Abstract
Polydimethylsiloxane (PDMS) has numerous desirable properties for fabricating microfluidic devices, including optical transparency, flexibility, biocompatibility, and fabrication by casting; however, partitioning of small hydrophobic molecules into the bulk of PDMS hinders industrial acceptance of PDMS microfluidic devices for chemical processing and drug development applications. Here we describe an attractive alternative material that is similar to PDMS in terms of optical transparency, flexibility and castability, but that is also resistant to absorption of small hydrophobic molecules.
Introduction
Microfluidic devices offer a major advantage over existing macroscale systems in terms of reduced operation cost due to use of small volumes of critical reagents. This property enables miniaturization of complex chemistries and processing of biological samples for diagnostic and analytical applications.1 The recent application of microfluidic approaches to create human ‘organs-on-chips’ in which microchannels are lined by living human cells also has opened an entirely new avenue of potential applications in which these biochips can be used as replacements for animal testing of pharmaceuticals, chemicals, cosmetics and toxins.2, 3 However, one of the potential limitations of use of microfluidics is that as microchannel volumes and reservoir sizes are reduced, diffusion times necessary for molecules to reach a channel wall become progressively shorter and, as a result, interactions between solutes and the wetted channel surfaces becomes dominant. If, for example, the channel material strongly absorbs solutes, they can be quickly depleted from the entire solution. Because most drugs and many fluorescent markers are small hydrophobic molecules, their absorption by the walls of the microchannels can result in reduction of effective drug concentration and inaccurate dose-response interpretation, cross-contamination, lower detection sensitivity, and higher background fluorescence. When these processes take place, the usefulness of the devices are greatly reduced due to lack of accuracy and reliability of the assays.
Polydimethylsiloxane (PDMS) is commonly used for fabrication of organs-on-chip microfluidic devices because it is biocompatible, flexible, optically clear and easily molded.4 But because PDMS strongly absorbs small hydrophobic molecules5, microfluidic devices fabricated from this material likely will not be broadly accepted by the pharmaceutical and chemical industries. While there are some substitute materials such as perfluoropolyether elastomers that exhibit superior resistance to organic solvents, acids, and oxidizing agents compared to PDMS, they still suffer from absorption of small hydrophobic molecules.6
Polyurethanes are a very broad class of polymers that have been used with success in many application areas, including medicine. In microfluidics, thin polyurethane films have been integrated into PDMS or rigid polymer devices for cell culture applications.7, 8 In a recent preliminary report, we described the ability to cast microfluidic devices from flexible polyurethanes.9 Here, we show that these polyurethane materials perform similar to PDMS in terms of their optical transparency, biocompatibility, flexibility, and ability to be formed into microdevices by replica molding that can be used for cell culture, but they are much more resistant to the absorption of small hydrophobic molecules. After curing, the polymer also resists degradation from water and ultra-violet light, and its surface can be activated by corona discharge or oxygen plasma allowing for strong bonding to itself, glass, and PDMS. Thus, this polyurethane could be used as a replacement for PDMS in the fabrication of microfluidic devices and organs-on-chips which require low absorption of small hydrophobic molecules, and allow for construction of polyurethane/PDMS/glass hybrid devices.
Materials and Methods
Polyurethane elastomer plaques and devices
The GS polyurethane elastomer (55 – 65 Shore A hardness) used was a castable two-component polymer GSP 1552-2 (GS Polymers, Inc.). Component 1552-2A is composed of dicyclohexylmethane-4,4’-diisocyanate (up to 85% by weight) and a prepolymer of dicyclohexylmethane-4,4’-diisocynate (15–20%). Component 1552-2B is a mixture of a proprietary polyol blend (up to 99.9%) and the catalyst, dibutyltin dilaurate (< 0.5%). Prior to mixing, polyurethane components were degassed separately for two minutes using a planetary atmospheric pressure centrifugal mixer (ARE-310, Thinky). Then, using the mixer, components were mixed together at 1:1 weight ratio.
The ClearFlex polyurethane elastomer (50 Shore A hardness) that was investigated in the leaching tests was ClearFlex 50 polyurethane (Smooth-On, Inc.). Component A contains dicyclohexylmethane-4, 4’- diisocyanate (75–85% by weight). Component B contains polyol/plasticizer blend (99.6–99.8%) and the catalyst, phenylmercuric neodecanoate (0.1–0.15%). Components A and B were mixed at 1:2 ratio by weight as recommended by the manufacturer. Because ClearFlex 50 polyurethane is considerably easier to degas than the GS polyurethane, degassing was carried out after the components were mixed and poured into the mold.
To fabricate test plaques for optical, mechanical, and cell adhesion characterization, polymer mixtures were poured onto a non-treated mirror-polished (Ra ~ 1.5 µm) aluminium surface with vertical barriers to generate a layer ~2 mm in thickness. Direct casting of the GS polyurethane onto silanized silicon wafers and SU-8 on silicon masters was not used due to higher adhesion of polyurethane to the surface and more difficult demolding. Immediately after pouring, the polymer was degassed in a dessicator at approximately 720 Torr for 30 minutes and cured at atmospheric pressure and room temperature overnight followed by thermal treatment at 60°C for two hours. After curing, polyurethane sheets were peeled off from the aluminium surface and samples of desired geometry were cut out for various tests. Oblong 25.4 × 4.0 mm samples were punched for optical characterization while 50.8 × 6.4 mm oblong samples were used to characterize bond strength, Young’s modulus, and plastic deformation. Circular 9.5 mm diameter discs were punched for cell adhesion experiments in multiwell tissue culture plates, and rectangular samples were used to determine water contact angle. The polymer side that was in contact with mirror-polished aluminium during casting was used as an active surface for bonding and water contact angle measurements. Before testing, all polyurethane samples as well as glass slides used in the bonding tests were cleaned with a detergent solution (Natural Dish Liquid, Seventh Generation, Inc.) in a standard ultrasonic cleaner. The active ingredients of the detergent were sodium lauryl sulfate, caprylyl/myristyl glucoside, and lauramine oxide. The samples were then properly rinsed in MilliQ water and blow-dried. Glass slides were further rinsed with acetone.
Polyurethane devices for dye absorption investigation were assembled from two polyurethane blocks (each was 50 × 7 × 3 mm) – see Fig. 1a. The top block contained a serpentine channel (0.025 × 0.300 × 242 mm) on its bottom side that was cast from silanized mold made from Smooth-Cast 310® polymer (Smooth-On, Inc.). The mold was prepared using an SU-8 master, as described previously.10 Prior to and after silanization, the Smooth-Cast 310 mold was baked at 60°C for 30 minutes, which was found to improve quality of the polyurethane parts. Because thicker layers of the GS polyurethane are difficult to degas, polymer pouring and degassing were completed in stages. First a layer of polymer (~1 mm thick) was poured into the mold and degassed for 5 minutes. Then, the remaining amount of the polymer was poured into the mold and degassing was repeated.
Fig. 1.
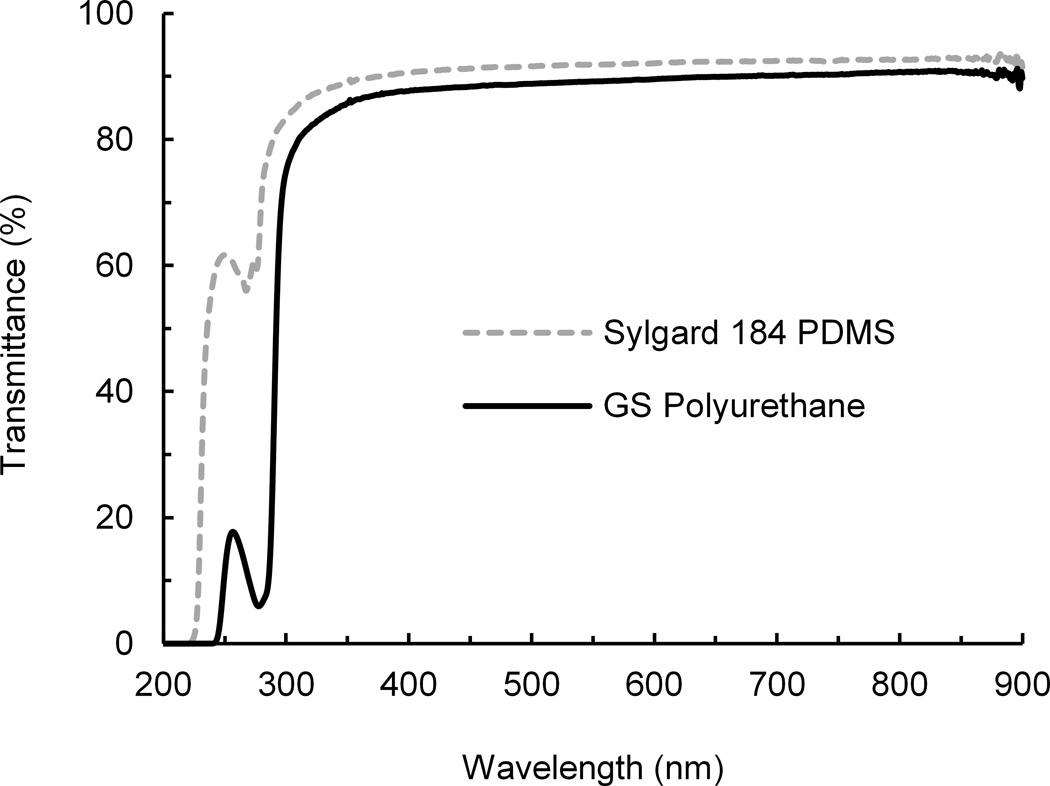
Photograph of two polyurethane (PU) devices with serpentine channels. (a) Comparison of optical transmittance of PU and PDMS over the UV-Visible-NIR spectrum. The plots are average values of two PU and two PDMS samples of ~2 mm in thickness. N=2. (b)
Blank bottom polyurethane blocks without any features were prepared by pouring the polymer onto non-treated aluminium surface and degassed using the multistep procedure described above. After curing, the polymer sheet was manually cut into individual blocks and holes facilitating connections to the channel inlet and outlet were bored in the top block with a disposable biopsy punch. Both blocks were then cleaned by sonication in a detergent solution, rinsed in MilliQ water, blow-dried, and bonded together forming a device. Two sets of bonded devices were reversibly attached to a 60 × 25 × 0.19 mm glass cover slip.
PDMS plaques and devices
PDMS plaques composed of Sylgard 184 (Dow Corning, Inc.) that were used for optical and mechanical characterization were prepared in the same way as the polyurethane plaques. The silicone elastomer base and silicone elastomer curing agent were mixed in a 10:1 weight ratio in an ARE-310 mixer. To make PDMS devices for dye absorption tests, PDMS was poured directly onto a silanized SU-8-on-silicon master placed in an aluminium dish, degassed in a dessicator, and cured in the same manner as polyurethane. Blank bottom PDMS blocks were cast on a silanized silicon wafer placed in an aluminium dish. Device cutting, boring, cleaning, and bonding were done in the same way as with polyurethane devices. Both polyurethane and PDMS devices were plasma activated and bonded a day before the dye absorption tests.
Corona, plasma, and UV ozone surface treatment
Surface treatments of polyurethane and PDMS were performed with corona discharge, air and oxygen plasma, and UV ozone. Corona discharge was done with a high-frequency corona generator BD-20AC (Electro-Technic Products, Inc.). The corona generator tip was scanned for two minutes, 5 mm above both surfaces to be bonded. Air plasma treatment of plaques for shear bond strength evaluation was performed with SPI Plasma-Prep II Plasma Etcher (SPI Supplies, Inc.) at the pressure of 0.38 Torr of air and power of 10 W. For the dye absorption tests, polymer surfaces were activated with oxygen plasma for 30 seconds with PE-100 Plasma Etch Benchtop System (Plasma Etch, Inc.) at an oxygen flow of 19 ccm, power of 100 W, and process pressure of 0.22 Torr. Ultraviolet ozone treatment of samples was done with UVO-cleaner 342 (Jelight Company, Inc.) equipped with a low-pressure mercury vapour grid. Samples were placed with test surfaces facing up at the distance of 5 mm from the UV lamp as recommended by the manufacturer. At this distance, the stated intensities at 184.9-nm and 253.7-nm wavelengths were ~ 6 mW/cm2 and 30 mW/cm2, respectively.
Optical transmittance
Optical transmittance of polyurethane and PDMS samples was measured in the range from 190 to 900 nm with a Cary 300 UV-VIS spectrophotometer (Agilent Technologies, Inc.). Thickness of the samples was approximately 2 mm.
Cyclic Mechanical Loading
Polyurethane and PDMS samples were cyclically stretched to 10% elongation for 200 cycles with 8-second periodicity. The samples were cut from 2 mm thick sheets with a 50.8 × 6.4 mm punch. The width and thickness of each sample were verified with calipers before the test to determine cross-sectional area for stress calculations. Samples were loaded into the grips of a tensiometer 5566 (Instron Industrial Products) with a 1 kN load cell such that 12.5 mm of the sample was clamped by the top grip and 12.5 mm of the sample was clamped by the bottom grip, leaving a functional testing area of 25 mm between the grips. Metal spacers, 0.4 mm in thickness, were placed on either side of the sample in the grips before they were clamped. In this manner, a constant gripping force was achieved for the highly deformable samples, which prevented material slippage from the grips (in the case of under-tightening the grips) and material shearing at the grips (in the case of over-tightening the grips).
Peak force was recorded for each cycle and normalized by the sample cross-sectional area to obtain peak stresses. These stresses were then normalized by the initial peak stress for each sample (after the first cycle), and ten-point data binning was performed to reduce background noise. Samples were tested in triplicate and the means of the normalized peak stresses were reported as with their associated standard deviation (S.D.).
Elastic modulus determination
Samples with the same oblong geometry were also used to obtain the elastic modulus. The polyurethane and PDMS samples were secured in the grips with 0.4 mm spacers and pulled apart at a rate of 30 mm/min. Moduli were determined from the stress-strain curves by finding the slopes of the linear sections, via linear regression, between 10 and 25 % strain.
Shear bond strength evaluation
Immediately after surface treatment, 50.8 mm × 6.4 mm oblong samples were overlapped by ~25 × 6.4 mm and pressed together with pressure of ~1 kPa. Samples were then placed in 40°C and 60°C ovens with appropriate weights placed on top of the overlapping bonding regions to achieve bonding pressures of 3.5 kPa, 7 kPa, and 14 kPa, respectively. The bonded samples were allowed to cool down after removal from the oven, and bond strength testing in shear was performed with a tensiometer 5544 (Instron Industrial Products). The free ends of the partially overlapped and bonded oblong samples were clamped in the tensiometer by two pneumatic grips and the samples were stretched at a separation rate of 0.2 mm/sec until the bond failed. The maximum load supported by the bond was recorded and normalized by the bond area to give the bond strength of each sample in N/m2. If a sample failed at a location other than the bond area, then the minimum value for the bond strength was given.
Water contact angle characterization
Water contact angle was measured with the static sessile drop method using an instrument built in-house. Small rectangular polymer samples were placed on a horizontal platform of a XYZ stage. A 1.5-µL water droplet was dispensed with a pipette on the polymer surface, illuminated with a gooseneck light source through a diffuser, and imaged immediately after dispensing with stereo microscope Discovery V8 (Carl Zeiss, Inc.). Digital images of droplets were analyzed with ImageJ software and the DropSnake module.
Dye absorption evaluation
To compare dye absorption in polyurethane and PDMS devices, a solution of rhodamine B (1 µM in phosphate buffered saline) was pumped through the devices. Samples of the solution were collected at the device outlets and inlets and their fluorescence intensity was quantified with a fluorometer to determine dye loss. To perfuse the devices, dye was ejected from 1 ml polypropylene syringes (Becton Dickinson) at the flow rate of 10 µL/hour using NanoJet syringe drives (Chemyx, Inc.). Devices were connected to the syringes using polyethylene tubing (PE 20, BD Intramedic) and the 10-µl samples of the dye solutions that passed through the devices were collected in polypropylene pipette tips inserted into the devices. Following collection, samples were transferred into a 384-well plate and analyzed with Synergy Neo HTS multi-mode microplate reader (BioTek Instruments, Inc.). Outlet fluorescence intensity was normalized to inlet fluorescence intensity to provide relative concentration (outlet/inlet). Average inlet fluorescent intensity measured at the end of the experiment was 43757 +/− 6911 arbitrary units.
Evaluation of the effect of polymer extracts
Shredded pieces of cast polyurethane and PDMS sheets (~2 × 2 × 2 mm) were sterilized by gamma irradiation in glass vials with a 25 kGy dose. After sterilization, samples of the shredded pieces from each polymer (1 gm by weight) were immersed in 3 mL of Baker analyzed LC/MS reagent water and incubated in glass vials at 37°C for 9 days. As a control, LC/MS reagent water was incubated in a separate glass vial with the other samples. After incubation, liquid was aspirated from the glass vials and used for dosing human umbilical vein endothelial cells (HUVEC) (Lonza, Inc.; passage 4) plated into a polystyrene 96-well plate and grown to confluence. For viability testing, polymer extracts were mixed with complete HUVEC culture medium (EGM-2 with BulletKit) (Lonza, Inc.) in ratios of 1:3,1:6, 1:9 and 1:27 in a 100 µL total culture volume. Heat-inactivated fetal bovine serum (FBS) (Gibco/Invitrogen, Inc.) was added to each extract to match the manufacturer's recommended serum concentration (5%). Cells were incubated at 37°C under 5% CO2 for 24 hours. After incubation, cell viability was measured using an MTT assay (ATCC), and absorbance at 570 nm was measured the following day on a SpectraMax M5 microplate reader (Molecular Devices, Inc.) per manufacturer's instructions. All samples were tested in triplicate. All viability results are presented as percent viability compared to medium-only cell culture controls on tissue culture polystyrene.
Cell culture on GS polyurethane elastomer
For cell culture experiments, GS polyurethane discs (9.5 mm diameter, 2–3 mm thick) were cleaned by sonication in a liquid detergent solution, rinsed in MilliQ water, and blow-dried. The discs were sterilized by UV ozone for 20 and 300 seconds. Following the sterilization, the discs were snugly fit into a polystyrene 48-well cell culture plate and immersed in 0.5 mL of 20 µg/mL fibronectin in 50 mM, pH 9.3 carbonate buffer. After 24-hour incubation at 4°C, the solution was aspirated and replaced with 0.5 mL of EGM-2 endothelial growth medium and incubated overnight at 37°C. The medium was then aspirated and HUVEC cells (passage 3 to 8) were seeded at a density of 2 × 105 per well and cultured for several days at 37°C under 5% CO2.
Results and Discussion
Optical clarity
One of the most unique properties of PDMS is its high optical clarity. Pieces fabricated from PDMS and polyurethane both appear optically clear by eye (Fig. 1a). More rigorous analysis of optical transmittance curves of polyurethane and PDMS elastomers revealed that although PDMS had higher transmittance than polyurethane for wavelengths below 300 nm, the transmittance of the materials was similar for wavelengths above 350 nm (Fig. 1b). This is important because these higher wavelengths are more relevant for assays that use fluorescence microscopy or spectroscopy, which are critical for drug development and chemical analysis applications. The optical clarity of the material is unchanged by plasma treatment and bonding at 60°C for 8 hours, as shown by the polyurethane transmittance data (see Supplementary Figs S1 and S2, ESI†).
Mechanical Performance
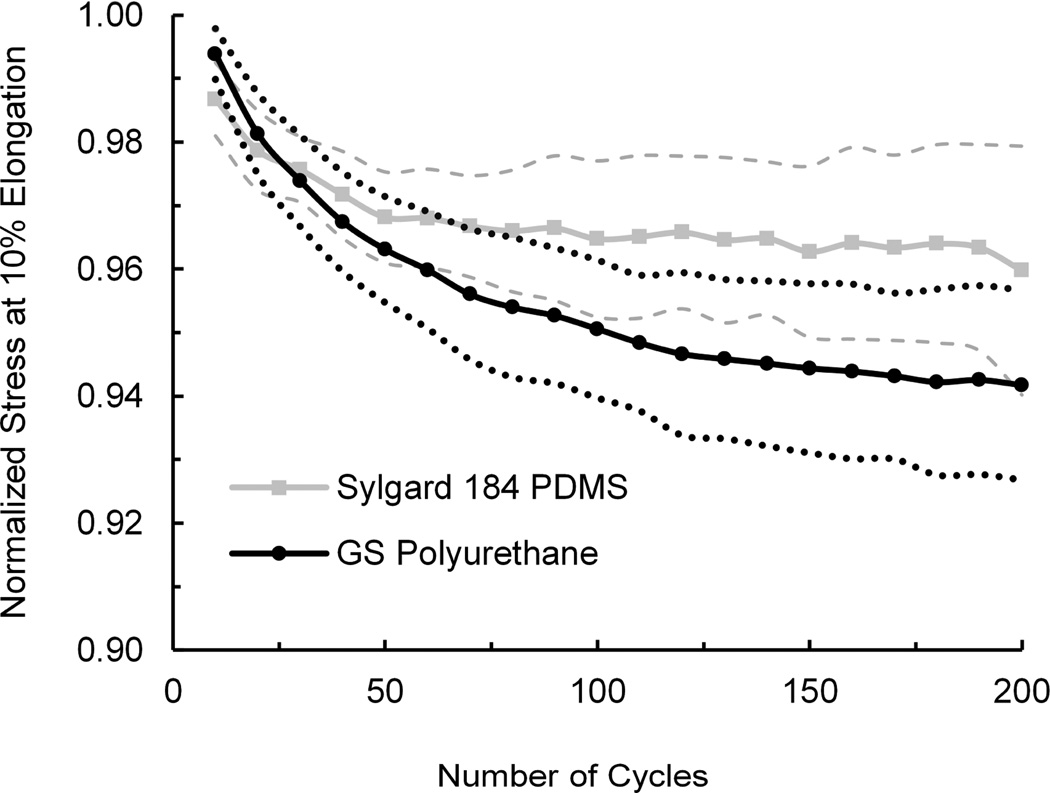
In organs-on-chip models of the lung11, intestine12 or other mechanically-active organs, key physiological conditions (e.g., cyclic breathing and peristalsis-like motions, respectively) are reconstituted by applying cyclic mechanical strain to cells cultured on a porous elastic membrane within a microfluidic device. The exerted strain is typically 5 – 15% with frequency of 0.1 – 2 Hz. Because strain is typically applied for an extended period of time, it is important that the elastomeric membrane does not suffer from inelastic deformation (stress relaxation). To mimic the conditions exerted on the polymer in an organ-on-a-chip microdevice, we subjected a sample to cyclic mechanical load. These studies revealed that after 200 stretch cycles with 8-second periodicity, normalized stress measured for polyurethane at 10% elongation does not significantly differ from the PDMS material (Fig. 2). Thus, at least under these test conditions, the GS polyurethane exhibits similar elastomeric properties to Sylgard 184 PDMS.
Fig. 2.
Performance of PU and PDMS samples subjected to cyclic mechanical strain. Average stresses at 10% elongation over time were normalized by the initial stress at 10% elongation. Dashed and dotted lines represent one standard deviation above and below the normalized average stresses for PDMS and polyurethane, respectively. Cycle time was 8 seconds. N=3.
When tensile failure tests were carried out, we found that in all cases, the materials failed at the grips, indicating that the shear forces due to clamping influenced material failure (see Supplementary Figs S3 and S4, ESI†). Linear regression analysis of the stress-strain curve for each sample between 10 and 25% strain revealed that the elastic modulus of this polyurethane is ~2.56 MPa, which is significantly higher (p < 0.01) than the modulus measured for PDMS (~1.22 MPa, see Supplementary Fig. S5, ESI†).
Optimization of material bonding
Optimization of conditions for bonding polyurethane to polyurethane was done in two stages. First, we tested several corona surface pre-treatment conditions and bonding times and qualitatively evaluated changes in sample appearance and bond strength. This was done by visual evaluation of polymer yellowing and separating the bonded discs by hand. Second, we maintained the corona surface pre-treatment time constant and quantified the shear bond strength as a function of bonding pressure, bonding temperature, and a narrower range of bonding time. When we varied corona pre-treatment time and bonding time while keeping the bonding pressure and temperature constant at 14 kPa and 60°C, we observed that both longer corona treatment times (~5 min) and bonding times (> 24 hours) led to a strong bond, but there was a pronounced yellowing of polyurethane. In contrast, shorter corona pre-treatment times (~1 min) combined with longer bonding times (>24 hours) resulted in formation of a weak bond. The best results were obtained with a 2-minute corona pre-treatment and 2 hours of bonding that created a strong bond without the yellowing effects (see Supplementary Table 1, ESI†).
Next, we quantitatively determined minimum bonding pressure, lowest bonding temperature and shortest bonding time because establishing these conditions is essential to avoid material degradation and distortion of imprinted features. After performing a set of preliminary tests, we investigated bonding pressures of 3.5 kPa, 7 kPa, and 14 kPa; bonding temperatures of 23.7°C, 40°C, and 60°C; and bonding times of 2, 4, and 8 hours. After the bonding, all 27 possible permutations of these three parameters were evaluated by measuring shear bond strength. These measurements revealed that varying the bonding pressure within the investigated range does not have a significant effect on the bond strength (see Supplementary Table 2, ESI†); however, samples bonded at 60°C had the strongest bond of the three investigated temperatures, followed by bonding at 40°C, and room temperature bonding yielded the weakest bond. Thus, longer bonding times resulted in a stronger bond. But there seems to be some saturation of bond strength with bonding time because the 8 hour bonding yielded only a 3% stronger bond than the 4-hour treatment, while the 4-hour bonding was 7% stronger than 2-hour treatment.
To explore the possibility of incorporating glass optical windows and fabricating hybrid microfluidic devices from different polymers, we also investigated bonding of polyurethane to glass and polyurethane to PDMS. For comparison, we also measured shear bond strength between PDMS and itself and between PDMS and glass. In addition, because plasma surface pre-treatment is also commonly used for bonding PDMS and other polymers, we investigated the bond strength of both air plasma-treated and corona pre-treated polyurethane bonded to itself, glass, and PDMS using the conditions optimized for the corona pre-treated samples.
Our results are shown together with water contact angle data in Table 1. Water contact angle (measured immediately after every surface treatment) is listed in the table because it is one of the important parameters that characterize pre-bonding surface functionalization, and thus affect the bond strength. We found that a strong bond can be achieved between polyurethane and itself, polyurethane and glass, and polyurethane and PDMS. This provides a useful flexibility for fabricating hybrid polyurethane microfluidic devices. Corona pre-treatment also outperformed air plasma pre-treatment for bonding polyurethane to itself, but it was not as effective for bonding polyurethane to glass, even though water contact angles of the polyurethane surfaces were about the same (60 – 61°) in both cases. Further, for corona pre-treated samples, the bond strength of polyurethane bonded to itself was stronger than the bond strength of polyurethane to PDMS, and it was even stronger than PDMS bonded to itself.
Table 1.
Water contact angle and shear bond strength of polyurethane and PDMS samples pre-treated either with corona discharge for 120 seconds or air plasma for 30 seconds. Bonding pressure was 5 kPa, bonding temperature 60°C, and bonding time 8 hours. Water contact angles of untreated polyurethane and PDMS were (82.9±3.0)˚ and (103.1±12)˚, respectively. Errors are standard deviations. N=3.
| Material Combination | Treatment of Both Surfaces |
Water Contact Angle of the Polymer after Treatment (°) |
Bond Strength (kPa) |
|---|---|---|---|
|
Polyurethane- Polyurethane |
Corona | 60.9 ± 2.5 | 110.0 ± 1.0 |
| Polyurethane-Glass | 119.4 ± 15.0 | ||
|
Polyurethane- Polyurethane |
Air Plasma | 59.8 ± 2.3 | 68.4 ± 6.9 |
| Polyurethane-Glass | 195.3 ± 42.2 | ||
| PDMS-PDMS | Corona | 44.9 ± 3.3 | 23.2 ± 1.8 |
| PDMS-Glass | >31.2 ± 4.1* | ||
| PDMS-PDMS | Air Plasma | 4.1 ± 1.0 | >36.6 ± 0.3* |
| PDMS-Glass | >42.9 ± 6.0* | ||
| Polyurethane-PDMS | Corona | see above | 30.3 ±1.0 |
| Air Plasma | >38.1 ± 1.2* |
PDMS broke outside the bond area prior to separation of the two samples.
Effect of UV ozone sterilization
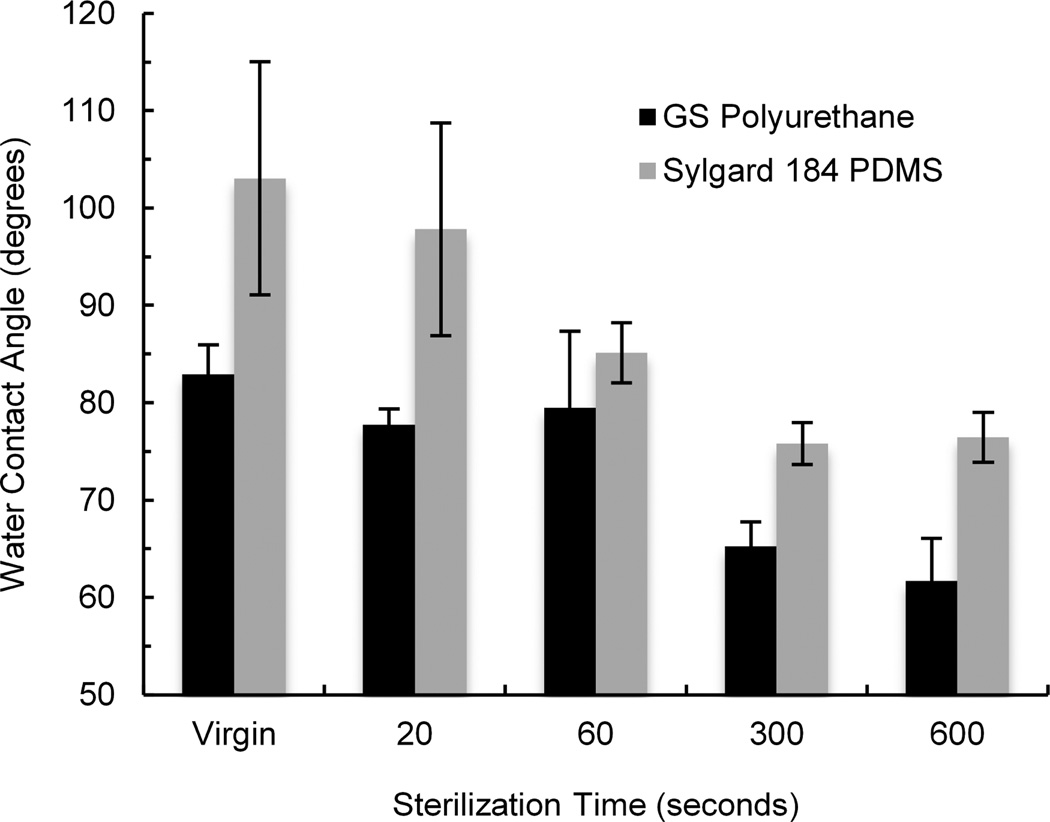
A usual procedure step for preparing polymer scaffolds for cell culture is sterilization and coating the surface with an extracellular matrix (ECM) protein. Because sterilization temperature by autoclaving (121 – 134°C) is too high for the GS polyurethane, we sterilized the samples with UV ozone. The sterilization time recommended by the manufacturer of the UV ozone cleaner is 20 s for samples positioned 5 mm or less from the lamp. However, because this exposure did not significantly decrease water contact angle (Fig. 3), we used longer treatment times (5 or 10 minutes) that resulted in a marked water contact angle decrease and more favorable conditions for cell attachment.
Fig. 3.
Effect of UV-ozone sterilization of polyurethane and PDMS on water contact angle. Error bars are standard deviations. N=3.
Effects of surface treatments on hydrophobicity
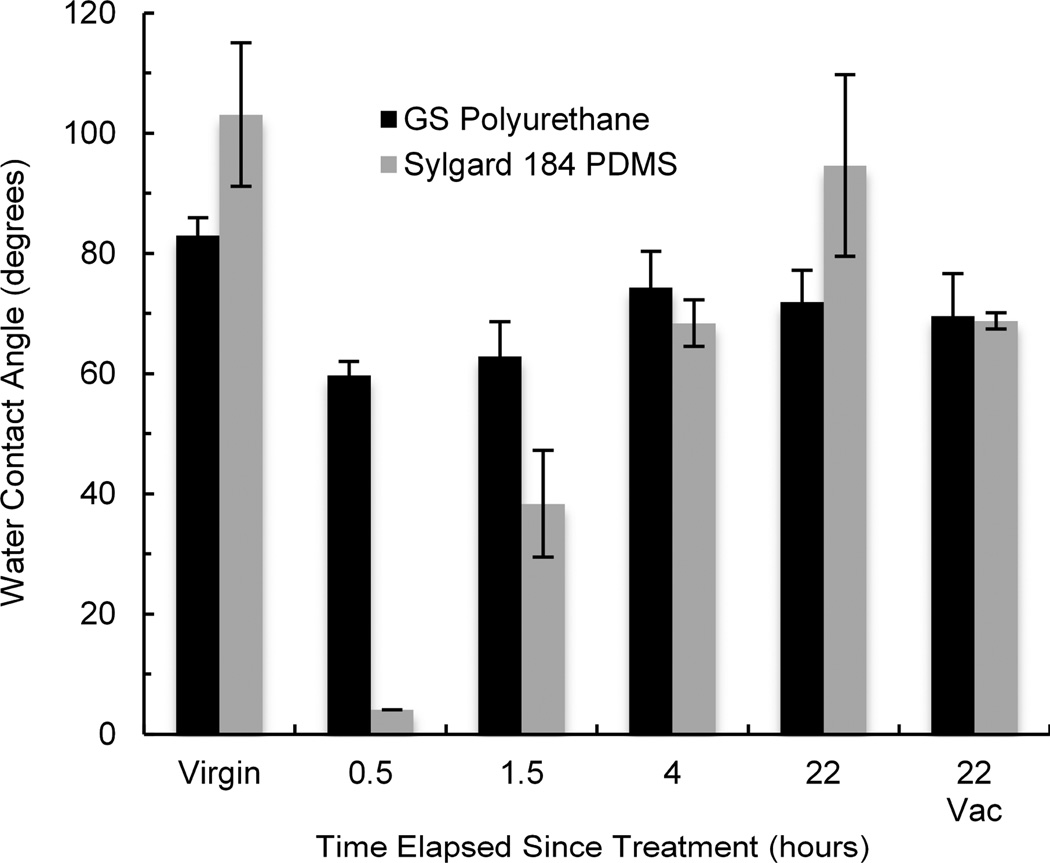
We found that for the specific treatment parameters used, plasma treatment reduced water contact angle of polyurethane by ~31% while PDMS was reduced by ~96% (Fig. 4). Following the surface treatment, many polymers tend to recover to their hydrophobic state mainly due to relaxation of polar groups and diffusion of oligomers to the surface. This process has been well documented for PDMS.13–15 Hydrophobic recovery is clearly noticeable in Fig. 4 for plasma treated PDMS, as the water contact angle increased from approximately 4 degrees to 95 degrees over 22 hours. Hydrophobic recovery seems to occur in polyurethane as well, albeit to a smaller degree. Because there is no statistically significant difference between water contact angles of polyurethane samples recovering in ambient air and under 635 Torr of vacuum, it would suggest that similarly to PDMS13, hydrophobic recovery of polyurethane is not significantly influenced by the interaction with the surrounding atmosphere. For the effect of corona and UV ozone treatments on water contact angle of polyurethane and PDMS, as well as their hydrophobic recoveries, see Supplementary Fig. S6, ESI†.
Fig. 4.
Comparison of hydrophobic recovery of polyurethane and PDMS after treatment with plasma for 30 seconds. Error bars are standard deviations. N=3.
Device fabrication
Challenges of fabricating microfluidic devices from GS polyurethane include more difficult degassing due to a high surface tension and demolding due to a stronger adhesion. Because one of the components contains diisocyanate, processing should be done in a chemical hood to avoid exposure. When components are mixed together, it is important to ensure 1:1 stochiometry to prevent the presence of unreacted components. This is critical for cell culture applications in particular. Compared to PDMS, the gel time of the GS polyurethane is significantly shorter (~30 – 60 minutes). These challenges can be addressed by mixing smaller batches and by proper preparation for molding. Components of castable polyurethanes are moisture sensitive and thus, require storage in hermetically sealed containers with a dry gas in the headspace.
Characterization of dye absorption
Orally administered drugs often require optimal solubility both in water and fat because they have to pass through the gastrointestinal tract, cross the intestinal wall, be transported by blood, and penetrate the lipid membrane of cells. Two important factors affecting solubility in polymers are the relative lipophilic/hydrophobic behaviour of the chemical compound, which is typically reported as the logarithm of the octanol-water partition coefficient LogP and the molecular mass.
Rhodamine 6G and diazepam that exhibit LogP values of 2.62 and 2.8, respectively, have been shown to absorb into PDMS significantly more than the less hydrophobic compounds, mannitol, dexamethasone, and phenytoin (LogP of -3.10, 1.83, and 2.47).16 For example, in three different past studies of representative oral drugs, the mean calculated LogP values were 2.3, 2.5, and 2.5, and mean molecular masses were 333, 337, and 344 Da.17 To match these typical LogP values, we chose rhodamine B with a reported log P value of 2.43 – 2.44 and a molecular mass of 479 Da as a representative drug surrogate for comparing the absorption of a small hydrophobic compound by polyurethane versus PDMS.18, 19
We recently showed in qualitative dye absorption experiments involving static immersion of PDMS and polyurethane polymer discs (10 mm diameter, 4 mm in thick) in solutions of rhodamine B and Nile red, that there was much stronger absorption of these small hydrophobic molecules by PDMS compared to GS polyurethane.9 However, in most applications, it is more practical to know the level of drug depletion from the solution than the drug present in the polymer. Depending on how the device is utilized in an assay, a drug solution can, for example, be stagnant in a reservoir well of the device for a prolonged period of time and then be pumped through a microchannel in the device to a chamber with cells. The key is then to know the effective drug concentration the cells are exposed to. To mimic the situation, we compared depletion of rhodamine B in GS polyurethane versus PDMS by flowing the dye solution through long microchannels in devices made from the two materials and measuring fluorescence of the solution at the device outlets. Geometrical dimensions of the serpentine channel were chosen to maximize surface to volume ratio and thus, maximize the interaction of the dye with the polymer (Fig. 5). Specifically, this was accomplished by molding a very long and shallow channel with a surface to volume ratio of ~87. To further enhance dye interaction, the solution was pumped only at10 µL/hr, which corresponds to a dye residence time in the device of approximately 11 minutes.
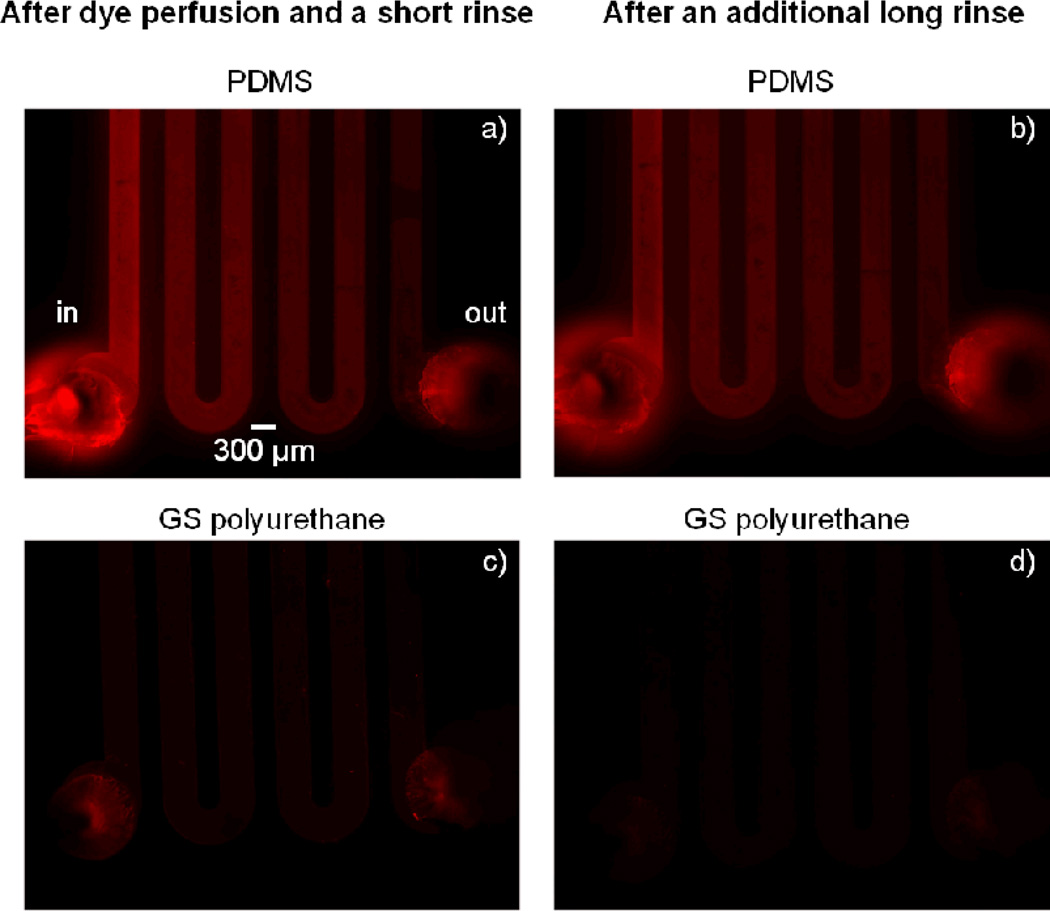
Fig. 5.
Absorption of rhodamine B solution in PDMS and PU devices. Images on the left were taken after a 26-hour perfusion of rhodamine B solution, a 3-minute PBS perfusion rinse, and air-drying. Images on the right show devices after an additional PBS perfusion rinse for 28 hours and air-drying.
First, we again evaluated presence of the dye in the polymers after perfusing rhodamine B through the serpentine channel and rinsing the channel with PBS. Large differences between polyurethane and PDMS were readily noticeable when viewed by fluorescence microscopy (Fig. 5). While only a faint residue of the dye was visible in the polyurethane device channel, the PDMS device displayed bright fluorescence surrounding the channel, and there was a large decrease in brightness along the fluid flow path from the inlet to the outlet. The difference between dye interaction with polyurethane and PDMS was even more apparent after an additional 28-hour perfusion rinse with PBS. While the washed PDMS device still retained large amounts of the dye and exhibited bright fluorescence staining, the polyurethane device did not show any detectable sign of dye absorption.
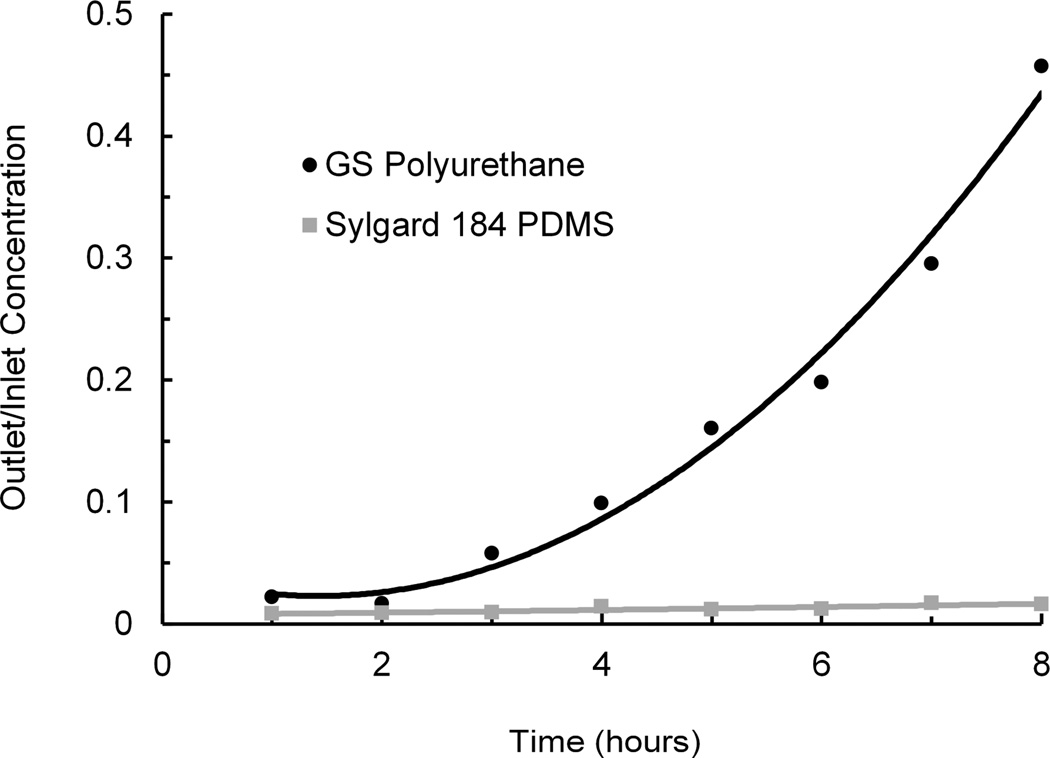
Next, rhodamine B effluent concentrations at the outlets of polyurethane and PDMS devices were measured, compared to the concentrations at the inlets, and plotted as a function of time (Fig. 6). The dye at the PDMS device outlet remained almost completely depleted due to adsorption and absorption for the duration of the 8-hour experiment. In contrast, the dye at the polyurethane device outlet was depleted only initially. As the experiment progressed, the outlet fluorescent signal increased significantly over time (20-fold higher than PDMS at 8 hours), suggesting a drastically lower combined rate of adsorption and absorption compared to PDMS.
Fig. 6.
Relative concentration (outlet/inlet) at the outlets of serpentine channels in devices fabricated from PU and PDMS. Fluorescent dye (rhodamine B) solution was perfused at 10 µL/h and collected at the device outlet to monitor dye loss during flow through a microchannel. Graph shows average values of two PU devices and four PDMS devices.
Effect of polyurethane on cultured cells
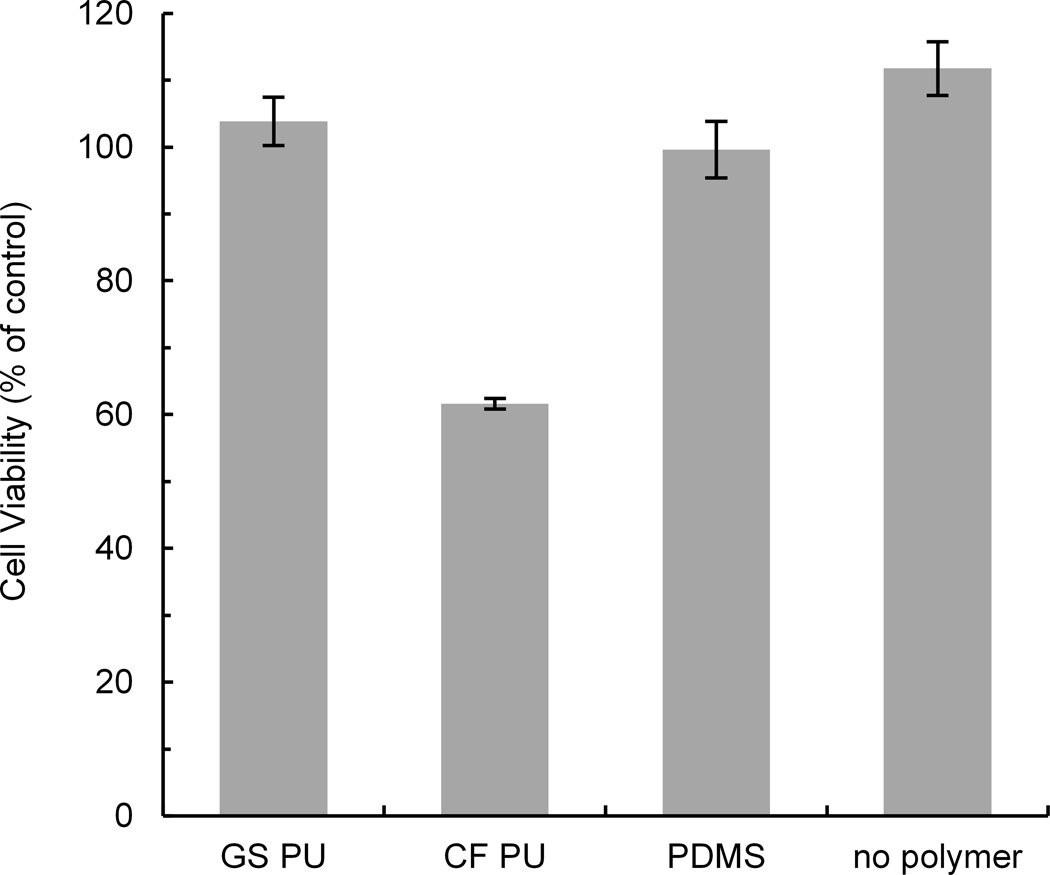
Initially, we identified castable ClearFlex 50 polyurethane as a possible replacement of PDMS for applications that cannot tolerate major absorption of small hydrophobic molecules. ClearFlex 50 did extremely well in the dye absorption tests, was very easy to cast on SU-8/Si masters, and to degas and demold. However, when we cultured cells on the polymer in a standard multiwell tissue culture plate, the cells would not attach and grow, regardless of the surface treatment and extracellular protein used to coat the polymer (see Supplementary Figs S7 and S8, ESI†). To investigate if toxic compounds were leaching from the polymer, we grew HUVE cells on tissue culture-treated polystyrene dishes and exposed them to extracts from shredded ClearFlex 50 polyurethane, GS polyurethane or PDMS. HUVE cells were chosen for the cytotoxity tests because these cells are known for their high sensitivity to cytokines, and they been used in microfluidic devices such as the PDMS lung-on-a-chip11. When the cells were exposed to a 1:3 dilution of extracts from GS polyurethane elastomer and PDMS materials, there was no significant reduction of cell viability with either extract (Fig. 7). In contrast, a 1:3 dilution of the extract from ClearFlex 50 polyurethane elastomer reduced cell viability by ~40% compared to medium-only cell culture controls on tissue culture-treated polystyrene.
Fig. 7.
Toxicity of extracts from GS polyurethane, ClearFlex 50 polyurethane, and Sylgard 184 PDMS on viability of adherent endothelial cells. Extracts were obtained by incubating shredded polymers in water at 37°C for 9 days. Cytotoxicity was evaluated using two independent MTT assays with all samples in triplicate. Error bars are standard deviations calculated from the two averages.
Based on the information provided by manufacturers, component A of both polyurethanes is dicyclohexylmethane-4,4’-diisocyanate (HDI). However, ClearFlex 50 contains phenylmercuric neodecanoate catalyst while GS polyurethane contains dibutyltin dilaurate. Component B of both formulations contains proprietary polyol blends. Liquid chromatography-mass spectrometry showed presence of a molecule with 58 atomic mass units in the extracts from ClearFlex 50, indicative of polypropylene glycol. The same signature was detected in the polyol B component of ClearFlex 50 polyurethane (see Supplementary Figs. S9 and S10, ESI†). A previous study of elastomeric polyurethane membranes synthesized for immunoisolation applications from toluene diisocyanate (TDI), a polyol, butanediol-extended prepolymer, and dibutyl tin dilaurate catalyst showed that when polytetratmethylene glycol was used as a polyol, the cured polyurethane membrane did not have any detectable cytotoxic effects. However, when polypropylene glycol was used as a polyol in the synthesis (with the same catalyst), polyurethane elastomer membranes with differing hard segment content exhibited cytotoxic effects either under direct contact (ASTM F813-83) with L929 fibroblasts or when the cells were exposed to extracts from the cured membranes.20 Detrimental effects of 0.5 – 1.0% polypropylene glycol on natural killer cells and neutrophil function also have been reported.21 Therefore, it is likely that the cytotoxic effects of ClearFlex 50 are caused by polyol blend. Importantly, the GS polyurethane extract did not show detectable levels of polyol by LC-MS analysis, and this was consistent with our finding that HUVE cell viability was not affected by addition of the polymer extract.
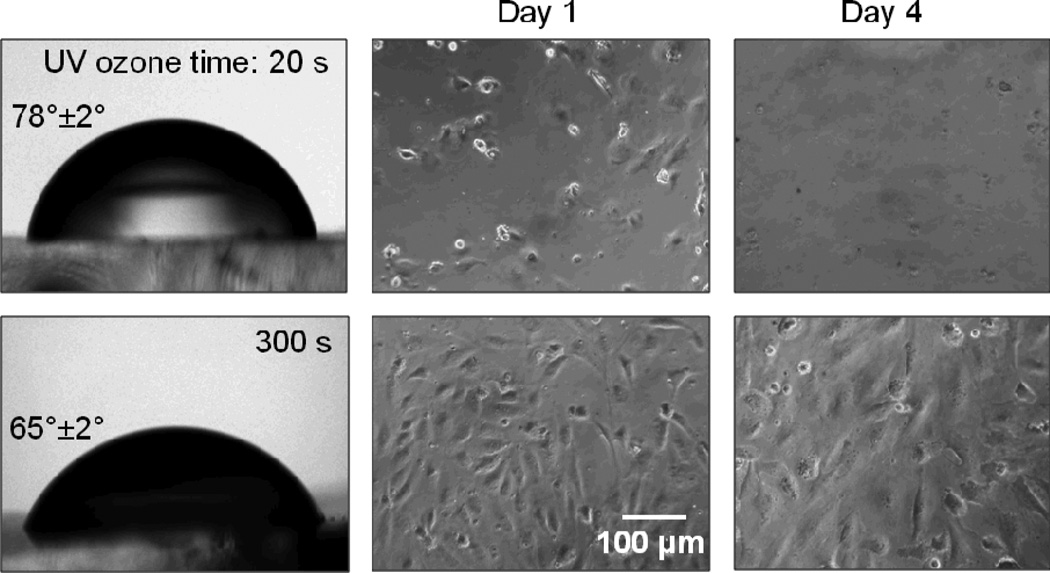
Based on our initial findings that GS polyurethane is non-toxic, we cultured HUVE cells on its surface after exposing the material to UV ozone for different times and coating it with fibronectin. These studies revealed that a longer time (≥ 5 min) of UV ozone surface treatment was necessary to achieve appropriate cell attachment, polygonal morphology, proliferation, and formation of endothelial monolayers on these polyurethane culture substrates (Fig. 8), much as they do on standard polystyrene culture dishes. Other methods of surface functionalization of polyurethane, such as by photoinduced grafting of bioactive molecules have also been demonstrated. For example, poly(methacrylic acid) has been grafted onto polyurethane surface by UV irradiation followed by immobilization of gelatin or RGD peptide for better cytocompatibility.22
Fig. 8.
Culture of HUVECs on fibronectin-coated polyurethane. Top and bottom row images show cells cultured on polyurethane treated with UV ozone for 20 and 300 seconds, respectively. Images on the left depict water contact angles for the two treatment times.
Permeation of atmospheric gasses such as oxygen, nitrogen, and carbon dioxide through polyurethane is approximately two orders of magnitude lower than through PDMS.23 Therefore, oxygenation of cells cultured in polyurethane microfluidic devices must be accomplished by other means. Possible alternatives include pre-oxygenation of cell culture medium entering the polyurethane device, as is generally done for thicker PDMS devices, or oxygenation through a thin gas permeable membrane in the device, if necessary.
Conclusions
These data show that when composition is judiciously selected, castable polyurethane elastomers can be suitable for fabricating microfluidic devices for cell culture and drug development applications. We demonstrated attractive optical and mechanical properties of a specific clear polyurethane elastomer from GS Polymers, and developed a procedure for forming a strong bond to itself, glass, and PDMS. By flowing a drug surrogate solution through the polyurethane and PDMS devices and measuring its fluorescence at the device outlet, we also demonstrated that the tested polyurethane is much more resistant to absorption of a small hydrophobic compound. While the polyurethane has a number of superior properties compared to Sylgard 184 PDMS, there are some downsides: it is more difficult to degas and mold, requires processing in a chemical hood, and its components must be stored in hermetically sealed containers with dry gas in the headspace. But we have developed methods and protocols to address all of these concerns. Thus, in applications in which PDMS cannot be utilized due to its absorptive properties, polyurethane can be a very attractive alternative.
Supplementary Material
Acknowledgements
This work was supported by a grant (U01 NS073474) from the NIH Common Fund, through the Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI), Office of the Director, NIH and by the Food and Drug Administration (FDA). Additional funds also were provided by the Defense Advanced Research Projects Agency (DARPA) under Cooperative Agreement Number W911NF-12-2-0036. The content of the information does not necessarily reflect the position or the policy of DARPA or the U.S. Government and no official endorsement should be inferred. We also thank Dan Shea for his help with preparing samples, Norman Wen for measuring optical transmittance, Ralft Jungmann for AFM imaging, and Christopher Johnson for his help with the LC-MS analysis.
Footnotes
Electronic supplementary information (ESI) available.
References
- 1.Whitesides GM. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 2.Huh D, Torisawa Y-s, Hamilton GA, Kim HJ, Ingber DE. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer AD, van den Berg A. Integr. Biol. 2012;4:461–470. doi: 10.1039/c2ib00176d. [DOI] [PubMed] [Google Scholar]
- 4.Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 5.Toepke MW, Beebe DJ. Lab Chip. 2006;6:1484–1486. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- 6.Devaraju NSGK, Unger MA. Lab on a Chip. 2011;11:1961–1967. doi: 10.1039/c0lc00274g. [DOI] [PubMed] [Google Scholar]
- 7.Moraes C, Kagoma YK, Beca BM, Tonelli-Zasarsky RLM, Sun Y, Simmons CA. Biomaterials. 2009;30:5214–5250. doi: 10.1016/j.biomaterials.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 8.Mehta G, Lee J, Cha W, Tung Y-C, Linderman JJ, Takayama S. Analytical Chemistry. 2009;81:3714–3722. doi: 10.1021/ac802178u. [DOI] [PubMed] [Google Scholar]
- 9.Domansky K, Leslie DC, Fraser JP, Hamilton GA, Ingbern DE. MicroTAS. Seattle, USA: The Society for Chemistry and Micro-Nano Systems (CHEMINAS); 2011. pp. 1831–1833. [Google Scholar]
- 10.Desai SP, Freeman DM, Voldman J. Lab on a Chip. 2009;9:1631–1637. doi: 10.1039/b822081f. [DOI] [PubMed] [Google Scholar]
- 11.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Huh D, Hamilton G, Ingber DE. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 13.Hillborg H, Ankner JF, Gedde UW, Smith GD, Yasuka HK, Wikstrom K. Polymer. 2000;41:6851–6863. [Google Scholar]
- 14.Hillborg H, Sandelin M, Gedde UW. Polymer. 2001;42:7349–7362. [Google Scholar]
- 15.Olah A, Hillborg H, Vansco Applied Surface Science. 2005;239:410–423. [Google Scholar]
- 16.Wang JD, Douville NJ, Takayama S, ElSayed M. Annals of Biomedical Engineering. 2012;40:1862–1873. doi: 10.1007/s10439-012-0562-z. [DOI] [PubMed] [Google Scholar]
- 17.Leeson PD, Springthorpe B. Nature Reviews/Drug Discovery. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 18.Kochhar JS, Zou S, Chan SY, Kang L. International Journal of Nanomedicine. 2012;7:3143–3154. doi: 10.2147/IJN.S32000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahnstein K, Schmehl T, Rusch U, Rieger M, Seeger W, Gessler T. International Journal of Pharmaceutics. 2008;351:158–164. doi: 10.1016/j.ijpharm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 20.George S, Nair PD, Risbud MV, Bhonde RR. Journal of Biomaterials Applications. 2002;16:327–340. doi: 10.1106/088532802024249. [DOI] [PubMed] [Google Scholar]
- 21.Denning DW, Webster AD. Journal of Pharmacy and Pharmacology. 1987;39:236–238. doi: 10.1111/j.2042-7158.1987.tb06258.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao C, Guan J, Zhu Y, Shen J. Macromol. Biosci. 2003;3:157–162. [Google Scholar]
- 23.Sturm P, Leuenberger M, Sirignano C, Neubert REM, Meijer HAJ, Langenfelds R, Brand WA, Tohjima Y. Journal of Geophysical Research. 2004;109:D04309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.