Abstract
Sphingomonas paucimobilis SYK-6 degrades syringate to 3-O-methylgallate (3MGA), which is finally converted to pyruvate and oxaloacetate via multiple pathways in which protocatechuate 4,5-dioxygenase, 3MGA dioxygenase, and gallate dioxygenase are involved. Here we isolated the syringate O-demethylase gene (desA), which complemented the growth deficiency on syringate of a Tn5 mutant of the SYK-6 derivative strain. The desA gene is located 929 bp downstream of ferA, encoding feruloyl-coenzyme A synthetase, and consists of a 1,386-bp open reading frame encoding a polypeptide with a molecular mass of 50,721 Da. The deduced amino acid sequence of desA showed 26% identity in a 325-amino-acid overlap with that of gcvT of Escherichia coli, which encodes the tetrahydrofolate (H4folate)-dependent aminomethyltransferase involved in glycine cleavage. The cell extract of E. coli carrying desA converted syringate to 3MGA only when H4folate was added to the reaction mixture. DesA catalyzes the transfer of the methyl moiety of syringate to H4folate, forming 5-methyl-H4folate. Vanillate and 3MGA were also used as substrates for DesA; however, the relative activities toward them were 3 and 0.4% of that toward syringate, respectively. Disruption of desA in SYK-6 resulted in a growth defect on syringate but did not affect growth on vanillate, indicating that desA is essential to syringate degradation. In a previous study the ligH gene, which complements the growth deficiency on vanillate and syringate of a chemical-induced mutant of SYK-6, DC-49, was isolated (S. Nishikawa, T. Sonoki, T. Kasahara, T. Obi, S. Kubota, S. Kawai, N. Morohoshi, and Y. Katayama, Appl. Environ. Microbiol. 64:836-842, 1998). Disruption of ligH resulted in the same phenotype as DC-49; its cell extract, however, was found to be able to convert vanillate and syringate in the presence of H4folate. The possible role of ligH is discussed.
Lignin is the most abundant aromatic compound in nature, and the utilization of lignin for production of chemicals has been expected. One of the practical procedures for utilizing lignin is its conversion to valuable intermediate metabolites using the microbial lignin degradation enzyme systems (22). It is known that the degradation of native lignin is initiated by the attack by lignin peroxidase, manganese peroxidase, and laccase secreted by white rot fungi (14), and bacteria contribute to the process of mineralization of the abundant lignin-derived compounds found in soil (44, 47). In microbial degradation of lignin-derived compounds, vanillate and syringate are the important intermediate metabolites. Sphingomonas paucimobilis SYK-6 is able to utilize these compounds and various lignin-derived biaryls as the sole source of carbon and energy (20-22, 29, 30). Vanillate and syringate are O demethylated by this strain to produce protocatechuate (PCA) and 3-O-metylgallate (3MGA), respectively. PCA is further degraded through the PCA 4,5-cleavage pathway. In contrast, it has been found that 3MGA is degraded via multiple pathways in which the PCA 4,5-dioxygenase (LigAB), 3MGA dioxygenase (DesZ), and an unidentified gallate dioxygenase are involved (D. Kasai, E. Masai, K. Miyauchi, Y. Katayama, and M. Fukuda, unpublished data). Our investigators have characterized the structures and functions of all the genes involved in the PCA 4,5-cleavage pathway (15, 16, 23, 24, 27). However, details regarding each of the O-demethylation steps of vanillate and syringate are largely unknown.
We have previously obtained a chemical-induced mutant strain of SYK-6, DC-49, that lacks the ability to grow on vanillate and syringate (26). The resting cells of DC-49 are not able to convert vanillate and syringate; as such, the gene involved in the O demethylation of vanillate and syringate seems to be mutated in this strain. The ligH gene, which complements the growth deficiency of DC-49 on vanillate and syringate, has been isolated, with its deduced amino acid sequence showing ca. 60% identity with 10-formyltetrahydrofolate (10-formyl-H4folate) synthetase of Moorella thermoacetica. H4folate was found to be required for the O-demethylation activity of the cell extract of SYK-6 toward vanillate and syringate; H4folate-dependent O-demethylase therefore appears to be involved in this reaction. However, the ligH gene product expressed in Escherichia coli did not show O-demethylase activity toward either vanillate or syringate in the presence of H4folate, and the actual function of this gene has not been established.
Two types of aromatic demethylation systems have been documented. One is vanillate demethylase (VanA and VanB), which is a class IA oxygenase composed of an oxygenase containing an iron-binding site and a Rieske-type [2Fe-2S] cluster, and a reductase containing a flavin and a [2Fe-2S] redox center. This type of demethylase is involved in vanillate degradation by all the vanillate-utilizing aerobic bacteria, such as Pseudomonas and Acinetobacter, reported thus far (8, 10, 36, 43). Another type is H4folate-dependent aromatic O-demethylase reported in anaerobic bacteria, including Acetobacterium dehalogenans (19), Acetobacterium woodii (4), and M. thermoacetica (25). Vanillate O-demethylase of A. dehalogenans is composed of four distinct proteins. In its vanillate-degradation reaction, a methyl transferase I catalyzes transfer of the methyl moiety of vanillate to a corrinoid protein. A methyl transferase II catalyzes the subsequent transfer of the methyl group from the corrinoid protein to H4folate. The fourth protein is thought to be an activation protein that reduces the accidentally oxidized corrinoid.
In the present study, we isolated a novel type of the H4folate-dependent syringate O-demethylase gene from S. paucimobilis SYK-6. We characterized the function and roles of this gene in the syringate catabolism in SYK-6, and the insertion mutant of ligH was also characterized to gain insight into the function of ligH.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. S. paucimobilis SYK-6 was grown in W minimal salt medium (29) containing a 10 mM concentration of syringate, vanillate, PCA, or 0.2% yeast extract or in Luria-Bertani (LB) medium. In the case of using PCA as the sole source of carbon and energy, 50 mg of l-methionine/liter was added to the medium. The SYK-6 derivative strains DB, KDB-4, and DKDA and DKLH were grown in LB media containing 300 mg of carbenicillin/liter, 300 mg of carbenicillin and 50 mg of kanamycin (KAN)/liter, and 50 mg of KAN/liter, respectively.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| S. paucimobilis strains | ||
| SYK-6 | Wild type; Nalr Smr | 18 |
| DB | SYK-6 derivative; ligB::bla; syringate+ vanillate−; Nalr Smr Cbr | D. Kasai |
| KDB-4 | A Tn5 mutant of DB; 3MGA+ vanillate− syringate−; Nalr Smr Cbr Kmr | This study |
| DKDA | SYK-6 derivative; desA::kan; vanillate+ syringate−; Nalr Smr Kmr | This study |
| DKLH | SYK-6 derivative; ligH::kan; vanillate− syringate−; Nalr Smr Kmr | This study |
| E. coli strains | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thiΔ(lac-proAB) F′[traD36 proAB+ lacIqlacZΔM15] | 46 |
| BL21 (DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3); T7 RNA polymerase gene under the control of the lacUV5 promoter | 40 |
| HB101 | supE44 hsd20(rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 6 |
| S17-1 | recA; harboring the tra genes of plasmid RP4 in the chromosome; proA thi | 38 |
| Plasmids | ||
| pUC18 and -19 | Cloning vectors; Apr | 46 |
| pBluescript II KS(+) and SK(+) | Cloning vectors; Apr | 37 |
| pUC119 | Cloning vector; Apr | 45 |
| pET21(+) | Expression vector; Apr T7 promoter | Novagen |
| pSUP5011 | pBR322::Tn5-mob Apr Kmr Cmr | 38 |
| pVK100 | Broad-host-range cosmid vector; Kmr Tetr | 12 |
| pRK2013 | Kmr Tra+ Mob+ | 13 |
| pUC4K | Apr Kmr | 41 |
| pIK03 | KS(+) with a 1.3-kb EcoRV fragment carrying kan of pUC4K | This study |
| pK19mobsacB | oriT sacB Kmr | 34 |
| pL2 | pVK100 with an approximately 20-kb fragment carrying ferBA and desA | This study |
| pSS8 | pVK100 with 4.0- and 4.8-kb SalI fragments carrying desA and part of ferA | This study |
| pK401 | KS(+) with a 4.0-kb SalI fragment of pSS8 | This study |
| pK402 | KS(+) carrying the same fragment as pK401 in the opposite direction | This study |
| pUC401 | pUC18 with a 4.8-kb SalI fragment of pSS8 | This study |
| pUC402 | pUC18 carrying the same fragment as pUC401 in the opposite direction | This study |
| pBXN | SK(+) with a 0.44-kb XhoI-NotI fragment of pSS8 | This study |
| pDSA | pET21(+) with a 1.5-kb EcoRI-NotI fragment carrying desA | This study |
| pUId2 | pUC19 with a 3.0-kb MunI fragment of pSS8 | This study |
| pUKD | pUId2 with an insertion of kan of pIK03 replacing a 0.24-kb BstXI fragment | This study |
| pSSAC | pK19mobsacB with a 4.1-kb SphI-XbaI fragment of pUKD | This study |
| pUEX2.0 | pUC119 with a 2.0-kb fragment carrying ligH | 26 |
| pUDLH01 | pUEX2.0 with an insertion of kan of pUC4K into XhoI site | This study |
| pSONH | pK19mobsacB with a 3.3-kb XbaI-EcoRI fragment of pUDLH01 | This study |
Abbreviations: Nalr, Smr, Cbr, Kmr, Apr, Cmr, and Tetr, resistance to nalidixic acid, streptomycin, carbenicillin, kanamycin, ampicillin, chloramphenicol, and tetracycline, respectively.
Chemicals.
3MGA was synthesized chemically according to the method of Scheline (35). Syringate, vanillate, syringaldehyde, vanillin, sinapinic acid, and ferulic acid were purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). H4folate and 5-methyl-H4folate were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Tn5 mutagenesis of the ligB insertion mutant of SYK-6.
Tn5 insertion mutants of the ligB insertion mutant of SYK-6 strain DB (D. Kasai et al., unpublished) were generated by using pSUP5011, which was transferred from E. coli S17-1 to DB by conjugation. Tn5 insertion mutants of DB were grown in W medium containing 10 mM syringate, 25 mg of nalidixic acid/liter, and 50 mg of KAN/liter until the turbidity of the culture at 600 nm reached 0.3 to 0.2. The target mutants unable to grow with syringate were enriched by the method of penicillin screening as described previously (20). The ability of the resulting cells to grow on syringate was tested on the plate, and then six mutants were obtained. The transformation activities of the whole cells of the mutants toward syringate and 3MGA were spectrophotometrically analyzed by using a DU-7500 spectrophotometer (Beckman, Fullerton, Calif.)
The pVK100 cosmid library carrying the partially SalI-digested fragments of SYK-6 total DNA was introduced from E. coli HB101 to one of the Tn5 insertion mutants, KDB-4, by triparental mating. The resulting transconjugants were plated on W medium containing 10 mM syringate.
DNA manipulations and nucleotide sequencing.
DNA manipulations were carried out as described in references 2 and 32. Nucleotide sequence was determined by the dideoxy termination method with an ALFexpress DNA sequencer (Pharmacia Biotech., Milwaukee, Wis.). A Sanger reaction (33) was carried out by using the Thermosequenase fluorescent-labeled primer cycle sequencing kit with 7-deaza-dGTP (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Sequence analysis was carried out with the GeneWorks program (Intelligenetics, Inc., Mountain View, Calif.). Homology search was done with the DDBJ database by using the BLAST program.
Expression of desA in E. coli and preparation of cell extracts.
The 1.5-kb EcoRI-NotI fragment carrying desA of pSS8 was cloned into pET21(+) to construct pDSA. E. coli BL21(DE3) cells harboring pDSA were grown in LB medium containing 100 mg of ampicillin/liter at 37°C. The expression of desA was induced for 4 h by adding 1 mM isopropyl-β-d-thiogalactopyranoside when the turbidity of the culture at 600 nm reached 0.5. Cells were harvested by centrifugation at 3,000 × g for 10 min, suspended by 100 mM sodium phosphate buffer (pH 8.0), and washed twice with the same buffer. Cells suspended in the buffer were sonicated, and the cell lysate was centrifuged at 15,000 × g for 15 min. The resulting supernatant was used as the cell extract.
Protein determination and polyacrylamide gel electrophoresis.
The protein concentration was determined by the method of Bradford (7). The expression of the gene was determined by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. Gel staining was carried out with Coomassie brilliant blue.
Identification of the reaction product.
The 1-ml assay mixture contained 20 mM Tris-HCl buffer (pH 7.5), 100 μM syringate, 0.5 mM H4folate, and the cell extract of E. coli BL21(DE3) cells harboring pDSA (3 mg of protein). The reaction was carried out at 30°C for 3 h. The reaction mixture was acidified and extracted by ethyl acetate, and then the extract was trimethylsilylated (TMS). The resultant TMS derivative was analyzed by gas chromatography-mass spectrometry (MS) using a model 5971A apparatus with an Ultra-2 capillary column (50 m by 0.2 mm; Agilent Technologies, Palo Alto, Calif.). The analytical condition was the same as described previously (24).
For detection of the one-carbon (C1) derivative of H4folate generated during O demethylation of syringate catalyzed by DesA, electrospray ionization (ESI)-MS was employed. The 1-ml assay mixture contained 100 mM Tris-HCl buffer (pH 8.0), 5 mM syringate, 5 mM H4folate, and the cell extract of E. coli BL21(DE3) cells harboring pDSA (1 mg of protein). The reaction was carried out at 30°C and stopped by the addition of methanol (final concentration, 25%) at 10 min. Precipitated protein was removed by centrifugation (15,000 × g for 15 min), and the supernatant was analyzed by ESI-MS (HP1100 series LC-MSD; Agilent Technologies). In this analysis, mass spectra were obtained by negative-mode ESI, with a needle voltage of −3.5 kV and a source temperature of 350°C. The mobile phase was a mixture of water (89%), methanol (10%), and acetic acid (1%), and the flow rate was 0.2 ml/min.
Quantification of 5-methyl-H4folate formed and syringate degraded during the O demethylation of syringate catalyzed by DesA was done as follows. The 1-ml assay mixture contained 100 mM sodium phosphate buffer (pH 8.0), 100 μM syringate, 1 mM H4folate, and the cell extract of E. coli BL21(DE3) cells harboring pDSA (100 μg of protein). A portion of the reaction mixture was taken at sampling points, and reactions were stopped by the addition of methanol (final concentration, 25%). The reaction mixture was centrifuged at 15,000 × g for 15 min and filtered. The supernatant was analyzed by using a high-pressure liquid chromatography (HPLC) system (Alliance 2690 separations module; Waters, Milford, Mass.) equipped with a TSKgel ODS-80TM column (6 by 150 mm; Tosoh, Tokyo, Japan). The mobile phase was a mixture of water (93%), acetonitrile (6%), and phosphoric acid (1%), and the flow rate was 1 ml/min. Syringate and 5-methyl-H4folate were detected at 275 and 290 nm, respectively.
Enzyme assay.
The O-demethylase activities of the cell extracts toward syringate, vanillate, 3MGA, syringaldehyde, vanillin, ferulic acid, and sinapinic acid were determined by measuring the decrease in substrates by using the HPLC system. The 1-ml assay mixture contained 100 mM sodium phosphate buffer (pH 8.0), 100 μM substrate, 1 mM H4folate, and the cell extract of E. coli BL21(DE3) cells harboring pDSA (100 μg of protein). A portion of the reaction mixture was taken at sampling points and analyzed by HPLC. For analysis of the conversion of syringate, vanillate, and 3MGA, the mobile phase was a mixture of water (84%), acetonitrile (15%), and phosphoric acid (1%), and the flow rate was 1 ml/min. Syringate, vanillate, and 3MGA were detected at 275 nm, and their retention times were 12.4, 11.9, and 6.7 min, respectively. For analysis of the conversion of vanillin, syringaldehyde, ferulic acid, and sinapinic acid, the mobile phase was a mixture of water (69%), acetonitrile (30%), and phosphoric acid (1%). Compounds were detected as follows: vanillin, 280 nm; syringaldehyde, 305 nm; ferulic acid and sinapinic acid, 320 nm. The retention times of vanillin, syringaldehyde, ferulic acid, and sinapinic acid were 6.9, 6.9, 6.7, and 6.4 min, respectively.
Construction of insertion mutants of S. paucimobilis SYK-6.
The 3.0-kb MunI fragment carrying desA of pSS8 was cloned into the SmaI site of pUC19 to generate pUId2, and the 0.24-kb BstXI fragment was deleted for desA disruption. The 1.3-kb EcoRV fragment carrying the KAN resistance gene (kan) from pIK03 was inserted into the BstXI site of the 2.76-kb MunI fragment to construct pUKD. pUKD was digested with SphI and XbaI, and the insert was cloned into pK19mobsacB to generate pSSAC. The 1.3-kb SalI fragment carrying kan from pUC4K was inserted into the XhoI site of the 2.0-kb fragment carrying ligH in pUEX2.0 to construct pUDLH01. The 3.3-kb XbaI-EcoRI fragment of pUDLH01 was cloned into pK19mobsacB to generate pSONH. Each plasmid was introduced into SYK-6 cells by electroporation, and the candidates for mutants of desA and ligH were screened by the same method described in a previous study (24). Southern hybridization analysis was done to examine the disruption of desA and ligH by using the digoxigenin system (Roche Molecular Biochemicals, Mannheim, Germany). The total DNAs of candidates for desA and ligH mutants were digested with SacI and BamHI, respectively. The 1.5-kb EcoRI-NotI fragment carrying desA, the 2.0-kb fragment carrying ligH, and the 1.3-kb EcoRV fragment carrying kan were labeled with the digoxigenin system and used as probes.
Preparation of cell extracts of SYK-6 and insertion mutants.
To determine the syringate and vanillate O-demethylase activities of the cell extracts of SYK-6 and its insertion mutants, these cells were grown in W medium containing 0.2% yeast extract. Cells grown on yeast extract until the turbidity of the culture at 600 nm reached 0.8 were harvested by centrifugation (5,000 × g for 20 min), washed twice with W medium, and suspended with the same medium. To induce the O-demethylase activities, these cells were inoculated to W medium containing 10 mM syringate or vanillate to a turbidity at 600 nm of 0.5 and incubated for 12 h. Syringate and vanillate O-demethylase activities of the cell extracts prepared from these cells (400 μg of protein/ml) and uninduced cells grown on yeast extract (2 mg of protein/ml) were determined. Preparation of the cell extracts and the enzyme assay were essentially the same as described above.
RT-PCR.
S. paucimobilis SYK-6 grown on yeast extract was incubated with 10 mM syringate or sucrose as described above. Total RNA was prepared from 500 ml of culture as essentially described in reference 2. A cDNA library was obtained by reverse transcription (RT) reaction using Revertra Ace (Toyobo, Osaka, Japan) and a random 9-mer. The cDNA was used as a template for subsequent PCRs with specific primers which amplify the boundaries of ferB-ferA-orf1-desA. The primers used were as follows: ferB-forward (nucleotide positions 1132 to 1151 in the sequence AB110975) and ferA-reverse (positions 1466 to 1485); ferA-forward (positions 3366 to 3384) and orf1-reverse (positions 3647 to 3665); orf1-forward (positions 4060 to 4079) and desA-reverse (positions 4579 to 4598). Control samples in which reverse transcriptase was omitted in the RT-PCR were run in parallel with RT-PCR.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper was deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB110975.
RESULTS
Cloning of the syringate O-demethylase gene. To obtain the mutants that cause defects in the genes involved in the syringate catabolic pathway, the ligB insertion mutant of SYK-6, strain DB, was subjected to Tn5 mutagenesis, because 3MGA is degraded through multiple pathways, including the PCA 4,5-cleavage pathway (15). Six mutants lacking the ability to grow on syringate were obtained. The whole cells of all these mutants lost their transformation activity toward syringate but retained that toward 3MGA. The cells therefore seemed to be mutated in the gene involved in the O demethylation of syringate. The SYK-6 gene library constructed with pVK100 was introduced into one of the mutants, KDB-4, by triparental mating. Four cosmid clones were isolated, as they complemented the growth deficiency of KDB-4 on syringate. When one of the clones, pL2, was used as a probe, Southern hybridization analysis indicated that all the cosmid clones contained the common 4.8- and 4.0-kb SalI fragments. A subcloning experiment of pL2 showed that pSS8 containing the 8.8-kb DNA fragment carrying these two SalI fragments was able to complement the growth deficiency of KDB-4 on syringate, suggesting that the mutated gene was located in the 8.8-kb DNA fragment.
Nucleotide sequence of the syringate O-demethylase gene.
A series of deletion clones of the 4.8- and 4.0-kb SalI fragments were generated, and the nucleotide sequences of these fragments and the overlapping 0.4-kb XhoI-NotI fragment were determined. The nucleotide sequence of the 8,734-bp DNA fragment revealed the partial sequence of the ferA gene encoding feruloyl-coenzyme A (CoA) synthetase at the 5′ end of the fragment, which was characterized in a previous study (20). Downstream of ferA, two open reading frames (ORFs) (orf1 and orf2) of 750 and 1,386 bp and three ORFs (orf3, orf4, and orf5) of 747, 876, and 1,614 bp that were oriented in the same and opposite directions relative to ferA, respectively, were found (Fig. 1). The deduced amino acid sequence of orf2 revealed 26, 23, 26, and 23% identity with aminomethyltransferase (GcvT) of E. coli (in a 325-amino-acid overlap) (28), dimethylglycine dehydrogenase of humans (in a 369-amino-acid overlap) (5), sarcosine dehydrogenase of rats (in a 246-amino-acid overlap) (3), and the α-subunit of sarcosine oxidase of Corynebacterium sp. strain P1 (in a 202-amino-acid overlap) (9), respectively. All these enzymes catalyze the transfer of an aminomethyl or methyl moiety from the substrates to H4folate. Because it is known that the O-demethylase activity of SYK-6 toward vanillate and syringate depends on the presence of H4folate (26), orf2 seemed to encode the syringate O-demethylase that requires H4folate as a C1 acceptor. We designated orf2 as desA.
FIG. 1.
Restriction maps of the 10.3-kb fragment carrying desA (A) and the 6.5-kb fragment carrying ligH (B). ferB, ferA, orf1, desA, orf3, orf4, orf5, metF, and ligH are indicated by the filled arrows. Vertical bars above the restriction maps indicate the positions of the kan gene insertion of the desA mutant (DKDA) and the ligH mutant (DKLH). Double-headed arrows indicate the locations of the amplified RT-PCR products shown in Fig. 6. Abbreviations for restriction enzymes: B, BamHI; Bx, BstXI; E, EcoRI; Ev, EcoRV; M, MunI; N, NotI; P, PstI; S, SalI; Sc, SacI; Sm, SmaI; X, XhoI.
The deduced amino acid sequences of orf1, orf3, orf4, and orf5 revealed 28, 24, 32, and 41% identity with those of the genes for 3-oxoadipate enol-lactone hydrolase/4-carboxymuconolactone decarboxylase (PcaL) of Streptomyces sp. strain 2065 (in a 204-amino-acid overlap) (17), an IclR-type transcriptional regulator PcaR of Pseudomonas putida PRS1 (in a 245-amino-acid overlap) (31), a functionally unknown ORF (ORFR1) of Sphingomonas sp. strain RW1 (in a 149-amino-acid overlap) (1), and benzoylformate decarboxylase of P. putida ATCC 12633 (in a 528-amino-acid overlap) (42), respectively. On the basis of the functions of these genes, other ORFs found in the 8.8-kb SalI fragment other than desA seemed not to be involved in O demethylation of syringate.
DesA catalyzes the conversion of syringate to 3MGA.
The 1.5-kb EcoRI-NotI fragment carrying desA was cloned in pET21(+) to construct pDSA. Overexpression of desA in E. coli BL21(DE3) cells harboring pDSA yielded a protein with an apparent molecular mass of 49 kDa, similar to the predicted molecular mass of the gene product of desA (Mr, 50,721). To examine whether desA actually encodes syringate O-demethylase, the reaction product from syringate catalyzed by the crude DesA enzyme was determined by gas chromatography-MS. Only when H4folate was added to the reaction mixture did the crude DesA enzyme completely convert syringate to 3MGA after 3 h of incubation (Fig. 2). This result indicated that DesA encodes the H4folate-dependent syringate O-demethylase.
FIG. 2.
Conversion of syringate to 3MGA by DesA. Cell extract of E. coli BL21(DE3) cells harboring pDSA (3 mg of protein/ml) was incubated with 100 μM syringate in the presence of 0.5 mM H4folate. (A and B) Gas chromatograms of the TMS derivatives of the reaction product at 0 and 3 h of incubation, respectively. (C) Mass spectrum of the peak with a retention time of 29.3 min in the gas chromatogram of panel B.
The optimum pH of DesA was examined in the pH range 6.0 to 9.0 by using buffers consisting of 100 mM sodium phosphate buffer (pH 6.0 to 8.0) and 100 mM Tris-HCl buffer (pH 7.5 to 9.0). The optimum concentration of H4folate was also examined in the millimolar range, 0.5 to 5.0. The enzyme exhibited the highest activity at pH 8.0 in the presence of 1 mM H4folate.
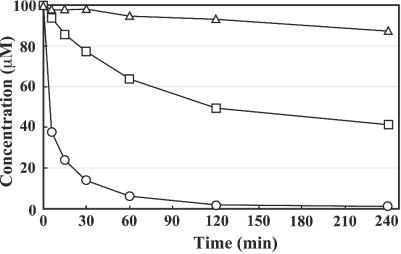
The O-demethylase activities of DesA toward syringaldehyde, 3MGA, vanillate, vanillin, sinapinic acid, and ferulic acid were also examined by measuring with HPLC the decreases in the amounts of the substrates. DesA converted vanillate and 3MGA in addition to syringate. Crude DesA (100 μg of protein/ml) converted ca. 50 and 7% of 100 μM vanillate and 3MGA, respectively, for 2 h, while syringate was completely transformed (Fig. 3). The transformation activity of DesA toward vanillate and 3MGA measured in a 1-min reaction was 3 and 0.4% of the activity toward syringate (260 mU/mg of protein), respectively.
FIG. 3.
Time course of degradation of syringate, vanillate, and 3MGA by DesA. Cell extract of E. coli BL21(DE3) cells harboring pDSA (100 μg of protein/ml) was incubated with 100 μM syringate (circles), vanillate (squares), and 3MGA (triangles) in the presence of 1 mM H4folate. The concentrations of each substrate were determined by HPLC.
Identification and quantification of C1-H4folate.
To identify the C1-H4folate generated in the syringate O demethylation, the reaction products from syringate and H4folate catalyzed by the crude DesA enzyme were analyzed by negative-mode ESI-MS (Fig. 4). After 10 min of reaction, the intensity of the fragments at m/z 197 and 444 corresponding to the deprotonated molecular ions ([M-H]−) of syringate and H4folate, respectively, decreased and the generation of the fragment at m/z 183 corresponding to [M-H]− of 3MGA and that at m/z 458 was observed. Because m/z 458 corresponded to the [M-H]− of 5-methyl-H4folate, the results strongly suggested that H4folate was converted to 5-methyl-H4folate. Formation of 5-methyl-H4folate was further confirmed by comparing the retention times between the reaction product and the authentic 5-methyl-H4folate analyzed by HPLC. The rates for syringate degradation and the formation of 5-methyl-H4folate determined by HPLC were indicated to be equivalent (Fig. 5).
FIG. 4.
Identification of C1-H4folate generated in O demethylation of syringate catalyzed by DesA. Cell extract of E. coli BL21(DE3) cells harboring pDSA (1 mg of protein/ml) was incubated with 5 mM syringate and H4folate. Results shown are negative-ion ESI-MS spectra of the reaction mixtures at 10 min of incubation without (A) or with (B) enzyme, respectively.
FIG. 5.
Kinetics of 5-methyl-H4folate formation from syringate catalyzed by DesA. Cell extract of E. coli BL21(DE3) cells harboring pDSA (100 μg of protein/ml) was incubated with 100 μM syringate and 1 mM H4folate. The concentrations of syringate (circles) and 5-methyl-H4folate (squares) were determined by HPLC.
RT-PCR analysis of the ferB-ferA-orf1-desA gene cluster.
ferB, which encodes feruloyl-CoA hydratase-lyase, can be found upstream from ferA, and each of the gene products of ferA and ferB catalyzes the conversion of ferulic acid to feruloyl-CoA and of feruloyl-CoA to vanillin, respectively (20). The distances between ferB and ferA, ferA and orf1, and orf1 and desA were 119, 151, and 22 bp, respectively; they were therefore expected to be transcribed in an operon. RT-PCR experiments were carried out with total RNA isolated from SYK-6 cells incubated with syringate and primers complementary to neighboring ORFs. Agarose gel electrophoresis showed the amplification products of ferB-ferA (350 bp), ferA-orf1 (300 bp), and orf1-desA (540 bp), indicating that these genes constitute an operon (Fig. 6). In the case of using total RNA isolated from the cells incubated with sucrose, the same result was obtained (data not shown). However, the cell extract of SYK-6 cells incubated with syringate showed approximately 10 times higher syringate O-demethylase activity (15 mU/mg) than that incubated with sucrose. Thus, this operon seemed to be transcribed to some extent in the uninduced cells, but its transcription was more strongly induced in the cells grown on syringate.
FIG. 6.
Agarose gel electrophoresis of RT-PCR products across the boundaries of ferB-ferA-orf1-desA genes. The sizes of the molecular weight markers in lane M are indicated on the left. Even-numbered lanes correspond to controls without reverse transcriptase. Lanes: 1 and 2, orf1-desA intergenic region (expected size, 539 bp); 3 and 4, ferA-orf1 intergenic region (expected size, 300 bp); 5 and 6, ferB-ferA intergenic region (expected size, 354 bp).
Disruption of desA and ligH in S. paucimobilis SYK-6.
To examine the roles of desA and ligH in the O demethylation of syringate, each of the genes in SYK-6 was disrupted by the gene replacement technique with the desA- and ligH-disrupted plasmids pSSAC and pSONH, respectively, whose desA and ligH were inactivated by insertion of the kan gene. Southern hybridization analysis of the desA and ligH mutants using the desA, ligH, and kan gene probes revealed that each of desA and ligH was inactivated by homologous recombination through the double crossover (Fig. 7A). The resultant insertion mutants of desA and ligH were designated strains DKDA and DKLH, respectively (Fig. 1).
FIG. 7.
Syringate and vanillate O-demethylase activities of the desA mutant (DKDA) and ligH mutant (DKLH). (A) Southern hybridization analysis of the insertion mutants. Lanes 1 and 3, total DNA of SYK-6 digested with SacI; 2 and 4, total DNA of DKDA digested with SacI; 5 and 7, total DNA of SYK-6 digested with BamHI; 6 and 8, total DNA of DKLH digested with BamHI. The 1.3-kb EcoRV fragment carrying kan (lanes 3, 4, 7, and 8), the 1.5-kb EcoRI-NotI fragment carrying desA (lanes 1 and 2), and the 2.0-kb fragment carrying ligH (lanes 5 and 6) were used as probes. (B and D) The time course of the degradation of syringate (B) and vanillate (D) by cell extracts (2 mg of protein/ml) of SYK-6 (circles), DKDA (triangles), and DKLH (squares) cells grown on yeast extract. (C and E) The time course of the degradation of syringate (C) and vanillate (E) by cell extract (400 μg of protein/ml) of SYK-6 (circles), DKDA (triangles), and DKLH (squares) incubated with 10 mM syringate (C) or vanillate (E). Each cell extract was incubated with 100 μM syringate or vanillate in the presence of 1 mM H4folate. HPLC was used to monitor the time course of the substrate removal. Each value is the average ± standard deviation (error bar) of at least three measurements.
DKDA completely lost the ability to grow on syringate, but it grew on a plate containing vanillate as well as the wild type. The transformation activities toward syringate and vanillate of the cell extract of DKDA were examined. DKDA was grown in W medium containing 0.2% yeast extract, and the cells were harvested and incubated with syringate or vanillate to induce the enzyme. The cell extract of DKDA incubated with syringate did not show syringate O-demethylase activity (Fig. 7C), but that incubated with vanillate retained the vanillate transformation activity (Fig. 7E). These results indicated that desA is essential to the O demethylation of syringate, while desA appears to be not involved in O demethylation of vanillate. In contrast, DKLH was not able to grow on either syringate or vanillate, as indicated by the chemical-induced mutant DC-49, whose ligH was mutated (26). However, the cell extract of DKLH grown on yeast extract showed O-demethylation activity toward both syringate and vanillate in the presence of H4folate (Fig. 7B and D), though the enzyme activities of DKLH were considerably lower than those of the wild type when cells incubated with syringate or vanillate were used (Fig. 7C and E).
DISCUSSION
In the 8.8-kb DNA fragment, which conferred the growth ability of the Tn5 mutant KDB-4 on syringate, we found a new type of O-demethylase gene involved in syringate degradation. The H4folate-dependent multicomponent O-demethylation system in anaerobic bacteria (4, 19, 25) and the class IA oxygenase system in aerobic bacteria (8, 10, 36, 43) have thus far been reported for the aromatic demethylases. DesA did not show any similarity to these systems but was similar to aminomethyltransferase (T-protein), dimethylglycine dehydrogenase, sarcosine dehydrogenase, and the α-subunit of sarcosine oxidase. All these enzymes catalyze transfer of the aminomethyl or methyl moiety from the substrates to H4folate to form 5,10-methylene-H4folate. Amino acid sequence alignments of T-protein and the carboxy-terminal half of dimethylglycine dehydrogenase with the carboxy-terminal half of the α-subunit of Corynebacterium sarcosine oxidase revealed an evolutionary relationship among these enzymes (9). DesA transformed syringate to 3MGA only in the presence of H4folate, and 5-methyl-H4folate was identified as the product from H4folate. In the reaction catalyzed by the H4folate-dependent multicomponent system of anaerobic bacteria, 5-methyl-H4folate is produced. However, the methyl moiety of the substrate was once transferred to the corrinoid protein by the action of methyl transferase I, and the methyl moiety was further transferred from the corrinoid protein to H4folate by the action of methyl transferase II. Accordingly, DesA and the H4folate-dependent multicomponent system of anaerobic bacteria are completely different types of O-demethylase. S. paucimobilis SYK-6 seems to have evolved with a simpler O-demethylation system that catalyzes direct transfer of the methyl moiety of the substrates to H4folate. The similar function and sequence of DesA with T-protein, dimethylglycine dehydrogenase, sarcosine dehydrogenase, and the α-subunit of sarcosine oxidase may suggest an evolutionary relationship among these enzymes.
RT-PCR analysis indicated that ferB, ferA, orf1, and desA were transcribed in an operon. An interpretation of this cotranscription of desA with ferB and ferA is that these genes are involved in sinapinic acid degradation, as syringate seems to be an intermediate metabolite of sinapinic acid. The enzyme mixture of FerA and FerB produced in E. coli indeed demonstrated an ability to convert sinapinic acid to syringaldehyde (20). However, the growth of SYK-6 was poor on sinapinic acid and syringaldehyde. Further research is necessary to address the transcriptional regulation of the ferB-ferA-orf1-desA operon.
Gene disruption of desA indicated that this gene is essential to the growth of SYK-6 on syringate but not on vanillate. The cell extract of DKDA indeed showed vanillate O-demethylase activity; however, it completely lost its syringate O-demethylase activity (Fig. 7C and E). This result is consistent with the significantly higher specific activity of DesA toward syringate than toward vanillate. These results suggest that desA is essential to syringate degradation and that another H4folate-dependent O-demethylase is involved in vanillate degradation. The reason for the higher vanillate O-demethylase activity of DKDA (and DKLH) than that of wild type when the cells were grown on yeast extract is unknown at present.
To gain insight into the actual function of ligH, we constructed a ligH insertion mutant. DKLH completely lost its ability to grow on vanillate and syringate, but the cell extract of DKLH grown on yeast extract transformed both vanillate and syringate in the presence of H4folate (Fig. 7B and D). This result indicates that ligH is not directly involved in the O demethylation of vanillate and syringate. High sequence similarity (60% identity) between LigH and 10-formyl-H4folate synthetase (FTHFS) of M. thermoacetica raised the possibility that LigH is involved in H4folate-mediated C1 metabolism as FTHFS (Fig. 8). Recently, the metF gene, which encodes 5,10-methylene-H4folate reductase, was found just upstream of ligH (Fig. 1) (39). Disruption of metF in SYK-6 resulted in the growth deficiency on vanillate and syringate, but the cell extract of the metF mutant was able to transform vanillate and syringate in the presence of H4folate. In this reaction, a significant amount of 5-methyl-H4folate accumulated, whereas such an accumulation was not observed in the reaction mixture containing the wild-type cell extract. The tandem location of metF and ligH in SYK-6 and the similar phenotype between the metF and ligH mutants may support the hypothesis that ligH encodes FTHFS. It is most likely that DKLH lost its growth ability on vanillate and syringate because of the deficiency of the regeneration of H4folate from 10-formyl-H4folate (Fig. 8). The low level of O-demethylase activities in the cell extracts of DKLH grown in the presence of syringate or vanillate (Fig. 7C and E) may have been caused by the lack of induction of desA and the vanillate O-demethylase gene expressions. Expression of these genes may be induced by the metabolite(s) of syringate and vanillate. However, further studies are needed to address this notion. In a previous study, the O-demethylase activities toward vanillate and syringate of the cell extracts of the ligH mutant DC49 were not detected in the presence of H4folate (26). The low levels of O-demethylase activities in DC-49 incubated with syringate or vanillate seems to have led to an incorrect conclusion.
FIG. 8.
Proposed syringate O-demethylation system linked with H4folate-mediated C1 metabolism in S. paucimobilis SYK-6. The metF gene just upstream of ligH (Fig. 1) was previously suggested to catalyze the oxidation of 5-methyl-H4folate (39). The function of ligH was deduced from the sequence similarity with FTHFS and the results obtained in this study. The reactions indicated by gray arrows have not been confirmed.
It has been a long-standing question as to why S. paucimobilis SYK-6 shows only poor growth on PCA, which is the O-demethylation product of vanillate, as the sole source of carbon and energy. If 5-methyl-H4folate generated during the O demethylation of vanillate and syringate were the major source of C1-H4folate in SYK-6, this strain would be expected to show auxotrophy for the purines, N-formyl-methionyl tRNA, thymidylate, and methione, whose synthesis requires C1-H4folate when SYK-6 is grown on PCA. Our preliminary experiment revealed that both SYK-6 and DKLH were able to grow well on PCA in the presence of 50 mg of methionine per liter (data not shown). This result suggested that the O demethylation of syringate and vanillate is important to SYK-6 as not only an essential degradation step for these compounds but also to supply the 5-methyl-H4folate required for methionine biosynthesis. C1-H4folate required for the synthesis of other than methionine might be supplied from 5,10-methylene-H4folate, which is ordinarily generated by the conversion of serine to glycine and from glycine cleavage (11). Further investigations are needed to clarify the details of C1 metabolism in SYK-6.
Acknowledgments
We thank K. Okamura-Ikeda for helpful suggestions regarding preparation of H4folate. We also thank H. Hara for assistance with the ESI-MS analysis and A. Ichimura for construction of pIK03.
REFERENCES
- 1.Armengaud, J., and K. N. Timmis. 1998. The reductase RedA2 of the multi-component dioxin dioxygenase system of Sphingomonas sp. RW1 is related to class-I cytochrome P450-type reductases. Eur. J. Biochem. 253:437-444. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bergeron, F., A. Otto, P. Blache, R. Day, L. Denoroy, R. Brandsch, and D. Bataille. 1998. Molecular cloning and tissue distribution of rat sarcosine dehydrogenase. Eur. J. Biochem. 257:556-561. [DOI] [PubMed] [Google Scholar]
- 4.Berman, M. H., and A. C. Frazer. 1992. Importance of tetrahydrofolate and ATP in the anaerobic O-demethylation reaction for phenylmethylethers. Appl. Environ. Microbiol. 58:925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binzak, B. A., R. A. Wevers, S. H. Moolenaar, Y. M. Lee, W. L. Hwu, J. Poggi-Bach, U. F. Engelke, H. M. Hoard, J. G. Vockley, and J. Vockley. 2001. Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency. Am. J. Hum. Genet. 68:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolivar, F., and K. Backman. 1979. Plasmids of Escherichia coli as cloning vector. Methods Enzymol. 68:245-267. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Brunel, F., and J. Davison. 1988. Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J. Bacteriol. 170:4924-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlumsky, L. J., L. Zhang, and M. S. Jorns. 1995. Sequence analysis of sarcosine oxidase and nearby genes reveals homologies with key enzymes of folate one-carbon metabolism. J. Biol. Chem. 270:18252-18259. [DOI] [PubMed] [Google Scholar]
- 10.Civolani, C., P. Barghini, A. R. Roncetti, M. Ruzzi, and A. Schiesser. 2000. Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13. Appl. Environ. Microbiol. 66:2311-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dev, I. K., and R. J. Harvey. 1982. Sources of one-carbon units in the folate pathway of Escherichia coli. J. Biol. Chem. 257:1980-1986. [PubMed] [Google Scholar]
- 12.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold, M. H., and M. Alic. 1993. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol. Rev. 57:605-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara, H., E. Masai, Y. Katayama, and M. Fukuda. 2000. The 4-oxalomesaconate hydratase gene, involved in the protocatechuate 4,5-cleavage pathway, is essential to vanillate and syringate degradation in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 182:6950-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara, H., E. Masai, K. Miyauchi, Y. Katayama, and M. Fukuda. 2003. Characterization of the 4-carboxy-4-hydroxy-2-oxoadipate aldolase gene and operon structure of the protocatechuate 4,5-cleavage pathway genes in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 185:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwagami, S. G., K. Yang, and J. Davies. 2000. Characterization of the protocatechuic acid catabolic gene cluster from Streptomyces sp. strain 2065. Appl. Environ. Microbiol. 66:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama, Y., S. Nishikawa, M. Nakamura, K. Yano, M. Yamasaki, N. Morohoshi, and T. Haraguchi. 1987. Cloning and expression of Pseudomonas paucimobilis SYK-6 genes involved in the degradation of vanillate and protocatechuate in P. putida. Mokuzai Gakkaishi 33:77-79. [Google Scholar]
- 19.Kaufmann, F., G. Wohlfarth, and G. Diekert. 1998. O-Demethylase from Acetobacterium dehalogenans substrate specificity and function of the participating proteins. Eur. J. Biochem. 253:706-711. [DOI] [PubMed] [Google Scholar]
- 20.Masai, E., K. Harada, X. Peng, H. Kitayama, Y. Katayama, and M. Fukuda. 2002. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 68:4416-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masai, E., A. Ichimura, Y. Sato, K. Miyauchi, Y. Katayama, and M. Fukuda. 2003. Roles of the enantioselective glutathione S-transferases in cleavage of β-aryl ether. J. Bacteriol. 185:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masai, E., Y. Katayama, S. Nishikawa, and M. Fukuda. 1999. Characterization of Sphingomonas paucimobilis SYK-6 genes involved in degradation of lignin-related compounds. J. Ind. Microbiol. Biotechnol. 23:364-373. [DOI] [PubMed] [Google Scholar]
- 23.Masai, E., K. Momose, H. Hara, S. Nishikawa, Y. Katayama, and M. Fukuda. 2000. Genetic and biochemical characterization of 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase and its role in the protocatechuate 4,5-cleavage pathway in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 182:6651-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masai, E., S. Shinohara, H. Hara, S. Nishikawa, Y. Katayama, and M. Fukuda. 1999. Genetic and biochemical characterization of a 2-pyrone-4,6-dicarboxylic acid hydrolase involved in the protocatechuate 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6. J. Bacteriol. 181:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidu, D., and S. W. Ragsdale. 2001. Characterization of a three-component vanillate O-demethylase from Moorella thermoacetica. J. Bacteriol. 183:3276-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikawa, S., T. Sonoki, T. Kasahara, T. Obi, S. Kubota, S. Kawai, N. Morohoshi, and Y. Katayama. 1998. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demethylation of vanillate and syringate. Appl. Environ. Microbiol. 64:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda, Y., S. Nishikawa, K. Shiozuka, H. Kadokura, H. Nakajima, K. Yoda, Y. Katayama, N. Morohoshi, T. Haraguchi, and M. Yamasaki. 1990. Molecular cloning of the protocatechuate 4,5-dioxygenase genes of Pseudomonas paucimobilis. J. Bacteriol. 172:2704-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura-Ikeda, K., Y. Ohmura, K. Fujiwara, and Y. Motokawa. 1993. Cloning and nucleotide sequence of the gcv operon encoding the Escherichia coli glycine-cleavage system. Eur. J. Biochem. 216:539-548. [DOI] [PubMed] [Google Scholar]
- 29.Peng, X., T. Egashira, K. Hanashiro, E. Masai, S. Nishikawa, Y. Katayama, K. Kimbara, and M. Fukuda. 1998. Cloning of a Sphingomonas paucimobilis SYK-6 gene encoding a novel oxygenase that cleaves lignin-related biphenyl and characterization of the enzyme. Appl. Environ. Microbiol. 64:2520-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng, X., E. Masai, H. Kitayama, K. Harada, Y. Katayama, and M. Fukuda. 2002. Characterization of the 5-carboxyvanillate decarboxylase gene and its role in lignin-related biphenyl catabolism in Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 68:4407-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Steiner, S., R. E. Parales, C. S. Harwood, and J. E. Houghton. 1994. Characterization of the pcaR regulatory gene from Pseudomonas putida, which is required for the complete degradation of p-hydroxybenzoate. J. Bacteriol. 176:5771-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 35.Scheline, R. R. 1966. A rapid synthesis of 3-O-methylgallic acid. Acta Chem. Scand. 20:1182. [Google Scholar]
- 36.Segura, A., P. V. Bunz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 181:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Short, J. M., J. M. Fernandez, J. A. Sorge, and W. D. Huse. 1988. λ ZAP: a bacteriophage λ expression vector with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon, R. 1984. High frequency mobilization of gram-negative bacterial replicons by the in vivo constructed Tn-5 mob transposon. Mol. Gen. Genet. 196:413-420. [DOI] [PubMed] [Google Scholar]
- 39.Sonoki, T., Y. Otsuka, S. Ikeda, E. Masai, S. Kajita, and Y. Katayama. 2002. Tetrahydrofolate-dependent vanillate and syringate O-demethylation links tightly to one-carbon metabolic pathway associated with amino acid synthesis and DNA methylation in the lignin metabolism of Sphingomonas paucimobilis SYK-6. J. Wood Sci. 48:434-439. [Google Scholar]
- 40.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsou, A. Y., S. C. Ransom, J. A. Gerlt, D. D. Buechter, P. C. Babbitt, and G. L. Kenyon. 1990. Mandelate pathway of Pseudomonas putida: sequence relationships involving mandelate racemase, (S)-mandelate dehydrogenase, and benzoylformate decarboxylase and expression of benzoylformate decarboxylase in Escherichia coli. Biochemistry 29:9856-9862. [DOI] [PubMed] [Google Scholar]
- 43.Venturi, V., F. Zennaro, G. Degrassi, B. C. Okeke, and C. V. Bruschi. 1998. Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 144:965-973. [DOI] [PubMed] [Google Scholar]
- 44.Vicuña, R. 1988. Bacterial degradation of lignin. Enzyme Microbiol. Technol. 10:646-655. [Google Scholar]
- 45.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 46.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann, W. 1990. Degradation of lignin by bacteria. J. Biotechnol. 13:119-130. [Google Scholar]