Abstract
BACKGROUND
Glutamatergic modulation of γ-aminobutyric acid (GABA) interneurons via the NR2A subunit of the Nmethyl-D-aspartate (NMDA) receptor in the cerebral cortex contributes to the pathophysiology of schizophrenia and bipolar disorder. Previously, we found that, in the anterior cingulate cortex (ACCx), the number of GABA cells that expressed the mRNA for the NMDA NR2A subunit was significantly decreased in subjects with schizophrenia and bipolar disorder and that this decrease occurred most prominently in layer 2. In this study, we hypothesized that the subset of GABA interneurons that contained the calcium-binding protein calbindin (CB), by virtue of their preferential localization to layer 2, might be particularly affected.
METHODS
We simultaneously labeled the mRNA for the NMDA NR2A subunit with [35S] and the mRNA for CB with digoxigenin using an immunoperoxidase procedure.
RESULTS
We found that, in the normal human ACCx, only approximately 10% of all CB-containing cells expressed NR2A mRNA. However, compared to the normal control subjects and subjects with bipolar disorder, the density of CB+/NR2A+ neurons in layer 2 was increased by 41–44 % in subjects with schizophrenia whereas the amount of NR2A mRNA per CB+ neurons was unchanged.
CONCLUSIONS
These observations suggest that, in schizophrenia, a number of CB-containing cells that normally do not express NR2A may become NR2A-expressing or, perhaps not mutually exclusively, the number of CB-expressing cells may be increased and these cells express NR2A. The findings of this study highlight the notion that glutamatergic innervation of subsets of GABA cells may be differentially altered in schizophrenia and bipolar disorder.
INTRODUCTION
Genetic, molecular and clinical evidence increasingly implicates the N-methyl-D-aspartate (NMDA) class of glutamate receptors as playing a major role in the pathophysiology of schizophrenia (1–6). More specifically, it has been postulated that, in schizophrenia, hypofunction of the NMDA receptors that are located on γ-aminobutyric acid (GABA)ergic interneurons in the corticolimbic system may instigate a sequence of downstream events that may include neuronal injury and apoptosis (7, 8). Consistent with this idea, we recently reported that, in layer 2 of the anterior cingulate cortex (ACCx), the expression of the messenger RNA (mRNA) for the NR2A subunit of the NMDA receptor was decreased to an experimentally undetectable level in as many as 73% of the GABA interneurons in subjects with schizophrenia (9). Interestingly, similar changes were also observed in subjects with bipolar disorder (9), raising the possibility that altered NMDA receptor function on inhibitory interneurons may represent a shared pathophysiologic cascade for the two disorders.
Because connectionally and functionally distinct subpopulations of GABA interneurons regulate different aspects of information flow in the cerebral cortex (10–13), an important question that needs to be addressed in order to gain further insight into the pathophysiologic consequences of reduced NR2A expression is the identity of the GABA cells that are affected. Subpopulations of GABA neurons can be distinguished by their differential expression of distinct calcium binding proteins (13–18). For instance, the subset of GABA cells that express a high level of the calcium binding protein calbindin (CB) tends to be concentrated in the upper layers, especially layer 2, of the cortex, although cells that are weakly immunoreactive for CB, which may also include a small number of pyramidal cells, seem to spread across all layers (16, 19, 20). Because we previously found that, in the ACCx in schizophrenia and bipolar disorder, the decrease in the density of NR2A-expressing GABA cells occurred most prominently in layer 2 (9), we postulated that the expression of NR2A mRNA in CB-containing neurons in this layer would be decreased in these disorders. Contrary to this hypothesis, we instead found that the density of NR2A mRNA-expressing CB neurons might actually be increased in layer 2 of the ACCx in schizophrenia, but it was unchanged in bipolar disorder.
METHODS
Subjects
A cohort of 60 human brains from 20 subjects with schizophrenia, 20 subjects with bipolar disorder, and 20 normal control subjects were obtained from the Harvard Brain Tissue Resource Center at McLean Hospital, Belmont, MA (Supplemental Table 1). Each group of subjects with schizophrenia or bipolar disorder was matched to a normal control group on the basis of age, postmortem interval (PMI), and whenever possible, sex and side of hemisphere. The mean freezer storage time (days±S.D.) of brains did not differ between the normal control (1,407±555), schizophrenia (1,680±630), and bipolar disorder (1,500±834) groups. The mean pH (±S.D.) also did not differ between the 3 groups of subjects (normal control: 6.51±0.24; schizophrenia: 6.53±0.23; bipolar: 6.48±0.23).
Psychiatric diagnoses were established using a retrospective review of medical records and an extensive family questionnaire that included the medical, psychiatric and social history of the subjects. For the diagnosis of schizophrenia, the criteria of Feighner et al (21) were used and the diagnoses of schizoaffective and bipolar disorder were made according to DMS-III-R criteria. Four of the 20 subjects with schizophrenia were not on antipsychotic medications at the time of death. In the bipolar group, 13 subjects were on antipsychotic medications at the time of death and the medication status of 2 subjects is not known. The average dosage of antipsychotics that the bipolar subjects (213.3±282.8 mg) were receiving (expressed as chlorpromazine-equivalent dose or CED) was approximately half that of the schizophrenia subjects (412.2±465.7 mg). Some subjects in both disease groups were also on concomitant psychotropic medications, such as mood stabilizers, antidepressants or anxiolytics (see Supplemental Table 1). No subjects in the normal control group were receiving any psychotropics at the time of death.
Tissue Preparation
Tissue blocks (3 mm in thickness) from Brodmann’s area 24 were removed from fresh brain specimens at the level of the rostrum of the anterior cingulate gyrus between the points at which the gyrus curves above and below the corpus callosum (22). The blocks were immediately fixed in 0.1% paraformaldehyde in ice-cold 0.1 M phosphate buffer (pH 7.4) for 90 minutes, immersed in 30% sucrose in the same buffer overnight, and then frozen in Tissue Tek OCT® (Sakura Finetek, Torrance, CA) on dry ice. Tissue blocks were then sectioned at a thickness of 10 µm on a cryostat. Two sections per subject and therefore 6 sections per matched triplet were used for in situ hybridization. The six sections from each triplet were mounted on 3 slides as follows: 1) normal control + schizophrenia, 2) normal control + bipolar, and 3) schizophrenia + bipolar. This method of mounting sections controls for potential variability of hybridization signals between slides. All mounted sections were stored at −70 °C until riboprobe labeling was performed.
Double in situ Hybridication
Riboprobe preparation
Radiolabeled cRNA Probe for NR2A mRNA
The cRNA probes for the NR2A subunit (kindly provided by Dr. Christine Konradi) were transcribed in vitro from linearized cDNA subclones encoding the rat NMDA NR2A subunit, which is 89% identical to the human sequence. The probe was derived from a cDNA spanning nucleotides 1185–2154 (Genbank Accession #NM91561) within the coding region of the subunit. A corresponding sense probe was used as negative control. Radiolabeled cRNA probe was prepared by first drying down [35S]UTP (500 µCi/ml of probe, New England Nuclear, Boston, MA) in a DNA Speed-Vac (Savant, Farmingdale, NY). 100 ng/µl of the cDNA template, 0.1 M dithiothreitol (DTT), 3 U/µl RNasin, 5mM NTPs, 0.8 U/µl of T3 or T7 RNA polymerases (for antisense and sense probe, respectively), and 5X transcription buffer were then added. The transcription mixture was subsequently incubated at 37 °C for 1 hour. The cDNA template was digested by incubating the mixture with R1Q DNase at 37 °C for 15 minutes. Unincorporated NTPs were removed by running the mixture through a Stratagene Nuc-Trap (La Jolla, CA) push column. The eluate was collected and probe concentration was determined by scintillation counting. The probe was stored at −20 °C until use.
DIG-Labeled CB mRNA Probe
DIG-UTP-labeled cRNA probes were transcribed using 100 ng of linearized human cDNA subclones encoding nucleotides 289–937 (Genbank Accession # NM004929.2) of the human CB protein in the presence of 0.1 M DTT, 3 U/µl Rnasin, 0.8 U/µl of T3 and T7 RNA polymerases,10 mM of ATP, CTP, and GTP, 6.5 mM of UTP, and 3.5 mM of DIG-labeled UTP (Boehringer Mannheim, Indianapolis, IN). The mixture was incubated at 37 °C for 1 hour. cDNA template was digested with RQ1 DNase. Probe concentration was determined using a standard with known concentrations. A corresponding sense probe was used as negative control.
Hybridization
Probes were reconstituted in a hybridization buffer consisting of 50% formamide, 0.1% yeast tRNA, 10% dextran sulfate, 1X Dehardt’s solution, 0.5 M ethylenediamine tetraacetic acid (EDTA), 0.02% sodium dodecyl sulfate (SDS), 4X saline-sodium citrate (SSC) buffer, 10 mM DTT, and 0.1% ssDNA, at a final concentration of 0.4 ng probe/µl hybridization buffer. Before hybridization, mounted tissue sections were air dried and warmed to room temperature. They were then post-fixed in 4% paraformaldehyde for 10 minutes and incubated in 0.1 M tetraethylammonium (TEA) for 5 minutes at room temperature before being dehydrated in a graded series of ethanol. Probes were then added to slides for hybridization in a prewarmed, humidified dish. Sections were covered with coverslips and incubated at 57 °C for 12 hours. At the end of hybridization, coverslips were soaked off in 4X SSC in the presence of 100 µl of βMer. Tissue was then incubated in 0.5 M NaCl/0.05 M PB for 10 minutes, 0.5 M NaCl with 0.025 mg/ml RNaseA at 37 °C for 30 minutes, followed by a high stringency wash with a solution containing 50% formamide, 0.5 M NaCl/0.05 M PB, and 100 µl βMer at 63 °C for 30 minutes. Sections were finally washed overnight in 0.5X SSC with 20 mM βMer ETOH at room temperature.
Visualization of DIG labeling
After incubation in blocking buffer (100 mM Tris-HCL, 150 mM NaCl pH 7.5, 3% normal goat serum (NGS), 0.3% Triton X100, sections were incubated overnight at 4 °C in buffer containing 1:200 dilution of sheep anti-DIG antibody (Roche Diagnostics, Indianapolis, IN). Processing was then completed by using the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine (DAB).
Emulsion autoradiography
It was determined that sufficient autoradiographic signal had developed after the slides were apposed to X-ray film (Kodak Biomax MS) for 5 days. The slides were then dipped in emulsion (Kodak NTB-2), air dried, and stored at 4 °C in dark for 3 weeks. After development in the dark with Kodak D-19 developer, slides were counterstained with creysl violet and coverslipped.
Quantification of NR2A mRNA Expression
All microscopic analyses were conducted under strictly blind condition by one investigator (DL) as previously described (9, 23). The entire quantification process was completed in 10 weeks. [35S]-labeling of NR2A mRNA appeared as clusters of silver grains after processing for emulsion autoradiography. DIG labeling, in the form of DAB reaction product, was visualized under a brightfield microscope. Neurons that were single-labeled with DIG or [35S] and those that were double-labeled with DIG + [35S] were identified on images captured on a computer screen using a Leica Laborlux microscope, which was equipped with a solid state CCD video camera connected to a Bioquant Nova Image Analysis System (R&M Biometrics, Memphis, TN). Using a 100X oil immersion objective lens at a final magnification of 1,000X, the distribution of both single- and double-labeled neurons within a 250µm-wide column extending from the pial surface to the border between layer 6 and the subcortical white matter were obtained for each section. Neighboring sections were stained with cresyl violet for determination of laminar boundaries. Densities of single- and double-labeled neurons for each cortical layer were then obtained by dividing cell counts by laminar areas. Intra-rater reliability, as assessed by counting and recounting profiles within the same column, was established to be 93-97 % before the actual data collection process was begun.
To quantify the expression level of NR2A mRNA in individual CB mRNA+ cells, the area occupied by each grain cluster was outlined using a cursor displayed on the computer monitor. The cluster area was measured by highlighting the grains with a thresholding subroutine. The light intensity was subsequently adjusted to ensure that the size of the grains was neither under- nor over-saturated. The threshold and light intensity were held constant throughout the entire study. The area covered by autoradiographic grains within the cluster area was automatically computed based on the threshold value and was represented as a pixel count for NR2A transcript expression level. The pixel count was expressed as a function of cluster area (numerical density). By subtracting the background grain density (i.e. pixel count of the area covered by autoradiographic grains per unit area in µm2 in the white matter), the corrected NR2A expression level was obtained. The average NR2A expression level in CB mRNA+ neurons for each cortical layer for each case was then computed. Intra-rater reliability in grain density measurements, which was accessed by repeating the procedures described above on the same clusters, was determined to be consistently above 95% before the actual data collection process.
Statistical Analyses
The densities of CB+ single- labeled neurons were compared between subject groups across layers 2 through 6 using repeated-measures analysis of variance (ANOVA), with diagnosis as the between-groups factor, layer as the within-group factor, and repeated measures on layer. For the CB+/NR2A+ double-labeled neurons, to test the a priori hypothesis that the density of these neurons would be significantly decreased in layer 2 in subjects with schizophrenia and bipolar disorder, a one-way ANOVA was performed. A repeated-measures ANOVA was then carried out on layers 3–6. In order to evaluate the potential effects of confounding variables such as age, PMI, brain pH, freezer storage time and exposure to antipsychotic medications (expressed as the chlorpromazine-equivalent dose or CED), simple Pearson correlations were obtained for the individual groups and when the control group was combined with the schizophrenia and bipolar group, respectively. If any of these analyses revealed any statistically significant correlation, an analysis of covariance (ANCOVA) would be performed in order to understand how these confounding variables might have influenced our results. Effects of categorical variables such as hemispheric laterality and sex on our findings were evaluated by using two-tailed unpaired t-tests to compare the measures from the two hemispheres and sexes both within individual groups and when cases from the control group were combined with those from the schizophrenia and bipolar group, respectively.
RESULTS
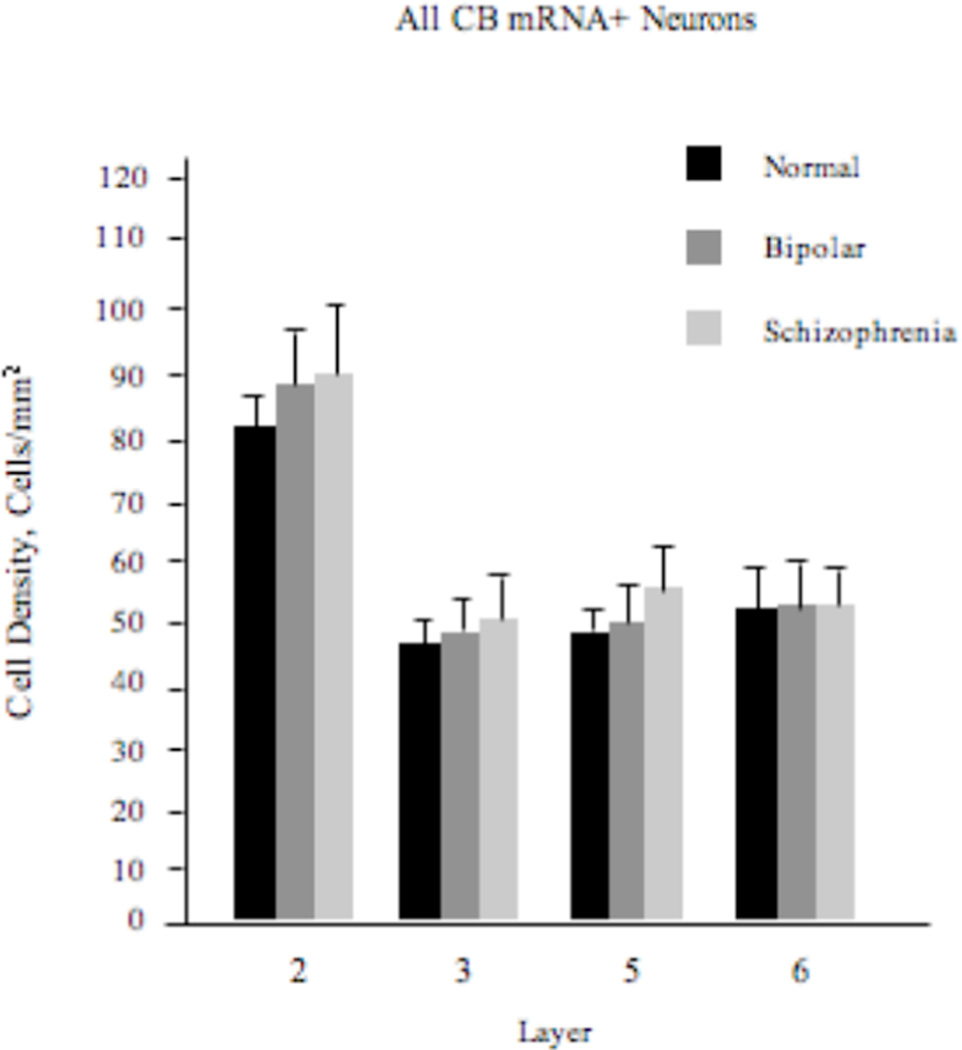
Density of All CB+ Neurons
Our repeated-measures ANOVA model failed to reveal any significant diagnosis effect (F2,57=1.09; p=0.34) on the density of these neurons (Figure 1).
Figure 1.
Density (mean±SE) of all CB+ neurons in the ACCx is unchanged in schizophrenia or bipolar disorder.
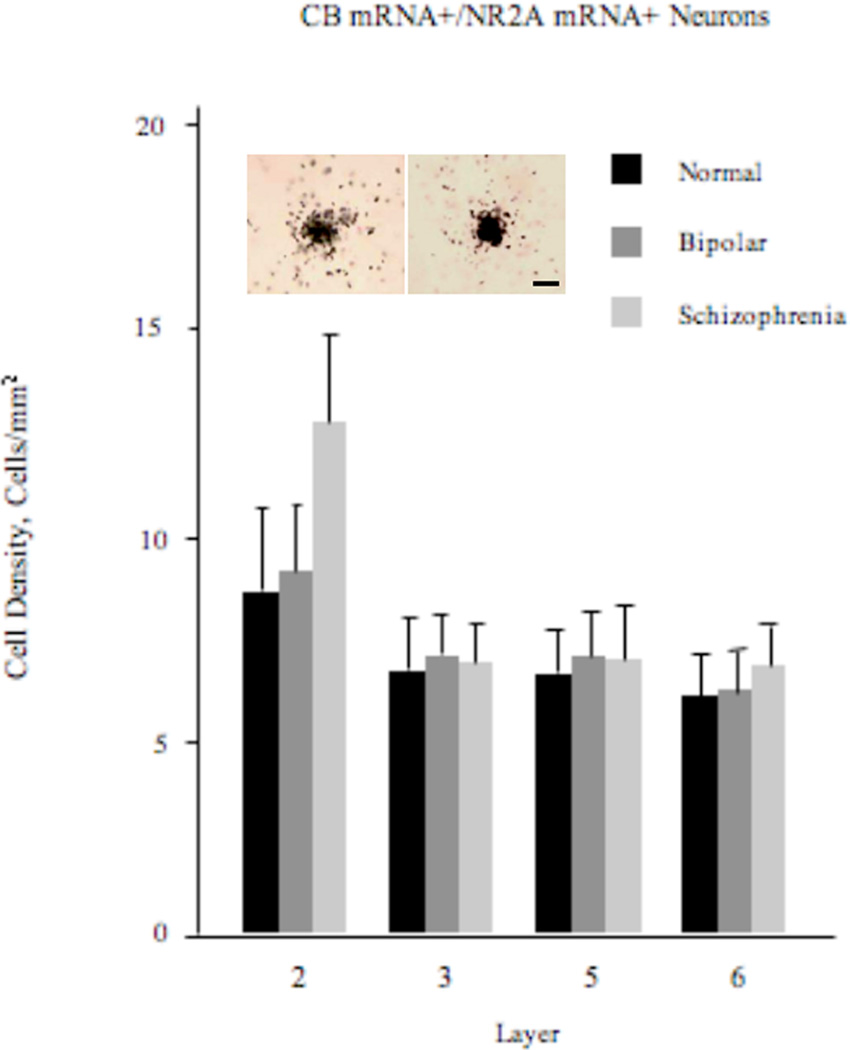
Density of CB+/NR2A+ Neurons
The ANOVA performed on layer 2 revealed that the effect of diagnosis was highly significant (F2,57=4.33; p=0.018; Figure 2). Post-hoc unpaired t-tests further revealed that the density of these neurons was significantly increased in schizophrenia by 41 and 44%, respectively, compared to the normal control (p=0.012) and bipolar disorder (p=0.016) groups. The repeated-measures ANOVA model carried out on layers 3–6 revealed no statistically significant diagnosis effect (F2,57=0.31; p=0.74).
Figure 2.
Density (mean±SE) of CB+/NR2A+ neurons in layer 2 of the ACCx appears to be increased in schizophrenia, but not in bipolar disorder. Also shown is a photomicrograph of a CB+/NR2A+ neuron (left) and, for comparison, that of a NR2A single-labeled neuron (right). Scale bar=10 µm.
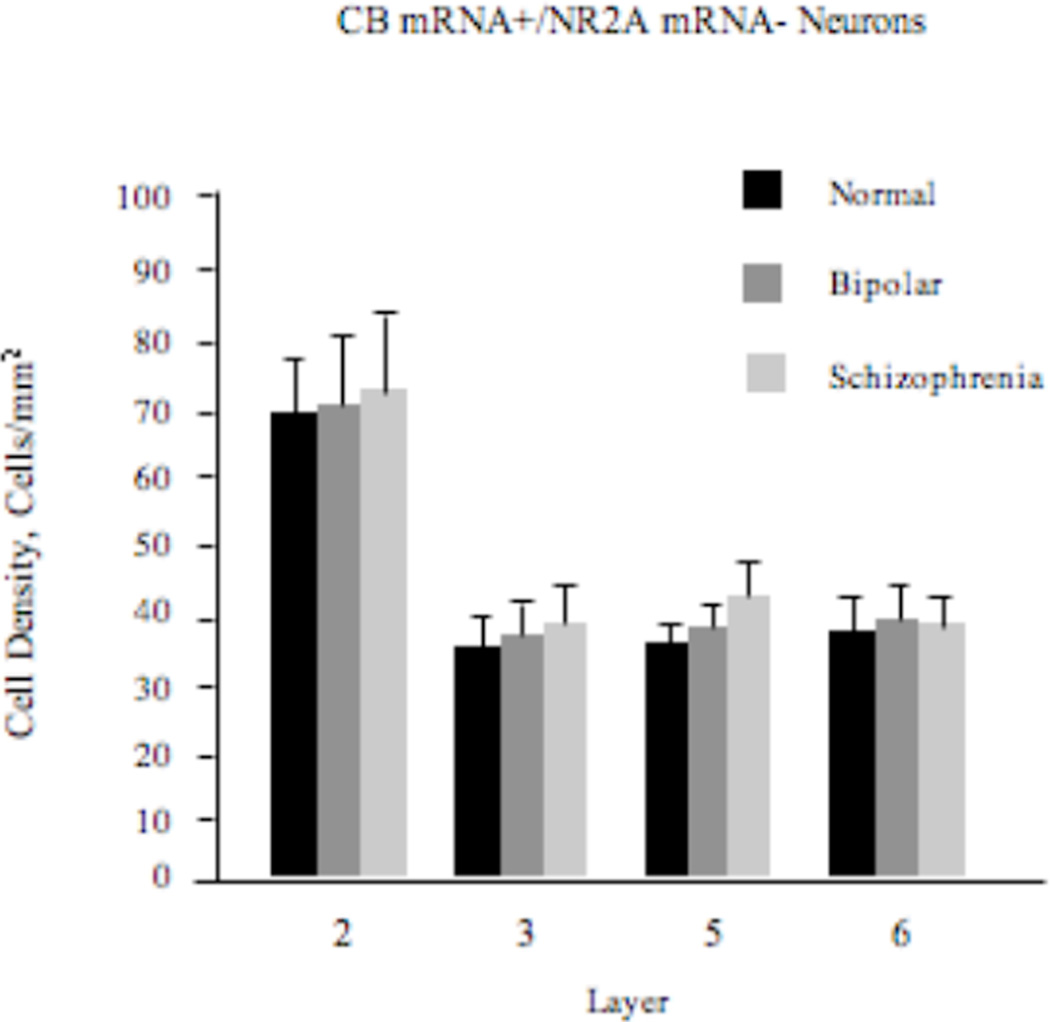
Density of CB+/NR2A-Neurons
There was no statistically significant effect of diagnosis on the density of these neurons (F2,57=0.66; p=0.52; Figure 3).
Figure 3.
Density (mean±SE) of CB+/NR2A- neurons in the ACCx is unchanged in schizophrenia or bipolar disorder.
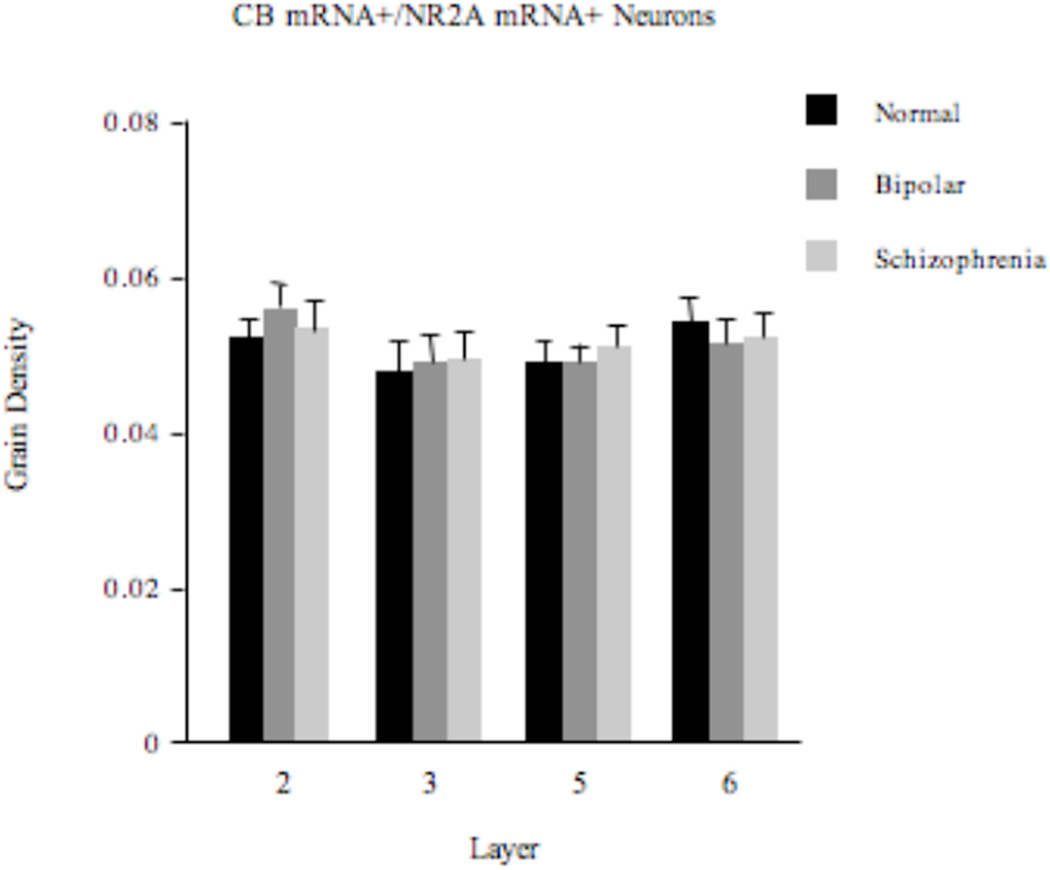
Expression Level of NR2A mRNA
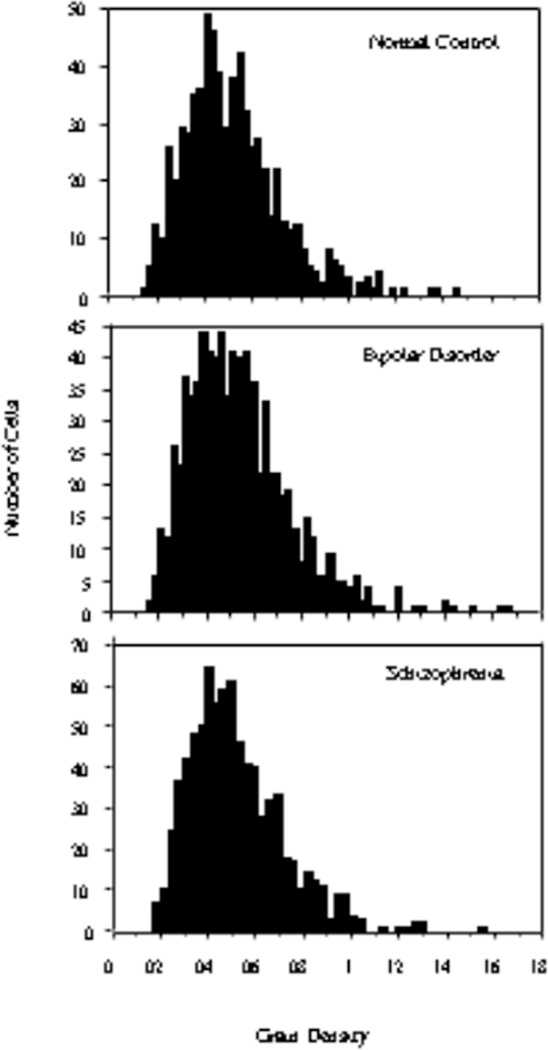
There were no statistically significantly differences (F2,57=0.26; p=0.77) in the density of silver grains on CB+ neurons between the 3 diagnosis groups (Figure 4). Because of the apparent increase in the density of CB+/NR2A+ cells in layer 2 in subjects with schizophrenia, we plotted the histogram distributions of grain densities within this layer for the 3 diagnosis groups and did not observe any shift in grain density distribution in the schizophrenia group (Figure 5). This was confirmed by a Kruskal-Wallis test. Therefore, it appears that, in schizophrenia, some of the CB-expressing cells that normally do not express NR2A mRNA or express it at an experimentally undetectable level become NR2A mRNA-expressing.
Figure 4.
Density (mean±SE) of silver grains over CB+ neurons in the ACCx is unchanged in schizophrenia or bipolar disorder.
Figure 5.
There is no discernable shift in grain density distribution in layer 2 of the ACCx in schizophrenia or bipolar disorder.
Cortical thickness
There are no differences in cortical thickness between the 3 subject groups (F=0.0057; P=0.99), indicating that the observation of increased density of CB+/NR2A+ neurons was not an artifact of reduced cortical volume in schizophrenia.
Potential Confounding Variables
We examined the potential confounding effects of variables such as age, PMI, brain pH, hemispheric laterality and antipsychotic exposure, on our findings. None of these factors appear to have influenced our results. Among these variables, pH is perhaps particularly important because it is often considered an indirect indicator of the integrity of mRNA (24–26). In our statistical analysis, we found no correlation between pH and any of our density measures either in individual diagnosis groups or when subjects from the disease groups and those from the normal control group were combined. An ANCOVA incorporating pH as a covariate also did not significantly alter the effect of diagnosis on the cell density and grain density measurements. Similar analyses with CED also revealed that exposure to antipsychotic medications was not significantly correlated with any of our measurements, nor did it contribute to the observed differences in the neuronal density measurements between diagnosis groups. Finally, cigarette smoking is highly prevalent in schizophrenia patients (27, 28). Unfortunately, the clinical information we have about the subjects used in this study does not allow us to quantify the amount of nicotine intake and hence we were unable to statistically assess any possible effect of nicotine on our findings. However, a recent study has shown that chronic exposure to nicotine appears to have no effect on CB expression in the anterior cingulate cortex in rats, although it does appear to increase the expression of calretinin and parvalbumin (29).
DISCUSSION
We have previously found that, in layer 2 of the ACCx, the expression of NR2A mRNA in approximately 53% and 35% of the GABA cells in schizophrenia and bipolar disorder, respectively, appears to be decreased to a level that is no longer experimentally detectable (9). Because CB-containing GABA cells tend to be more heavily localized to layer 2 of the cortex (16, 19, 20), we hypothesized that they would be among the GABA neurons that are affected. Contrary to this hypothesis, our data suggest that the density of the CB-containing cells that express NR2A mRNA seems to be unchanged in bipolar disorder. Furthermore, it appears that the density of these cells may actually be increased in schizophrenia.
There are at least two scenarios that could explain the observation of increased density of CB+/NR2A+ neurons in schizophrenia. First, this finding may indicate that some of the CB-containing cells that normally do not express NR2A become NR2A-expressing in schizophrenia. In this case, the number of CB+/NR2A- cells would be expected to decrease correspondingly, which our analysis failed to detect. However, it should be noted that because only about 10% of all CB mRNA-containing cells express NR2A mRNA, a ~40% increase in the density of these neurons would amount to only about a 4% decrease in the CB+/NR2A- neurons. Such a small magnitude of change is below the detection sensitivity of in situ hybridization. A second possible explanation of our finding is that the number of CB mRNA-expressing cells may actually be increased in schizophrenia and, additionally, these cells express NR2A. In this scenario, according to the discussion above, the density of the entire population of CB mRNA-expressing cells would increase by only ~4%, which, again, is unlikely to be detectable by in situ hybridization. An alternative methodology with superior sensitivity, such as single cell real-time polymerase chain reaction, will be required before we can distinguish between these two scenarios, which also need not be mutually exclusive. Although to our knowledge there have been no previous studies examining the expression of CB mRNA in schizophrenia, several studies have found changes in the density of CB-immunoreactive neurons. For example, Daviss and Lewis have shown that the density CB-immunoreactive neurons may be increased in the dorsolateral prefrontal cortex in subjects with schizophrenia (30); this observation may therefore seem to lend support to one of the scenarios discussed above about a possible increase in the density of CB mRNA-expressing cells in the ACCx. However, in another study, the density of CB-immunoreactive cells in layer 2 of the ACCx has actually been found to be decreased in subjects with schizophrenia (19). Similarly, a decrease in the density of these cells has also been observed in the temporal cortex (31). Nevertheless, transcript and protein expression do not necessarily always correlate (32); it is conceivable that, in schizophrenia, one mechanism to compensate for decreased CB protein expression is by increasing transcription.
Although the majority of the CB-expressing cells are GABA neurons; a small number of pyramidal cells (mostly in the lower layers) in the normal cortex also contain CB (16). Therefore, it is possible that at least some of the NR2A-expressing CB-containing cells that are affected may be pyramidal cells. Although this possibility cannot be ruled out, it is not supported by our observation that the average area (mean±S.D.) of grain clusters on CB+ cells, which can be considered a reflection of somal size, in layer 2 of the ACCx was found to be significantly (t=1.98; p=0.048) smaller in subjects with schizophrenia (158.3±62.1 µm2) compared to the normal control subjects (164.9±65.5 µm2). Importantly, this difference was not observed in any of the other cortical layers (data not shown). This finding would seem to support the argument that the majority of the CB+/NR2A+ cells that are affected in schizophrenia may in fact be GABA interneurons, which have smaller somal areas than pyramidal cells.
Specific subsets of GABA interneurons subserve distinct functions to ensure the integrity of information processing in the cerebral cortex (33). Many of the CB-containing GABA cells, for instance, are thought to be double-bouquet cells, which selectively target the distal dendrites of pyramidal neurons (20). The firing of the distal dendrite-targeting inhibitory neurons may play a very important role in phasing the activity of the pyramidal cells they innervate to temporally match the spike pattern of the incoming excitatory stimuli in order to facilitate activity-dependent synaptic plasticity (34). Furthermore, there is also evidence suggesting that activation of these neurons may be very important in regulating the stability of network activity by filtering out distracting stimuli (11). Our finding of increased density of CB+/NR2A+ cells implies that glutamatergic inputs to the CB-containing neuronal network in layer 2 of the ACCx may be altered in schizophrenia, although the exact nature of this alteration cannot at present be determined. A possible pathophysiologic manifestation of altered glutamatergic modulation of CB-containing neurons is that the inhibition provided by these neurons to time excitatory inputs to pyramidal cells may be compromised and hence the process of activity-dependent synaptic plasticity on pyramidal cells may be disturbed. In addition, in functional terms, these pyramidal cells may also become more susceptible to the distracting effects of stimuli that are context-irrelevant, rendering the pyramidal cell network more easily destabilized. Together these mechanisms may contribute to aberrant information processing in the ACCx in schizophrenia. Finally, it would be of significant interest to identify the source of the glutamatergic inputs that may be altered in schizophrenia; one of the possibilities is that they may represent, at least in part, inputs from the amygdala, as layer 2 is one of the main layers where the axonal projections of this structure terminate (35–38). Interestingly, amygdalocortical projections to the ACCx appear to undergo progressive sprouting throughout the adolescent period, achieving the adult pattern of innervation only during early adulthood (35), which coincides with the period of time when the symptomatology of schizophrenia typically begins to emerge. Together these observations raise an interesting possibility that disturbances of the periadolescent maturation of the amygdalocortical innervation of CB-containing neurons in the ACCx, which may, at least in part, be mediated by stress, may play a role in the pathophysiology of the onset of schizophrenia.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institutes of Health (MH/NS31862, MH00423 and MH42261).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors reported no biomedical financial interest or potential conflicts of interest.
REFERENCES
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 4.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 5.Meador-Woodruff JH, Clinton SM, Beneyto M, McCullumsmith RE. Molecular abnormalities of the glutamate synapse in the thalamus in schizophrenia. Ann N Y Acad Sci. 2003;1003:75–93. doi: 10.1196/annals.1300.005. [DOI] [PubMed] [Google Scholar]
- 6.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- 7.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 8.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 9.Woo TUW, Walsh JP, Benes FM. Density of Glutamic Acid Decarboxylase 67 Messenger RNA-Containing Neurons That Express the N-Methyl-D-Aspartate Receptor Subunit NR2A in the Anterior Cingulate Cortex in Schizophrenia and Bipolar Disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287(5451):273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 11.Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101(5):1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 13.Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17(10):3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cerebral Cortex. 1993;3(4):273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex--development and deficits in schizophrenia. Journal of Chemical Neuroanatomy. 2001;22(1–2):95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- 16.DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. Journal of Chemical Neuroanatomy. 1997;14(1):1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- 17.Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. Journal of Comparative Neurology. 1994;341(1):95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7(6):476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 19.Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51(5):377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- 20.Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J Neurocytol. 1997;26(12):779–797. doi: 10.1023/a:1018518515982. [DOI] [PubMed] [Google Scholar]
- 21.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26(1):57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 22.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biological Psychiatry. 2001;50(6):395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 23.Woo TUW, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res. 2007;96(1–3):46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28(2):311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- 25.Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200(3):151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 26.Eastwood SL, Kerwin RW, Harrison PJ. Immunoautoradiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biological Psychiatry. 1997;41(6):636–643. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- 27.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- 28.Dalack GW, Meador-Woodruff JH. Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res. 1996;22(2):133–141. doi: 10.1016/s0920-9964(96)80441-5. [DOI] [PubMed] [Google Scholar]
- 29.Liu JJ, Mohila CA, Gong Y, Govindarajan N, Onn SP. Chronic nicotine exposure during adolescence differentially influences calcium-binding proteins in rat anterior cingulate cortex. Eur J Neurosci. 2005;22(10):2462–2474. doi: 10.1111/j.1460-9568.2005.04423.x. [DOI] [PubMed] [Google Scholar]
- 30.Daviss SR, Lewis DA. Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Research. 1995;59(1–2):81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- 31.Chance SA, Walker M, Crow TJ. Reduced density of calbindin-immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res. 2005;1046(1–2):32–37. doi: 10.1016/j.brainres.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 32.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19(3):1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltesz I. Diversity in the Neuronal Machine. New York: Oxford University Press; 2005. [Google Scholar]
- 34.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27(31):8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 36.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 38.Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198(1):121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.