positions of MOK and faint nonspecific bands (asterisk) are indicated on the right.
To find a clue to the regulation of Cdc37 by CK2, we examined the effect of mutations in the CK2 phosphorylation site on Cdc37 function. FLAG-MOK was expressed in COS7 cells concomitantly with Cdc37(WT), Cdc37(13SA), or Cdc37(13SD). The association of Cdc37 with MOK was examined by coimmunoprecipitation experiments. As is the case for endogenous Cdc37 (Fig. 3) (32), expressed HA-Cdc37 bound specifically to MOK (Fig. 4B, lane 3). Importantly, the association of nonphosphorylatable Cdc37 with MOK was markedly low compared to that of Cdc37(WT) (Fig. 4B, lanes 4 and 5). The amounts of immunoprecipitated MOK were equal for all of the immunoprecipitates (Fig. 4C, lanes 2 to 5). This result indicates that Ser13 is required for MOK binding activity of Cdc37. The same conclusion was obtained from a reverse coimmunoprecipitation experiment in which Cdc37 was immunoprecipitated and the association of MOK was examined by Western blotting (Fig. 4D; compare lane 3 with lanes 4 and 5).
Ser13 of Cdc37 is essential for the association of Cdc37 with Raf1.
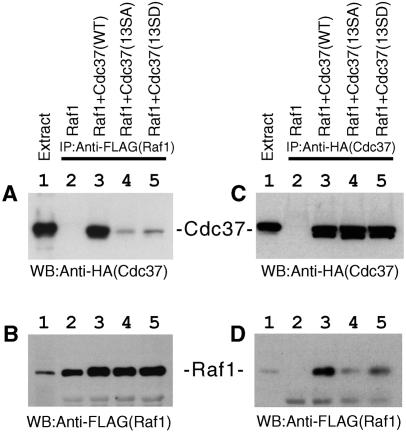
Next, we examined the effect of Ser13 mutations on the association of Cdc37 with Raf1, which belongs to the MAP kinase kinase kinase family. Raf1 was immunoprecipitated from cell lysates, and the associated Cdc37 was detected by Western blotting. The Raf1-binding activity of Cdc37 was dramatically diminished when Ser13 was mutated to Ala or Asp (Fig. 5A, compare lane 3 with lanes 4 and 5). The amount of Raf1 was the same for all of the immunoprecipitates (Fig. 5B, lanes 3 to 5). The same conclusion was obtained from a reverse coimmunoprecipitation experiment in which Cdc37 was immunoprecipitated and the association of Raf1 was examined by Western blotting. Whereas equal amounts of Cdc37 were immunoprecipitated (Fig. 5C), the association of Raf1 was diminished by Ser13 mutations of Cdc37 (Fig. 5D). These results indicate that Ser13 is required for the Raf1-binding activity of Cdc37. In these experiments for MOK and Raf1, the mutation of Ser to Asp did not make Cdc37 constitutively active (Fig. 4 and 5, lane 5), indicating that simply introducing a negatively charged amino acid does not mimic phosphorylation of Cdc37.
FIG. 5.
Disruption of the CK2 phosphorylation site of Cdc37 abolished the association of Cdc37 with Raf1. The binding of Raf1 to Cdc37 was examined by coimmunoprecipitation experiments. (A) Raf1 was immunoprecipitated from cotransfected cell lysates, and the association of Cdc37 was revealed by anti-HA Western blotting (WB). Lane 1, cell extract; lane 2, Raf1 only; lane 3, Raf1 plus Cdc37(WT); lane 4, Raf1 plus Cdc37(SA); lane 5, Raf1 plus Cdc37(SD). (B) The equal amount of Raf1 was immunoprecipitated from cotransfected cell lysates. Lane marks are the same as those for panel A. (C) Cdc37 or its mutants were immunoprecipitated from cotransfected cell lysates. The amounts of immunoprecipitated Cdc37 were revealed by Western blotting with anti-HA antibody. Lane marks are the same as those for panel A. (D) Cdc37 or its mutants were immunoprecipitated, and the amounts of Cdc37-associated Raf1 were examined by Western blotting with anti-FLAG antibody. Lane marks are the same as those for panel A.
Ser13 of Cdc37 is important for the recruitment of Hsp90 to protein kinase-Cdc37 complexes.
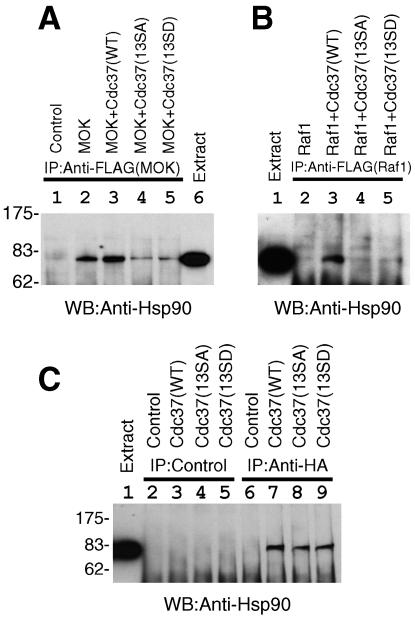
Cdc37 has been reported to have a function to recruit Hsp90 to certain protein kinases (15, 18, 27, 53). We thus examined the effect of Ser13 mutations of Cdc37 on Hsp90 recruitment to kinase complexes. MOK or Raf1 was expressed and immunoprecipitated, and the amounts of endogenous Hsp90 in the kinase immunocomplexes were analyzed by Western blotting. A specific association of Hsp90 with MOK-Cdc37(WT) complexes (Fig. 6A) or with Raf1-Cdc37(WT) complexes (Fig. 6B) was observed (lane 3). The association of Hsp90 with MOK or Raf1 was severely impaired when the CK2 phosphorylation site of Cdc37 was mutated (Fig. 6A and B, compare lane 3 with lanes 4 and 5). The amounts of immunoprecipitated MOK or Raf1 were equal for all of the immunoprecipitates (Fig. 4C and 5B). The results suggested that the CK2-dependent phosphorylation site Ser13 of Cdc37 is important for the recruitment of Hsp90 to protein kinase-Cdc37 complexes.
FIG. 6.
Phosphorylation of Ser13 of Cdc37 by CK2 is important for the Hsp90 recruitment, but not for the Hsp90 binding, of Cdc37. (A) Ser13 mutations of Cdc37 diminished the association of Hsp90 with MOK. MOK and Cdc37 were cotransfected, and MOK was immunoprecipitated. The association of endogenous Hsp90 with Cdc37-MOK complexes was examined by anti-Hsp90 Western blotting (WB). Lane 1, control; lane 2, MOK only; lane 3, MOK plus Cdc37(WT); lane 4, MOK plus Cdc37(SA); lane 5, MOK plus Cdc37(13SD); lane 6, cell extract to show the position of Hsp90. (B) Ser13 mutations of Cdc37 abolished the association of Hsp90 with Raf1. The association of Hsp90 with Cdc37-Raf1 complexes was examined by anti-Hsp90 Western blotting of Raf1 immunoprecipitates. Lane 1, cell extract; lane 2, Raf1 only; lane 3, Raf1 plus Cdc37(WT); lane 4, Raf1 plus Cdc37(13SA); lane 5, Raf1 plus Cdc37(13SD). (C) Phosphorylation on Ser13 of Cdc37 was not important for Hsp90-binding activity of Cdc37. Cdc37(WT) (lanes 3 and 7), Cdc37(13SA) (lanes 4 and 8), or Cdc37(13SD) (lanes 5 and 9) with an HA tag was expressed in COS7 cells and immunoprecipitated with control antibody (lanes 2 to 5) or with anti-HA antibody (lanes 6 to 9). Nontransfected cells were used as controls (lanes 2 and 6). The coimmunoprecipitation of Hsp90 with Cdc37 was examined by anti-Hsp90 Western blotting. Cell extract was included in lane 1 to show the migration position of Hsp90. The positions of molecular weight markers are shown on the left.
The reduction of the Hsp90 recruitment activity of Cdc37 by Ser13 mutations observed as described above might be a consequence of less Hsp90-binding activity of Ser13 mutants of Cdc37. We directly examined the Hsp90-binding activity of Cdc37 by coimmunoprecipitation experiments. Cdc37 was immunoprecipitated, and the association of endogenous Hsp90 was revealed by Western blotting. We observed that the binding of Cdc37 to Hsp90 was not affected by the Ser13 mutations (Fig. 6C, lanes 7 to 9). Thus, it is concluded that nonphosphorylatable Cdc37 mutants can bind Hsp90 but cannot fetch Hsp90 for protein kinases as a result of the reduced kinase binding of Cdc37.
Inhibition of CK2 in vivo decreased the intracellular amounts of Cdc37-dependent protein kinases.
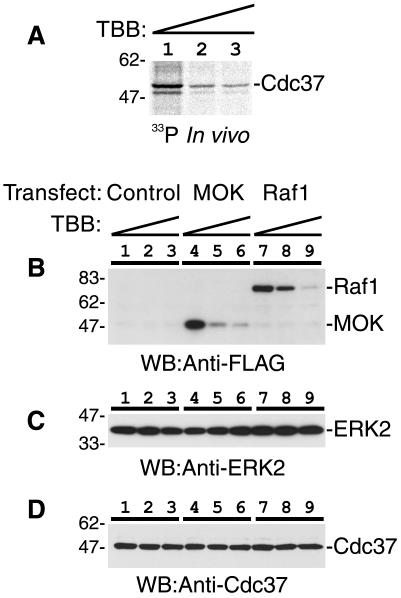
Next, we examined whether specific inhibition of CK2 in vivo suppresses the Cdc37 phosphorylation and reduces the amounts of protein kinases that are dependent on the molecular chaperone activity of Cdc37. To inhibit CK2 activity in vivo, we treated cells with TBB, a specific chemical inhibitor of CK2. Specificity and effective doses of TBB on CK2 activity both in vivo and in vitro were previously precisely described (44, 45). COS7 cells were treated with increasing concentrations of TBB for 16 h and then labeled with [33P]orthophosphate. In vivo phosphorylation of Cdc37 was determined by immunoprecipitation of Cdc37 followed by SDS-PAGE and autoradiography. As shown in Fig. 7A, the phosphorylation of Cdc37 in vivo was dramatically suppressed by the inhibition of CK2. The amount of Cdc37 was not affected by TBB treatment (Fig. 7D), indicating that the net phosphorylation of Cdc37 was suppressed by CK2 inhibition. This result clearly indicates that CK2 is mainly responsible for the in vivo phosphorylation of Cdc37; however, we could not exclude a possibility that a minor part of Cdc37 might be phosphorylated by other protein kinases in vivo.
FIG. 7.
Inhibition of CK2 in vivo induced dephosphorylation of Cdc37 and destabilization of Cdc37 target kinases. (A) Suppression of Cdc37 phosphorylation in vivo by CK2 inhibition. Cells were treated with 0 (lane 1), 66 (lane 2), or 166 (lane 3) μM TBB for 16 h. The phosphorylation of Cdc37 in vivo was determined by immunoprecipitation followed by SDS-PAGE and autoradiography. (B) Inhibition of CK2 in vivo induced destabilization of Cdc37 target kinases MOK and Raf1. MOK or Raf1 was expressed in COS7 cells, and the amounts of MOK (lanes 4 to 6) and Raf1 (lanes 7 to 9) after treatment of cells with 0 (lanes 1, 4, and 7), 66 (lanes 2, 5, and 8), or 166 (lanes 3, 6, and 9) μM TBB for 16 h were determined by Western blotting (WB). (C and D) The amounts of ERK2 (C) and Cdc37 (D) were determined by Western blotting with corresponding antibodies. Lane marks are the same as those for panel B. The positions of molecular weight markers (left) and corresponding proteins (right) are shown.
Molecular chaperone activities are required for intracellular stability of many signaling kinases. Next, we determined the amounts of protein kinases whose expression levels are dependent on Hsp90/Cdc37 function. COS7 cells expressing MOK or Raf1 were treated with CK2 inhibitor TBB, and then the amounts of MOK and Raf1 in cells were determined by Western blotting. As shown in Fig. 7B, MOK and Raf1 were dramatically diminished by TBB treatment in a dose-dependent manner. The amounts of ERK2 were not affected by TBB treatment (Fig. 7C), suggesting that the effect of TBB was specific for functional Cdc37-dependent protein kinases such as MOK and Raf1. This result clearly shows that CK2-dependent phosphorylation of Cdc37 is necessary for the chaperone activity of Cdc37 that is required for the stable existence of target kinases. Moreover, the result directly indicates that CK2 controls the intracellular levels of Cdc37-dependent protein kinases, and the control mechanism may well be explained by the CK2-dependent phosphorylation of Cdc37 on Ser13 that is a prerequisite for proper protein kinase binding activity of Cdc37.
CK2 phosphorylation of Cdc37 is essential for the optimal binding of Cdc37 toward various protein kinases.
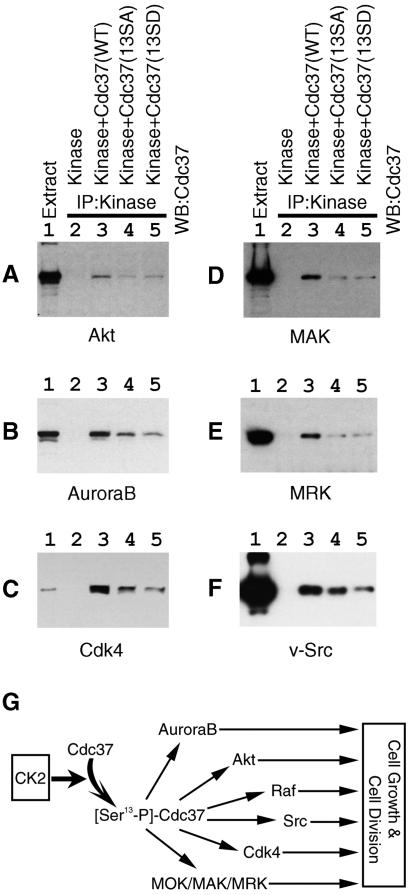
Since CK2 plays roles in cell division, cell cycle progression, and cell survival, we postulated that the regulation of Cdc37 by CK2 could generally be observed for Cdc37 target kinases other than MOK and Raf1. Finally, we set up a system to examine the specific association of Cdc37(WT) with various protein kinases by coimmunoprecipitation (Fig. 8A to F, lane 3). Very importantly, in this system, nonphosphorylatable forms of Cdc37 showed lower binding activities toward all of these kinases examined, including Akt, Aurora-B, Cdk4, MAK, MRK, and v-Src (Fig. 8, lanes 4 and 5), than that of Cdc37(WT) (lane 3). Thus, we concluded that CK2 phosphorylates Cdc37 on Ser13 and up-regulates the chaperone activity of Cdc37 to target protein kinases universally, thus affecting pleiotropic downstream cellular functions (Fig. 8G).
FIG. 8.
The CK2 phosphorylation site of Cdc37 is essential for efficient binding to multiple signaling protein kinases. Protein kinases were expressed in COS7 cells with Cdc37(WT), Cdc37(13SA), or Cdc37(13SD). Protein kinases were immunoprecipitated, and the binding of Cdc37 was revealed by Western blotting. (A) Akt; (B) Aurora-B; (C) Cdk4; (D) MAK; (E) MRK; (F) v-Src. Lane 1, cell extract to show the position of Cdc37; lane 2, control (kinase only); lane 3, kinase plus Cdc37(WT); lane 4, kinase plus Cdc37(SA); lane 5, kinase plus Cdc37(SD). (G) A schematic illustration of the expanding signal transduction from CK2 to multiple protein kinases via Cdc37 is shown.
DISCUSSION
The specific activities of many protein kinases are controlled by various mechanisms such as activating phosphorylation and association with regulatory subunits. In addition, the amounts of protein kinases in cells should be strictly controlled by regulating the balance between their synthesis and degradation. The intimate collaboration between Cdc37 and Hsp90 in the quality and quantity control of protein kinases has been revealed, both genetically and biochemically (9, 20, 53). Initially Cdc37 was simply considered a kinase-targeting subunit for Hsp90; however, recent advances disclosed that the relationship among Cdc37, Hsp90, and kinases is more complicated than was previously believed (27). In this study, we have clearly shown that phosphorylation by CK2 regulates the function of Cdc37. The results suggest that Cdc37 is not a static bridge or glue but is rather a dynamic functional component of protein kinase complexes.
We observed that CK2-dependent phosphorylation of Cdc37 regulates the protein kinase-binding activity, but not the Hsp90-binding activity of Cdc37. We identified the phosphorylation site as Ser13 in the N-terminal region of Cdc37 both in vivo and in vitro. Previously, it was proposed that the N-terminal half of Cdc37 is responsible for the kinase binding, while the middle region of Cdc37 is required for the Hsp90-binding activity of Cdc37 (15, 47, 49, 50). This domain mapping agrees well with our present results that the modification of Cdc37 at the N-terminal extremity affected only the protein kinase binding activity of Cdc37. The mutation in Ser13 completely abolished the phosphorylation of Cdc37 in COS7 cells (Fig. 2E), indicating that no other site of Cdc37 is phosphorylated in vivo. It should be interesting to see whether phosphorylation of other sites of Cdc37 by other kinases can be observed in certain types of cells or after triggering cells by certain stimulations, such as growth factor treatment. Previously, it was reported that CK2 binds and phosphorylates Hsp90 (33, 34). In addition, CK2 phosphorylates another Hsp90 cochaperone, FKBP52, and disrupts the association between FKBP52 and Hsp90 (31). Most of the steroid hormone receptor complexes contain Hsp90 and FKBP52 but not Cdc37, while protein kinase complexes include Hsp90 and Cdc37 but not FKBP52. Altogether, the chaperone complexes seem to be dynamically regulated by phosphorylation and dephosphorylation.
Although the association of Cdc37 and Hsp90 with protein kinases has been well documented, a stereoscopic view of the complexes has not yet been clarified. In this report, we determined that the protein kinase domain of MOK is responsible for the binding of Cdc37. This has been reported to also be the case for Raf1 kinase (15). In addition, the protein kinase domain of Raf1 (51) and MOK (32) is essential for the Hsp90 binding. Thus, Hsp90 and Cdc37 may both bind to the protein kinase domain independently. The other possibility is that the associations of Cdc37 and Hsp90 to protein kinases are mutually interdependent or cooperative; i.e., the binding of Cdc37 or Hsp90 to the protein kinase domain is a prerequisite for the efficient association of the other. In this paper, we have observed that the disruption of the protein kinase binding activity of Cdc37 by Ser13 mutations inhibited the association of Hsp90 with kinases, whereas the association between Hsp90 and Cdc37 was not affected. The simplest explanation for this finding is that Cdc37 bridges between kinases and Hsp90; however, the precise structural analysis of the complexes will be required to solve the stereoscopic relationship among Cdc37, HSP90, and kinases.
CK2 is one of the most conserved protein kinases (16), and the CK2 phosphorylation site of Cdc37 is evolutionarily conserved in all eukaryotic species (Fig. 2A). Thus, the regulation of Cdc37 by CK2-mediated phosphorylation should be conserved from S. cerevisiae to human. In fact, a yeast strain with the cdc37-34 allele was reported to encode a Cdc37 mutant where Ser14 of Cdc37 (corresponding to Ser13 of mammalian Cdc37) was replaced by leucine, and this mutation was reported to cause growth arrest at a nonpermissive temperature (11). The cdc37-34 allele was also shown to be defective in the activation of the MAP kinase kinase kinase family member Ste11 (1) and that of the tyrosine kinase v-Src (11). Bandhakavi et al. reported the identification of Cdc37 as a multicopy suppressor of a temperature-sensitive allele of CK2 in yeast and analyzed their genetic interaction in detail (4). All of these genetic observations in yeast can well be explained by our present finding showing that CK2-dependent phosphorylation of Ser13 is of conserved and physiological importance for the molecular chaperone activity of Cdc37. Shao et al. reported that Ser13 of Cdc37 is phosphorylated and the mutation of Ser13 disrupts Cdc37 recruitment to complexes between Hsp90 and heme-regulated eIF2α kinase in rabbit reticulocyte lysate (48). Thus, in combination with our data for MOK, Raf1, Akt, Aurora-B, Cdk4, MAK, MRK, and v-Src, it is natural to consider that all of the kinases that are targets for Cdc37 should be regulated by CK2-dependent phosphorylation of Cdc37 on Ser13 in various types of cells.
Many cellular functions modulated by CK2 have been described (2, 16, 26). Our results here identify a regulatory mechanism of molecular chaperone Cdc37 by CK2. This implicates a novel type of expanding signaling in which multiple kinases are regulated indirectly by CK2 via Cdc37 (Fig. 8G). The number of signaling protein kinases and other functional molecules that are dependent on Cdc37 has been increasing (27). Thus, we suggest an intriguing possibility that CK2 influences pleiotropic cellular functions by modulating numerous cellular Cdc37-dependent protein kinases by phosphorylating Ser13 of Cdc37. The enhanced activity of CK2 induces increased phosphorylation of Cdc37, which is essential for the chaperone activity of Cdc37 toward a variety of targets, leading to higher stabilities and activities of many protein kinases whose functions are dependent on functional Cdc37. Consequently, Cdc37 target kinases can be fully activatable by appropriate specific activators. This may be a reason why CK2 is apparently involved in many cellular signaling systems. A large number of cellular substrates that are phosphorylated by CK2 have been reported (29), and substrates other than Cdc37 should also be involved in the pleiotropy of physiological roles of CK2.
It is notable that the overexpression of Cdc37 (52, 54) or CK2 (56) can be oncogenic in mice. As the list of Cdc37-dependent proteins includes many tumorigenic kinases, such as Src, Raf1, and Akt, higher activity of CK2 would indirectly support the neoplastic activity of these protein kinases via Cdc37. The inhibition of multiple Hsp90-dependent kinases by a specific inhibitor, geldanamycin, has been demonstrated to be effective for anticancer chemotherapy (28, 36). Thus, direct inhibition of Cdc37, or inhibition of Cdc37 indirectly by inactivating CK2, can be a possible way to suppress multiple oncogenic target kinases simultaneously to achieve an anticancer effect.
Acknowledgments
We thank T. Aoki and M. Nakagawa for excellent technical assistance, L. A. Pinna and F. Meggio for providing CK2 inhibitor TBB, M. MacLean and D. Picard (Université de Genève) for sending us an excellent review on Cdc37 before publication, Y. Kimura (Tokyo Metropolitan Institute of Medical Science) for discussion and suggestion, and F. Toyoshima (Kyoto University), C. Sherr (Howard Hughes Medical Institute and St. Jude Children's Research Hospital), M. Shibuya (University of Tokyo), Y. Ikawa (Tokyo Medical and Dental University/RIKEN), S. Hattori (University of Tokyo), and H. Sabe (Osaka Bioscience Institute) for cDNA of Aurora-B, Cdk4, MAK, MRK, Raf1, and v-Src, respectively.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, and D. Picard. 2000. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 467:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, K., D. A. Gerber, and C. Cochet. 2002. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 12:226-230. [DOI] [PubMed] [Google Scholar]
- 3.Akten, B., E. Jauch, G. K. Genova, E. Y. Kim, I. Edery, T. Raabe, and F. R. Jackson. 2003. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 6:251-257. [DOI] [PubMed] [Google Scholar]
- 4.Bandhakavi, S., R. McCann, D. Hanna, and C. Glover. 2003. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J. Biol. Chem. 278:2829-2836. [DOI] [PubMed] [Google Scholar]
- 5.Basso, A. D., D. B. Solit, G. Chiosis, B. Giri, P. Tsichlis, and N. Rosen. 2002. Akt forms an intracellular complex with Hsp90 and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 277:39858-39866. [DOI] [PubMed] [Google Scholar]
- 6.Buchner, J. 1999. Hsp90 & co.—a holding for folding. Trends Biochem. Sci. 24:136-141. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G., P. Cao, and D. V. Goeddel. 2002. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9:401-410. [DOI] [PubMed] [Google Scholar]
- 8.Csermely, P., T. Schnaider, C. Soti, Z. Prohaszka, and G. Nardai. 1998. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 79:129-168. [DOI] [PubMed] [Google Scholar]
- 9.Cutforth, T., and G. M. Rubin. 1994. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77:1027-1036. [DOI] [PubMed] [Google Scholar]
- 10.Dai, K., R. Kobayashi, and D. Beach. 1996. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 271:22030-22034. [DOI] [PubMed] [Google Scholar]
- 11.Dey, B., J. J. Lightbody, and F. Boschelli. 1996. CDC37 is required for p60v-src activity in yeast. Mol. Biol. Cell 7:1405-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell, A., and D. O. Morgan. 2000. Cdc37 promotes the stability of protein kinases Cdc28 and Cak1. Mol. Cell. Biol. 20:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fliss, A. E., Y. Fang, F. Boschelli, and A. J. Caplan. 1997. Differential in vivo regulation of steroid hormone receptor activation by Cdc37p. Mol. Biol. Cell 8:2501-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber, M. R., A. Farrell, R. J. Deshaies, I. Herskowitz, and D. O. Morgan. 1995. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc. Natl. Acad. Sci. USA 92:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grammatikakis, N., J. H. Lin, A. Grammatikakis, P. N. Tsichlis, and B. H. Cochran. 1999. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 19:1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra, B., and O. G. Issinger. 1999. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20:391-408. [DOI] [PubMed] [Google Scholar]
- 17.Hartson, S. D., A. D. Irwin, J. Shao, B. T. Scroggins, L. Volk, W. Huang, and R. L. Matts. 2000. p50cdc37 is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry 39:7631-7644. [DOI] [PubMed] [Google Scholar]
- 18.Hunter, T., and R. Y. C. Poon. 1997. Cdc37: a protein kinase chaperone? Trends Cell Biol. 7:157-161. [DOI] [PubMed] [Google Scholar]
- 19.Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, M. Gotta, A. Kanapin, N. Le Bot, S. Moreno, M. Sohrmann, D. P. Welchman, P. Zipperlen, and J. Ahringer. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:220-221. [DOI] [PubMed] [Google Scholar]
- 20.Kimura, Y., S. L. Rutherford, Y. Miyata, I. Yahara, B. C. Freeman, L. Yue, R. I. Morimoto, and S. Lindquist. 1997. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 11:1775-1785. [DOI] [PubMed] [Google Scholar]
- 21.Koyasu, S., E. Nishida, T. Kadowaki, F. Matsuzaki, K. Iida, F. Harada, M. Kasuga, H. Sakai, and I. Yahara. 1986. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc. Natl. Acad. Sci. USA 83:8054-8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamphere, L., F. Fiore, X. Xu, L. Brizuela, S. Keezer, C. Sardet, G. F. Draetta, and J. Gyuris. 1997. Interaction between Cdc37 and Cdk4 in human cells. Oncogene 14:1999-2004. [DOI] [PubMed] [Google Scholar]
- 23.Lange, B. M., E. Rebollo, A. Herold, and C. Gonzalez. 2002. Cdc37 is essential for chromosome segregation and cytokinesis in higher eukaryotes. EMBO J. 21:5364-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, P., J. Rao, A. Fliss, E. Yang, S. Garrett, and A. J. Caplan. 2002. The Cdc37 protein kinase-binding domain is sufficient for protein kinase activity and cell viability. J. Cell Biol. 159:1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, J. M., V. L. Kilman, K. Keegan, B. Paddock, M. Emery-Le, M. Rosbash, and R. Allada. 2002. A role for casein kinase 2α in the Drosophila circadian clock. Nature 420:816-820. [DOI] [PubMed] [Google Scholar]
- 26.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLean, M., and D. Picard. 2003. Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones 8:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maloney, A., and P. Workman. 2002. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin. Biol. Ther. 2:3-24. [DOI] [PubMed] [Google Scholar]
- 29.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17:349-368. [DOI] [PubMed] [Google Scholar]
- 30.Miyata, Y., M. Akashi, and E. Nishida. 1999. Molecular cloning and characterization of a novel member of the MAP kinase superfamily. Genes Cells 4:299-309. [DOI] [PubMed] [Google Scholar]
- 31.Miyata, Y., B. Chambraud, C. Radanyi, J. Leclerc, M.-C. Lebeau, J.-M. Renoir, R. Shirai, M.-G. Catelli, I. Yahara, and E.-E. Baulieu. 1997. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II (CK2): regulation of HSP90-binding activity of FKBP52. Proc. Natl. Acad. Sci. USA 94:14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyata, Y., Y. Ikawa, M. Shibuya, and E. Nishida. 2001. Specific association of a set of molecular chaperones including HSP90 and Cdc37 with MOK, a member of the MAP kinase superfamily. J. Biol. Chem. 276:21841-21848. [DOI] [PubMed] [Google Scholar]
- 33.Miyata, Y., and I. Yahara. 1992. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 267:7042-7047. [PubMed] [Google Scholar]
- 34.Miyata, Y., and I. Yahara. 1995. Interaction between casein kinase II and the 90-kDa stress protein, HSP90. Biochemistry 34:8123-8129. [DOI] [PubMed] [Google Scholar]
- 35.Miyata, Y., and I. Yahara. 2000. p53-independent association between SV40 large T antigen and the major cytosolic heat shock protein, HSP90. Oncogene 19:1477-1484. [DOI] [PubMed] [Google Scholar]
- 36.Neckers, L. 2002. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 8:S55-S61. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki, T., K. Irie, and S. Sakiyama. 1995. Molecular cloning and cell cycle-dependent expression of a novel gene that is homologous to cdc37. DNA Cell Biol. 14:1017-1023. [DOI] [PubMed] [Google Scholar]
- 38.Perdew, G. H., H. Wiegand, J. P. Vanden Heuvel, C. Mitchell, and S. S. Singh. 1997. A 50 kilodalton protein associated with raf and pp60v-src protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry 36:3600-3607. [DOI] [PubMed] [Google Scholar]
- 39.Pinna, L. A. 2002. Protein kinase CK2: a challenge to canons. J. Cell Sci. 115:3873-3878. [DOI] [PubMed] [Google Scholar]
- 40.Pinna, L. A., and F. Meggio. 1997. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 3:77-97. [DOI] [PubMed] [Google Scholar]
- 41.Pratt, W. B., and D. O. Toft. 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228:111-133. [DOI] [PubMed] [Google Scholar]
- 42.Rao, J., P. Lee, S. Benzeno, C. Cardozo, J. Allbertus, D. M. Robins, and A. J. Caplan. 2001. Functional interaction of human Cdc37 with the androgen receptor but not with the glucocorticoid receptor. J. Biol. Chem. 276:5814-5820. [DOI] [PubMed] [Google Scholar]
- 43.Reed, S. I. 1980. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95:561-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruzzene, M., D. Penzo, and L. A. Pinna. 2002. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem. J. 364:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarno, S., H. Reddy, F. Meggio, M. Ruzzene, S. P. Davies, A. Donella-Deana, D. Shugar, and L. A. Pinna. 2001. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (“casein kinase-2”). FEBS Lett. 496:44-48. [DOI] [PubMed] [Google Scholar]
- 46.Seldin, D. C., and P. Leder. 1995. Casein kinase II α transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267:894-897. [DOI] [PubMed] [Google Scholar]
- 47.Shao, J., N. Grammatikakis, B. T. Scroggins, S. Uma, W. Huang, J.-J. Chen, S. D. Hartson, and R. L. Matts. 2001. Hsp90 regulates p50cdc37 function during the biogenesis of the active conformation of the heme-regulated eIF2α kinase. J. Biol. Chem. 276:206-214. [DOI] [PubMed] [Google Scholar]
- 48.Shao, J., T. Prince, S. D. Hartson, and R. L. Matts. 2003. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J. Biol. Chem. 278:38117-38220. [DOI] [PubMed] [Google Scholar]
- 49.Siligardi, G., B. Panaretou, P. Meyer, S. Singh, D. N. Woolfson, P. W. Piper, L. H. Pearl, and C. Prodromou. 2002. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277:20151-20159. [DOI] [PubMed] [Google Scholar]
- 50.Silverstein, A. M., N. Grammatikakis, B. H. Cochran, M. Chinkers, and W. B. Pratt. 1998. p50cdc37 binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J. Biol. Chem. 273:20090-20095. [DOI] [PubMed] [Google Scholar]
- 51.Stancato, L. F., Y.-H. Chow, K. A. Hutchison, G. H. Perdew, R. Jove, and W. B. Pratt. 1993. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J. Biol. Chem. 268:21711-21716. [PubMed] [Google Scholar]
- 52.Stepanova, L., M. Finegold, F. DeMayo, E. Schmidt, and J. W. Harper. 2000. The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues. Mol. Cell. Biol. 20:4462-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stepanova, L., X. Leng, S. B. Parker, and J. W. Harper. 1996. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10:1491-1502. [DOI] [PubMed] [Google Scholar]
- 54.Stepanova, L., G. Yang, F. DeMayo, T. M. Wheeler, M. Finegold, T. C. Thompson, and J. W. Harper. 2000. Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia. Oncogene 27:2186-2193. [DOI] [PubMed] [Google Scholar]
- 55.Wang, X., N. Grammatikakis, and J. Hu. 2002. Role of p50/Cdc37 in hepadnavirus assembly and replication. J. Biol. Chem. 277:24361-24367. [DOI] [PubMed] [Google Scholar]
- 56.Xu, X., E. Landesman-Bollag, P. L. Channavajhala, and D. C. Seldin. 1999. Murine protein kinase CK2: gene and oncogene. Mol. Cell. Biochem. 191:65-74. [PubMed] [Google Scholar]
- 57.Yahara, I., Y. Minami, and Y. Miyata. 1998. The 90-kDa stress protein, Hsp90, is a novel molecular chaperone. Ann. N. Y. Acad. Sci. 851:54-60. [DOI] [PubMed] [Google Scholar]