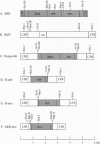
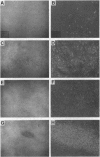
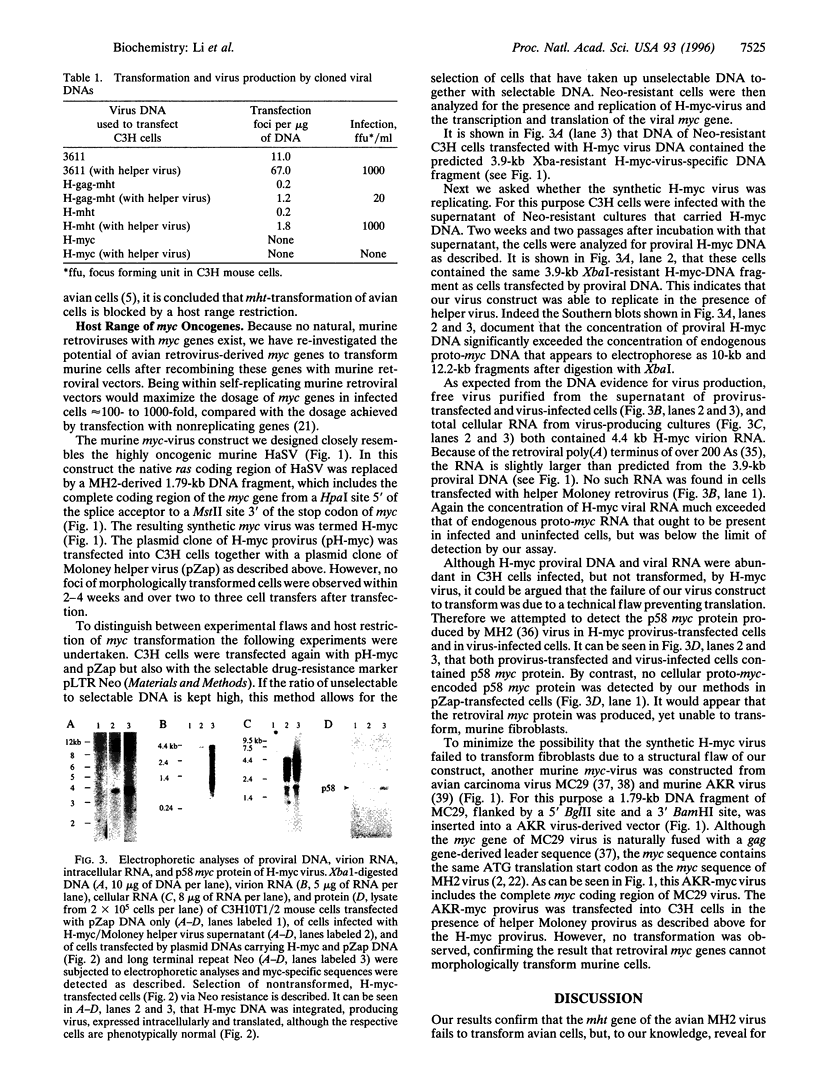
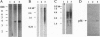Abstract
The host range of retroviral oncogenes is naturally limited by the host range of the retroviral vector. The question of whether the transforming host range of retroviral oncogenes is also restricted by the host species has not been directly addressed. Here we have tested in avian and murine host species the transforming host range of two retroviral onc genes, myc of avian carcinoma viruses MH2 and MC29 and mht/raf of avian carcinoma virus MH2 and murine sarcoma virus MSV 3611. Virus vector-mediated host restriction was bypassed by recombining viral oncogenes with retroviral vectors that can readily infect the host to be tested. It was found that, despite high expression, transforming function of retroviral myc genes is restricted to avian cells, and that of retroviral mht/raf genes is restricted to murine cells. Since retroviral oncogenes encode the same proteins as certain cellular genes, termed protooncogenes, our data must also be relevant to the oncogene hypothesis of cancer. According to this hypothesis, cancer is caused by mutation of protooncogenes. Because protooncogenes are conserved in evolution and are presumed to have conserved functions, the oncogene hypothesis assumes no host range restriction of transforming function. For example, mutated human proto-myc is postulated to cause Burkitt lymphoma, because avian retroviruses with myc genes cause cancer in birds. But there is no evidence that known mutated protooncogenes can transform human cells. The findings reported here indicate that host range restriction appears to be one of the reasons (in addition to insufficient transcriptional activation) why known, mutated protooncogenes lack transforming function in human cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman B. K., Pattengale P. K., Fan H. Generation and characterization of a recombinant Moloney murine leukemia virus containing the v-myc oncogene of avian MC29 virus: in vitro transformation and in vivo pathogenesis. J Virol. 1986 Oct;60(1):68–81. doi: 10.1128/jvi.60.1.68-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A. K., Cichutek K., Duesberg P. H. Transforming function of proto-ras genes depends on heterologous promoters and is enhanced by specific point mutations. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2217–2221. doi: 10.1073/pnas.88.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Oncogenes and cancer. Science. 1995 Mar 10;267(5203):1407–1408. doi: 10.1126/science.7794335. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Schwartz J. R. Latent viruses and mutated oncogenes: no evidence for pathogenicity. Prog Nucleic Acid Res Mol Biol. 1992;43:135–204. doi: 10.1016/s0079-6603(08)61047-8. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Feuerman M. H., Davis B. R., Pattengale P. K., Fan H. Generation of a recombinant Moloney murine leukemia virus carrying the v-src gene of avian sarcoma virus: transformation in vitro and pathogenesis in vivo. J Virol. 1985 Jun;54(3):804–816. doi: 10.1128/jvi.54.3.804-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerman M. H., Lee W. T., Pattengale P. K., Fan H. Comparison of three recombinant murine leukemia viruses carrying the v-src oncogene of avian sarcoma virus: differences in in vitro transformation and in vivo pathogenicity. Mol Carcinog. 1988;1(1):57–66. doi: 10.1002/mc.2940010112. [DOI] [PubMed] [Google Scholar]
- Haugen A., Ryberg D., Hansteen I. L., Amstad P. Neoplastic transformation of a human kidney epithelial cell line transfected with v-Ha-ras oncogene. Int J Cancer. 1990 Mar 15;45(3):572–577. doi: 10.1002/ijc.2910450333. [DOI] [PubMed] [Google Scholar]
- Hjelle B., Liu E., Bishop J. M. Oncogene v-src transforms and establishes embryonic rodent fibroblasts but not diploid human fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4355–4359. doi: 10.1073/pnas.85.12.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. W., Bister K. Nucleotide sequence analysis of the chicken gene c-mil, the progenitor of the retroviral oncogene v-mil. Virology. 1985 Jun;143(2):359–367. doi: 10.1016/0042-6822(85)90376-9. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Garon C. F., Duesberg P. H., Papas T. S. Avian carcinoma virus MH2 contains a transformation-specific sequence, mht, and shares the myc sequence with MC29, CMII, and OK10 viruses. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6566–6570. doi: 10.1073/pnas.80.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. A common onc gene sequence transduced by avian carcinoma virus MH2 and by murine sarcoma virus 3611. Science. 1984 Feb 24;223(4638):813–816. doi: 10.1126/science.6320371. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. Nucleotide sequence of avian carcinoma virus MH2: two potential onc genes, one related to avian virus MC29 and the other related to murine sarcoma virus 3611. Proc Natl Acad Sci U S A. 1984 May;81(10):3000–3004. doi: 10.1073/pnas.81.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Repaske R., Garon C. F., Chan H. W., Rowe W. P., Martin M. A. Characterization of proviruses cloned from mink cell focus-forming virus-infected cellular DNA. J Virol. 1982 Feb;41(2):435–448. doi: 10.1128/jvi.41.2.435-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella A. R., Fiszer-Maliszewska L., Mitchell E. L., Guo Y. P., Fox M., Scott D. Introduction of the activated N-ras oncogene into human fibroblasts by retroviral vector induces morphological transformation and tumorigenicity. Carcinogenesis. 1990 Oct;11(10):1803–1809. doi: 10.1093/carcin/11.10.1803. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl E. C., England L. J., DeClue J. E., Martin G. S. Host range mutants of v-src: alterations in kinase activity and substrate interactions. J Virol. 1992 Jul;66(7):4315–4324. doi: 10.1128/jvi.66.7.4315-4324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubsky S., Dunn T. M., Diamond L., Hagag N. Transformation of diploid human lung fibroblasts with oncogene ras increases the frequency of abnormal mitoses. Cytobios. 1994;80(322):161–178. [PubMed] [Google Scholar]
- Mitsialis S. A., Caplan S., Guntaka R. V. An upstream regulatory domain of avian tumor virus long terminal repeat is required for the expression of a procaryotic neomycin gene in eucaryotic cells. Mol Cell Biol. 1983 Nov;3(11):1975–1984. doi: 10.1128/mcb.3.11.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark P. Viral oncogene permutations. Nature. 1983 Dec 1;306(5942):426–426. doi: 10.1038/306426a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G. M., Moscovici G., Moscovici C., Bishop J. M. Neoplastic transformation and tumorigenesis by the human protooncogene MYC. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2102–2106. doi: 10.1073/pnas.87.6.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Fredrickson T. N., Holmes K. L., Morse H. C., 3rd, Jansen H. W., Patschinsky T., Bister K. Rapid induction of hemopoietic neoplasms in newborn mice by a raf(mil)/myc recombinant murine retrovirus. J Virol. 1985 Jul;55(1):23–33. doi: 10.1128/jvi.55.1.23-33.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. R., Duesberg S., Duesberg P. H. DNA recombination is sufficient for retroviral transduction. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2460–2464. doi: 10.1073/pnas.92.7.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. R., Duesberg S., Duesberg P. H. DNA recombination is sufficient for retroviral transduction. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2460–2464. doi: 10.1073/pnas.92.7.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C., Hoffman J., Goff S. P., Baltimore D. Intramolecular integration within Moloney murine leukemia virus DNA. J Virol. 1981 Oct;40(1):164–172. doi: 10.1128/jvi.40.1.164-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J. Human tumor suppressor genes. Annu Rev Genet. 1990;24:615–657. doi: 10.1146/annurev.ge.24.120190.003151. [DOI] [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Vass W. C., Lowy D. R., Tambourin P. E. Harvey murine sarcoma virus: influences of coding and noncoding sequences on cell transformation in vitro and oncogenicity in vivo. J Virol. 1989 Mar;63(3):1384–1392. doi: 10.1128/jvi.63.3.1384-1392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Kahn P., Adkins B., Enrietto P., Hayman M. J., Graf T., Luciw P. Transformation of mammalian fibroblasts and macrophages in vitro by a murine retrovirus encoding an avian v-myc oncogene. EMBO J. 1984 Dec 20;3(13):3223–3229. doi: 10.1002/j.1460-2075.1984.tb02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderame M. F., Varmus H. E. Highly conserved amino acids in the SH2 and catalytic domains of v-src are altered in naturally occurring, transformation-defective alleles. Oncogene. 1994 Jan;9(1):175–182. [PubMed] [Google Scholar]
- Watson D. K., Reddy E. P., Duesberg P. H., Papas T. S. Nucleotide sequence analysis of the chicken c-myc gene reveals homologous and unique coding regions by comparison with the transforming gene of avian myelocytomatosis virus MC29, delta gag-myc. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2146–2150. doi: 10.1073/pnas.80.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M., Julius M. A., Cerni C., Marcu K. B. Elevated expression of an exogenous c-myc gene is insufficient for transformation and tumorigenic conversion of established fibroblasts. Oncogene. 1987 Mar;1(1):19–27. [PubMed] [Google Scholar]
- Zhou R. P., Duesberg P. H. myc protooncogene linked to retroviral promoter, but not to enhancer, transforms embryo cells. Proc Natl Acad Sci U S A. 1988 May;85(9):2924–2928. doi: 10.1073/pnas.85.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. P., Kan N., Papas T., Duesberg P. Mutagenesis of avian carcinoma virus MH2: only one of two potential transforming genes (delta gag-myc) transforms fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6389–6393. doi: 10.1073/pnas.82.19.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]