Abstract
Context:
The onset of labor appears to involve the activation of myometrial inflammatory pathways, and transcription factors such as nuclear factor-κB (NF-κB) control expression of the contraction-associated proteins required to induce a procontractile phenotype. These responses might involve CRH, which integrates immune and neuroendocrine systems.
Objectives:
In human myometrium we investigated cyclooxygenase 2 (PGHS2) expression and regulation by CRH and the proinflammatory cytokine IL-1β before and after labor.
Design:
Myometrial tissues obtained from pregnant women at term before (n = 12) or during labor (n = 10) and pathological cases of choriamnionitis-associated term labor (n = 5) were used to isolate primary myocytes and investigate in vitro, CRH effects on basal and IL-1β regulated p65 activation and PGHS2 expression.
Results:
In nonlaboring myometrial cells, CRH was unable to induce NF-κB nuclear translocation; however, it altered the temporal dynamics of IL-1β-driven NF-κB nuclear entry by initially delaying entry and subsequently prolonging retention. These CRH-R1-driven effects were associated with a modest inhibitory action in the early phase (within 2 hours) of IL-1β stimulated PGHS2 mRNA expression, whereas prolonged stimulation for 6–18 hours augmented the IL-1β effects. The early-phase effect required intact protein kinase A activity and was diminished after the onset of labor. The presence of chorioamnionitis led to exaggerated PGHS2 mRNA responses to IL-1β but diminished effects of CRH.
Conclusions:
CRH is involved in the inflammatory regulation of PGHS2 expression before and during labor; these actions might be important in priming and preparing the myometrium for labor and cellular adaptive responses to inflammatory mediators.
At the end of human pregnancy, myometrial cells undergo a series of functional and structural changes that switch their quiescent phenotype, which is active throughout pregnancy and prevents inappropriate activation of the uterus, to a procontractile one that allows cells to respond to hormonal signals and mechanical forces that initiate active myometrial contractions and labor (1). In some pregnancies, this transition is inappropriately induced by infection before term, resulting in premature labor, which is associated with high fetal morbidity and mortality, especially if it occurs before 32 weeks of gestation.
It is now evident that labor, either term or preterm, involves activation of inflammatory responses (1, 2). Even in the absence of detectable infection, increased levels of proinflammatory cytokines such as IL-6, TNFα, and IL-1β are detected in the amnion, choriodecidua, and myometrium (3), possibly due to increased infiltration of the myometrium, cervix, and fetal membranes by neutrophils and macrophages (4). These invading immune cells secrete cytokines and chemokines to induce a sterile inflammatory process (5), activating nuclear factor-κB (NF-κB) and other proinflammatory transcription factors in the myometrium (6). Myometrial cells also appear to be involved in the development of the inflammatory phenotype and immunolocalization studies detected IL-1β expression in myocytes (7). NF-κB auto- or paracrine activation by proinflammatory cytokines increases expression of a cassette of genes encoding proteins that promote myometrial contractility (contraction associated proteins) such as the prostaglandin F2 receptor, the oxytocin receptor, and cyclooxygenase-2 (PGHS2) (8–11).
Several proinflammatory cytokines stimulate PGHS2 expression and downstream prostaglandin synthesis prior to parturition (12), with IL-1β actions most extensively investigated. The central role of this pathway is highlighted by the numerous mechanisms that control its activity: for example, proquiescent molecules such as progesterone receptor act to suppress it by rapid induction of inhibitory-κBα, a protein that blocks NF-κB transactivation (13). In addition, IL-1β activated NF-κB can regulate activity of the cAMP/protein kinase A (PKA) cascade by repressing myometrial Gαs and up-regulation of cAMP-phosphodiesterase-4 (14, 15) and thus switch signaling balance from myometrial quiescence toward contractility. Interestingly, a positive regulation of myometrial PGHS2 expression appears to involve cAMP-driven pathways (16), raising the possibility of additional roles for cAMP that might support development of myometrial contractility.
During pregnancy one potential target of IL-1β might be CRH. Placental CRH, which is under the control of IL-1β, has been implicated in the endocrine control of labor (17). Through the activation of CRH receptors (CRH-Rs) (18), placental CRH exerts important biological roles in the fetomaternal unit (19). In the myometrium (20–22), CRH, acting primarily via the type 1 CRH receptor (CRH-R1), appears to activate signaling pathways that promote relaxation, suggesting a role in the prevention of premature uterine activation. The myometrium also expresses type 2 CRH-R, and during labor its expression is significantly increased in the uterine fundus. Activation of this receptor leads to procontractile signals suggesting that locally produced CRH and urocortins might have multifaceted roles in the development of human parturition; before term CRH might act to prevent premature activation of the myometrium, whereas at term urocortins might coordinate the transition of the uterus from a state of relaxation to one of contraction (23–25).
There is increasing evidence suggesting cross talk mechanisms between the CRH/CRH-R system and mediators of myometrial inflammatory response. For example, IL-1β was identified as a regulator of myometrial CRH-R1 gene expression and functional activity (26). In addition, CRH has been shown to attenuate IL-1β in vitro effects on myometrial cell prostaglandin (PG) production (22). Most importantly the Extremely Low Gestational Age Newborns study, designed to determine whether placental CRH expression changes in extreme preterm labor, reported strong association between inflammation and low placental CRH expression (27). In this study, we investigated potential CRH-driven signaling mechanisms and transcriptional effects regulating PGHS2 expression in primary myometrial cell cultures as well as cross talk with proinflammatory mediators such as IL-1β. Myocytes were prepared from biopsies obtained from pregnant women at term before or during labor as well as myometrium from pathological cases of choriamnionitis-associated term labor.
Materials and Methods
Chemicals
Human/rat CRH was obtained from Bachem (United Kingdom) Ltd (Merseyside, United Kingdom). Mouse monoclonal vimentin antibody and antimouse IgG-tetramethylrhodamine isothiocyanate conjugate were obtained from Sigma Chemical Co (Poole, United Kingdom). Mouse monoclonal muscle-actin antibody was obtained from DAKO Ltd (Crawley, United Kingdom). Primary antibodies for p65, PGHS2, and β-tubulin were from Santa Cruz Biotechnology Ltd (Wembley, Middlesex, United Kingdom) and glyceraldehyde-3-phosphatedehydrogenase (GAPDH) was from MorphoSys UK Ltd (Kidlington, Oxford, United Kingdom). IL-1β was from Merck Biosciences (Nottingham, United Kingdom). The nuclear extraction kit was from Active Motif (Carlsbad, California). Secondary antibodies were from Dako Cytomation (Eye, United Kingdom); Alexa-Fluor 594, Alexa-Fluor 488-phalloidin, and Slow Fade gold antifade reagent with 4′,6′-diamidino-2-phenylindole were from Invitrogen (Life Technologies, Paisley, United Kingdom). Vectashield Hard Set TM mounting medium for fluorescence was from Vector Laboratories (Burlingame, California). Mounting solution without 4′,6′-diamidino-2-phenylindole was from Vector Laboratories (Peterborough, United Kingdom). Cell culture media were from Invitrogen. CellTiter 96 AQueous One Solution cell proliferation assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt], RNasin, avian myeloblastosis virus, random hexamers, and oligo-deoxytocopherol transfer protein were from Promega (Madison, Wisconsin), and deoxynucleotide triphosphates, Taq polymerase, and the DNA ladder were purchased from Bioline (London, United Kingdom). SYBR Green I and the PCR mixture for quantitative PCR were from BioGene (Kimbolton, United Kingdom). Primers were purchased from TANG (Gateshead, United Kingdom). Serine 536-phosphorylated p65 antibody was from Cell Signaling Technology Inc (Danvers, Massachusetts). The RNeasy plant total RNA kit for polyadenylated RNA isolation was obtained from QIAGEN Ltd (Crawley, United Kingdom). CRH-R1 small interfering RNA (siRNA), CRH-R antagonists antisauvagine 30, K41498 and NBI 27914, and all other chemicals were purchased from Sigma Chemical Company Ltd. The anti-CD68 antibody was from DAKO UK Ltd (Ely, Cambridgeshire, United Kingdom), and the anti-CD45 antibody was from AbD Serotec (Kidlington, United Kingdom).
Experimental subjects and sample preparations
Myometrial tissues obtained from pregnant women undergoing elective caesarean section for nonmaternal problems (fetal distress) at term before (n = 12) or during the onset of labor (n = 10) or pathological cases of choriamnionitis-associated term labor (n = 5). The presence of infection and chorioamnionitis during labor was diagnosed on the basis of clinical findings, namely fever, uterine fundal tenderness, maternal tachycardia (>100 m/min), fetal tachycardia (>160 m/min), raised C-reactive protein, and maternal leukocytosis. The biopsy site was standardized to the upper margin of the lower segment of the uterus in the midline. The relative content of myometrial and fibrous tissue in these biopsies was identified by immunofluorescent labeling using selective smooth muscle cell and fibroblast markers (actin and vimentin, respectively). The biopsies were immediately processed for myocyte cell culture. Ethical approval was obtained from the local ethical committee and written informed consent to the study was obtained from all patients.
Immunohistochemistry-quantification of inflammatory cell density
Leukocytes and macrophages were identified using primary antibodies directed against CD45 (the common leukocyte antigen) and CD68, respectively, in myometrial biopsy obtained before and during labor as previously described (7). Sections were 8 μm thick and were fixed in cold acetone. The antibodies (1:20 and 1:10 for CD68 and CD45, respectively) were incubated for 1 hour at room temperature, and the antigen was localized as a brown end product using the Novolink Polymer detection system (Leica Microsystems, Milton Keynes, United Kingdom) following the manufacturers' instructions. Negative controls included slides incubated without the primary antibody.
Following immunohistochemistry, the inflammatory cells were identified by histological analysis. The number of cell transects in 10 randomly selected high power fields (×400 objective magnification) was quantified by 2 independent observers for each specimen blinded as to whether the tissues were from a laboring or nonlaboring source. Inflammatory cells within the blood vessels were not included in the counts. The median density of positive cells for each specimen was calculated.
Preparation of myometrial cell cultures and explants: treatments
Myocytes were prepared by enzymatic dispersion as previously described (25). The cells were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2 until confluent (approximately 2 weeks). The purity of myometrial muscle cells was assessed by immunocytochemical staining (25). To minimize fibroblast contamination, the myocyte preparation was repurified 48 hours before the experiments using 0.5% trypsin. Cells were transferred to media lacking fetal calf serum 18 hours prior to treatments. The cells were treated with various concentrations of CRH in the presence or absence of 1 ng/mL IL-1β for 2, 6, 12, or 18 hours. In some experiments, cells were preincubated with 1 μM PKA inhibitor 14–22 amide (myristoylated) for 30 minutes or the CRH-R antagonists, antisauvagine 30, or NBI 27914 (1 μM for 1 hour) or K41498 (500 nM for 2 hours). Experiments on myometrial explants were carried out using previously described protocols (28). Myometrial tissue samples were dissected into explants of 3 mm3 and placed onto 0.2-μm polycarbonate filters floating on 2 mL of appropriate media in 6-well plates and cultured overnight at 37°C in 5% CO2, 95% air (5 explants per well). Explant viability was confirmed by histological examination for necrotic cells and maintenance of smooth muscle specific marker expression and RNA integrity (data not shown).
RNA extraction and real-time RT-PCR
Total RNA was extracted from myometrial cell cultures by an RNeasy total RNA kit and reverse transcribed to synthesize cDNA by using RNase H Reverse Transcriptase (Life Technologies). Quantitative PCR was used to determine the relative expression levels of CRH-R1 mRNA between groups as previously described (26) on a TaqMan gene expression assay (Applied Biosystems, Warrington, United Kingdom). The oligonucleotide primers and TaqMan probe were predesigned from the GenBank database by mySciences (Applied Biosystems). The primers/probe combination used amplified a 61-bp sequence overlapping exons 8–9 of CRH-R1 mRNA. Real-time RT-PCRs were performed using the ABI PRISM 7000 sequence detection system (Applied Biosystems) in a total volume of 30 μL reaction mixture following the protocol of the manufacturer, using the SYBR Green Universal 2 × PCR master mix (Applied Biosystems) and 0.1 μM of each primer using the dissociation protocol for the amplification of CRH-R1 mRNA. Negative controls containing water instead of first-strand cDNA were also used. Each sample was normalized on the basis of its 18S ribosomal RNA content (housekeeping gene, assay identification number Hs 99999901_s1). The 18S quantification was performed with a TaqMan rRNA reagent kit (Applied Biosystems) using the protocol of the manufacturer. All samples were run in triplicate, and results were calculated with reference to the amplification of 18S rRNA using comparative threshold cycle (CT) method for relative quantitation (ABI Prism 7000 SDS, version 1.1 software; Applied Biosystems). The results were expressed as mean ± SEM.
Relative gene expression of target mRNA was normalized to a calibrator that was chosen to be the control or basal condition (untreated sample). Results were calculated with the ΔΔCT method; they were expressed as the n-fold differences in gene expression relative to 18S rRNA and calibrator and were determined as follows: n-fold = 2-(ΔCT sample − ΔCT calibrator), where the parameter CT is defined as the fractional cycle number at which the PCR reporter signal passes a fixed threshold. The ΔCT values of the sample and calibrator were determined by subtracting the average CT value of the transcript under investigation from the average CT value of the 18S rRNA gene for each sample.
For PGHS2 mRNA expression, the Roche Light Cycler system (Roche Molecular Biochemicals, Mannheim, Germany) was used. The PCR was performed in a 10-μL reaction mixture containing 5 μL of PCR 2× master mix with 2 mM MgCl2, 0.5 μL of Light Cycler DNA Master SYBER Green I (Roche, Mannheim, Germany), 1 μL of each primer (2 ng/μL), and 1 μL of cDNA. The PCR protocol consisted of a denaturation step at 95°C for 15 seconds, followed by 40 cycles of amplification at 95°C for 5 seconds, 58°C for 10 seconds, and 72°C for 15 seconds, and finally by a melting-curve analysis step at 56°C for 15 seconds. For analysis, quantitative amounts of gene of interest were standardized against the housekeeping gene β-actin. The sequence of PGHS2 mRNA primers used were as follows: forward, 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′; and reverse, 5′-AGATCATCTCTGCCTGAGT ATCTT-3′. The β-actin primers were as follows: forward, 5′-AAGAGAGGCATCCTCACCCT-3′; and reverse, 5′-TACATGGCTGGGGTGTTGAA-5′. As negative controls, preparations lacking RNA or reverse transcriptase were used. RNAs from at least 3 independent biological replicates were assayed in triplicate. The RNA levels were expressed as a ratio, using the ΔΔCT method for comparing relative expression results between treatments in real-time PCR. In preliminary validation experiments, the efficiency of the PCR amplification reaction was determined for both target and control (housekeeping) genes, using a dilution series of cDNA and the average amplification approach as previously described (29, 30).
The CT values were plotted against cDNA input and efficiency calculated from the slope of the linear regression line according to the equation E = 10(−1/slope). The calculated amplification efficiencies were 1.96 ± 0.01 for CRH-R1, 1.98 ± 0.02 for PGHS2, 2.07 ± 0.02 for 18S, and 1.99 ± 0.01 for β-actin, with R2 greater than 0.988 for all PCRs. In parallel experiments the linearity of the PCR amplification of all targets and control genes was confirmed by using a series of 5-log dilutions of the cDNA template (1:5–1:50 000) from both untreated and treated cells. The analytical specificity of the PCR reactions and ability to detect the appropriate target sequence rather than nonspecific targets was determined in house via either in silico specificity screen by using BLAST nucleic acid database searches from the National Centre for Biotechnology Information, or agar gel electrophoresis of PCR products that demonstrated the absence of amplification of additional nonspecific DNA fragments (data not shown). The identity of amplified DNA was confirmed by direct sequencing in an automated DNA sequencer, and the sequence data were analyzed using Blast nucleic acid database searches from the National Centre for Biotechnology Information.
CRH-R1 gene silencing
Knockdown of CRH-R1 was performed on cells at 60% confluency cultured in 6-well plates, using oligonucleotides probes according to the manufacturer's protocols. Three separate 21-nucleotide siRNA probes annealing to nucleotide sequences 1428–1448, 1934–954, and 1125–1145, respectively (final concentration 0.05 μM) were individually mixed with 3 μL Lipofectamine 2000 (Invitrogen) and transfected into myocytes. A negative siRNA with no homology to any known gene sequence was used as a control. After 4 hours the transfection medium was replaced by fresh culture medium and cells were incubated for further 48h and then the efficacy of CRH-R1 knockdown was assessed by quantitative RT-PCR as described above. Cell toxicity effects were monitored by the CellTiter 96 AQueous One Solution cell proliferation assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; Promega]; in a typical transfection experiment, cell viability was greater than 80% (data not shown).
Western blot analysis and confocal microscopy
Proteins from cell lysates were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride filter. The filter was then blocked in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% milk (wt/vol), for 1 hour at room temperature. After a brief wash with TBS-0.1% Tween 20, the polyvinylidene difluoride membranes were incubated with primary antisera. The primary antibody for PGHS2 and p65 Ser536 was used at a 1:1000 dilution in TBS-0.1% Tween 20 at room temperature for 1 hour. Anti-GAPDH was used in 1:40 000 dilution in TBS-0.1% Tween 20 at room temperature for 1 hour. The filters were washed thoroughly for 30 minutes with TBS-0.1% Tween 20 before incubation with the appropriate secondary antibody-horseradish peroxidase (1:2000) for 1 hour at room temperature and further washing for 30 minutes with TBS-0.1% Tween 20. Antibody complexes were visualized using the enhanced chemiluminescence reagent. In some experiments, nuclear extracts were prepared from myocytes by using the nuclear extract kit and following the manufacturer's instructions (Active Motif). Expression of p65 and TATA-binding protein (TBP) was determined by Western blotting using overnight incubation at 4°C with a selective antibody (1:100 and 1:500, respectively). Potential contamination of the nuclear extract with cytoplasmic proteins was assessed by Western blotting of β-tubulin using overnight incubation at 4°C with a selective antibody (1:500). For confocal microscopy, myocytes were grown on polylysine-treated glass coverslips, and p65 immunoreactivity was detected as previously described (26).
Statistical analysis
Data are shown as the means ± SEM of each measurement. Data were tested for homogeneity, and comparison between group means was performed by 1- or 2-way ANOVA. P < .05 was considered significant.
Results
Expression profiling of CRH-R1 and PGHS2 mRNA in laboring myometrium associated with fetal membrane infection
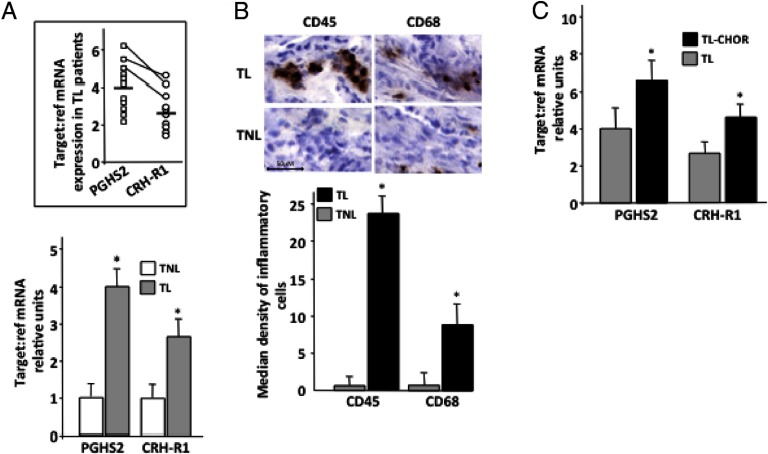
In agreement with previous studies (26, 31), real-time RT-PCR experiments in myometrial term tissue samples obtained from patients in labor (n = 10), demonstrated increased expression of both CRH-R1 and PGHS2 mRNAs compared with myometrium obtained from nonlaboring patients (n = 12) (Figure 1A). Interestingly, in the cohort of patients tested, there was a close correlation in the mRNA expression levels, and patients with the highest CRH-R1 mRNA exhibited parallel very high PGHS2 mRNA expression levels (Figure 1A, inset). Both leukocyte and macrophages were identified in all laboring biopsies examined, whereas the nonlaboring myometrial biopsies exhibited very little or no staining for the specific antigens CD45 and CD68 (Figure 1B). Formal comparison of cell numbers in 10 biopsies per group showed that total leukocyte and macrophage density was substantially greater in laboring vs nonlaboring biopsies.
Figure 1.
Myometrial CRH-R1 and PGHS2 mRNA expression and inflammatory cell infiltration levels in relation to progression toward the onset of labor (A and B) or in infection-associated labor (C). Relative expression was determined in myometrial tissues obtained from pregnant women undergoing elective cesarean section for nonmaternal problems at term before (n = 12) or during the onset of labor (n = 10) or pathological cases of choriamnionitis-associated term labor (n = 5). For real-time quantitative RT-PCR (A and C) experiments, Taqman (Applied Biosystems) probes and specific primers for CRH-R1 and the Roche LightCycler (Roche Molecular Biochemicals) for PGHS2 mRNA determination were used. RNAs from at least 3 independent biological replicates were assayed in triplicate. The RNA levels were expressed as a ratio, using the δ-δ method for comparing relative expression in real-time PCR. Results are expressed as the group's mean ± SEM of relative RNA expression normalized against the relevant reference gene, 18sRNA (for CRH-R1) or β-actin (for PGHS2). *, P < .05 compared with term nonlaboring (A) or laboring without infection (C). The mean results of nonlaboring term myometrium (A) or laboring term myometrium without infection (C) were arbitrarily assigned the value 1. TL, term laboring; TL-CHO, term laboring with chorioamnionitis; TNL, term nonlaboring. Inset, Individual CRH-R1 and PGHS2 mRNA expression levels in TL myometria in relation to the group's mean. Connecting lines identify results from the same patient. B, Inflammatory (leukocyte, macrophage) cell density using antibodies against CD45 or CD68 was determined in biopsies before or after the onset of labor. The background is hematoxylin counterstain. The antigen was localized using 1 mg/mL diaminobenzidene tetrahydrochloride (DAB), which appears as a brown end product. Sections were then counterstained with Harris hematoxylin (Sigma). After immunohistochemistry, the number of inflammatory cells were identified by histological analysis and was quantified in 10 randomly selected high power fields (×400 objective magnification). The median density of positive cells for each specimen was calculated. Representative images are shown. The data represent the mean ± SEM of 3 estimations from 10 patients. *, P < .05 compared with nonlaboring myometrium. Scale bar, 50 μm.
The presence of chorioamnionitis during labor, a condition previously known to alter CRH and CRH-R expression in fetal-maternal tissues and myometrial PGHS2 expression (32, 33), was associated with a further increase in the expression of both mRNAs in the laboring myometrium (Figure 1C).
Effects of CRH on p65 activation and transcriptional regulation of PGHS2 expression
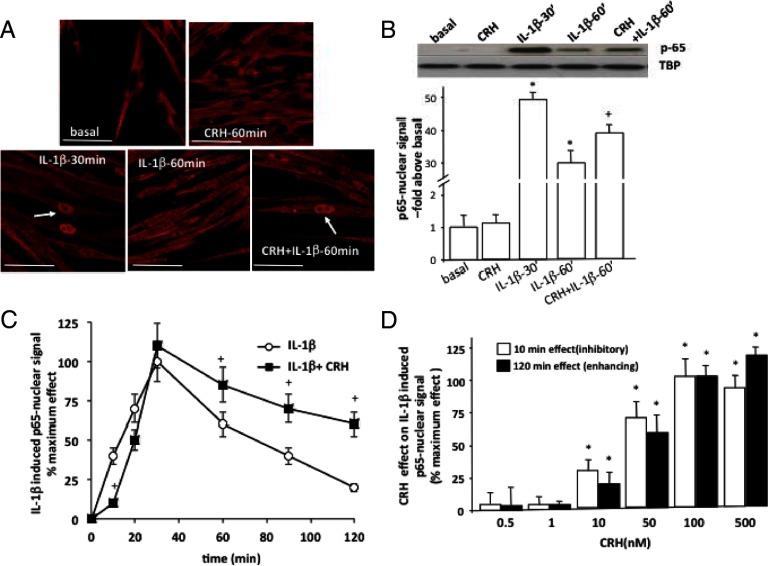
It has previously been shown that NF-κB is a key regulator of myometrial PGHS2 and CRH-R1 mRNA expression, up-regulated in response to proinflammatory signals such as IL-1β (26, 34, 35). Potential direct effects of CRH on myometrial NF-κB activation were next investigated by monitoring p65 (RelA) nuclear localization with confocal microscopy in primary smooth muscle cells prepared from term myometrial samples from nonlaboring patients. These experiments showed that in untreated cells, p65 immunoreactivity was primarily found in the cytoplasm and nuclear staining was minimal (Figure 2A, top). Exposure of myometrial cells to CRH (100 nM) for up to 1 hour failed to stimulate nuclear translocation dynamics of p65. We next investigated whether CRH was able to modify activated p65 nuclear translocation after IL-1β stimulation. As previously shown (26), IL-1β (1 ng/mL) treatment of primary myocytes for 30 minutes induced a robust increase in p65 translocation to the nucleus. This effect was evident for at least 60 minutes, although p65 nuclear signal in the confocal images appeared reduced compared with 30 minutes immunostaining signal. Qualitative inspection of cells simultaneously treated with IL-1β and CRH suggested an amplified p65 nuclear retention (Figure 2A, bottom). Immunodetection of p65 signal in nuclear extracts was also used to provide a quantitative assessment of these effects (Figure 2B); in untreated cells, nuclear p65 immunoreactivity was almost undetectable and substantially increased after IL-1β treatment. After 60 minutes of stimulation, significantly more p65 was retained in the nucleus when myocytes were simultaneously treated with IL-1β (1 ng/mL) and 100 nM CRH compared with IL-1β alone. A detailed investigation of the temporal characteristics of the p65 signal in nuclear extracts from myocytes treated with either IL-1β alone or IL-1β with CRH for 10–120 minutes demonstrated that at early time points (10–20 minutes), CRH delayed p65 nuclear translocation, and although it did not alter maximum nuclear localization (achieved at 30 minutes), it significantly prolonged the amount of p65 retained in the nucleus for up to 2 hours (Figure 2C). Both early inhibitory and potentiating effects of CRH were dose dependent and were detectable at concentrations greater than 10 nM and reached maximum at similar agonist concentrations around 100 nM (Figure 2D). This concentration range is significantly higher than circulating CRH levels during pregnancy; however, CRH is also produced by the human myometrial cells (36), and therefore, it is possible that the local CRH concentrations of the myometrial microenvironment are within the concentration range experimentally tested.
Figure 2.
Role of CRH on basal and IL-1β-induced NF-κB activation in term nonlaboring myometrial cells. Primary cells were isolated from myometrial tissues obtained from pregnant women undergoing elective cesarean section for nonmaternal problems at term before the onset of labor. Cells were treated with CRH (100 nM) alone or with IL-1β (1 ng/mL) for various time intervals (0–120 minutes). A, p65 (RelA) nuclear translocation was monitored by indirect immunofluorescence confocal microscopy as described in Materials and Methods. White arrows indicate examples of increased p65 nuclear signal. In some experiments (panels B–D), p65 was detected by immunoblotting in nuclear cell extracts from cells treated with IL-1β (1 ng/mL) alone or in combination with CRH (100 nM). The TBP protein was used as a loading control. In some experiments (panel D), cells were treated with different concentrations of CRH (0.5–500 nM) for 0–120 minutes. Representative images (panels A) or Western blots (panel B) are shown. The data represent the mean ± SEM of 3 estimations from at least 5 patients. *, P < .05 compared with basal (untreated); +, P < .05 compared with IL-1β alone. Scale bar, 20 μm.
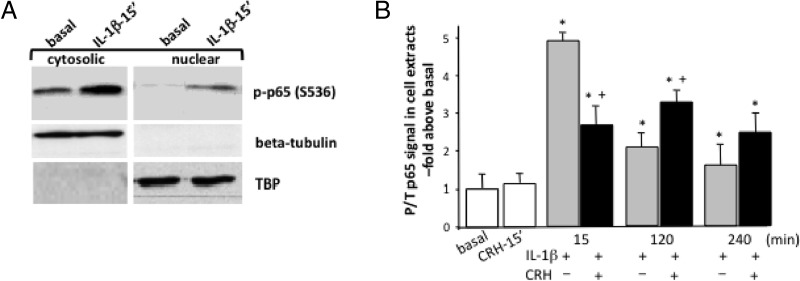
Because phosphorylation of p65 at the specific phosphoacceptor residues including serine 536 is an important modification for subsequent NF-κB transcriptional activity (37), we investigated CRH effects on basal and IL-1β-induced phosphorylation of p65. Although CRH had no effect on p65 S356 phosphorylation (Figure 3A), IL-1β treatment of myometrial cells led to a robust phosphorylation of p65 within 15 minutes. IL-1β-induced phospho-p65 was primarily localized in the cytoplasm, although some immunoreactivity was also detected in the nuclear fraction. Investigation of the temporal characteristics of p65 phosphorylation at S536 in whole-cell extracts from cells treated with IL-1β revealed detectable phospho-p65 signal after 2 and 4 hours, although the immunoreactive signal was reduced by 50%–60% compared with 15 minutes signal (Figure 3B). The presence of CRH significantly reduced by 40%–50% IL-1β effects on early (15 minutes) p65 phosphorylation levels and increased by 30% the amount of phosphorylated p65 present at the 2-hour time point (Figure 3B), suggesting prolonged activation. In contrast, there was no difference in p65 phosphorylation at S536 after 4 hours of stimulation with either IL-1β or IL-1β+CRH.
Figure 3.
Role of CRH on IL-1β-induced p65 phosphorylation at Ser536 in term nonlaboring myometrial cells. Primary cells were isolated from myometrial tissues obtained from pregnant women undergoing elective cesarean section for nonmaternal problems at term before the onset of labor. Cells were treated with CRH (100 nM) or IL-1β (1 ng/mL) or both for various time intervals (15 or 120 minutes); p65 S536 phosphorylation was monitored in nuclear or cytosolic fractions (A) or total cell extracts (B) by immunoblotting using specific antibodies as described in Materials and Methods. Total p65 was detected by immunoblotting. The nuclear protein TBP-protein was used as a loading control, and β-tubulin was used as a marker of cytoplasmic protein contamination. Representative Western blots are shown. The data represent the mean ± SEM of 3 estimations from at least 5 patients. *, P < .05 compared with basal (untreated); +, P < .05 compared with IL-1β alone.
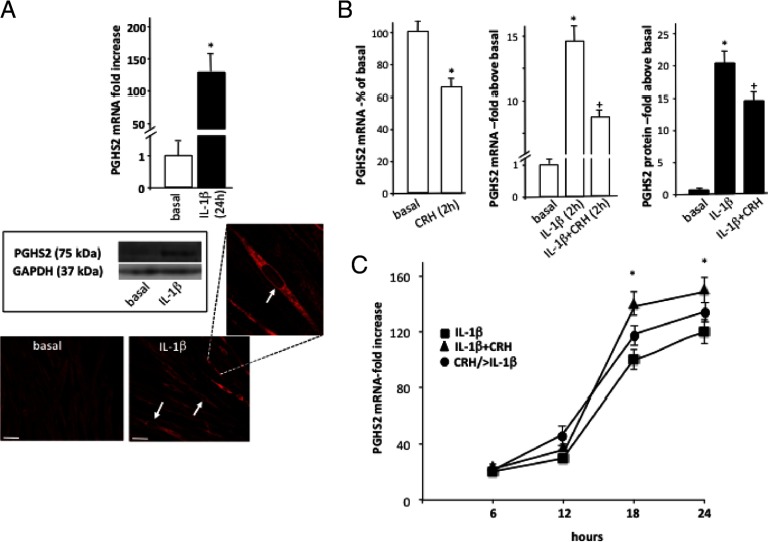
The effects of CRH on p65 nuclear localization and S536 phosphorylation prompted us to investigate the impact on the temporal characteristics of IL-1β regulation of myometrial PGHS2 expression. In agreement with previous studies (26), quantitative RT-PCR and indirect immunofluorescent confocal microscopy confirmed that treatment of myocytes with IL-1β (1 ng/mL) for 18 hours led to a substantial up-regulation of PGHS2 mRNA and protein, evident by the substantial increase of PGHS2 intracellular immunofluorescent signal (Figure 4A). PGHS2 immunoreactivity was primarily cytoplasmic, although IL-1β treatment induced strong PGHS2 staining in the perinuclear region. This pattern of localization has been previously reported in various types of cells (38) and appears to be important for PG synthesis. Short treatment of cells with 100 nM of CRH for 1–2 hours attenuated basal and IL-1β-stimulated PGHS2 mRNA by 50% (Figure 4B). Similar inhibitory actions were observed in PGHS2 protein levels, although the CRH effect was modest (25% reduction) (Figure 4B, right). In contrast, prolonged exposure of cells to 100 nM CRH for 6–18 hours had no effect on basal PGHS2 mRNA expression (data not shown), although simultaneous stimulation of cells with CRH and IL-1β revealed a strong potentiating effect on IL-1β-induced PGHS2 expression by 60%–90% (Figure 4C). Moreover, this effect did not require simultaneous activation of CRH and IL-1β signaling pathways because priming of myocytes by pretreatment with 100 nM CRH for 20 hours before stimulation with IL-1β also led to enhanced IL-1β responses (Figure 4C).
Figure 4.
Regulation of PGHS2 expression by IL-1β and CRH in human term nonlaboring myometrial cells. Primary cells were isolated from myometrial tissues obtained from pregnant women undergoing elective cesarean section for nonmaternal problems at term before the onset of labor. Subsequently cells were treated with IL-1β (1 ng/mL) for 18 hours (A), and PGHS2 mRNA levels were determined by real-time quantitative RT-PCR. Relative mRNA expression levels were normalized against β-actin mRNA. Data are expressed as mean values ± SEM of 3 estimations from at least 4 patients. *, P < 0.05 compared with basal (untreated) values. PGHS2 protein expression was also determined by immunoblotting (inset) in cell extracts and cellular distribution by indirect confocal microscopy using Alexa-Fluor 594 secondary antibody (red). White arrows indicate examples of perinuclear staining. GAPDH was used as a protein loading control. Similar results were obtained in 4 independent cell preparations. Individual groups of cells were identically processed, and the microscope settings were identical throughout. In some experiments (B), cells were stimulated with 100 nM CRH, IL-1β (1 ng/mL), or both for 2 hours, and PGHS2 mRNA and protein were determined by quantitative RT-PCR or Western blotting. Alternatively, PGHS2 mRNA expression was determined in cells stimulated with IL-1β (1 ng/mL), with or without 100 nM CRH, for 6–24 hours or in cells pretreated with CRH for 20 hours before stimulation with IL-1β (1 ng/mL) for 6–24 hours (CRH/> IL-1β) (C). The data represent the mean ± SEM of 3 estimations from 5 independent primary cell preparations. *, P < .05 compared with basal (untreated); +, P < .05 compared with IL-1β alone.
CRH-R subtypes and signaling molecules mediating CRH effects on transcriptional regulation of PGHS2 expression
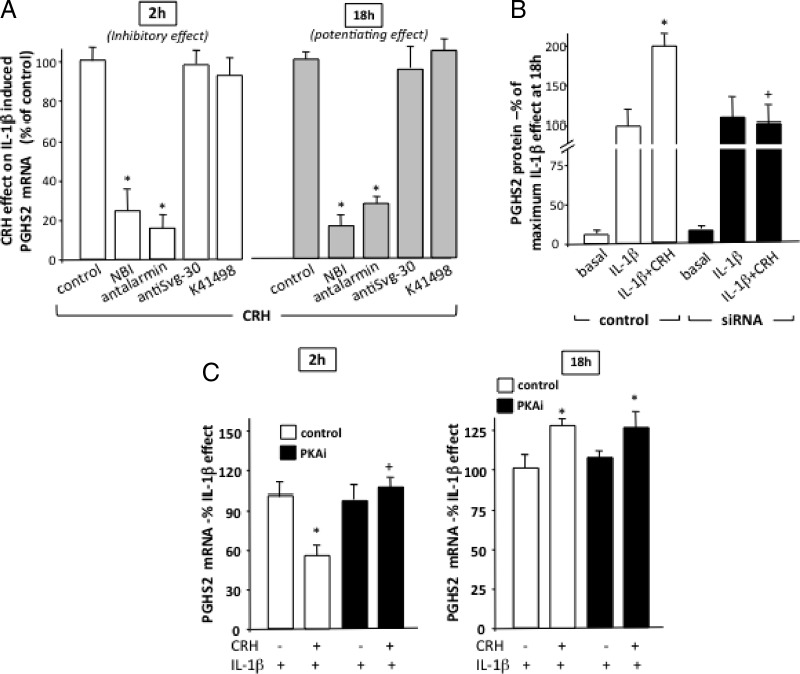
To identify the specific CRH-R subtype(s) that mediate CRH effects, myocytes were pretreated with specific CRH-R1 nonpeptide antagonist NBI 27914 and the CRH-R2 antagonists antisauvagine 30 and K41498. Both CRH-R1 antagonists substantially impaired the early-phase inhibitory action of CRH (Figure 5A, left) as well as the potentiating effects on PGHS2 mRNA expression after prolonged exposure to IL-1β (Figure 5A, right). In contrast, neither antisauvagine 30 nor K41498 treatment affected CRH actions, suggesting that CRH-R1 is the primary receptor subtype involved in the regulation of myometrial PGHS2 mRNA expression. To confirm these findings, a complementary approach was used to knock down CRH-R1 expression by overexpression of siRNA oligonucleotide duplexes into cultured myometrial cells. In transfected cells there was a 60%–80% reduction in CRH-R1 mRNA expression 48 hours after transfection (data not shown), and CRH effects on IL-1β-induced PGHS2 protein expression were substantially impaired (Figure 5B), confirming involvement of CRH-R1.
Figure 5.
CRH-R subtypes and PKA involved in regulation of IL-1β-induced PGHS2 expression in human term nonlaboring myometrial cells. Primary cells were isolated from myometrial tissues obtained from pregnant women at term before the onset of labor. Subsequently, cells were pretreated with specific CRH-R antagonists for 1–2 hours (A), specific CRH-R1 siRNA oligonucleotide probes as described in Materials and Methods (B), or the specific PKA inhibitor peptide 14-22 amide (myristoylated) (1 μM for 1 hour). Subsequently, cells were treated with IL-1β (1 ng/mL) alone or with 100 nM CRH for the indicated time period, and PGHS2 mRNA and protein levels were determined by real-time quantitative RT-PCR or immunoblotting. Relative mRNA expression levels were normalized against β-actin mRNA and GAPDH was used as a protein loading control. Data are expressed as mean values ± SEM of 3 estimations from at least 4 patients and are presented as a percentage change of maximum CRH effect on IL-1β-induced PGHS2 mRNA (inhibitory at 2 hours or potentiating at 18 hours)(A) or a percentage change of maximum IL-1β effect on PGHS2 protein (B) or mRNA (C). *, P < 0.05 compared with control (no inhibitor) (A) or IL-1β alone (B and C) values; +, P < .05 compared with IL-1β alone.
Because myometrial CRH-R1s are coupled to the cAMP/PKA signaling cascade, the role of this pathway on CRH/IL-1β interactions was further investigated. Under conditions of PKA inhibition (induced by cell treatment with the selective PKA inhibitor, 14-22 amide myristoylated), CRH was unable to attenuate the early-phase stimulation of PGHS2 mRNA by IL-1β (Figure 5C, left). However, PKA inhibition had no effect on the CRH potentiating effects on PGHS2 mRNA expression during the prolonged exposure to IL-1β (Figure 5C, right).
CRH-R regulation of PGHS2 expression in laboring myometrium
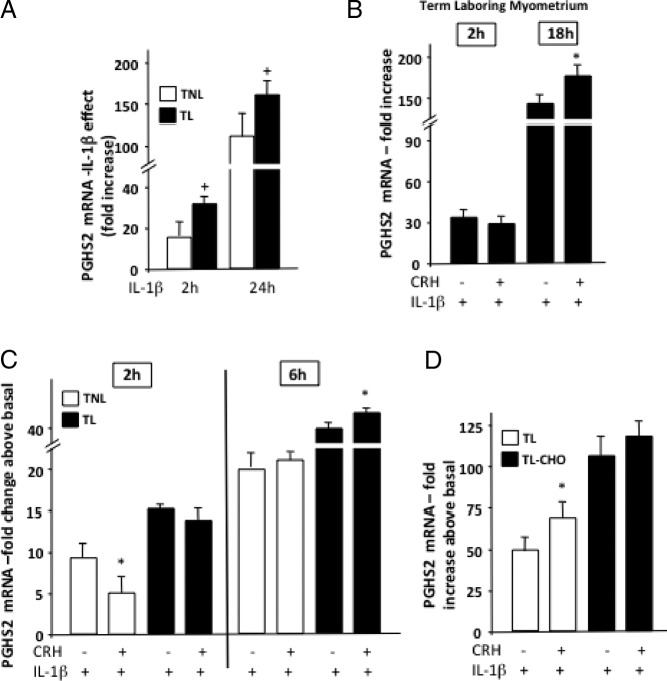
The onset of labor alters the CRH-R1 pattern of expression and ability to activate the cAMP/PKA cascade (23, 26, 39). Therefore, we explored the possibility that in laboring myocytes, CRH exerts distinct effects on PGHS2 expression. In primary myometrial cultures prepared from laboring tissue, IL-1β was significantly more potent in stimulating PGHS2 mRNA expression compared with nonlaboring myocytes (Figure 6A). Investigating CRH/IL-1β interactions in laboring myocytes identified important differences in PGHS2 responses: although potentiating actions on IL-1β effects were still present, CRH exhibited very small nonsignificant inhibitory effects during the early phase of IL-1β-induced PGHS2 mRNA expression (Figure 6B). Similar results were obtained when myometrial explants from nonlaboring and laboring myometrium were used (26) and treated with IL-1β alone or with CRH for either 2 or 6 hours (Figure 6C). Interestingly, myometrial explants from pathological cases of choriamnionitis-associated term labor demonstrated exaggerated PGHS2 mRNA responses to IL-1β but diminished effects of CRH (Figure 6D) compared with term laboring explants.
Figure 6.
Effect of CRH on IL-1β-induced regulation of PGHS2 expression in human term nonlaboring and laboring myometrial cells. Primary cells were isolated from myometrial tissues obtained from pregnant women at term before or after the onset of labor (A and B). In some experiments myometrial explants were prepared as described in Materials and Methods from term nonlaboring or laboring biopsies with or without chorioamnionitis (C and D). Cells or explants were stimulated with IL-1β (1 ng/mL) alone or with 100 nM CRH for the indicated time period, and PGHS2 mRNA levels were determined by real-time quantitative RT-PCR. Data are expressed as mean values ± SEM of 3 estimations from at least 3 patients and are presented as fold increase above basal (A–D). *, P < 0.05 compared with IL-1β alone (B–D) values; +, P < .05 compared with nonlaboring (A).
Discussion
Mediators of sterile inflammatory responses are emerging as important components of the pathophysiological mechanisms controlling myometrial contractile machinery (1, 2, 5). In agreement with this, we found that laboring myometrium is characterized by increased expression of PGHS2 and parallel increases in CRH-R1 expression, a receptor thought to be involved in the regulation of myometrial inflammatory responses. Further increases in expression levels were observed in myometria from infection (chorioamnionitis)-complicated labor, suggesting responses to peripartum proinflammatory phenomena. However, the appropriate interpretation of these data is complicated by the finding that labor is associated with increased levels of mononuclear inflammatory cells present in myometrium such as leukocytes and macrophages, cells known to express CRH-R and inflammatory machinery, raising the possibility that the observed differences could be related to the relative amounts of invading inflammatory cells. We also investigated the potential of CRH to modulate inflammatory signaling cascades and downstream expression of inflammation-sensitive contraction-associated proteins in isolated primary myometrial cells. Inflammatory molecules such as IL-1β are detected in myocytes and autocrine or paracrine stimulation by proinflammatory cytokines leads to the up-regulation of PGHS2 expression and downstream prostaglandin synthesis prior to parturition (12).
The first novel finding is that CRH was unable to directly activate the NF-κB cascade. This is in agreement with previous studies that failed to detect a role for CRH in the control of proinflammatory cytokine and prostaglandin expression in human pregnant myometrium (22, 40). Despite the inability to induce basal p65 phosphorylation at S536 and nuclear translocation, CRH altered the temporal characteristics of p65 activation (phosphorylation) and nuclear translocation and retention, exerting actions that initially delayed IL-1β induced early p65 nuclear entry at 10–20 minutes and enhanced p65 retention in the nucleus during prolonged IL-1β stimulation. In parallel, CRH altered the profile of IL-1β induced S536 phosphorylation of p65. Alterations in the temporal dynamics and patterns of oscillations in the p65 nuclear content are a key mechanism that controls duration, intensity, and selectivity of downstream biological responses (41). This modification appeared to have important functional consequences for NF-κB-mediated transcriptional activity of IL-1β and in particular in the regulation of myometrial PGHS2 expression.
CRH actions on IL-1β-induced p65 nuclear entry mirrored effects on PGHS2 expression by attenuating early activation of PGHS2 mRNA and protein expression and acting in a complementary fashion to enhance later IL-1β transcriptional effects through activation of CRH-R1. At present, the relationship between the temporal control of IL-1β-induced nuclear p65 entry and functional consequences for development of a myometrial acute inflammation gene expression signature is not well understood. It is conceivable that this switch to a procontractile phenotype is critical for cells ability to respond to hormonal signals and mechanical forces. In vitro studies identified at least 198 transcriptional targets of IL-1β in myometrial cells that are rapidly up-regulated within 1 hour of IL-1β exposure (42). These include transcription factors such as NF-κB and inflammatory response genes, up-regulation of chemokines, and synthesis of prostaglandins and extracellular matrix remodeling signaling molecules. However, during this stage, expression of key molecules required for contraction (ie, oxytocin receptor and connexin 43) does not change, suggesting that the early stages of IL-1β action promote an intermediate cellular phenotype between the quiescent and activated states and a cellular environment favorable for myometrial cell contraction.
Our results implicate CRH in this process and suggest important, albeit indirect, roles that possibly allow myometrial cells to adapt to increasing activity of inflammatory cytokines that induce expression of PGHS2 expression, which represents a major step toward myometrial prostaglandin formation. In fact, PGHS2 expression is not the only enzyme regulated by IL-1β, which appears to target the myometrial prostaglandin biosynthetic pathways by stimulating the expression of cytosolic phospholipase A2 and cytosolic PGE synthases (cPGES or PGES-1), enzymes whose expression has been found to increase in human myometrium with the onset of labor (34). The observed dual acute inhibitory-late enhancing action of CRH on IL-1β-induced PGHS2 expression might allow the controlled increase in PGHS2 expression because the uterus is preparing for the onset of labor to prevent uncontrolled expression, especially in the presence of active proinflammatory signals. It should be noted that the pattern of placental CRH secretion suggests that because the myometrium is exposed to increasing levels of CRH at the end of pregnancy, this is most likely associated with persistent activation of CRH-R1. Hence, the short term (inhibitory) effects of CRH might represent a more transient response, possibly relevant only during the myometrial priming before the onset of labor when the cAMP/PKA pathway is still active. Our studies on isolated cells and explants from laboring myometrium suggest that during active labor, the inhibitory actions of CRH are abolished, whereas the potentiating effects of CRH are still intact and enhance IL-1β effects. By up-regulating myometrial CRH-R1 expression (26), IL-1β can further augment these interactions through development of a positive feedback loop.
These observations identified a crucial role for PKA for guiding signaling direction of CRH toward attenuation of early-phase IL-1β induction of PGHS2 mRNA and might involve the association of myometrial p65 (RelA) with PKAcα (as well as IκBα) through an interaction that regulates the phosphorylation of p65 as previously suggested (43). This association might control the p65 active-inactive state until the cell is exposed to p65-activating stimuli such as IL-1β. Interestingly, recent reports suggest a positive regulation of myometrial PGHS2 expression via cAMP-driven but PKA-independent pathways (16), which highlights the complexity of the signaling pathways involved in the transcriptional control of PGHS2. We have previously shown that myometrial CRH-R immunoreactivity is localized in discrete microdomains (26); therefore, it is likely that the spatial characteristics and local availability of appropriate components of the signaling machinery such as PKA would determine signal propagation and overall cellular responses. After the onset of labor, CRH-inhibitory effects are diminished to promote enhanced activity of IL-1β on PGHS2 expression, this might be directly relevant to the reduced particulate type II PKA expression and activity observed in laboring myometrium (44), although altered patterns of CRH-R subtype expression and G-proteins activation associated with the onset of labor (45) might also play a contributing role. Such molecular interactions might contribute in the exaggerated responses that increase CRH-R1 mRNA expression in choriamnionitis-associated pathological myometrium. The presence of infection such as in cases of chorioamnionitis appears to increase CRH-R1 and CRH expression in other fetomaternal tissues (32). Hence, although clinical studies such as the Extremely Low Gestational Age Newborns study reported reduced placental CRH expression in inflammation, increased receptor expression might increase tissue sensitivity and contribute to the development of the local inflammatory phenomena, especially during active labor.
In conclusion, although CRH does not exert direct effects on myometrial NF-κB activation, it acts via the CRH-R1 to play complex modulatory roles on IL-1β-mediated actions by altering the temporal dynamics of p65 phosphorylation, nuclear localization, and transcriptional regulation of downstream targets such as PGHS2. The physiological significance of these actions is not well understood; the actions of CRH might be important for priming and preparing the myometrium for the onset of labor by regulating cell adaptation to increasing activity of inflammatory cytokines, and switch to a procontractile phenotype with increased responsiveness to hormonal signals and mechanical forces.
Acknowledgments
This work was supported by the Wellcome Trust and the Birmingham-Warwick Science City Research Alliance.
Disclosure Summary: The authors have no potential financial conflicts of interest to disclose.
Footnotes
- CRH-R
- CRH receptor
- CRH-R1
- type 1 CRH receptor
- CT
- comparative threshold cycle
- GAPDH
- glyceraldehyde-3-phosphatedehydrogenase
- NF-κB
- nuclear factor-κB
- PG
- prostaglandin
- PGHS2
- PG cyclooxygenase-2
- PKA
- protein kinase A
- siRNA
- small interfering RNA
- TBP
- TATA-binding protein
- TBS
- Tris-buffered saline.
References
- 1. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett R. Nuclear factor-κB is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod. 2001;7:787–790 [DOI] [PubMed] [Google Scholar]
- 4. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236 [PubMed] [Google Scholar]
- 5. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in labouring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775 [DOI] [PubMed] [Google Scholar]
- 7. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449 [DOI] [PubMed] [Google Scholar]
- 8. Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17:717–730 [DOI] [PubMed] [Google Scholar]
- 9. Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–741 [DOI] [PubMed] [Google Scholar]
- 11. Soloff MS., Cook DL, Jr, Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of COX-2 and IL-8 in cultured human myometrial cells. Endocrinology. 2004;145:1248–1254 [DOI] [PubMed] [Google Scholar]
- 12. Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Postaglandin endoperoxide-H synthase-1 and -2 messenger ribonucleic acid levels in human amnion with spontaneous labor onset. J Clin Endocrinol Metab. 1995;80:517–523 [DOI] [PubMed] [Google Scholar]
- 13. Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 14. Oger S, Mehats C, Dallot E, Ferre F, Leroy MJ. Interleukin-1β induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E2- and cyclic adenosine 3′,5′-monophosphate-dependent pathway. J Clin Endocrinol Metab. 2002;87:5524–5531 [DOI] [PubMed] [Google Scholar]
- 15. Chapman NR, Smyrnias I, Anumba DO, Europe-Finner GN, Robson SC. Expression of the GTP-binding protein (Gαs) is repressed by the nuclear factor κB RelA subunit in human myometrium. Endocrinology. 2005;146:4994–5002 [DOI] [PubMed] [Google Scholar]
- 16. Chen L, Sooranna SR, Lei K, et al. Cyclic AMP increases COX-2 expression via mitogen activated kinase in human myometrial cells. J Cell Mol Med. 2012;16:1447–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uh A, Nicholson RC, Gonzalez GV, et al. Lipopolysaccharide stimulation of trophoblasts induces corticotropin-releasing hormone expression through MyD88. Am J Obstet Gynecol. 2008;199:317.e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hillhouse EW, Grammatopoulos D, Milton N, Quartero H. The identification of a human myometrial corticotropin-releasing hormone receptor which increases in affinity during pregnancy. J. Clin. Endocrinol. Metab. 1993;76:736–741 [DOI] [PubMed] [Google Scholar]
- 19. Grammatopoulos D, Hillhouse EW. Role of corticotropin-releasing hormone in the onset of labour. Lancet. 1999;353:1546–1549 [DOI] [PubMed] [Google Scholar]
- 20. Aggelidou E, Hillhouse EW, Grammatopoulos DK. Up-regulation of nitric oxide synthase and modulation of the guanylate cyclase activity by corticotropin releasing hormone in cultured human pregnant myometrial cells. Proc Natl Acad Sci USA. 2002;99:3300–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mignot TM, Paris B, Carbonne B, Vauge C, Ferre F, Vaiman D. Corticotropin-releasing hormone effects on human pregnant vs. nonpregnant myometrium explants estimated from a mathematical model of uterine contraction. J Appl Physiol. 2005;99:1157–1163 [DOI] [PubMed] [Google Scholar]
- 22. Grammatopoulos D, Hillhouse EW. Basal and interleukin-1β stimulated prostaglandin production from cultured human myometrial cells: differential regulation by corticotropin-releasing hormone. J Clin Endocrinol Metab. 1999;84:2204–2211 [DOI] [PubMed] [Google Scholar]
- 23. Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20:432–438 [DOI] [PubMed] [Google Scholar]
- 24. Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3(6):e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karteris E, Hillhouse EW, Grammatopoulos D. Urocortin II is expressed in human pregnant myometrial cells and regulates myosin light chain phosphorylation: potential role of the type-2 corticotropin-releasing hormone receptor in the control of myometrial contractility. Endocrinology. 2004;145:890–900 [DOI] [PubMed] [Google Scholar]
- 26. Markovic D, Vatish M, Gu M, et al. The onset of labor alters corticotropin-releasing hormone type 1 receptor variant expression in human myometrium: putative role of interleukin-1β. Endocrinology. 2007;148:3205–3213 [DOI] [PubMed] [Google Scholar]
- 27. Trivedi S, Joachim M, McElrath T, et al. ; Extremely Low Gestational Age Newborns (ELGAN) study investigators Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstet Gynecol. 2012;206:236.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welsh T, Johnson M, Yi L, et al. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERα activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012;212:227–238 [DOI] [PubMed] [Google Scholar]
- 29. Regier N, Frey B. Experimental comparison of relative RT-qPCR quantification approaches for gene expression studies in poplar. BMC Mol Biol. 2010;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfaffl MW. Quantification strategies in real-time PCR. In: Bustin SA, ed. A-Z of Quantitative PCR. La Jolla, California: International University Line; 2004:87–112 [Google Scholar]
- 31. Sooranna SR, Grigsby PL, Engineer N, et al. Myometrial prostaglandin E2 synthetic enzyme mRNA expression: spatial and temporal variations with pregnancy and labour. Mol Hum Reprod. 2006;12:625–631 [DOI] [PubMed] [Google Scholar]
- 32. Torricelli M, Novembri R, Bloise E, De Bonis M, Challis JR, Petraglia F. Changes in placental CRH, urocortins, and CRH-receptor mRNA expression associated with preterm delivery and chorioamnionitis. J Clin Endocrinol Metab. 2011;96(2):534–540 [DOI] [PubMed] [Google Scholar]
- 33. Havelock JC, Keller P, Muleba N, et al. Human myometrial gene expression before and during parturition. Biol Reprod. 2005;72:707–719 [DOI] [PubMed] [Google Scholar]
- 34. Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-β. J Clin Endocrinol Metab. 2005;90:3517–3527 [DOI] [PubMed] [Google Scholar]
- 35. Khanjani S, Kandola MK, Lindstrom TM, et al. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J Cell Mol Med. 2011;15:809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clifton VL, Telfer JF, Thompson AJ, et al. Corticotropin-releasing hormone and proopiomelanocortin-derived peptides are present in human myometrium. J Clin Endocrinol Metab. 1998;83:3716–3721 [DOI] [PubMed] [Google Scholar]
- 37. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52 [DOI] [PubMed] [Google Scholar]
- 38. Murakami M, Das S, Kim YJ, Cho W, Kudo I. Perinuclear localization of cytosolic phospholipase A(2)α is important but not obligatory for coupling with cyclooxygenases. FEBS Lett. 2003;546:251–256 [DOI] [PubMed] [Google Scholar]
- 39. Grammatopoulos D, Stirrat GM, Williams SA, Hillhouse EW. The biological activity of the corticotropin-releasing hormone receptor-adenylate cyclase complex in human myometrium is reduced at the end of pregnancy. J Clin Endocrinol Metab. 1996;81:745–751 [DOI] [PubMed] [Google Scholar]
- 40. Sehringer B, Schäfer WR, Wetzka B, et al. Formation of proinflammatory cytokines in human term myometrium is stimulated by lipopolysaccharide but not by corticotropin-releasing hormone. J Clin Endocrinol Metab. 2000;85:4859–4865 [DOI] [PubMed] [Google Scholar]
- 41. Ashall L, Horton CA, Nelson DE, et al. Pulsatile stimulation determines timing and specificity of NF-κB-dependent transcription. Science. 2009;324:242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1β regulated genes in uterine smooth muscle cells. Reproduction. 2007;134:811–822 [DOI] [PubMed] [Google Scholar]
- 43. Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424 [DOI] [PubMed] [Google Scholar]
- 44. MacDougall MW, Europe-Finner GN, Robson SC. Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII α) protein kinase A holoenzyme. J Clin Endocrinol Metab. 2003;88:2194–2205 [DOI] [PubMed] [Google Scholar]
- 45. Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286 [DOI] [PubMed] [Google Scholar]