This work presents a genetic screen for early components of singlet oxygen signaling using the unicellular green alga Chlamydomonas and reports the identification of a small zinc finger protein, MBS, that accumulates in distinct cytosolic granules and is required for induction of singlet oxygen–dependent gene expression in Chlamydomonas and Arabidopsis.
Abstract
All cells produce reactive oxygen species (ROS) as by-products of their metabolism. In addition to being cytotoxic, ROS act as regulators of a wide range of developmental and physiological processes. Little is known about the molecular mechanisms underlying the perception of ROS and initiation of cellular responses in eukaryotes. Using the unicellular green alga Chlamydomonas reinhardtii, we developed a genetic screen for early components of singlet oxygen signaling. Here, we report the identification of a small zinc finger protein, METHYLENE BLUE SENSITIVITY (MBS), that is required for induction of singlet oxygen–dependent gene expression and, upon oxidative stress, accumulates in distinct granules in the cytosol. Loss-of-function mbs mutants produce singlet oxygen but are unable to fully respond to it at the level of gene expression. Knockout or knockdown of the homologous genes in the higher plant model Arabidopsis thaliana results in mutants that are hypersensitive to photooxidative stress, whereas overexpression produces plants with elevated stress tolerance. Together, our data indicate an important and evolutionarily conserved role of the MBS protein in ROS signaling and provide a strategy for engineering stress-tolerant plants.
INTRODUCTION
Phototrophic organisms produce a range of different reactive oxygen species (ROS) either by energy transfer or electron transfer reactions. While electron transfer produces superoxide (O2−) radical ions that subsequently can be converted to hydrogen peroxide (H2O2) or hydroxyl radicals, energy transfer to molecular oxygen results in the generation of singlet oxygen (1O2; Apel and Hirt, 2004). Plant cells produce ROS as unavoidable byproducts of aerobic metabolism, with the photosynthetic electron transport chain being a major source (Mullineaux and Karpinski, 2002; Foyer and Noctor, 2003; Wagner et al., 2004; Mubarakshina et al., 2010). Efficient enzymatic and nonenzymatic detoxification mechanisms are in place to prevent accumulation of excessive amounts of ROS and the resultant oxidative damage to biomolecules, especially proteins and lipids (op den Camp et al., 2003; Apel and Hirt, 2004; Henmi et al., 2004; Scheller and Haldrup, 2005; Krieger-Liszkay and Trebst, 2006). Over the past two decades, it has become increasingly clear that, even under nonstressed conditions, plants continuously produce ROS and that ROS do not merely act as cytotoxic agents, but also can be employed as signaling molecules. In vascular plants, ROS signaling has been implicated in a variety of physiological and developmental processes, including pathogen defense, abscisic acid–induced stomatal closure, maintenance of ion homeostasis, root development and gravitropism, as well as retrograde signaling from organelles to the nucleus (Lam et al., 2001; Apel and Hirt, 2004; Fischer et al., 2007; Pogson et al., 2008; Galvez-Valdivieso and Mullineaux, 2010; Jaspers and Kangasjärvi, 2010; Tsukagoshi et al., 2010; Jiang et al., 2012).
1O2 is produced mainly by photosystem II (PSII), especially upon absorption of excess light energy (Krieger-Liszkay and Trebst, 2006). It is generated via energy transfer from the triplet state of chlorophyll in the PSII reaction center to molecular oxygen in its ground state (3O2). Release of 1O2 results in the rapid induction of the expression of a specific set of nuclear genes (including transcription factors), as elegantly revealed by genetic studies in the conditional Arabidopsis thaliana mutant fluorescent (flu; op den Camp et al., 2003; Laloi et al., 2007; Kim et al., 2009). This mutant accumulates high levels of the photosensitizer protochlorophyllide in the dark and, following a shift to the light, produces massive amounts of 1O2. Interestingly, the resulting growth arrest and cell death responses are not directly caused by 1O2 toxicity but result from a genetic program (that is dependent on the EXECUTER proteins; Wagner et al., 2004; Lee et al., 2007; Kim et al., 2012).
Apart from the 1O2-mediated cell death pathway, only a few components of the cellular machinery involved in 1O2 sensing and signaling have been identified to date. Recent biochemical work has demonstrated that carotenoid oxidation products (especially β-cyclocitral) act as oxidative stress signals that mediate responses to 1O2 in higher plants, including the early transcriptional responses at the level of nuclear gene expression (Ramel et al., 2012a). In addition, several protein factors, including a bZIP-type putative transcription factor (apparently specific to green algae; Fischer et al., 2012) and a member of the P-subunit of photosystem II family (Brzezowski et al., 2012), have been implicated in 1O2 signaling. However, what connects 1O2 and/or the primary chemical signals generated by 1O2 (oxidized carotenoids and, perhaps, oxidized lipids; Fischer et al., 2012; Ramel et al., 2012a) with the early transcription factors mediating the downstream changes in gene expression, is currently unknown. It is conceivable that sensor proteins monitor the levels of 1O2 either directly or indirectly by measuring the concentration of reaction products generated at or close to the site of 1O2 production. A better understanding of the molecular mechanisms of 1O2 sensing, signaling, and detoxification is not only relevant to basic research, but also is essential for improving the tolerance of crops plant to photooxidative stress conditions.
The unicellular green alga Chlamydomonas reinhardtii has become a useful model organism for a wide range of biological questions. It combines a powerful classical genetics with the availability of excellent genetic and genomic resources. The completion of the C. reinhardtii genome sequencing project (Merchant et al., 2007) along with the development of new molecular tools (Schroda et al., 2000; Shao and Bock, 2008; Neupert et al., 2009; Ramundo et al., 2013) made it possible to conduct reporter gene-based genetic screens for new components of signal transduction pathways, including ROS signaling (Fischer et al., 2010, 2012; Brzezowski et al., 2012).
Here, we combined a previously developed sensitive reporter of gene expression in C. reinhardtii (Shao and Bock, 2008) with a previously characterized ROS-responsive promoter (Shao et al., 2007) and an efficient insertional mutagenesis strategy (Gonzalez-Ballester et al., 2011) to set up a new screen for algal mutants defective in early cellular 1O2 responses. We report the identification of a small zinc finger protein (METHYLENE BLUE SENSITIVITY [MBS]) that plays a crucial role in mediating 1O2-responsive gene expression in C. reinhardtii. Importantly, characterization of the homologous genes in the higher plant model Arabidopsis revealed a critical role of the MBS proteins in mediating 1O2 signaling and determining oxidative stress tolerance, indicating evolutionary conservation of the pathway from green algae to vascular plants.
RESULTS
A Genetic Screen for ROS Signaling Mutants in C. reinhardtii
We previously reported that a codon-optimized version of the luciferase gene from the copepod Gaussia princeps provides a sensitive reporter of gene expression in the unicellular model alga C. reinhardtii (Shao and Bock, 2008). The availability of a sensitive reporter gene has opened up the possibility to exploit the convenience of C. reinhardtii genetics for the isolation of regulators of gene expression by forward genetics. This provides a particularly attractive alternative to higher plant models, where complex multicellular organization makes mutant screens difficult or impossible. Light stress–induced ROS signaling is a case in point because the primary responses are largely confined to photosynthetically active tissues and therefore affect only a small number of the many cell types present in higher plants.
We used the codon-optimized G. princeps luciferase gene in combination with the 1O2-responsive PHSP70A promoter (Shao et al., 2007) to set up a genetic screen for mutants defective in 1O2 sensing and/or signaling (Figure 1A). To this end, an algal strain harboring the PHSP70A-driven reporter cassette was subjected to large-scale insertional mutagenesis based on random insertion of a hygromycin resistance gene cassette (Berthold et al., 2002; Figure 1A). A total of 4890 insertion mutants were generated by transformation and screened for their inability to induce luciferase expression in response to 1O2 stress. 1O2 stress was applied by exposure of algal cultures to methylene blue (MB), a photosensitizer that is readily taken up by C. reinhardtii cells and triggers the production of 1O2 in the light (Shao et al., 2007). Seventeen mutants were identified that lacked luciferase induction upon application of 1O2 stress (Figure 1B). To distinguish between mutants that are specifically defective in their response to 1O2 and mutants with general defects in expression of the luciferase reporter, heat stress experiments were performed. The HSP70A promoter driving the luciferase reporter is not only responsive to 1O2, but it is also strongly inducible by heat shock. As the heat response is genetically distinct from the response to 1O2 (Shao et al., 2007; Lodha et al., 2008; Shao and Bock, 2008), any mutant specifically affected in 1O2 responses should still show wild-type-like luciferase induction by heat. This was the case for 12 of the algal mutants identified in the MB screen (Figure 1C), suggesting that these 12 mutants are neither generally affected in luciferase expression nor generally defective in their response to abiotic stresses.
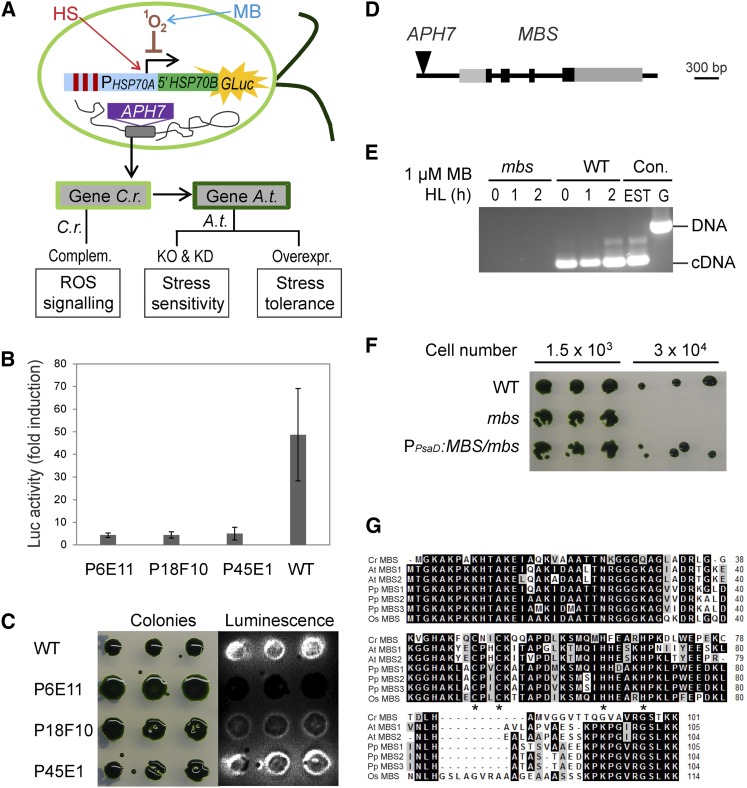
Figure 1.
Screen for ROS Signaling Mutants in C. reinhardtii and Identification of the MBS Gene.
(A) Schematic overview of the experimental strategy for isolating regulators of ROS signaling. High-throughput genetic screens with a ROS-inducible reporter gene in C. reinhardtii are combined with reverse genetic analyses in Arabidopsis. An algal strain expressing a 1O2-inducible luciferase reporter gene (PHSP70A-5′HSP70B-GLuc; Shao and Bock, 2008) was subjected to insertional mutagenesis with the hygromycin resistance gene APH7 (Berthold et al., 2002). Screening for mutants defective in 1O2-mediated signaling was performed by treatment of cultures with the photosensitizer MB that generates 1O2 in the light. As the HSP70A promoter also responds to heat stress (HS; heat stress elements indicated as red bars), ROS signaling mutants can be identified as strains displaying luciferase induction upon heat stress, but lacking induction upon exposure to 1O2 stress. Assays performed on mutants and transgenic lines are indicated in the boxes at the bottom of the panel. C.r., C. reinhardtii; A.t., Arabidopsis; Complem., complementation; KO, knockout; KD, knockdown; Overexpr., overexpression.
(B) Example of three insertion mutants (P6E11, P18F10, and P45E1) that do not respond to 1O2 stress generated by HL (1000 µE m−2 s−1) in the presence of 1 µM MB (monitored by luciferase bioluminescence). Data are mean values ± sd of three biological replicates. WT, wild type.
(C) Heat stress response in the three insertion mutants P6E11, P18F10, and P45E1. Growing algal colonies were shifted from 23 to 40°C for 1 h. While the P45E1 mutant has a normal heat stress response (as evidenced by induction of luciferase luminescence), the P6E11 and P18F10 mutants show an impaired response to heat stress compared with the wild type.
(D) Model of the MBS gene and insertion site of the APH7 marker in the mbs mutant of C. reinhardtii. Black boxes represent exons, and gray boxes represent 5′ and 3′ untranslated regions as confirmed by EST sequencing.
(E) RT-PCR analysis demonstrating the absence of MBS transcripts in mutant P45E1 (mbs). The 300-bp MBS gene-specific RT-PCR product is obtained with wild-type cDNA as template after 0, 1, and 2 h of HL treatment (1000 µE m−2 s−1) in the presence of 1 µM MB but cannot be amplified from cDNA of the mbs mutant. PCR products amplified from an EST clone (AV621044) and genomic DNA (G) of the wild type are shown as controls (Con.).
(F) MB-sensitive phenotype of the mbs mutant (P45E1) compared with the wild type and the complemented PPsaD:MBS/mbs strain.
(G) Alignment of the amino acid sequences of the MBS proteins from the alga C. reinhardtii (Cr), the moss P. patens (Pp), and the higher plants Arabidopsis (At) and rice (Os). Putative MBS sequences were retrieved from the full genome sequences and the alignment was produced using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The asterisks indicate the zinc finger motif of the C2H2 type. Amino acids highlighted in black indicate identical residues in all sequences, and residues in gray denote similar amino acids.
Identification of the MBS Gene
The algal mutants for the genetic screen were generated by insertional mutagenesis to facilitate identification of the mutated gene based on tagging with the selectable marker gene cassette (Figure 1A). However, previous work has shown that only ∼50% of the mutations are tagged by the transforming DNA (Dent et al., 2005). Moreover, the integration of transforming DNA into the C. reinhardtii nuclear genome is often accompanied by larger deletions at the site of insertion, thus additionally complicating gene identification. When isolation of flanking sequences by inverse PCR was attempted for four of the mutants that proved to be 1O2 specific, only one of the mutants (P45E1; Figures 1B and 1C) turned out to be tagged and was devoid of a genomic deletion accompanying marker insertion. The mutant harbored a single-copy insertion of the hygromycin resistance cassette in the putative promoter region of a small gene of unknown function (insertion site: chr_9:4592978-4593500; Joint Genome Initiative C. reinhardtii genome version 4.0). The gene, subsequently referred to as MBS (Figure 1D) is interrupted by three introns, as revealed by comparison of the genomic sequence with a full-length clone in the expressed sequence tag (EST) database and further confirmed by RT-PCR and DNA sequencing (Figure 1E). RT-PCR assays also indicated that the insertion of the hygromycin cassette into the promoter completely abolishes MBS expression in the isolated mbs mutant (Figures 1D and 1E), suggesting that the mutant represents a null allele.
To confirm that the tagged locus is indeed responsible for the mutant phenotype, the mutant strain was complemented by genetic transformation with a cDNA expression construct. The resulting transgenic strains (PPsaD:MBS/mbs) showed wild-type-like tolerance to MB under conditions where the mbs mutant died, presumably due to lethal photooxidative damage (Figure 1F).
The putative MBS protein is a small protein of 10.7 kD. It has homologs in all lineages of land plants. While it is encoded by a single-copy nuclear gene in C. reinhardtii and rice (Oryza sativa), two (highly similar) copies are found in the Arabidopsis genome and three copies in the genome of the model moss Physcomitrella patens (Figure 1G). The only salient feature identifiable upon bioinformatics analysis of the amino acid sequence was a single zinc finger motif of the C2H2 type (Figure 1G).
Isolation and Characterization of mbs Mutants in Arabidopsis
To assess the possible involvement of the MBS homologs in 1O2 sensing and/or signaling in embryophytes, we sought to isolate mutants of the two genes in Arabidopsis, tentatively named MBS1 (AT3G02790) and MBS2 (AT5G16470). Neither of the two genes displays a pronounced tissue-specific or developmental stage-specific expression pattern, with the exception of a moderate upregulation of MBS2 during senescence, a condition known to involve substantial production of ROS (see Supplemental Figure 1 online).
Searches of the collections of T-DNA–tagged Arabidopsis lines revealed three candidate mutants, two of them harboring insertions upstream of MBS1 (mbs1-1 and mbs1-2) and one carrying a T-DNA insertion upstream of MBS2 (mbs2-1; Figure 2A). Sequencing of the insertion sites confirmed the location of the T-DNA inserts. To determine the effects of the T-DNA insertions on MBS expression, homozygous mutant lines were isolated and analyzed by nonquantitative RT-PCR assays. The results revealed that, while the mbs1-1 mutant showed no detectable expression and therefore is probably equivalent to a knockout mutant, the mbs1-2 and mbs2-1 mutants had wild-type-like expression levels (Figure 2B). This finding is consistent with the T-DNA insertion in the mbs1-1 mutant being much closer to the coding region than in the other two mutants (Figure 2A).
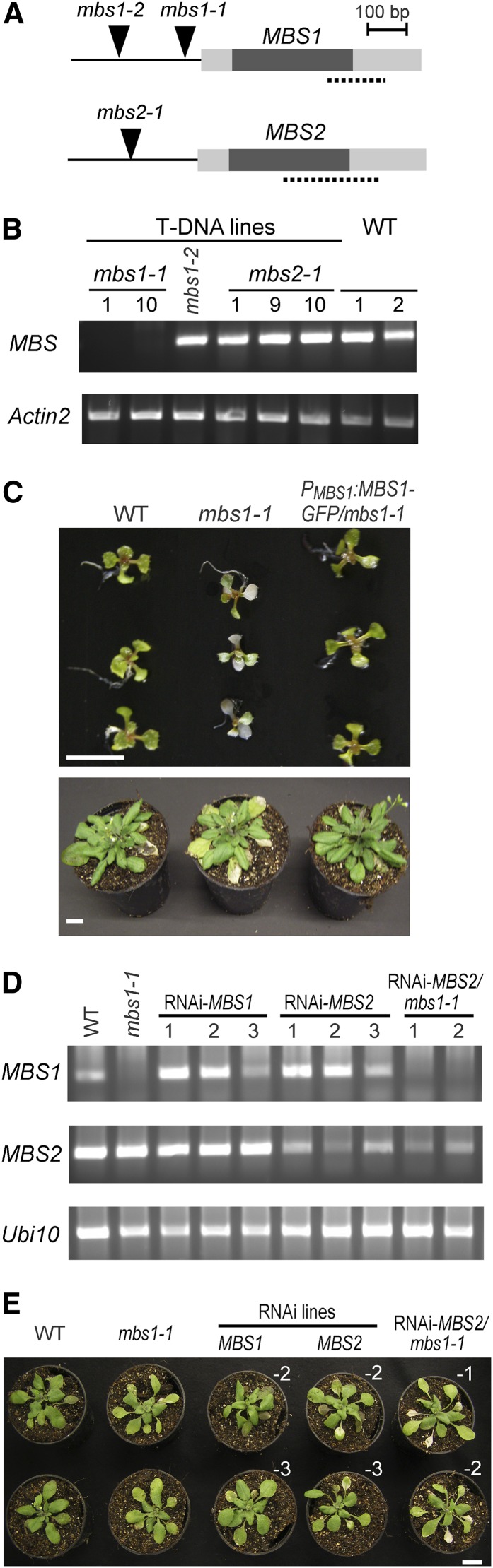
Figure 2.
Characterization of Arabidopsis MBS Genes, Isolation of mbs Mutants, and Generation of RNAi Lines.
(A) Schematic structure of the MBS1 and MBS2 genes in Arabidopsis and identification of T-DNA insertion sites (arrowheads) in mbs1 and mbs2 mutant alleles. Dark boxes indicate coding regions, and gray boxes denote the 5′ and 3′ untranslated regions. The dotted lines indicate the gene-specific tags used for the construction of RNAi lines.
(B) Expression analysis of MBS1 and MBS2 in T-DNA insertion mutants. RT-PCR analysis demonstrates the absence of MBS1 transcripts from the mbs1-1 T-DNA line (1 and 10 indicate two different homozygous plants isolated), whereas MBS1 transcripts are detectable in the homozygous mbs1-2 line and MBS2 transcripts are detectable in the mbs2-1 plants. Actin2 was used as an internal control. WT, wild type.
(C) Sensitivity of mbs1-1 mutant plants to HL stress. Twelve-day-old seedlings grown on synthetic medium were exposed to 900 µE m−2 s−1 for 2 d (top panel), and 4-week-old plants grown in soil were exposed to 1000 µE m−2 s−1 for 3 d and then transferred back to 600 µE m−2 s−1 for 4 d (bottom panel; cf. Supplemental Figure 2 online). The wild type, the mbs1-1 mutant, and the complemented line PMBS1:MBS1-GFP/mbs1-1 were analyzed (for three additional independently generated complemented lines, see Supplemental Figure 2 online). Bars = 1 cm.
(D) RT-PCR analysis of MBS1 and MBS2 mRNA accumulation in the wild type, RNAi-MBS1 lines, RNAi-MBS2 lines, and RNAi-MBS2 mbs1-1 double mutant lines. The Ubi10 mRNA served as internal control.
(E) Increased sensitivity of the RNAi-MBS2 mbs1-1 double mutant to HL stress compared with the mbs1-1 single mutant and the RNAi lines against MBS1 and MBS2. Plants were exposed to 1000 µE m−2 s−1 for 3 d and then transferred back to 600 µE m−2 s−1 for 2 d. Independently generated lines are indicated by white Roman numerals. Bar = 1 cm.
The mbs1-1 mutant did not display a discernible phenotype under standard growth conditions. To preliminarily test for involvement of Arabidopsis MBS1 in plant responses to 1O2, the sensitivity of mbs1-1 mutant plants to high-light (HL) stress was examined, a condition known to trigger massive production of 1O2 by PSII (Krieger-Liszkay and Trebst, 2006). Indeed, mbs1-1 mutant plants showed severe symptoms of photooxidative damage under excess light (Figure 2C), consistent with an involvement of the Arabidopsis MBS1 protein in ROS responses. At the rosette stage, the phenotype was characterized by early senescence of the older leaves, indicative of the accumulation of light-induced oxidative damage over time.
In the absence of a second independent T-DNA mutant for MBS1, complementation of the mbs1-1 mutant with an MBS1-GFP (for green fluorescent protein) fusion gene under the control of the native MBS1 promoter was attempted. Transgenic lines (PMBS1:MBS1-GFP/mbs1-1 lines) were initially identified by their GFP fluorescence and subsequently assayed for complementation of the HL-sensitive mbs1-1 phenotype. Four independently generated lines showed accumulation of the MBS1-GFP fusion protein and displayed wild-type-like tolerance to HL stress (Figures 2C; see Supplemental Figure 2 online), thus proving that the phenotype of the mbs1-1 mutant is indeed caused by the T-DNA insertion and, at the same time, demonstrating that the MBS1-GFP fusion protein is functional.
In the absence of both a mutant for MBS2 and a second independent T-DNA allele for MBS1, mutant lines for both genes were produced by RNA interference (RNAi). To this end, gene-specific tags were inserted into RNAi vectors and stably transformed into the Arabidopsis genome. For both MBS1 and MBS2, RNAi lines could be isolated that showed varying amounts of gene-specific downregulation of expression at the mRNA level (Figure 2D). To generate a double mutant, the RNAi construct against MBS2 was transformed into the mbs1-1 mutant and homozygous RNAi-MBS2 mbs1-1 mutants were isolated. They showed lack of detectable MBS1 expression and low levels of MBS2 expression (Figure 2D). While the phenotype of the RNAi-MBS2 plants was similar to that of the mbs1-1 mutant, the double mutants clearly showed a more severe phenotype in that premature leaf senescence occurred already at the early rosette stage (Figure 2E).
Accumulation of MBS Proteins in Stress Granules and mRNA Processing Bodies
To determine the subcellular localization of the MBS proteins in both C. reinhardtii and Arabidopsis, gene fusions to yellow fluorescent protein (YFP) (C. reinhardtii) and GFP (Arabidopsis) were constructed and introduced into cells by stable and transient transformation, respectively. Visualization of YFP fluorescence in C. reinhardtii was possible using the recently generated UV mutagenesis (UVM) expression strains (Karcher et al., 2009; Neupert et al., 2009). The MBS-YFP fusion protein showed a clear cytosolic localization in the transgenic algal strains (Figure 3A). Similarly, the Arabidopsis MBS1-GFP fusion protein accumulated in the nucleocytosolic compartment of transiently transformed tobacco (Nicotiana tabacum) protoplasts (Figure 3B). Interestingly, the localization pattern changed markedly when the cells were exposed to 1O2 stress. The previously homogeneous distribution of the fluorescence in the cytosol was now confined to discrete spots (Figure 3C), raising the possibility that the perception of 1O2 involves a pronounced change of MBS1 protein distribution in the cell.
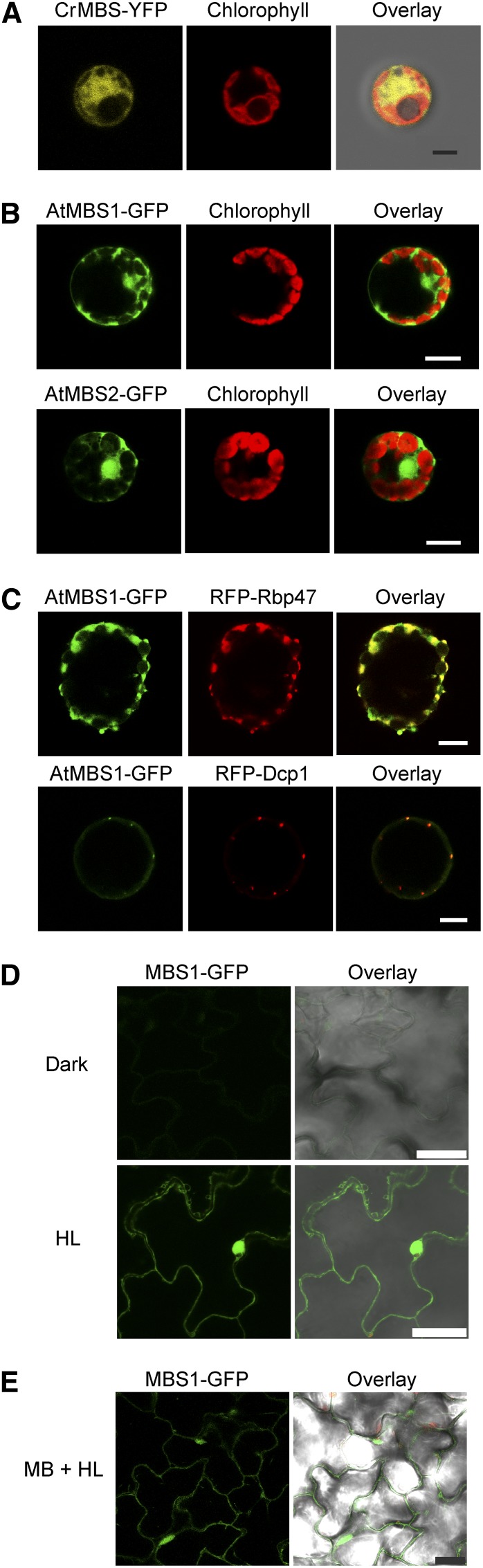
Figure 3.
Subcellular Localization of MBS1 under Normal Conditions and under 1O2 Stress.
(A) Localization of a Cr-MBS-YFP fusion protein in stably transformed C. reinhardtii cells. MBS is localized in the cytosol and the nucleus. YFP fluorescence, chlorophyll fluorescence, and the overlay of YFP fluorescence, chlorophyll fluorescence, and the bright-field image are shown. Bar = 3 µm.
(B) Localization of At-MBS1-GFP and At-MBS2-GFP fusion proteins in transiently transformed tobacco protoplasts. Both proteins are localized in the cytoplasm and the nucleus under nonstressed conditions. Images were obtained 48 h after protoplast transformation. Bars = 10 μm.
(C) At-MBS1 localizes to cytoplasmic SGs and PBs under 1O2 stress. AtMBS1-GFP was transiently coexpressed in tobacco protoplasts with either the SG marker Rbp47 or the PB marker Dcp1 (Weber et al., 2008). Colocalization in cytoplasmic foci was observed 30 min after induction of 1O2 stress by exposure to HL (500 µE m−2 s−1) in the presence of 1 µM MB. Note that PBs are much smaller than SGs, which is an observation that was consistently made in all protoplasts showing PB staining (cf. Supplemental Figure 3 online). The smaller size of the PBs correlates with lower levels of MBS1-GFP accumulation, possibly due to reduced stability of the protein molecules that are not incorporated into PBs. Bars = 10 μm.
(D) MBS1-associated cytoplasmic foci induced in cells of the upper epidermis by HL treatment (1000 µE m−2 s−1) for 3 h after 2 d of preincubation in the dark. The assays were performed with Arabidopsis seedlings that stably express the PMBS1:MBS1-GFP fusion gene in the mbs1-1 mutant background. Bars = 25 μm.
(E) Image from a movie of MBS1-associated cytoplasmic foci trafficking under 1O2 stress in PMBS1:MBS1-GFP/mbs1-1 seedlings (cf. Supplemental Movie 1 online). Bar = 25 μm.
Stress-related globular structures that are known to form in the cytosol include stress granules (SGs) and processing bodies (PBs). SGs accumulate under various stress conditions and represent large cytoplasmic aggregates of untranslated mRNAs bound to a specific set of proteins. As the mRNAs are stored in the form of stalled preinitiation complexes, translation initiation factors, such as RNA-binding protein47 (RBP47), can serve as molecular markers for SGs (Weber et al., 2008). PBs are stress-related cytoplasmic foci that contain enzymes involved in mRNA degradation. In plant cells, they can be distinguished from SGs using the DECAPPING1 (DCP1) subunit of the mRNA decapping complex as marker protein (Weber et al., 2008). To test for a possible association of MBS1 with SGs and/or PBs, constructs expressing fusions of the red fluorescent protein (RFP) to RBP47 or DCP1 (Weber et al., 2008) were employed in cotransformation experiments with the MBS1-GFP fusion construct. Interestingly, MBS1-GFP fluorescence showed clear overlap with both RFP-RBP47 and RFP-Dcp1 fluorescence, indicating that MBS1 associates with both SGs and PBs upon 1O2 stress (Figure 3C; see Supplemental Figure 3 online). Light stress–dependent accumulation of MBS1 (and its concentration in cytoplasmic granular structures) was also seen in transgenic plants stably expressing the MBS1-GFP fusion protein (Figures 3D and 3E; see Supplemental Movie 1 online).
To ultimately confirm that MBS1 protein is associated with cytosolic RNA-containing granules, we enriched these granules from plants expressing the MBS1-GFP fusion protein using a protocol developed for PB purification (Teixeira et al., 2005). This procedure resulted in the isolation of fluorescent bodies that could be readily observed by confocal laser scanning microscopy (see Supplemental Figure 3B online). Treatment with RNase A resulted in loss of the granular GFP fluorescence suggesting that it dissolved the granules or at least released MBS1-GFP protein from the granules (see Supplemental Figure 3B online).
Light Stress Sensitivity and Accumulation of 1O2 in mbs Mutants and Overexpression Lines
If mbs mutants are defective in 1O2 sensing or signaling, they should be incapable of efficient induction of the antioxidative defense system that detoxifies 1O2 after stress acclimation. To test for such defects, 1O2 imaging of light-stressed Arabidopsis seedlings was performed by staining leaves with the 1O2-specific fluorescent dye singlet oxygen sensor green (SOSG; Driever et al., 2009). Indeed, leaves of the mbs1-1 mutant showed much more intense staining with SOSG than the wild type (Figure 4A), suggesting that the mutant is deficient in inducing 1O2 detoxification reactions, presumable due to the lack of proper 1O2 signaling. The effect was specific to 1O2 in that staining for H2O2 accumulation with diamino benzidine revealed no significant difference between the wild type and the mutant (see Supplemental Figure 4 online).
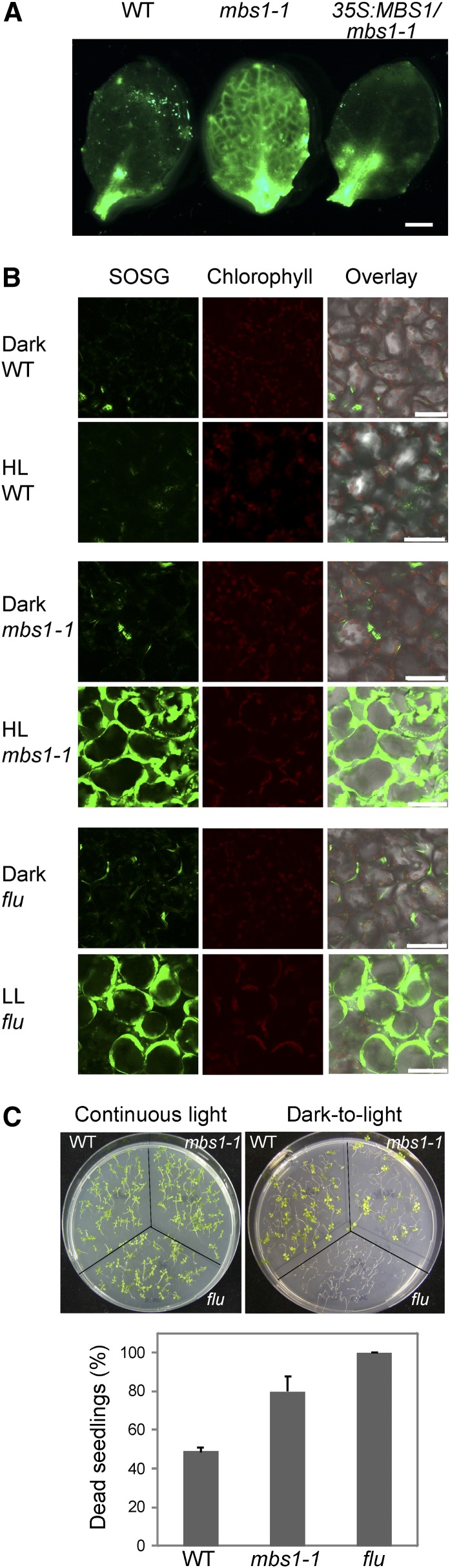
Figure 4.
1O2 Accumulation in the Arabidopsis mbs1-1 Mutant under Light Stress.
(A) 1O2 imaging of light-stressed Arabidopsis seedlings by staining of leaves with SOSG. Leaves of the wild type (WT), the mbs1-1 mutant, and the 35S:MBS1/mbs1-1 transgenic line were analyzed. For quantitation of SOSG fluorescence, see Supplemental Figure 9 online. Bar = 1 mm.
(B) Accumulation of 1O2 in chloroplasts of mesophyll cells in the wild type, the mbs1-1 mutant, and the flu mutant of Arabidopsis in response to light stress. Cells were stained with SOSG, and fluorescence was detected by confocal laser scanning microscopy. As a control, the flu mutant, which generates 1O2 upon transition from dark to light (op den Camp et al., 2003), was exposed to low light (LL). Bars = 50 μm.
(C) Light induction of the cell death response in seedlings of the mbs1-1 mutant and the flu mutant. Seedlings were either grown in continuous light (top left panel) or kept in the dark for 5 d followed by transfer to the light (8-h-dark/16-h-light diurnal cycle; top right panel) to trigger 1O2 production. The graph (bottom panel) shows the death rates of seedlings as scored in three independent dark-to-light shift experiments. Values represent means ± sd.
To study the effects of MBS1 overexpression, an additional set of transgenic lines was generated (in addition to the complemented PMBS1:MBS1-GFP/mbs1-1 lines; see above), in which the MBS1 transgene is driven by the strong constitutive cauliflower mosaic virus (CaMV) 35S promoter in the msb1-1 mutant background (35S:MBS1/mbs1-1 lines). As expected, these lines also showed complementation of the mbs1-1 mutant phenotype, including full restoration of 1O2 signaling and detoxification reactions in response to light stress (Figure 4A).
SOSG staining was also employed to investigate 1O2 accumulation at the subcellular level. As a control, the Arabidopsis flu mutant was included, which accumulates the photosensitizer protochlorophyllide in the dark and, after a dark-to-light shift, generates massive amounts of 1O2 in the chloroplast (op den Camp et al., 2003; Wagner et al., 2004). As expected, this was seen as intense SOSG fluorescence upon transfer of mutant plants to the light (Figure 4B). By contrast, wild-type plants did not show significant changes in SOSG fluorescence even upon transfer to HL, presumably because of the efficient induction of 1O2 signaling mechanisms and detoxification reactions. The mbs1-1 mutant, however, showed strong accumulation of 1O2 in chloroplasts in response to light stress (Figure 4B), confirming that the chloroplast is the main site of 1O2 production also in the mbs1-1 mutant and providing further evidence for this mutant not properly responding to 1O2.
Extensive work on the flu mutant has shown that, under low-level photooxidative stress, 1O2 production does not directly cause lethal cellular damage, but instead, activates a genetic program (dependent on the function of the EXECUTER1 protein) that ultimately results in cell death, seedling lethality, and inhibited plant growth (Wagner et al., 2004). At higher levels of photooxidative stress, this genetically controlled response is gradually overridden by the physiological cell death response triggered by 1O2 toxicity. When the sensitivity of the mbs1-1 mutant to photooxidative stress was analyzed in dark-light shift experiments, it was found to be highly sensitive (with ∼80% seedling lethality), albeit significantly less sensitive than the flu mutant (that showed 100% lethality; Figure 4C).
Light sensitivity of the mbs1-1 mutant was further confirmed by measurements of the maximum quantum efficiency of PSII, Fv/Fm. Upon HL stress, Fv/Fm declined sharply in the mutant compared with the wild type (see Supplemental Figure 5 online), suggestive of the accumulation of photooxidative damage in PSII. Altogether, these data are consistent with an important role of the MBS protein in triggering appropriate cellular responses to 1O2 stress.
Expression of ROS-Responsive Marker Genes in mbs Mutant Plants
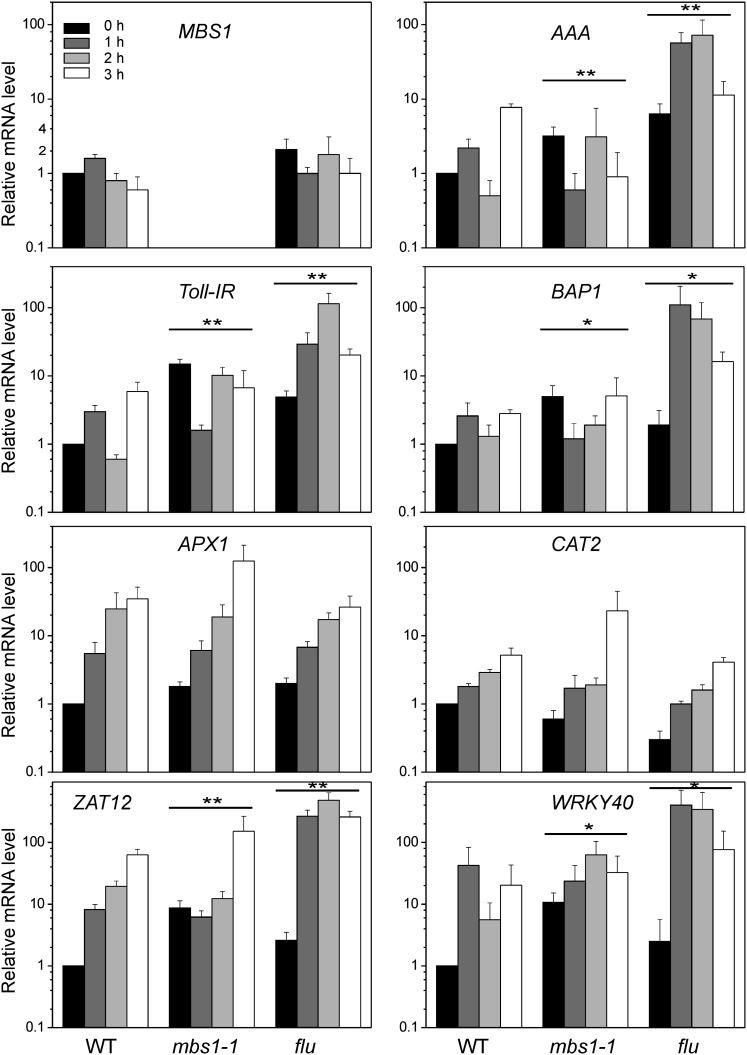
Taken together, the data on the C. reinhardtii and Arabidopsis mutants establish that mbs mutants produce 1O2 upon photooxidative stress, but cannot properly induce cellular defense responses. In C. reinhardtii, this is also evident at the level of 1O2-responsive gene expression (Figure 1). To test whether the Arabidopsis mbs1-1 mutant is defective in ROS-responsive gene expression, quantitative real-time RT-PCR (qRT-PCR) assays were performed for marker genes of oxidative stress (Figure 5). The Arabidopsis flu mutant was included as a control because it shows extreme induction of 1O2-responsive gene expression, due to the massive production of 1O2 after transition from darkness (or low light) to HL conditions (op den Camp et al., 2003; Wagner et al., 2004). Analysis of MBS1 expression in the wild type and the flu mutant revealed that MBS1 itself is not inducible at the mRNA level by 1O2 stress (Figure 5). qRT-PCR using specific primers for the 1O2 marker genes AAA (for AAA-ATPase; Baruah et al., 2009), BAP1 (for BON ASSOCIATION PROTEIN1; Baruah et al., 2009), and Toll-IR (for Toll-Interleukin-Resistance domain-containing protein; Ramel et al., 2012a) revealed rapid induction of expression in the flu mutant already 1 h after the shift to HL stress (which slightly decreased after 3 h) and moderate induction in the wild type after 1 h (followed by a decline after 2 h and another increase after 3 h). By contrast, no increase above the level at time point zero was seen in the expression of any of the three 1O2 marker genes in the mbs1-1 mutant (and overall, the expression levels tended to even decrease).
Figure 5.
Deregulation of 1O2-Inducible Genes in the mbs1-1 Mutant under Light Stress.
Expression of marker genes of oxidative stress responses in mbs1-1 is compared with the wild type (WT) and the flu mutant. The expression levels of ROS-responsive genes were measured over four time points by qRT-PCR using specific primers for the 1O2 marker genes AAA (At3g28580), BAP1 (At3g61190), and Toll-IR (At3g50970), the H2O2 marker genes APX1 (At1g07890) and CAT2 (At4g35090), and the two ROS-responsive transcription factors ZAT12 (At5g59820) and WRKY40 (At1g80840). mRNA levels were determined by the 2−ΔΔCT method relative to the wild-type sample at time point 0 h, which was assigned a value of 1. Data are mean values ± sd of three biological replicates. Statistical analysis of the data was performed by two-way analysis of variance. Differences between expression patterns in the mutants and the wild type that are significant at the level of **P ≤ 0.001 or *P ≤ 0.01.
To test whether or not other ROS responses are also impaired in the mbs1-1 mutant, the expression of two H2O2 marker genes was analyzed: ASCORBATE PEROXIDASE1 (APX1; Baruah et al., 2009) and CATALASE2 (CAT2; Du et al., 2008). Both genes showed similar induction behavior upon light stress in the wild type, the flu mutant, and the mbs1-1 mutant (Figure 5), indicating that the responses to H2O2 are largely unaffected. Finally, two general light stress–responsive transcription factors were investigated: ZAT12 (Baruah et al., 2009) and WRKY40 (Adhikari et al., 2011). Their induction by light stress was delayed or less pronounced in the mbs1-1 mutant compared with the wild type and the flu mutant, possibly due to the lack of the contribution of the 1O2 signal to the induction. Together, these data suggest that, at the level of gene expression, the mbs1-1 mutant does not respond properly to 1O2, while it shows largely unaffected responses to other ROS.
MBS1 Overexpression Leads to Enhanced Tolerance to Photooxidative Stress
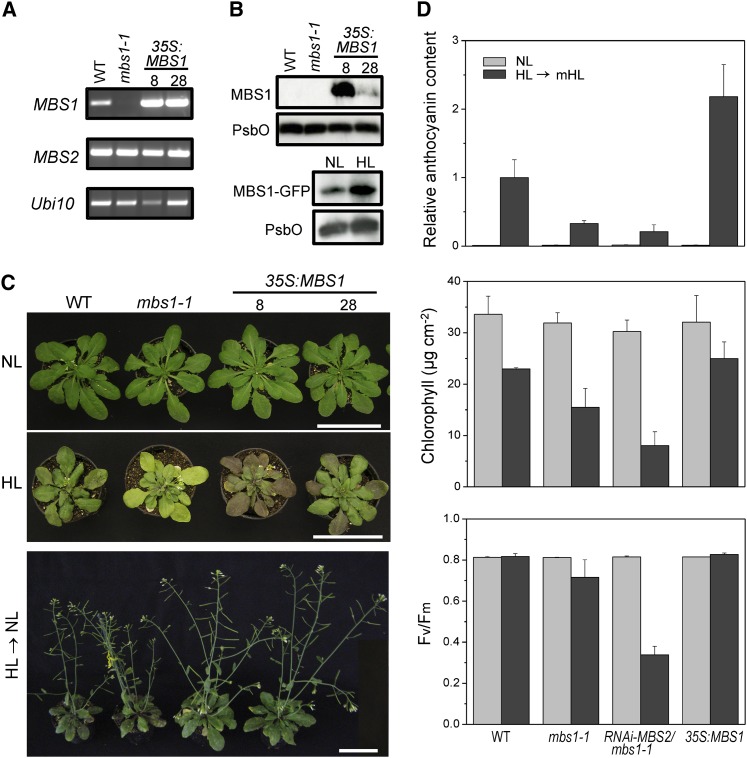
Using the 35S:MBS1 construct (see above), transgenic Arabidopsis lines overexpressing the MBS1 protein were generated. Nonquantitative RT-PCR analyses indicated that, at the mRNA level, substantial overexpression of the MBS1 gene could be achieved (Figure 6A). To be able to assess expression at the protein level, specific antibodies against the MBS1 protein were generated. The antibodies recognized the MBS1 protein with high specificity but were not sensitive enough to detect the protein in wild-type plants, possibly suggesting that the expression level is low or protein detection is difficult due to the small size of MBS1 (of only 11.3 kD). However, in two MBS1 overexpression lines (35S:MBS1-8 and 35S:MBS1-28; Figure 6B), the antibodies detected the MBS1 protein, demonstrating that strong overexpression was achieved also at the protein level. The MBS1 overexpression lines were visually indistinguishable from wild-type plants and mbs1-1 mutant plants under normal light conditions (120 µE m−2 s−1). However, when exposed to HL stress (at 1000 µE m−2 s−1; Figure 6C), the MBS1 overexpression lines showed a much stronger accumulation of the stress-protective pigment anthocyanin than the wild type, while the mbs1-1 mutant (and also the RNAi-MBS2 mbs1-1 mutant) showed a pale phenotype and lacked visible accumulation of anthocyanin (although some low-level induction of anthocyanin biosynthesis was measurable; Figures 6C and 6D). It is well established that anthocyanin synthesis is induced upon exposure to HL (and other types of oxidative stress), but this induction is largely inhibited by overaccumulation of ROS (Vanderauwera et al., 2005). Thus, the pattern of anthocyanin accumulation in the mbs mutants and overexpression lines confirms that 1O2 overaccumulation occurs in the mbs mutants and suggests that light stress responses are induced more efficiently in the MBS1 overexpression lines. The stronger protective response to HL stress translates into a pronounced growth advantage of the MBS1 overexpression lines after transfer back to normal light conditions (Figure 6C). High tolerance of the overexpression lines to light stress (and hypersensitivity of the mbs mutants) was further confirmed by measurements of the change in chlorophyll content (which declined less severely in the overexpression lines than in the wild type) and in the maximum quantum efficiency of PSII (which did not decline in the wild type and the overexpression lines) during the recovery from HL stress (Figure 6D).
Figure 6.
MBS1 Expression Levels Determine the HL Tolerance of Plants.
(A) MBS transcript levels in the wild type (WT), the mbs1-1 mutant, and two MBS1 overexpression lines (8 and 28). RT-PCR analysis demonstrates overexpression of MBS1 in the 35S:MBS1 transgenic lines. MBS2 and Ubi10 served as internal controls.
(B) MBS1 protein levels in the wild type, the mbs1-1 mutant, and the MBS1-overexpressing lines. While in the wild type, the 11.3-kD MBS1 protein is not expressed to levels detectable with the sensitivity of our anti-MBS1 antibody, the protein is readily detected in the 35S:MBS1 overexpression lines (top panel). The protein is also detectable in one of the complemented lines expressing MBS1-GFP from the endogenous MBS1 promoter (bottom panel). To measure expression in response to HL stress, 4-week-old PMBS1:MBS1-GFP plants grown in soil were treated with HL (1000 μE m−2 s−1) for 3 h and then transferred to normal light (NL; 120 μE m−2 s−1) for 45 min. Untreated plants were maintained in normal light (120 μE m−2 s−1). Fifteen micrograms of total protein per sample was loaded. The PSII protein PsbO was used as loading control.
(C) Comparison of the phenotypes of wild-type plants, the mbs1-1 mutant, and two 35S:MBS1 overexpression lines under normal light conditions (µE m−2 s−1; 5-week-old plants; top panel), under HL stress (4-week-old plants exposed to light stress at 1000 µE m−2 s−1 for 5 d in a 16-h-HL/8-h-dark cycle; middle panel), and after recovery from HL and continued growth for 21 d under normal light (HL → NL; bottom panel). Bars = 6 cm.
(D) Light stress acclimation in Arabidopsis MBS mutants and overexpression plants. Anthocyanin contents, chlorophyll contents, and PSII activities were compared under light stress conditions (HL → mHL; 1000 µE m−2 s−1 for 3 d and then transfer to 600 µE m−2 s−1 for 3 d; 16-h-light/8-h-dark regime) and normal light (120 µE m−2 s−1). The wild type, the mbs1-1 and RNAi-MBS2 mbs1-1 mutants, and a transgenic 35S:MBS1 overexpression line were analyzed. Anthocyanin contents are displayed as relative values to the wild type after the stress treatment, which was assigned a value of 1. Data represent mean values ± sd of three biological replicates.
The anti-MBS1 antibody also detected the MBS1-GFP protein in one of our complemented lines that were transformed with the MBS1-GFP construct under the control of the endogenous MBS1 promoter (Figure 6B). This allowed us to determine MBS1 protein accumulation in response to HL stress. Indeed, the protein accumulated to elevated level after transfer from normal light to HL (Figure 6B). As light stress does not significantly alter MBS1 mRNA levels (Figure 5), this finding suggests that MBS1 accumulation is regulated at the translational or posttranslational level. Light stress–induced MBS1-GFP accumulation also explains the strong increase in GFP fluorescence after transfer from the dark to HL conditions observed in our subcellular localization analyses (Figure 3D).
Assuming that MBS proteins act as components of 1O2 signaling, but do not function as a scavenger of 1O2, MBS-expressing bacterial strains should not show an elevated tolerance to 1O2 stress, as the MBS-overexpressing plants do. To confirm this, the MBS genes from C. reinhardtii (Cr-MBS) and Arabidopsis (At-MBS1) were expressed under the control of an isopropyl–β–d–thiogalactopyranosid–inducible promoter in Escherichia coli. When the strains were challenged with 1O2 stress induced by MB treatment, no increased tolerance was seen in the stains overexpressing Cr-MBS or At-MBS1 (see Supplemental Figure 6 online), suggesting that the MBS protein does not act as a 1O2 scavenger.
Global Analysis of ROS-Responsive Gene Expression in mbs Mutants and Overexpression Plants
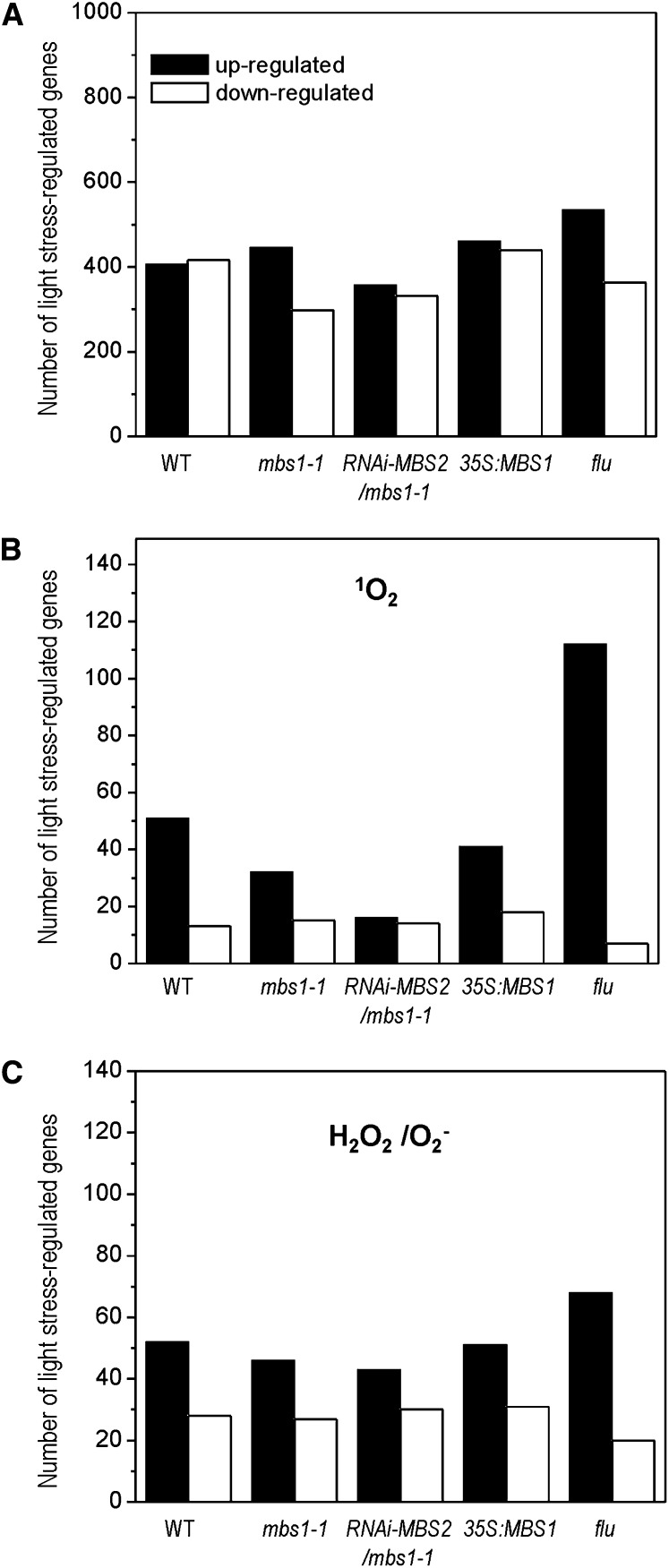
Transcriptome profiling experiments were performed for the following five lines: mbs1-1, RNAi-MBS2 mbs1-1, 35S:MBS1, flu, and the wild type. Plants were stressed by shifting them from a 3-h dark period to HL for 3 h and compared with unstressed control plants at the end of the 3-h dark period (see Methods) in three biological replicates using Affymetrix ATH1 microarrays. When the numbers of genes in the genome that displayed a more than fourfold significant change 3 h after the onset of light stress (P value threshold set to 0.05 based on Student’s t test with false discovery rate correction) were compared, the flu mutant showed the strongest change (Figure 7A). The number of induced genes was elevated compared with the wild type and the other plant lines investigated, consistent with the flu mutant suffering from severe photooxidative stress upon transfer from low light to light stress conditions.
Figure 7.
Light Stress–Responsive Gene Expression in the Wild Type, the mbs1-1 Mutant, the RNAi-MBS2 mbs1-1 Double Mutant, the 35S:MBS1 Overexpression Line and the flu Mutant.
The genome-wide response of the transcriptome to a sudden shift from low-light to HL conditions was determined by microarray analysis.
(A) Light stress response of all genes represented on the ATH1 arrays. Total numbers of genes in the five plant lines that displayed a significant and greater than fourfold change 3 h after the onset of light stress. WT, wild type.
(B) Light stress response of the subset of genes previously reported as responsive to 1O2 (op den Camp et al., 2003; Gadjev et al., 2006). Panel shows number of genes in the subset showing significant and greater than fourfold response in the microarray analysis (see Supplemental Data Set 2 online).
(C) Light stress response of the subset of genes previously reported as responsive to H2O2/O2− (Gadjev et al., 2006). Panel shows number of genes in the subset showing significant and greater than fourfold response in the microarray analysis (see Supplemental Data Set 2 online).
To assess the regulation of ROS-responsive genes, we extracted from the microarray data sets all Arabidopsis genes that had been reported as specifically responding to 1O2 or H2O2/O2− based on previously performed comprehensive transcriptomic studies using the same microarrays (Affymetrix ATH1; op den Camp et al., 2003; Gadjev et al., 2006). This gave a set of 625 1O2-responsive genes (=617 probe sets on the ATH1 array) and 638 H2O2/O2−-responsive genes (=597 probe sets; see Supplemental Data Set 1 online). These sets were then analyzed in the five plant lines (Figure 7; see Supplemental Figures 7 and 8 and Supplemental Data Set 2 online). As expected, the ROS-responsive gene expression pattern in the flu mutant differed most strongly from that of the other four lines. The wild type grouped together with the 35S:MBS1 overexpression line, and the two mbs mutant lines (mbs1-1 and RNAi-MBS2 mbs1-1) clustered together. Interestingly, in the mutant lines, the number of 1O2 marker genes significantly induced by more than fourfold by HL stress was much lower than in the wild type (Figure 7B). Whereas 51 1O2-responsive genes were upregulated in the wild type, only 16 were upregulated in the RNAi-MBS2 mbs1-1 double mutant (Figure 7B), supporting the idea that the MBS gene product is required to mediate 1O2 responses at the level of gene expression. By contrast, the number of H2O2/O2−-responsive genes induced in the mutants was only slightly lower than in the wild type and the 35S:MBS1 line, suggesting that these responses are not primarily affected in the mbs mutants (Figure 7C).
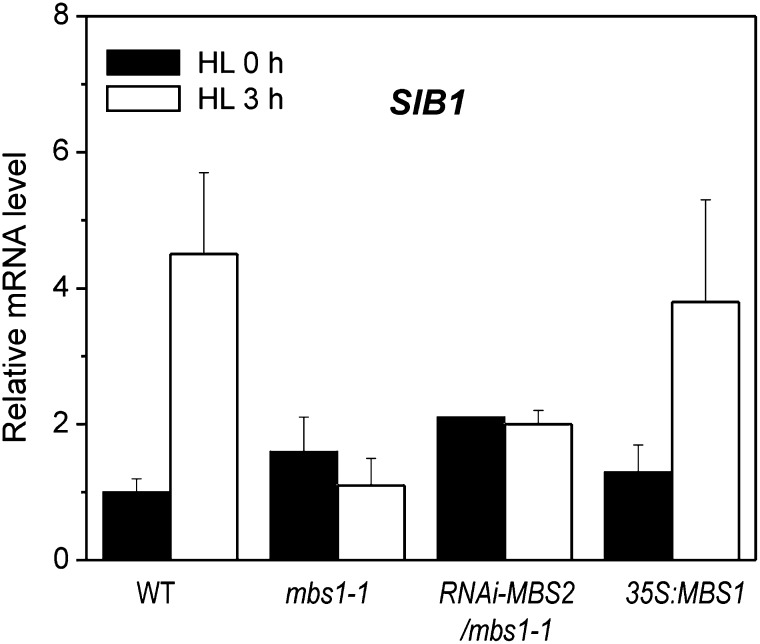
MBS accumulates in the nucleocytosolic compartment. 1O2 is mainly produced in the chloroplast as a by-product of photosynthetic electron transfer (Apel and Hirt, 2004). The chloroplast is also an important site where 1O2-mediated adaptation responses occur to scavenge 1O2 and minimize its production. The latter includes a transcriptional response of the chloroplast genome (Coll et al., 2009), which encodes many of the key components of the ROS-producing photosystems, especially the reaction center subunits of both photosystems which bind a large number of redox-active cofactors. Chloroplast photosynthesis genes are mainly transcribed by the plastid-encoded RNA polymerase, which resembles bacterial RNA polymerases and requires nuclear-encoded sigma factors for promoter recognition (Liere and Börner, 2007). Sigma factors, in turn, are regulated by sigma factor binding proteins (Lai et al., 2011). In Arabidopsis, the SIGMA FACTOR BINDING PROTEIN1 (SIB1) is rapidly induced by 1O2 stress (op den Camp et al., 2003) and by pathogen attack (Lai et al., 2011), the response to which also involves ROS production and extensive ROS-mediated signal transduction (Lam et al., 2001; Apel and Hirt, 2004). Our microarray experiments indicated that the transcriptional induction of the SIB1 gene in response to HL stress is abrogated in mbs mutants (see Supplemental Data Sets 1 and 2 online). In view of the key role that SIB1 and plastid sigma factors play in mediating primary stress responses in chloroplast gene expression (Coll et al., 2009; Lai et al., 2011), we sought to confirm this finding using an independent method. qRT-PCR analyses demonstrated that neither the mbs1-1 mutant nor the RNAi-MBS2 mbs1-1 mutant were capable of inducing the expression of the SIB1 gene in response to HL stress, whereas the 35S:MBS1 overexpression line showed a wild-type-like response (Figure 8). These data provide further evidence for mbs mutants being impaired in their response to 1O2 at the level of nuclear gene expression and, moreover, suggest that also the 1O2-induced remodeling of chloroplast gene expression is defective in the mutants.
Figure 8.
MBS1 Inactivation Affects the Light Stress Response of the Chloroplast-Localized SIB1.
Expression of SIB1 (At3g56710) was measured by qRT-PCR analysis in Arabidopsis wild-type plants (WT), the mbs1-1 mutant, the RNAi-MBS2 mbs1-1 mutant, and the 35S:MBS1 overexpression line. Twelve-day-old seedlings incubated under continuous light of 10 µE m−2 s−1 for 7 d on agar plates were exposed to HL stress (1000 µE m−2 s−1). Samples were collected prior to the onset of light stress (HL 0 h) and 3 h after transfer to HL (HL 3 h). The relative mRNA levels of SIB1 were quantified relative to the expression level in the wild type at 0 h, to which a value of 1 was assigned. Data are mean values ± sd of three biological replicates.
DISCUSSION
In this work, we used the model alga C. reinhardtii to isolate new components of ROS signaling in plants, employing a genetic screen based on the versatile reporter luciferase. This was made possible by the development of a new luciferase reporter (Shao and Bock, 2008) that, in spite of the often low transgene expression levels in C. reinhardtii, is sensitive enough to allow the identification of mutants that are defective in their capacity to induce reporter gene expression in response to an environmental stimulus.
For the genetic dissection of ROS signaling pathways, the green alga C. reinhardtii offers significant advantages over higher plants, most of which are related to its unicellularity. Multicellular eukaryotes sense stresses and respond to them by a highly complex network of tissue-specific, cell-specific, and developmentally regulated pathways. For example, while abscisic acid is often regarded as a general stress hormone in plants that mediates responses to nearly all types of abiotic stresses, its biosynthesis is largely confined to specific tissues and cell types, and dedicated trafficking pathways are required to transport the hormone from its site of synthesis to its sites of action (Galvez-Valdivieso et al., 2009; Antoni et al., 2011; Seo and Koshiba, 2011). By contrast, stress perception and stress responses in C. reinhardtii all occur in a single cell. An additional advantage of the alga’s unicellularity relates to the application of inhibitors or ROS-generating chemicals in genetic screens. Uptake of these compounds is much more homogeneous in a single-celled alga (compared with application by spraying or watering in a higher plant); consequently, the intracellular concentrations of these chemicals can be controlled precisely. This helps to avoid toxic effects from local overconcentration as well as problems with limited efficacy due to inefficient distribution within the plant. In view of the complexity of ROS signaling in multicellular eukaryotes (Gill and Tuteja, 2010; Møller and Sweetlove, 2010; Finkel, 2011; Mittler et al., 2011) and the need to keep the intracellular ROS concentrations tightly controlled to prevent initiation of cell death programs, genetic screens based on sensitive reporter genes in C. reinhardtii offer a powerful tool for the identification of new components of ROS sensing and signaling pathways. In this study, we demonstrated the validity of this approach by isolating a new component of 1O2 signaling. By characterizing the homologous genes in the model plant Arabidopsis, we have also shown that new knowledge acquired about ROS signaling in C. reinhardtii is directly transferable to higher plants.
The mbs mutants isolated in the course of this study are unique in that they produce 1O2 in response to photosensitizer treatment or HL stress but are unable to induce the appropriate cellular responses that ultimately help the organism to cope with the oxidative stress by inducing defense and detoxification mechanisms. The mbs mutants are blocked early in their 1O2 responses, as evident by the failure to induce expression of 1O2-responsive genes. Compared with the C. reinhardtii mbs mutant, 1O2-responsive gene expression is not completely abrogated in the Arabidopsis mbs1-1 mutant (cf. Figures 1 and 5), presumably due to the presence of a second homolog encoded by the MBS2 locus. Interestingly, genes encoding ROS-responsive transcription factors show much more pronounced changes over time in the flu mutant than in the mbs1-1 mutant, consistent with 1O2 overproduction in flu (op den Camp et al., 2003) and an MBS1 action early in 1O2 sensing or signaling, upstream of the induction of gene expression programs. Whether the MBS protein acts directly as a 1O2 sensor or rather as an early component in 1O2 signal transduction remains to be clarified. The single conserved zinc finger in the MBS proteins (Figure 1G) could confer a sensor-like property, in that it theoretically could react with 1O2 and, for example, trigger a conformational response. However, thorough in vitro studies with the purified MBS protein will be required to substantiate this hypothesis. Our observation that MBS1 associates with RNA granules (SG and PBs; Figure 3) in a stress-dependent manner suggests that it might control the stability and/or translation of a specific set of mRNAs encoding signaling components and/or antioxidative defense components downstream of MBS1.
1O2 is generally known to be short-lived. In conjunction with photosynthetic electron transfer being its major source of production, the short half-life of 1O2 has cast some doubt on its potential to leave the chloroplast and activate signaling pathways in the cytosol and/or the nucleus. However, based on the lifetime of 1O2 measured in animal cells (of ∼3 μs), it has been calculated that 5 to 10% of the 1O2 produced in the chloroplast should be able to diffuse over the average distance between the chloroplast and the nucleus in C. reinhardtii cells (Fischer et al., 2007, and references therein). Interestingly, photooxidative stress–induced gene expression profiles of the flu mutant (producing 1O2 in the stroma) and the ch1 mutant (producing 1O2 at PSII in the thylakoid membrane) are very similar (Ramel et al., 2013b), providing further support of the idea that 1O2 can diffuse at least over some distance. In this context, it is important to note that the possibility of 1O2 directly acting as a signaling molecule and the recently established signaling function of molecules derived from chemical reactions with 1O2 (such as the carotenoid oxidation product β-cyclocitral; Ramel et al., 2012a, 2012b) are not mutually exclusive, not the least because multiple 1O2-dependent signaling pathways are likely to coexist in plants (Lee et al., 2007; Kim et al., 2012; Ramel et al., 2013a).
An Arabidopsis mbs1/mbs2 double knockout is currently not available, and, due to the small size of the MBS genes, isolation of a T-DNA insertion line for MBS2 may be difficult to achieve. However, together with the high sequence similarity at the protein level and the presence of only one MBS gene in the genomes of C. reinhardtii and rice (Figure 1G), the aggravated phenotype of our RNAi-MBS2 mbs1-1 double mutants (Figure 2) strongly suggests that the two Arabidopsis MBS genes serve overlapping if not redundant functions. The genomic regions in which MBS1 and MBS2 are embedded on chromosome 3 and chromosome 5, respectively, contain several other duplicated genes, suggesting duplication of a larger chromosomal segment as the likely origin of the two MBS genes in Arabidopsis. Whether or not MBS gene retention in the duplicated region is the result of selection (i.e., reflects an adaptation to stressful environments) remains to be investigated. Interestingly, our overexpression analyses clearly indicate that the amount of MBS1 protein is positively correlated with tolerance to photooxidative stress (Figure 6), further supporting an important role of MBS1 in ROS signaling. Our results also suggest overexpression of MBS genes as a suitable strategy to improve the stress tolerance of crop plants.
Given the multifarious roles that ROS play in physiological and developmental processes (Jiang et al., 2012) as well as in abiotic stress signaling (Jaspers and Kangasjärvi, 2010), retrograde signaling (Pogson et al., 2008), and cell death programs (Lam, 2008), a better knowledge of the cellular processes controlling ROS production and homeostasis and a deeper understanding of the underlying signaling and response pathways are of utmost importance. The combination of sensitive genetic screens in C. reinhardtii with subsequent functional analyses in both algae and higher plants provides a powerful strategy to dissect ROS responses and identify new players in the cellular networks controlling ROS homeostasis, sensing, and signaling. The identification of MBS1 provides proof of principle for this approach, and the many other mutants isolated in our genetic screen will provide a rich source for future investigations into the mechanisms of 1O2 signaling.
METHODS
Algal Strains and Culture Conditions
Mutant screening was performed in a transgenic algal strain derived from Chlamydomonas reinhardtii strain 325 (CW15, mt+, arg7-8). The reporter gene cassette consisting of the codon-optimized luciferase gene from Gaussia princeps and the 1O2-inducible HSP70A promoter was described previously (Shao and Bock, 2008). Subcellular localization analyses were performed in the C. reinhardtii expression strain UVM11 (Neupert et al., 2009). Algal cultures were grown photoheterotrophically in Tris-acetate phosphate (TAP) medium (Harris, 1989) on a rotatory shaker at 23°C under continuous irradiation with white light (55 µEinstein m−2 s−1). To test the sensitivity of algal strains to 1O2, 1.5 × 103 and 3 × 104 cells were spotted in three replicates on an agar plate containing 2 µM of the photosensitizer MB (Merck) and incubated for 6 h under HL (1000 µE m−2 s−1), followed by recovery in normal light (55 µE m−2 s−1) for 20 d.
Insertional Mutagenesis and Screening for 1O2 Mutants in C. reinhardtii
To produce insertional mutants for screening, a C. reinhardtii strain harboring the 1O2-inducible G. princeps luciferase cassette (Shao and Bock, 2008) was transformed with a 1.7-kb HindIII restriction fragment carrying the PTUB:aph7″ hygromycin resistance cassette from vector pHyg3 (Berthold et al., 2002). To this end, samples of 108 cells were transformed with 30 ng of fragment DNA using the glass bead–assisted transformation method (Kindle, 1990; Neupert et al., 2012). Transformants were selected on TAP plates containing 10 μg/mL of hygromycin B. For 1O2 induction of reporter gene expression, transgenic colonies grown for 2 weeks on TAP plates in continuous light (55 μE m−2 s−1) at 23°C were resuspended in 50 μL of TAP medium with or without 1 µM MB in 96-well microtiter plates and treated with HL (1000 µE m−2 s−1) for 1 h. After recovery in normal light for 3 to 4 h at room temperature, bioluminescence of the samples was measured with a luminometer (MicroBeta TriLux; Shao and Bock, 2008), and the fold induction of luciferase activity was calculated by comparison with the control sample not exposed to HL treatment. To monitor heat shock induction of luciferase expression, luminescence images of algal colonies were recorded with an ultrasensitive photon-counting camera (Hamamatsu) after shifting the culture plates from 23 to 40°C for 1 h and recovery at room temperature for 1 h (Shao and Bock, 2008).
Construction of Transformation Vectors for C. reinhardtii
The reading frame of the MBS gene from C. reinhardtii (Cr-MBS) was amplified with or without its stop codon by PCR using the EST clone AV621044 as a template and specific primers (primer NdeI-ZF combined with ZF-EcoRI or ZF-SnaBEcoRI; see Supplemental Table 1 online). The PPsaD:MBS construct was cloned by inserting the Cr-MBS fragment with the stop codon as NdeI/EcoRI restriction fragment into the Cr-PsaD expression cassette (Fischer and Rochaix, 2001) of a vector harboring the paromomycin resistance gene aphVIII as selectable marker gene (Sizova et al., 2001). To produce an MBS-YFP fusion gene, the Cr-MBS fragment lacking the stop codon and containing an additional SnaBI site at the 3′ end was inserted into the PsaD expression cassette. Subsequently, a PCR-amplified YFP fragment with SnaBI-EcoRI ends (see Supplemental Table 1 online) was integrated between Cr-MBS and the PsaD terminator, generating the PPsaD:MBS-YFP expression construct.
Isolation of Genomic DNA from C. reinhardtii and Identification of Insertion Sites by Inverse PCR
Total genomic DNA from C. reinhardtii was extracted following published protocols (Schroda et al., 2001). Inverse PCR was performed according to standard procedures (Jong et al., 2002) with the modification that nested PCRs were performed. Briefly, 0.5- to 1-µg aliquots of genomic DNA were digested overnight with either PstI or PuvII at 37°C in a total volume of 50 µL. Following phenol extraction and ethanol precipitation, self-ligation by T4 DNA ligase was performed with 100 ng of purified DNA in a 100-μL reaction volume overnight at 4°C. The ligated DNA was then linearized by digestion with SacI, a restriction enzyme with a unique cleavage site in the PTUB:aph7″ expression cassette. The purified SacI-digested DNA was used in inverse PCR reactions with the primers Inv-Hyg-f and Inv-Hyg-r (see Supplemental Table 1 online). Nested PCR with primers LMS-Hyg-f and LMS-Hyg-r amplified the insertion site-specific band and, in the case of the P45E1 mutant (mbs mutant), gave a band of ∼2.5 kb that was purified from the agarose gel and sequenced. The sequences flanking the insertion site were extracted from the C. reinhardtii genome sequence (Joint Genome Initiative version 4.0; Merchant et al., 2007) and analyzed with respect to the predicted gene models. Specific primers for Cr-MBS (see Supplemental Table 1) were designed according to the sequence of EST clone AV621044.
Transformation of C. reinhardtii
Nuclear transformation of C. reinhardtii was performed using the glass bead method (Kindle, 1990). For complementation of the mbs mutant, 200 ng of linearized DNA of the PPsaD:MBS plasmid was transformed and transgenic strains were selected on agar-solidified TAP medium containing 10 μg/mL of paromomycin. Transformants were screened for their luciferase activity in response to 1O2 induction with 1 µM MB in HL (1000 µE m−2 s−1) for 1 h. To determine the subcellular localization of MBS, the linearized PPsaD:MBS-YFP plasmid was transformed into C. reinhardtii expression strain UVM11 (Neupert et al., 2009) followed by selection of transformants for paromomycin resistance.
Arabidopsis thaliana Lines, Growth Conditions, and Stress Treatments
Arabidopsis wild-type and mutant plants (all ecotype Columbia) were grown on half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) with 1% Suc for 12 d and then transferred to soil and cultivated under long-day conditions (16 h light/8 h dark, 22°C/18°C) at a light intensity of 120 μE m−2 s−1. HL stress treatments were performed under 900 to 1100 μE m−2 s−1 (16 h light/8 h dark) at 21°C and 55% relative humidity. Prior to HL stress treatments, plants were incubated in low light (10 to 20 µE m−2 s−1) for 7 d. The T-DNA insertion lines mbs1-1 (SAIL_661_B05), mbs1-2 (WiscDsLox337D07), and mbs2-1 (SALK_091719) were obtained from the Nottingham Arabidopsis Stock Centre. Homozygous T-DNA lines were identified by PCR using genomic DNA and T-DNA–specific primers (LB-SALK-b1.3, LB-Wisc, and LB-SAIL) in combination with gene-specific primers (see Supplemental Table 2 online). All PCR products were sequenced to confirm the T-DNA insertions. The Arabidopsis flu mutant (op den Camp et al., 2003) was grown from seeds in continuous light.
Plant Transformation Constructs and Generation of Stable Transgenic Arabidopsis Lines
The RNAi-MBS1 and RNAi-MBS2 vectors were constructed using gene-specific tags of MBS1 (pENTR207_CATMA3a01730) and MBS2 (pENTR207_CATMA5a14770) from the AGRIKOLA collection of RNAi vectors (Hilson et al., 2004; Figure 2A; see Supplemental Table 3 online). The gene-specific tag region of the entry clones was recombined into the RNAi vector pK7GWIWG2(I) (Karimi et al., 2002). To generate the 35S:MBS1 overexpression construct, the PCR-amplified MBS1 reading frame was inserted into a CaMV 35S expression cassette as a BamHI-XbaI fragment (for sequences of PCR primers BamH-3g02790 and At3g02790-XbaI, see Supplemental Table 4 online). To construct a transformation vector for expression of an MBS1-GFP fusion protein (PMBS1:MBS1-GFP construct), a 1252-bp fragment covering the region upstream of the MBS1 start codon and the MBS1 reading frame without its stop codon (obtained by PCR with primers SacI-3g02790 and At3g02790-NcoI followed by digestion with SacI and NcoI; see Supplemental Table 4 online) was ligated to the coding region of GFP followed by the octopine synthase terminator. Transformation of wild-type plants and mbs1-1 mutant plants was performed by the floral dip method (Clough and Bent, 1998) using Agrobacterium tumefaciens strain GV3101:pMP90:pSOUP.
Subcellular Localization and Microscopy Analyses
GFP fusion constructs of the full-length coding regions of At-MBS1 and At-MBS2 were transiently expressed in tobacco (Nicotiana tabacum) protoplasts by polyethylene glycol–mediated DNA uptake. To this end, the At-MBS genes were PCR amplified with gene-specific primers introducing restriction sites for XhoI and NcoI at the 5′ and 3′ ends, respectively (see Supplemental Table 4 online). After digestion with XhoI and NcoI, the amplification products were ligated to the GFP gene in vector pA7-GFP (Voelker et al., 2006), generating an in-frame translational fusion between the MBS gene and the coding sequence of GFP under the control of the CaMV 35S promoter. For subcellular localization analyses, plasmids were transiently transformed into tobacco protoplasts (Huang et al., 2002). To test for colocalization with PBs and SGs, the pA7-MBS1-GFP vector was cotransformed with the Dcp1 or Rbp47 reporter constructs (Weber et al., 2008) into protoplasts. 1O2 stress treatments were performed under HL in the presence of 1 µM MB for 30 min. Subcellular localization of reporter protein fluorescence was determined by confocal laser scanning microscopy (Leica TCS SP5) using a HCx PL APO ×63 W objective lens. Excitation wavelengths were 488 nm for GFP, 514 nm for YFP, 561 nm for RFP, and 488 nm for chlorophyll fluorescence.
Isolation of Nucleic Acids, cDNA Synthesis, and RT-PCR
Total plant DNA was isolated from fresh leaf material by a rapid mini-prep procedure (Doyle and Doyle, 1990). Total cellular RNA was extracted using the peqGold TriFast reagent (Peqlab). RNA samples were digested with DNase (TURBO DNA-free kit; Ambion), and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) and oligo(dT)18 primers according to the manufacturer’s instructions (for RT-PCR primers, see Supplemental Table 5 online). cDNAs from C. reinhardtii were amplified using TaKaRa LA Taq polymerase with GC buffer (Fisher Scientific) and specific primers (see Supplemental Table 5 online) under the following conditions: 94°C for 2 min, 30 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 30 s, followed by a final extension at 72°C for 5 min. For qRT-PCR analyses in Arabidopsis, Actin2 or Ubi10 were amplified as controls for cDNA quality. For the analysis of stress-responsive gene expression by qRT-PCR, 12-d-old Arabidopsis seedlings grown on agar plates and adapted to 10 µE m−2 s−1 continuous light for 7 d were exposed to HL stress (1000 µE m−2 s−1, followed by a 3-h dark period), and samples were collected at different time points. qRT-PCR assays were performed using the LightCycler 480 real-time PCR system (Roche Applied Science) and Absolute SYBR Green ROX mix (Thermo Scientific; for primers, see Supplemental Table 6 online). mRNA abundance of stress-related genes was normalized to the Profilin1 (At2g19760; Baruah et al., 2009) expression level. To confirm the suitability of Profilin1 as reference gene under light stress, the expression of CYP5 (At2g29960) was analyzed as an additional reference. The reference genes changed by ≤1.2 cycles under the conditions of our light stress experiments for initial qRT-PCR evaluation of stress marker gene expression and by ≤0.5 cycles in the light stress experiment used for microarray analysis (see below). Three biological replicates were analyzed with two technical replicates each. The 2−ΔΔCT (cycle threshold) method was used to determine relative transcript levels (Livak and Schmittgen, 2001). Statistical evaluation was performed by analysis of variance with Microsoft Office Excel 2010. Nonquantitative RT-PCR with Arabidopsis cDNAs was performed under the following conditions: 94°C for 2 min, 30 cycles of 94°C for 30 s, 50°C for 1 min, and 72°C for 30 s, followed by a final extension at 72°C for 5 min. For primer sequences, see Supplemental Table 5 online.
Expression of Recombinant At-MBS1 Protein and Generation of Anti-At-MBS1 Antibodies
To generate a vector for overexpression of At-MBS1 in Escherichia coli, the reading frame was amplified with gene-specific primers (NdeI-At3g02790 and At3g02790EcoRI; see Supplemental Table 4 online), introducing restriction sites for NdeI and EcoRI at the 5′ and 3′ end, respectively. The PCR product was digested with NdeI and EcoRI and ligated into expression vector pET21b (Novagene). Polyclonal anti-MBS1 antibodies were produced by immunizing rabbits (BioGenes) with purified At-MBS1 protein overexpressed in E. coli.
To test for a possible 1O2-scavenging function of MBS proteins in E. coli, Cr-MBS and At-MBS1 were overexpressed from pET vectors. As a control, a strain overexpressing a short fragment of ovalbumin (amino acids 280 to 362) was used. Protein expression was induced by addition of 0.1 mM isopropyl-β-d-thiogalactopyranosid to the cultures (at OD600 = 0.6) followed by incubation for 2 h at room temperature. 1O2 stress tolerance was analyzed by comparing the growth of the strains on Luria-Bertani medium containing 5 µM MB and release of 1O2 by light treatment (at 1000 µE m−2 s−1 for 4 h). A dilution series of cultures spotted onto the Luria-Bertani agar plates with or without MB was analyzed in triplicate.
Protein Isolation and Protein Gel Blots
Total plant protein was extracted using a phenol-based method described previously (Petersen and Bock, 2011). Protein extracts were electrophoretically separated in Tricine-SDS polyacrylamide gels (Schägger and von Jagow, 1987) and transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare) using a Trans-Blot cell (Bio-Rad) and a standard transfer buffer (192 mM Gly, 25 mm Tris, 20% methanol, and 2% SDS, pH 8.3). Immunoblot detection was performed with specific antibodies using the enhanced chemiluminescence system (ECL PLUS; GE Healthcare). Polyclonal antibodies against the O-subunit of photosystem II and a peroxidase-conjugated anti-rabbit IgG were purchased from Agrisera.
Enrichment of RNA Granules and RNase Treatment
Four-week-old plants grown in soil were treated with HL (1000 μE m−2 s−1) for 3 h and then transferred to the normal light (120 μE m−2 s−1) for 45 min. The leaves were harvested, quickly ground at 4°C, and then lysed by rotation in the presence of cold lysis buffer composed of 1% Triton X-100, 0.4 mM Tris, pH 7.5, 5 mM NaCl, 6.25 mM MgCl2, 10 µM DTT, and 1× protease inhibitor cOmplete Mini EDTA free (Roche). Lysis was performed for 15 min at 4°C in the presence or absence of 0.4 units/μL of RNase inhibitor. Lysates were cleared by brief centrifugation at 2000g for 2 min at 4°C. The supernatant was centrifuged at 10,000g for 10 min at 4°C, yielding a pellet of semipurified PBs (Teixeira et al., 2005). To test for RNA sensitivity, the resuspended pellet was treated with RNase A (1 μg/μL for 10 min at room temperature; Teixeira et al., 2005). For microscopy analysis, 5 μL of the PB-enriched pellet was examined directly with the confocal microscope.
Imaging of H2O2 and 1O2 in Arabidopsis Leaves
Detection of H2O2 and 1O2 by staining of intact leaves with diamino benzidine and SOSG, respectively, was performed according to published protocols (Driever et al., 2009). For SOSG imaging, 12-d-old Arabidopsis seedlings were vacuum infiltrated with 100 µM SOSG in the dark and then exposed to HL (1000 µE m−2 s−1) for 1 h, while the control plants were kept in the dark. SOSG fluorescence images were taken with a confocal laser scanning microscope (Leica TCS SP5; excitation at 496 nm) or a fluorescence stereomicroscope (Leica MZ16 FA; excitation at 470/510 nm).
Determination of Anthocyanin and Chlorophyll Contents and Chlorophyll Fluorescence Measurements
Determination of chlorophyll and anthocyanin contents in Arabidopsis leaves was performed following published protocols (Lichtenthaler, 1987; Lohmann et al., 2006). Chlorophyll a fluorescence was recorded using a pulse-amplitude modulated fluorometer (DUAL-PAM-100; Heinz Walz) on intact plants at room temperature as described previously (Schöttler et al., 2007a, 2007b). Plants were dark-adapted for 15 min prior to determination of the maximum photochemical efficiency of PSII (Fv/Fm). PSII photoinhibition was measured by the decrease in the chlorophyll fluorescence ratio Fv/Fm in detached leaves exposed to HL stress (1000 µE m−2 s−1) for 4 h followed by recovery at low light (20 µE m−2 s−1) for 4 h.
Microarray Experiments
Light stress–regulated gene expression profiles were analyzed with Affymetrix ATH1 microarrays. Five Arabidopsis genotypes were compared: the wild type, mbs1-1, RNAi-MBS2 mbs1-1, 35S:MBS1, and the flu mutant (op den Camp et al., 2003). Twelve-day-old Arabidopsis seedlings grown on agar plates with half-strength Murashige and Skoog medium (supplemented with 1% Suc) and adapted to 10 µE m−2 s−1 continuous light for 7 d. Following a 3-h dark period, seedlings were exposed to HL stress (1000 µE m−2 s−1). The leaves were immediately collected at 0 h (time point HL 0 h) and 3 h (HL 3 h) of light stress. Three biological replicates were analyzed for each time point.
Total cellular RNA was extracted using the NucleoSpin RNA Plant kit (Macherey-Nagel) according to the manufacturer’s instructions, without DNase digestion. The remaining DNA was digested using the Ambion DNA-free DNase Treatment and Removal Reagents (Invitrogen). RNA quality and quantity of samples were determined by analysis on an Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano kit (Agilent Technologies). Transcript profiles were analyzed with Affymetrix GeneChip ATH1 arrays (ATLAS Biolabs). Raw data were passed through quality assessment and statistical analysis using Robin (Lohse et al., 2010) and the R software package (http://www.r-project.org). Each array was background-adjusted and normalized by the robust multiarray average normalization method in the R package “affy” (Irizarry et al., 2003). For each probe, the average expression level of the three replicates was calculated. Microarray data sets were deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE49596; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE49596).
Arabidopsis genes specifically responding to 1O2 or H2O2/O2− were selected based on two previously published comprehensive studies on ROS signaling that also used Affymetrix ATH1 microarrays for transcript profiling (op den Camp et al., 2003; Gadjev et al., 2006; see Supplemental Data Set 1 online). To determine differentially expressed genes, log2-fold changes (HL 3 h/HL 0 h) were calculated for each probe set on the Affymetrix ATH1 array and each genotype. Genes whose P value was ≤0.05 and whose absolute value of log2-fold change (HL 3 h/HL 0 h) was ≥2 were selected; among them, the genes known to be responsive to 1O2 or H2O2/O2− (op den Camp et al., 2003; Gadjev et al., 2006) were identified (see Supplemental Data Set 2 online). False discovery rate correction (Benjamini and Hochberg, 1995) was performed by the function p.adjust in the R package “stats.” Heat map was plotted using the heatmap.2 function in gplots of the R package. For clustering, hierarchical cluster analysis was used.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Arabidopsis MBS1, AT3G02790; Arabidopsis MBS2, AT5G16470; and C. reinhardtii MBS, jgi|Chlre4|298256.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Expression Patterns of MBS1 and MBS2 in Different Tissues and Developmental Stages of Arabidopsis thaliana.
Supplemental Figure 2. Phenotypic Analysis of Three Additional Complemented Lines of the Arabidopsis mbs1-1 Mutant under High-Light Stress.
Supplemental Figure 3. At-MBS1 Localization to P-Bodies under 1O2 Stress and RNase Sensitivity of MBS-Containing Cytosolic Granules.
Supplemental Figure 4. Production of Hydrogen Peroxide in Response to High-Light Stress in Arabidopsis Wild-Type Seedlings and the mbs1-1 Mutant.
Supplemental Figure 5. Photosystem II Photoinhibition in Leaves of Arabidopsis Wild-Type Plants and mbs1-1 Mutant Plants Exposed to High-Light Stress.
Supplemental Figure 6. Test for a Possible 1O2-Scavenging Function of MBS Proteins in E. coli.
Supplemental Figure 7. Light Stress Response of Genes Previously Reported as Responsive to Reactive Oxygen Species.
Supplemental Figure 8. ROS-Responsive Genes That Are Significantly Changed in Their Expression under Light Stress in at Least One of the Plant Lines Analyzed.
Supplemental Figure 9. Quantitation of 1O2 Accumulation in Leaves of the Arabidopsis Wild Type, the mbs1-1 Mutant, and the 35S:MBS1/mbs1-1 Transgenic Line under Light Stress.
Supplemental Table 1. Oligonucleotides Used for Chlamydomonas reinhardtii.
Supplemental Table 2. Oligonucleotides for Genotyping of T-DNA Insertion Lines.
Supplemental Table 3. Transformation Vectors for the Generation of RNAi Lines and Their Gene-Specific Tags.
Supplemental Table 4. Oligonucleotides for Transgenic Constructs in Arabidopsis.
Supplemental Table 5. Oligonucleotides for RT-PCR.
Supplemental Table 6. Oligonucleotides for Quantitative Real-Time PCR.
Supplemental Data Set 1. List of Singlet Oxygen–Responsive and H2O2/O2−-Responsive Genes and Their Regulation in the Wild Type, mbs1-1, RNAi-MBS2 mbs1-1, 35S:MBS1, and flu under Light Stress.
Supplemental Data Set 2. List of Singlet Oxygen–Responsive and H2O2/O2−-Responsive Genes That Are Significantly Regulated under Light Stress in at Least One of the Plant Lines Analyzed (the Wild Type, mbs1-1, RNAi-MBS2 mbs1-1, 35S:MBS1, and flu).
Supplemental Movie 1. Movie of the Trafficking of MBS1-Associated Cytoplasmic Foci under 1O2 Stress.
Supplementary Material
Acknowledgments
We thank Markus Fauth (University of Frankfurt) for plasmids carrying PB and SG marker genes and Klaus Apel (Boyce Thompson Institute, Cornell University) for providing seeds of the flu mutant. We thank Michael Schroda (University of Kaiserslautern), Eric Lam (Rutgers University), Takuya Matsuo (Nagoya University), Jean-David Rochaix (University of Geneva), Klaus Apel, and Wenbin Zhou (Max-Planck-Institut für Molekulare Pflanzenphysiologie) for helpful discussions. We thank Eugenia Maximova for discussion and help with microscopy, Wenbin Zhou and Mark A. Schöttler for help with chlorophyll fluorescence measurements, Frederik Sommer for help with protein purification, and Annemarie Matthes and Christin Albus (all Max-Planck-Institut für Molekulare Pflanzenphysiologie) for help with optimizing MBS protein detection. This work was supported by grants from the Bundesministerium für Bildung und Forschung (Systems Biology Initiative FORSYS) and the Deutsche Forschungsgemeinschaft (FOR 804; BO 1482/15-2) to R.B.
AUTHOR CONTRIBUTIONS
N.S. designed and performed research, analyzed data, and provided input on the article. G.Y.D. analyzed data. R.B. designed research, analyzed data, and wrote the article.
Glossary
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- 1O2
singlet oxygen
- PSII
photosystem II
- MB
methylene blue
- GFP
green fluorescent protein
- RNAi
RNA interference
- YFP
yellow fluorescent protein
- SG
stress granule
- PB
processing body
- RFP
red fluorescent protein
- SOSG
singlet oxygen sensor green
- CaMV
cauliflower mosaic virus
- Fv/Fm
maximum quantum efficiency of PSII
- qRT-PCR
quantitative real-time RT-PCR
- O2−
superoxide
- TAP
Tris-acetate phosphate
- HL
high light
References
- Adhikari N.D., Froehlich J.E., Strand D.D., Buck S.M., Kramer D.M., Larkin R.M. (2011). GUN4-porphyrin complexes bind the ChlH/GUN5 subunit of Mg-Chelatase and promote chlorophyll biosynthesis in Arabidopsis. Plant Cell 23: 1449–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni R., Rodriguez L., Gonzalez-Guzman M., Pizzio G.A., Rodriguez P.L. (2011). News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr. Opin. Plant Biol. 14: 547–553 [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Baruah A., Simková K., Apel K., Laloi C. (2009). Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 70: 547–563 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300 [Google Scholar]
- Berthold P., Schmitt R., Mages W. (2002). An engineered Streptomyces hygroscopicus aph 7″ gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii. Protist 153: 401–412 [DOI] [PubMed] [Google Scholar]
- Brzezowski P., Wilson K.E., Gray G.R. (2012). The PSBP2 protein of Chlamydomonas reinhardtii is required for singlet oxygen-dependent signaling. Planta 236: 1289–1303 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coll N.S., Danon A., Meurer J., Cho W.K., Apel K. (2009). Characterization of soldat8, a suppressor of singlet oxygen-induced cell death in Arabidopsis seedlings. Plant Cell Physiol. 50: 707–718 [DOI] [PubMed] [Google Scholar]
- Dent R.M., Haglund C.M., Chin B.L., Kobayashi M.C., Niyogi K.K. (2005). Functional genomics of eukaryotic photosynthesis using insertional mutagenesis of Chlamydomonas reinhardtii. Plant Physiol. 137: 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J.J., Doyle J.L. (1990). Isolation of plant DNA from fresh tissue. Focus 12: 13–15 [Google Scholar]
- Driever S.M., Fryer M.J., Mullineaux P.M., Baker N.R. (2009). Imaging of reactive oxygen species in vivo. Methods Mol. Biol. 479: 109–116 [DOI] [PubMed] [Google Scholar]
- Du Y.-Y., Wang P.-C., Chen J., Song C.-P. (2008). Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 50: 1318–1326 [DOI] [PubMed] [Google Scholar]
- Finkel T. (2011). Signal transduction by reactive oxygen species. J. Cell Biol. 194: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B.B., Eggen R.I., Niyogi K.K. (2010). Characterization of singlet oxygen-accumulating mutants isolated in a screen for altered oxidative stress response in Chlamydomonas reinhardtii. BMC Plant Biol. 10: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B.B., Krieger-Liszkay A., Hideg É., Snyrychová I., Wiesendanger M., Eggen R.I.L. (2007). Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett. 581: 5555–5560 [DOI] [PubMed] [Google Scholar]
- Fischer B.B., Ledford H.K., Wakao S., Huang S.G., Casero D., Pellegrini M., Merchant S.S., Koller A., Eggen R.I.L., Niyogi K.K. (2012). SINGLET OXYGEN RESISTANT 1 links reactive electrophile signaling to singlet oxygen acclimation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 109: E1302–E1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N., Rochaix J.-D. (2001). The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genomics 265: 888–894 [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. (2003). Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119: 355–364 [Google Scholar]
- Gadjev I., Vanderauwera S., Gechev T.S., Laloi C., Minkov I.N., Shulaev V., Apel K., Inzé D., Mittler R., Van Breusegem F. (2006). Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G., Fryer M.J., Lawson T., Slattery K., Truman W., Smirnoff N., Asami T., Davies W.J., Jones A.M., Baker N.R., Mullineaux P.M. (2009). The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21: 2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G., Mullineaux P.M. (2010). The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant. 138: 430–439 [DOI] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48: 909–930 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ballester D., Pootakham W., Mus F., Yang W., Catalanotti C., Magneschi L., de Montaigu A., Higuera J.J., Prior M., Galván A., Fernandez E., Grossman A.R. (2011). Reverse genetics in Chlamydomonas: A platform for isolating insertional mutants. Plant Methods 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E.H. (1989). The Chlamydomonas Sourcebook. (San Diego, CA: Academic Press). [Google Scholar]
- Henmi T., Miyao M., Yamamoto Y. (2004). Release and reactive-oxygen-mediated damage of the oxygen-evolving complex subunits of PSII during photoinhibition. Plant Cell Physiol. 45: 243–250 [DOI] [PubMed] [Google Scholar]
- Hilson P., et al. (2004). Versatile gene-specific sequence tags for Arabidopsis functional genomics: Transcript profiling and reverse genetics applications. Genome Res. 14 (10B): 2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.-C., Klaus S.M.J., Herz S., Zou Z., Koop H.-U., Golds T.J. (2002). Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol. Genet. Genomics 268: 19–27 [DOI] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Jaspers P., Kangasjärvi J. (2010). Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 138: 405–413 [DOI] [PubMed] [Google Scholar]
- Jiang C., Belfield E.J., Mithani A., Visscher A., Ragoussis J., Mott R., Smith J.A.C., Harberd N.P. (2012). ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J. 31: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A.Y., T’ang A., Liu D.P., Huang S.H. (2002). Inverse PCR. Genomic DNA cloning. Methods Mol. Biol. 192: 301–307 [DOI] [PubMed] [Google Scholar]
- Karcher D., Köster D., Schadach A., Klevesath A., Bock R. (2009). The Chlamydomonas chloroplast HLP protein is required for nucleoid organization and genome maintenance. Mol. Plant 2: 1223–1232 [DOI] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Kim C., Lee K.P., Baruah A., Nater M., Göbel C., Feussner I., Apel K. (2009). (1)O2-mediated retrograde signaling during late embryogenesis predetermines plastid differentiation in seedlings by recruiting abscisic acid. Proc. Natl. Acad. Sci. USA 106: 9920–9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Meskauskiene R., Zhang S., Lee K.P., Lakshmanan Ashok M., Blajecka K., Herrfurth C., Feussner I., Apel K. (2012). Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24: 3026–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K.L. (1990). High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87: 1228–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A., Trebst A. (2006). Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 57: 1677–1684 [DOI] [PubMed] [Google Scholar]
- Lai Z., Li Y., Wang F., Cheng Y., Fan B., Yu J.Q., Chen Z. (2011). Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 23: 3824–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C., Stachowiak M., Pers-Kamczyc E., Warzych E., Murgia I., Apel K. (2007). Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E. (2008). Programmed cell death in plants: Orchestrating an intrinsic suicide program within walls. Crit. Rev. Plant Sci. 27: 413–423 [Google Scholar]
- Lam E., Kato N., Lawton M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411: 848–853 [DOI] [PubMed] [Google Scholar]
- Lee K.P., Kim C., Landgraf F., Apel K. (2007). EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 10270–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H.K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 148: 350–382 [Google Scholar]
- Liere K., Börner T. (2007). Transcription and transcriptional regulation in plastids. Top. Curr. Genet. 19: 121–174 [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lodha M., Schulz-Raffelt M., Schroda M. (2008). A new assay for promoter analysis in Chlamydomonas reveals roles for heat shock elements and the TATA box in HSP70A promoter-mediated activation of transgene expression. Eukaryot. Cell 7: 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann A., Schöttler M.A., Bréhélin C., Kessler F., Bock R., Cahoon E.B., Dörmann P. (2006). Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J. Biol. Chem. 281: 40461–40472 [DOI] [PubMed] [Google Scholar]
- Lohse M., et al. (2010). Robin: An intuitive wizard application for R-based expression microarray quality assessment and analysis. Plant Physiol. 153: 642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S.S., et al. (2007). The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. (2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Møller I.M., Sweetlove L.J. (2010). ROS signalling—Specificity is required. Trends Plant Sci. 15: 370–374 [DOI] [PubMed] [Google Scholar]
- Mubarakshina M.M., Ivanov B.N., Naydov I.A., Hillier W., Badger M.R., Krieger-Liszkay A. (2010). Production and diffusion of chloroplastic H2O2 and its implication to signalling. J. Exp. Bot. 61: 3577–3587 [DOI] [PubMed] [Google Scholar]
- Mullineaux P., Karpinski S. (2002). Signal transduction in response to excess light: Getting out of the chloroplast. Curr. Opin. Plant Biol. 5: 43–48 [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol. Plant. 15: 473–497 [Google Scholar]
- Neupert J., Karcher D., Bock R. (2009). Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 57: 1140–1150 [DOI] [PubMed] [Google Scholar]
- Neupert J., Shao N., Lu Y., Bock R. (2012). Genetic transformation of the model green alga Chlamydomonas reinhardtii. Methods Mol. Biol. 847: 35–47 [DOI] [PubMed] [Google Scholar]
- op den Camp R.G.L., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Göbel C., Feussner I., Nater M., Apel K. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K., Bock R. (2011). High-level expression of a suite of thermostable cell wall-degrading enzymes from the chloroplast genome. Plant Mol. Biol. 76: 311–321 [DOI] [PubMed] [Google Scholar]
- Pogson B.J., Woo N.S., Förster B., Small I.D. (2008). Plastid signalling to the nucleus and beyond. Trends Plant Sci. 13: 602–609 [DOI] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Cuiné S., Triantaphylidès C., Ravanat J.-L., Havaux M. (2012b). Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 158: 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylidès C., Havaux M. (2012a). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Ksas B., Akkari E., Mialoundama A.S., Monnet F., Krieger-Liszkay A., Ravanat J.-L., Mueller M.J., Bouvier F., Havaux M. (2013b). Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25: 1445–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Mialoundama A.S., Havaux M. (2013a). Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 64: 799–805 [DOI] [PubMed] [Google Scholar]
- Ramundo S., Rahire M., Schaad O., Rochaix J.-D. (2013). Repression of essential chloroplast genes reveals new signaling pathways and regulatory feedback loops in Chlamydomonas. Plant Cell 25: 167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166: 368–379 [DOI] [PubMed] [Google Scholar]
- Scheller H.V., Haldrup A. (2005). Photoinhibition of photosystem I. Planta 221: 5–8 [DOI] [PubMed] [Google Scholar]
- Schöttler M.A., Flügel C., Thiele W., Bock R. (2007a). Knock-out of the plastid-encoded PetL subunit results in reduced stability and accelerated leaf age-dependent loss of the cytochrome b6f complex. J. Biol. Chem. 282: 976–985 [DOI] [PubMed] [Google Scholar]
- Schöttler M.A., Flügel C., Thiele W., Stegemann S., Bock R. (2007b). The plastome-encoded PsaJ subunit is required for efficient photosystem I excitation, but not for plastocyanin oxidation in tobacco. Biochem. J. 403: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M., Blöcker D., Beck C.F. (2000). The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J. 21: 121–131 [DOI] [PubMed] [Google Scholar]
- Schroda M., Vallon O., Whitelegge J.P., Beck C.F., Wollman F.-A. (2001). The chloroplastic GrpE homolog of Chlamydomonas: Two isoforms generated by differential splicing. Plant Cell 13: 2823–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Koshiba T. (2011). Transport of ABA from the site of biosynthesis to the site of action. J. Plant Res. 124: 501–507 [DOI] [PubMed] [Google Scholar]
- Shao N., Bock R. (2008). A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Curr. Genet. 53: 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N., Krieger-Liszkay A., Schroda M., Beck C.F. (2007). A reporter system for the individual detection of hydrogen peroxide and singlet oxygen: Its use for the assay of reactive oxygen species produced in vivo. Plant J. 50: 475–487 [DOI] [PubMed] [Google Scholar]
- Sizova I., Fuhrmann M., Hegemann P. (2001). A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277: 221–229 [DOI] [PubMed] [Google Scholar]
- Teixeira D., Sheth U., Valencia-Sanchez M.A., Brengues M., Parker R. (2005). Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S., Zimmermann P., Rombauts S., Vandenabeele S., Langebartels C., Gruissem W., Inzé D., Van Breusegem F. (2005). Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 139: 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker C., Schmidt D., Mueller-Roeber B., Czempinski K. (2006). Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 48: 296–306 [DOI] [PubMed] [Google Scholar]
- Wagner D., Przybyla D., Op den Camp R., Kim C., Landgraf F., Lee K.P., Würsch M., Laloi C., Nater M., Hideg E., Apel K. (2004). The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Weber C., Nover L., Fauth M. (2008). Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 56: 517–530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.